Abstract
We report a case of an African patient with sickle cell trait who was diagnosed in Spain with B-cell lymphoma. Blood smears were negative for malaria, and no plasmodium antigens were detected in the blood. To treat his lymphoma, the patient underwent chemotherapy and autologous stem cell transplantation. Following a splenectomy due to a worsening condition, he developed clinical malaria with detectable parasitemia. This case suggests that the humoral response and parasite removal by the spleen may afford protection from overt disease and may even help maintain subclinical human reservoirs of the disease.
CASE REPORT
A 48-year-old man from Equatorial Guinea presented with asthenia, weight loss, generalized lymphadenopathy, a bulky abdominal mass, hepatosplenomegaly, and bone marrow involvement upon his arrival in Spain in January 2006. The patient's medical history included sickle cell trait, filariasis (treated), gastrointestinal parasites, and repeated malaria infections (the last infection occurred in 2005). Microbiological screening in February 2006 ruled out HIV, hepatitis A virus (HAV), hepatitis C virus (HCV), Epstein-Barr virus (EBV), cytomegalovirus (CMV), varicella-zoster virus (VZV), Leishmania, dengue virus, Treponema pallidum, Mycobacterium tuberculosis, and parasites in urine and feces, but anti-hepatitis B virus (anti-HBV) antibodies (only against core antigen) were detected. Blood films and repeated thin and thick smears showed no parasites, and a blood sample was negative for Plasmodium antigens (BinaxNow). Lymph node and bone marrow biopsy specimens revealed a diffuse large-B-cell lymphoma rich in T cells. A standard chemotherapy regimen was initiated. The response to therapy was partial, and splenomegaly persisted. In October 2006, the patient underwent autologous stem cell transplantation (ASCT), which dramatically diminished lymphadenopathy and splenomegaly.
In May 2007, after a visit to Equatorial Guinea, the patient reported transitory fever. Thin and thick blood smears and tests for Plasmodium antigens in the blood (BinaxNow), each repeated twice, were negative for malaria. Each time, two thick and thin films were examined and 500 fields viewed under high-power magnification. Microbiological screening ruled out Gram-positive and Gram-negative bacterial infections, HIV, HAV, HCV, EBV, CMV, or VZV infection, leishmaniasis, dengue fever, syphilis, Mycobacterium tuberculosis, Toxoplasma gondii, and parasites in urine and feces. Blood transfusion-related malaria was also ruled out based on the following: (i) malaria was officially eradicated in Spain in 1964, (ii) people who come from or have visited areas of endemicity are rejected as blood donors, and (iii) according to the patient's medical records, he had not recently had a blood transfusion. During his visit to Africa, the patient did not receive prophylactic treatment for malaria. Compared to the scintigraphy imaging performed after ASCT, the spleen was now enlarged and lymph nodes were further enlarged. In a bone marrow aspirate, a low proportion of tumoral lymphocytes was detected (1% of all cells), while a bone marrow biopsy specimen returned only unspecific reactive findings. Further clinical findings of interest at this stage were low blood counts, mildly elevated bilirubin (1.6 mg/dl), a high HBV load (over 108 IU/ml), and normal liver function, and the patient was started on lamivudine treatment for HBV infection. Based on the suspicion of lymphoma relapse in the spleen, the patient was splenectomized in July 2007, 8 months after concluding the last immunosuppressive treatment. However, histological examination of the excised spleen indicated no trace of lymphoma, only signs of reactive lymphoid hyperplasia. The patient suffered several febrile episodes but showed no evidence of lymphoma relapse or an infection other than hepatitis B.
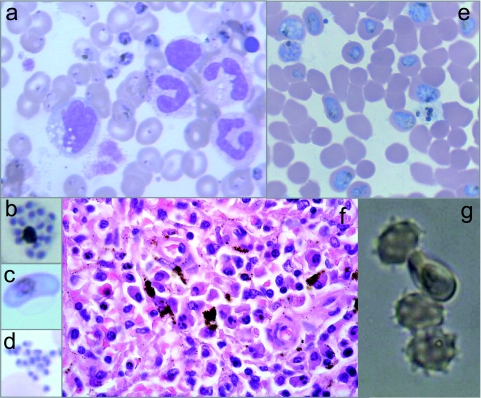
Eight weeks after the splenectomy, the patient suffered an acute crisis, including high fever (39.5°C) and chills, prostration, intense arthralgia, severe hemolytic anemia (hemoglobin, 6.6 g/dl; bilirubin, 12.38 mg/dl; lactate dehydrogenase, 434 IU/liter), and severe acidosis (pH 7.25). Blood smears revealed a massive Plasmodium falciparum infestation (49%) (Fig. 1a), including young and mature trophozoites, schizonts (Fig. 1b), and gametocytes (Fig. 1c), as well as ruptured schizonts (Fig. 1d). Plasmodium antigens were detected in the blood by an immunochromatographic assay, and identification of P. falciparum was confirmed by PCR (19).
Fig. 1.
Malaria diagnosis and characterization of the clinical isolate. (a to d) Giemsa-stained blood smears from the patient showing mostly ring and trophozoite forms of Plasmodium falciparum (a), but schizonts (b), gametocytes (c), and merozoites (d) were also observed. (e) Smear of the culture from the patient isolate showed optimal growth at high parasitemias. (f) Spleen slides (hematoxylin and eosin) showing local deposition of the malaria pigment hemozoin in macrophages. (g) Rosette formation by the P. falciparum isolate.
Intravenous quinine treatment was immediately given for 24 h only, since no neurological complications were observed. This treatment was followed by oral quinine (500 mg/8 h) and doxycycline (200 mg, twice daily) over a period of 9 days. After antimalarial treatment and before discharge, no parasites were observed in thick blood films or were immunologically detected in blood. However, the patient died some years later from a further episode of malaria contracted in a subsequent visit to his country.
In a retrospective examination, although P. falciparum could not be visualized in the spleen sections, hemozoin deposition was sporadically detected (Fig. 1f). In addition, stored blood smears corresponding to June 2007 revealed the presence of P. falciparum rings in extremely low numbers in thin films (<1/500 fields, parasitemia < 0.0016%).
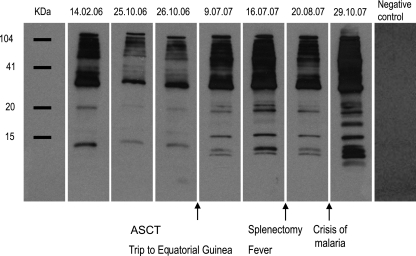
When a blood sample from the patient was cultured to a high parasitemia level (16), the observed features of the isolate were a short life cycle (42 to 46 h), rosette formation comparable to that produced in strain Dd2 (Fig. 1e and g), and at least two different parasite populations (i.e., multiplicity of infection [MOI] = 2) (5). The MOI is the minimal number of different P. falciparum infections detected in a single blood sample. Based on the patient's age, an MOI of 2 is considered low and suggests that some immunity has been mounted against the parasite (8). Through molecular analysis of the parasite's antimalarial markers, mutations potentially conferring resistance to pyrimethamine (P. falciparum dhfr [Pfdhfr], codons 108, 51, and 59), sulfadoxine (Pfdhps, codons 436 and 437), and chloroquine (Asn→Tyr mutation at codon 86 of the gene Pfmdr) were detected. No mutations were found in the genes Pfcytb and Pfcrt, which may lead to resistance against atovaquone and chloroquine, respectively (4, 9). These genotyped resistance genes (except for the case of sulfadoxine) were phenotypically confirmed in cultures of the patient isolate (13). Fifty percent inhibitory concentration (IC50) values obtained for pyrimethamine (95.71 ± 18 nM) and chloroquine (724 ± 27 nM) were above normal, but those for atovaquone (1.96 ± 1.41 nM) and artemisinin (1.00 ± 0.10 nM) were normal. During the 20-month period of disease progression (Fig. 2), the immunoreactivity of the patient's serum against his own schizonts revealed that specific antibodies had significantly decreased after his initial arrival in Spain, probably due either to gradually impaired parasite growth since some drugs used for the lymphoma treatment (e.g., vincristine) are known to inhibit P. falciparum growth (21) or to the waning of naturally acquired immunity as a consequence of staying outside the area of endemicity (11). Thus, the rise in specific antibodies detected after the patient's trip to Africa seems to indicate malaria reinfection. After splenectomy, a drop in specific antibodies was noted but the antibody levels then rose to maximum levels again during the acute malaria crisis (Fig. 2).
Fig. 2.
Retrospective study of immunoreactivity of the hidden malaria. Patient's immunoreactivity against total parasite protein extracts from the clinical isolate cultured at the schizont stage (Western blot) determined in serum samples obtained during the 20-month period of the patient's illness. In brief, the parasite isolate from the patient was grown until a high level of parasitemia was achieved as previously described (16). Next, aliquots of an extract of total parasite protein were separated by SDS-PAGE, transferred onto a nitrocellulose membrane, and separately incubated with stored patient sera obtained on the dates shown at the top of the figure (day/month/year). A horseradish peroxidase-conjugated anti-human IgG secondary antibody was used to detect patient IgGs reacting against specific P. falciparum proteins. SuperSignal (Pierce) was used as the chemiluminescent substrate for visualization.
P. falciparum is responsible for most of the deaths due to malaria and causes the most life-threatening complications of this infection. People living in areas where P. falciparum is endemic develop protective clinical immunity against malaria during childhood, and there is a gradual decline in clinical episodes in adults (11). However, a large percentage of persons with submicroscopic parasitemia are asymptomatic (19) due to reinfections that challenge the immune system, providing protection against clinical malaria (11). As in the present case, immunosuppression in patients with submicroscopic parasitemia leads to recrudescence of P. falciparum malaria (22). Although the patient was subjected to periodic examinations, there was no evidence of malaria infection until clinical symptoms suddenly appeared.
The clinical features of malaria can be misinterpreted in patients with a hematological malignancy, since questions about malaria in these patients are not routinely asked (18). Moreover, if malaria is suspected, it could be ruled out if thick and thin blood films prove negative. In patients from areas of endemicity, a differential diagnosis of malaria from lymphoma becomes a challenge when clinical presentation or potential relapse is accompanied by fever and splenomegaly (2). A few cases of malaria have been reported in patients with diffuse large-B-cell lymphoma (1, 15, 18), which is often associated with a splenectomy and with febrile neutropenia observed at the time of chemotherapy.
The normal incubation time of P. falciparum malaria is 7 to 15 days, but recrudescence is possible weeks to years after an untreated or inadequately treated infection (2, 7). According to the pattern of specific serum IgG levels in our patient, the most likely hypothesis is that he was reinfected during his brief return to Africa but that this clinical infection was contained due to his acquired immunity, as suggested by the high levels of specific antibodies that persisted until the acute malaria crisis. Besides this acquired immunity, his sickle cell trait would have also protected him against malaria (6, 12). We propose that the immunosuppressive effects of anti-CD20 treatment with rituximab, which depletes the organism of B cells and gamma globulins, along with the patient's diminished immunoresponse during ASCT therapy may have triggered malaria recrudescence, which was assisted by the splenectomy, which brought to an end the elimination of circulating parasites (10, 15).
An epidemiological correlation between malaria and lymphoma (e.g., with endemic Burkitt's lymphoma) suggests a link between high titers of malaria antibodies in children and a risk of Burkitt's lymphoma or that the joint actions of EBV and malaria infection promote lymphoma (14). Chronic or recurrent malaria infection, which continuously stimulates B lymphocytes, has been postulated to increase polyclonal B-cell proliferation, predisposing the person to malignant B-cell lymphoma associated with splenic lymphoma and perhaps to other subtypes of lymphoma (2). There is also evidence of an essential role of the B-cell response in malaria (11), and reactivation of HBV after chemotherapy, particularly with rituximab, is a known response (20). Interestingly, chronic liver disease due to hepatitis B may also be an additional risk for severe malaria (17). However, in our case, splenectomy may have played the greatest role in triggering the malaria crisis, given the immediacy of both events and the known increased susceptibility of splenectomized patients to severe malaria as a consequence of a compromised immune response and an inability to clear the parasite (3, 15). Therefore, it seems that before the infection outburst, two factors were able to synergistically control clinical malaria: the patient's active humoral response and the removal of circulating parasites by the spleen. This proposal is supported by our observation of parasites at every intraerythrocytic developmental stage in the peripheral blood smear from the patient, once splenectomized, which is a common observation in cases of severe malaria. The spleen is necessary for parasite antigen presentation on the infected erythrocyte surface and rosette formation. Thus, the ability of the patient isolate to form rosettes suggests that once this phenotype developed, it was not immediately lost after removal of the spleen (10).
In patients with a hematological malignancy who have lived in areas of endemicity, the risk of malaria upon immunosuppression and splenectomy should be considered, even if they have been away from such areas for a long time prior to becoming ill. Whenever possible, splenectomy should be avoided so that they can confront life-threatening complications in subsequent visits to tropical regions. Alternatively, strict antimalarial prophylaxis is recommended for splenectomized patients. The present case illustrates the efficacy of quinine and doxycycline (15) to treat resistant severe malaria and reveals that subclinical malaria can be easily missed in routine screenings. We recommend a full course of antimalarials before starting any chemotherapy treatment in patients from countries where malaria is endemic. Finally, our clinical case also highlights that humans with subclinical malaria may be a main reservoir of P. falciparum. Thus, travelers with asymptomatic infection may inadvertently transport the parasite and run the risk of provoking a malaria outbreak in a malaria-free area where the mosquito vector remains.
Acknowledgments
We are indebted to Ana Burton for reviewing, proofreading, and commenting on the paper.
This work was supported by grants from the Spanish Ministry of Science and Innovation (MICINN BIO2010-17039 ) and from the Santander-Universidad Complutense de Madrid Programme of Consolidated Research Groups (920267). M.L. holds a Ph.D. fellowship from Formación de Personal Investigador-MEC (AP20061576), and D.M. was supported by the Alban Programme (E06D101036CO), Universidad de Cartagena (CO) and Colfuturo.
Footnotes
Published ahead of print on 5 October 2011.
REFERENCES
- 1. Bates I., Bedu-Addo G. 1997. Chronic malaria and splenic lymphoma: clues to understanding lymphoma evolution. Leukemia 11: 2162–2167 [DOI] [PubMed] [Google Scholar]
- 2. Bidegain F., et al. 2005. Acute Plasmodium falciparum malaria following splenectomy for suspected lymphoma in 2 patients. Clin. Infect. Dis. 40: e97–e100 [DOI] [PubMed] [Google Scholar]
- 3. Buffet P. A., et al. 2011. The pathogenesis of Plasmodium falciparum malaria in humans: insights from splenic physiology. Blood 117: 381–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Duraisingh M. T., Curtis J., Warhurst D. C. 1998. Plasmodium falciparum: detection of polymorphisms in the dihydrofolate reductase and dihydropteroate synthetase genes by PCR and restriction digestion. Exp. Parasitol. 89: 1–8 [DOI] [PubMed] [Google Scholar]
- 5. Färnert A., et al. 2001. Genotyping of Plasmodium falciparum infections by PCR: a comparative multicentre study. Trans. R. Soc. Trop. Med. Hyg. 95: 225–232 [DOI] [PubMed] [Google Scholar]
- 6. Ferreira A., et al. 2011. Sickle hemoglobin confers tolerance to Plasmodium infection. Cell 145: 398–409 [DOI] [PubMed] [Google Scholar]
- 7. Giobbia M., et al. 2005. Late recrudescence of Plasmodium falciparum malaria in a pregnant woman: a case report. Int. J. Infect. Dis. 9: 234–235 [DOI] [PubMed] [Google Scholar]
- 8. Guerra-Neira A., et al. 2006. Plasmodium diversity in non-malaria individuals from the Bioko Island in Equatorial Guinea (West Central-Africa). Int. J. Health Geogr. 5: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Happi T. C., et al. 2003. Point mutations in the pfcrt and pfmdr-1 genes of Plasmodium falciparum and clinical response to chloroquine, among malaria patients from Nigeria. Ann. Trop. Med. Parasitol. 97: 439–451 [DOI] [PubMed] [Google Scholar]
- 10. Ho M., Bannister L. H., Looareesuwan S., Suntharasamai P. 1992. Cytoadherence and ultrastructure of Plasmodium falciparum-infected erythrocytes from a splenectomized patient. Infect. Immun. 60: 2225–2228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Langhorne J., Ndungu F. M., Sponaas A. M., Marsh K. 2008. Immunity to malaria: more questions than answers. Nat. Immunol. 9: 725–732 [DOI] [PubMed] [Google Scholar]
- 12. Luzzatto L., Nwachuku-Jarrett E. S., Reddy S. 1970. Increased sickling of parasitised erythrocytes as mechanism of resistance against malaria in the sickle-cell trait. Lancet i: 319–321 [DOI] [PubMed] [Google Scholar]
- 13. Moneriz C., Marin-Garcia P., Bautista J. M., Diez A., Puyet A. 2009. Haemoglobin interference and increased sensitivity of fluorimetric assays for quantification of low-parasitaemia Plasmodium infected erythrocytes. Malar. J. 8: 279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mutalima N., et al. 2008. Associations between Burkitt lymphoma among children in Malawi and infection with HIV, EBV and malaria: results from a case-control study. PLoS One 3: e2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Petithory J. C., Khelil A., Galeazzi G., Ardoin F. 2005. Malaria in splenectomized patients. Three fatal cases. Presse Med. 34: 519–521 [DOI] [PubMed] [Google Scholar]
- 16. Radfar A., et al. 2009. Synchronous culture of Plasmodium falciparum at high parasitemia levels. Nat. Protoc. 4: 1899–1915 [DOI] [PubMed] [Google Scholar]
- 17. Rai R. R., Jain P. 2009. Plasmodium falciparum and hepatitis B virus co-infection—a rare cause of acute hepatitis. J. Gastrointestin. Liver Dis. 18: 252–253 [PubMed] [Google Scholar]
- 18. Rapoport B. L., Uys A. 2008. Malaria parasitemia associated with febrile neutropenia in African patients undergoing chemotherapy for haematological malignancies. A report of three patients. Chemotherapy 54: 117–119 [DOI] [PubMed] [Google Scholar]
- 19. Rubio J. M., et al. 2002. Alternative polymerase chain reaction method to identify Plasmodium species in human blood samples: the semi-nested multiplex malaria PCR (SnM-PCR). Trans. R. Soc. Trop. Med. Hyg. 96 (Suppl. 1): S199–S204 [DOI] [PubMed] [Google Scholar]
- 20. Tsutsumi Y., et al. 2004. Hepatitis B virus reactivation in a case of non-Hodgkin's lymphoma treated with chemotherapy and rituximab: necessity of prophylaxis for hepatitis B virus reactivation in rituximab therapy. Leuk. Lymphoma 45: 627–629 [DOI] [PubMed] [Google Scholar]
- 21. Usanga E. A., O'Brien E., Luzzato L. 1986. Mitotic inhibitors arrest the growth of Plasmodium falciparum. FEBS Lett. 209: 23–27 [DOI] [PubMed] [Google Scholar]
- 22. Whitworth J., et al. 2000. Effect of HIV-1 and increasing immunosuppression on malaria parasitaemia and clinical episodes in adults in rural Uganda: a cohort study. Lancet 356: 1051–1056 [DOI] [PubMed] [Google Scholar]