Abstract
3′-5′-Cyclic AMP (cAMP) is an important second messenger which regulates neurite outgrowth. We demonstrate here that type VI adenylyl cyclase (AC6), an enzyme which catalyzes cAMP synthesis, regulates neurite outgrowth by direct interaction with a binding protein (Snapin) of Snap25 at the N terminus of AC6 (AC6-N). We first showed that AC6 expression increased during postnatal brain development. In primary hippocampal neurons and Neuro2A cells, elevated AC6 expression suppressed neurite outgrowth, whereas the downregulation or genetic removal of AC6 promoted neurite extension. An AC6 variant (AC6-N5) that contains the N terminus of AC5 had no effect, indicating the importance of AC6-N. The downregulation of endogenous Snapin or the overexpression of a Snapin mutant (SnapΔ33-51) that does not bind to AC6, or another Snapin mutant (SnapinS50A) that does not interact with Snap25, reversed the inhibitory effect of AC6. Pulldown assays and immunoprecipitation-AC assays revealed that the complex formation of AC6, Snapin, and Snap25 is dependent on AC6-N and the phosphorylation of Snapin. The overexpression of Snap25 completely reversed the action of AC6. Collectively, in addition to cAMP production, AC6 plays a complex role in modulating neurite outgrowth by redistributing localization of the SNARE apparatus via its interaction with Snapin.
INTRODUCTION
Membrane-bound adenylyl cyclases (ACs) belong to a superfamily of enzymes that convert ATP to cyclic AMP (cAMP) when Gsα-coupled receptors are stimulated (8, 60, 71). Among the nine ACs, AC6 is of particular interest because it exists in neurons and can be cross-regulated by multiple signaling pathways, including those involving calcium, protein kinase A (PKA), protein kinase C (PKC), tyrosine-mediated phosphorylation, and nitric oxide (7, 33, 44, 58, 74). In addition to its role in synthesizing cAMP, we previously demonstrated that Snapin binds to the N terminus of AC6 (AC6-N) and specifically abolishes the PKC-mediated suppression of AC6 (12). Snapin was first identified as a binding partner of the 25-kDa synaptosome-associated protein (Snap25) (28). It interacts with the assembled soluble N-ethylmaleimide-sensitive fusion protein attachment protein receptor (SNARE) core complex via binding to Snap25 through its C-terminal coiled-coil domain, and it assists in the synchronization of synaptic vesicle fusion (28, 46). Because the PKA-mediated phosphorylation of Snapin at Ser50 enhances its interaction with Snap25, Snapin is a PKA target that regulates neurotransmitter release (10, 28, 61, 63). The interaction between AC6 and Snapin might provide a fine-tuning mechanism for the cAMP production system via the vesicle-transporting machinery in the brain (68).
Evidence derived from organisms ranging from yeasts to humans indicates that the SNARE family acts as the conserved core protein machinery which is involved in all elements of vesicular trafficking, including neurite outgrowth (37, 43). The inhibition of Snap25 prevents neurite elongation and axonal growth (39, 42, 43), whereas the overexpression of Snap25 increases the number of neurites (55). In this study, we investigated the role of AC6 in regulating Snapin-mediated functions. Because AC6 is predominantly expressed in neuronal cells (35) and the Snapin-interacting protein, Snap25, plays a role in neurite outgrowth, we hypothesized that AC6 regulates neurite outgrowth through its interaction with Snapin. The growth and directionality of dendrites and axons involve the interplay of multiple cellular signaling pathways and dynamic changes in membrane trafficking (14). Membrane trafficking is essential for neurite outgrowth, because vesicle fusion and membrane expansion at the growing tips markedly contribute to neurite elongation (3, 59). Our results demonstrate that AC6 suppresses neurite extension by interfering with the localization of the SNARE machinery and therefore might play an essential role in neuronal development.
MATERIALS AND METHODS
Materials.
Forskolin and 3-isobutyl-1-methylxanthine (IBMX) were obtained from Sigma-Aldrich (St. Louis, MO). All cell culture reagents were purchased from Invitrogen (GibcoBRL, Carlsbad, CA). All other chemicals were obtained from Merck (Darmstadt, Germany) unless stated otherwise. In-house anti-AC6 and anti-Snapin rabbit antibodies were characterized and are described elsewhere (12, 33, 67). The anti-Snap25 goat antibody, anti-Syntaxin 1 mouse antibody, and anti-c-Myc mouse antibody were obtained from Santa Cruz Biotech (Santa Cruz, CA), and the antiactin mouse monoclonal antibody was from Chemicon International (Temecula, CA). The anti-Flag antibody was obtained from Sigma-Aldrich (St. Louis, MO), and the anti-Snap25 rabbit polyclonal antibody was obtained from Abcam (Cambridge Science Park, Cambridge, United Kingdom).
Plasmids.
The respective species for complementary DNAs (cDNAs) of AC6, Snapin, and Snap25 were rat, mouse, and mouse. Note that N termini of rat and mouse AC6 are highly homologous (96% identity in amino acids). The amino acid sequences of mouse and rat Snapin and mouse and rat Snap25 are identical. Plasmids containing cDNAs of AC6 variants (wild type [WT] and AC6-N5), of AC5-c-Myc, and of Snapin variants (WT, SnapinΔ33-51, and SnapinS50A) are described elsewhere (12, 30, 67). The expression construct of yellow fluorescent protein (YFP)-AC6 was generated by the digestion of pcDNA3-AC6 (30) to completion with HindIII and EcoRI. The excised DNA fragment encoding the full-length AC6 was subcloned into the pEYFP-C1 vector (Clontech, Mountain View, CA). The expression construct of the AC5 mutant (AC5-N6) was generated by the PCR amplification of the N terminus (amino acids 1 to 146) of rat AC6 using the primers 5′-GGAGACCCAAGCTTGGTACCG-3′ and 5′-CCGGTACCGTTCATCTGGAAGAAGTACCGCTG-3 and an expression construct of AC6 (pcDNA3-AC6 [30]) as the template. The amplicon then was digested using KpnI and subcloned into an expression construct of an N-terminal truncation mutant of AC5 (AC5Δ215). AC5Δ215 was created by amplifying the DNA fragment encoding amino acids 216 to 1262 of rat AC5 (67) using 5′-ATGATATTCCGCTCTAAGAAGTTCCG-3′ and 5′-ACTGAGGGGAGGCCCTCCAT-3′ as the primer set, which was subcloned into the pcDNA3.1 vector (Invitrogen). SnapinS50D was created by a two-step PCR technique (26) using standard molecular biological techniques and the following primers: 5′-GAGACCCAAGCTGGCTAGTTAAGC-3′, 5′-CTACTTGGTCTTCTCTGACCGCGTG-3′, 5′-CACGCGGTCAGAGAAGACCAAGTAG-3′, and 5′-GAGGCTGATCAGCGGGTTTAAAC-3′. Short hairpin RNA (shRNA) of mouse Snapin was subcloned into a pSUPER.neo+GFP vector (OligoEngine, Seattle, WA) according to the manufacturer's recommendations. Briefly, the oligonucleotide pair was annealed into a duplex of 5′-GATCCCCTGACAACCTAGCTACAGAATTCAAGAGATTCTGTAGCTAGGTTGTCATTTTTGGAAA-3′ and 5′-AGCTTAAAAATGACAACCTAGCTACAGAATCTCTTGAATTCTGTAGCTAGGTTGTCAGGG-3′ and ligated into the BglII and HindIII sites of the vector. The underlined sequences are the complementary target sequences of mouse Snapin. The shRNA (siAC6-1) against the homologous region of both rat and mouse AC6 was subcloned into a lentiviral vector (pLL3.7-UbiG-Ins) containing green fluorescent protein (GFP) using the following oligonucleotide pair: 5′-TGAAGAAGTATTCACGGAAATTCAAGAGATTTCCGTGAATACTTCTTCTTTTTTC-3′ and 5′-ACTTCTTCATAAGTGCCTTTAAGTTCTCTAAAGGCACTTATGAAGAAGAAAAAAGAGCT-3′. The underlined sequences are the complementary target sequences of both mouse and rat AC6. The pLKO.1 shRNA plasmids (siAC6-2, siSnap25-3, and siSnap25-7) were purchased from the National RNAi Core Facility, Taipei, Taiwan (see Table S1 in the supplemental material for comparisons of target sequence in the shRNA constructs used in the present study and target sequence in the corresponding genes). AC6D426A (20) was created by a two-step PCR technique as described previously (26) using the primers 5′-GGAGACCCAAGCTTGGTACCG-3′, 5′-CGACACACAGTAGTAACATGCTCCTAAGAT-3′, 5′-ATCTTAGGAGCATGTTACTACTGTGTGTCG-3′, and 5′-GGGCCCTCTAGATGCATGCTC-3′ and standard molecular biological techniques. cDNA encoding the full-length mouse Snap25 was generated by PCR amplification using the primers 5′-ATGCTAGCGCCACCATGGCCGAGGACGCA-3′ and 5′-CCACCACTTCCCAGCATC-3 from mouse brain cDNA and was cloned into the pcDNA3.1 vector (Invitrogen) by TA cloning according to the manufacturer's instructions. The pFLAG-Fc2 plasmid was a gift from Ruey-Bing Yang (Academia Sinica, Taipei, Taiwan). The expression construct of Flag-AC6-Fc was generated by the PCR amplification of AC6 using the primers 5′-CGAATTCAATGTCATGGTTTAGCGGCCT-3′ and 5′-TCTAGAACTGCTGGGGCCCCCATTG-3 from pcDNA3-AC6. The AC6 amplicon then was restriction digested with EcoRI and XbaI and cloned into pFlag-Fc2. The expression construct of Flag-AC6N5-Fc was generated by the PCR amplification of AC6N5 using 5′-CGAATTCAATGTCCGGCTCCAAAAGCG-3′ and 5′-CGAATTCGCACTGCTGGGGCCCC-3′ as the primers and pcDNA3-AC6N5 as the template. The AC6N5 amplicon then was digested with EcoRI and subcloned into pFlag-Fc2. Nucleotide sequences of all constructs used were verified by DNA sequencing.
Cell culture and transfection.
Primary hippocampal cultures were prepared from Sprague-Dawley rat brains on embryonic day 19 or from AC6 knockout (AC6-KO) mice (11) and littermate controls on embryonic day 18 as described elsewhere (4, 12). Briefly, dissected hippocampi were incubated with 2.5 mg/ml trypsin in Hanks' balanced salt solution (HBSS) at 37°C for 10 min and then neutralized with fetal bovine serum (FBS). After gently removing the enzyme-containing buffer, dissociated neurons were suspended in plating medium (modified Eagle's medium [MEM] plus an insulin-tranferrin-selenium supplement, 5% FBS, 5% horse serum, 0.6% glucose, and 0.5 mM l-glutamine) and plated on poly-d-lysine-coated coverslips (22 by 22 mm) at a density of 200 cells/mm2. One hour postplating, the plating medium was replaced with growth medium containing Neurobasal medium supplemented with B27, penicillin (100 U/ml), streptomycin (100 μg/ml), glutamate (12.5 μM), and l-glutamine (0.5 mM). At 4 days in vitro (DIV), cells were transfected using Optifect (Invitrogen, San Diego, CA) by following the manufacturer's instructions. Mouse neuroblastoma Neuro2A cells were cultured in MEM plus 10% FBS, penicillin (100 U/ml), streptomycin (100 μg/ml), l-glutamine (2 mM), nonessential amino acids (0.1 mM), and sodium pyruvate (1 mM) at 37°C with 5% CO2. Transfection was carried out using Lipofectamine 2000 (Invitrogen) based on the manufacturer's recommendations.
Neurite outgrowth assay.
Cells were transfected with the indicated construct(s) along with 1/6 of the molar amount of an enhanced green fluorescent protein (EGFP)-expressing construct. Primary hippocampal neurons were plated on poly-d-lysine-coated coverslips (22 by 22 mm) at a density of 200 cells/mm2. Neurons were transfected at 4 DIV, allowed to express the transfected protein for 72 h, fixed in 4% paraformaldehyde plus 4% sucrose in phosphate-buffered saline (PBS) at room temperature (RT) for 20 min, washed, mounted with Vectashield (Vector Laboratories, Burlingame, CA), and then used for the morphological analyses by using a scanning confocal microscope (LSM 510 meta; Carl Zeiss, Jena, Germany). Neuro2A cells were seeded at a density of 2 × 105 cells per 35-mm plate. At 16 h following plating, cells were transfected and incubated at 37°C for 4 to 6 h and then passed into fresh 6-well plates for 16 h at a 1/5 dilution. To induce differentiation, cells were exposed to a differentiation mix (MEM supplemented with 1% FBS and 10 μM retinoic acid [RA]) for 48 h and fixed as described above. Transfected cells were marked as EGFP-expressing cells under a fluorescence microscope (Axiovert 200 M; Carl Zeiss, Jena, Germany) with filter set 10 (BP 450-490). Neurite outgrowths were assessed for neurite length (primary hippocampal neurons) and neurite formation (Neuro2A cells) by an investigator blinded to the experimental conditions. The length of the longest neurite was quantified using ImageJ (National Institutes of Health, Bethesda, MD). To quantify neurite formation in Neuro2A cells, cells containing neurites of at least two cell body diameters in length were scored as neurite-bearing cells. Transfected cells that grew neurites were normalized to the number of total transfected cells and are presented as the percentage of neurite-bearing cells. Cell clumps containing more than five cells were prone to miscalculation and therefore were not included in the results. Usually, 30 to 50 transfected primary hippocampal neurons or 70 to 250 transfected Neuro2A cells were scored to obtain one data point in each experiment.
SDS-PAGE and Western blot analysis.
Protein concentrations were determined using the Bio-Rad protein assay dye reagent concentrate (Bio-Rad, Hercules, CA). Equal amounts of samples were separated by SDS-PAGE. Following electrophoresis, the gel was transferred to a polyvinylidene difluoride (PVDF) membrane (Millipore, Bedford, MA), blocked with 5% skim milk in PBST (0.1 M PBS and 0.1% Tween 20), and then incubated with the desired antibody at 4°C overnight (1:5,000 for the antiAC6 antibody, 1:5,000 for the anti-Snapin antibody, 1:1,000 for the anti-Snap25 antibody, 1:5,000 for the antiactin antibody, 1:1,000 for anti-c-Myc antibody, and 1:5,000 for anti-Flag antibody). After three 10-min washes in PBST, membranes were incubated with peroxidase-conjugated donkey anti-rabbit IgG, goat anti-mouse IgG (GE Healthcare Bio-Sciences AB, Uppsala, Sweden), or donkey anti-goat IgG (Santa Cruz Biotech) at a 1:5,000 dilution for 1 h at RT and washed six times with PBST. Immunoreactive bands were visualized using an enhanced chemiluminescence (ECL) method (PerkinElmer Life and Analytical Sciences, Boston, MA).
Preparation of the plasma membrane fraction.
Brains were collected from the indicated mice and homogenized in ice-cold homogenization buffer (20 mM HEPES [pH 7.4], 1 mM EDTA, 2 mM MgCl2, 150 mM NaCl, and protease inhibitors) using a MagNA lyser (Roche, Basel, Switzerland) at a setting of 5,000 amplitude vibrations for 30 s. The homogenate was centrifuged at 1,300 × g for 10 min to remove unbroken tissue clumps and nuclei. The supernatant was centrifuged at 50,000 ×g for 45 min to obtain the plasma membrane fraction. The protein concentration was measured using the Bio-Rad protein assay reagent.
cAMP assay.
The intracellular cAMP content was assayed as described before (9), with slight modifications. Neuro2A cells were seeded at 106 cells/100-mm plate and transfected with the indicated DNA(s) using Lipofectamine 2000 (Invitrogen). Seventy-two hours posttransfection, cells were lifted from the plates in aliquots of approximately 5 × 105 cells/tube in triplicate. Cells were washed twice with Ca2+-free Locke's solution (150 mM NaCl, 5.6 mM KCl, 5 mM glucose, 1 mM MgCl2, and 10 mM HEPES adjusted to pH 7.4) and resuspended in the same solution at 5 × 105 cells/300 μl. Cells then were treated with 0.5 mM IBMX with or without 10 μM forskolin for 20 min at RT. At the end of incubation, cells were rapidly washed once using ice-cold Locke's buffer. Cellular cAMP was extracted by adding 300 μl of 0.1 N HCl per 5 × 105 cells to each tube with gentle mixing for 10 min on ice. The cAMP content was assayed using a 125I-cAMP assay kit (PerkinElmer Life Sciences, Shelton, CT) according to the manufacturer's instructions.
Immunocytochemistry.
Primary neurons purified from the hippocampi of AC6-KO mice (11) or WT mice were fixed at 7 DIV in 4% (wt/vol) paraformaldehyde-4% (wt/vol) sucrose in PBS for 15 min, blocked with 3% bovine serum albumin for 1 h, stained with a monoclonal anti-Tuj1 antibody (1:1,000; Millipore, Bedford, MA) at 4°C overnight, and then incubated with a goat anti-mouse IgG antibody conjugated to Alexa Fluor 568 for 2 h. To analyze the cellular localization of Snap25, primary hippocampal neurons (DIV 4) were transfected with an expression construct encoding YFP, YFP-AC6, or AC5-c-Myc. YFP- or YFP-AC6-transfected neurons were fixed at DIV 7 and stained with anti-Syntaxin 1 (1:100; Santa Cruz) and anti-Snap25 antibodies (1:150; Abcam, Cambridge, United Kingdom) at 4°C overnight. AC5-Myc-transfected neurons were stained with anti-c-Myc-biotin (1:500; Sigma-Aldrich, St. Louis, MO), anti-Syntaxin 1, and anti-Snap25 antibodies at 4°C overnight. Snap25 was visualized with an Alexa Fluor 568 secondary antibody (red). Syntaxin 1 was visualized with an Alexa Fluor 647 secondary antibody (blue). AC5-c-Myc was visualized with an Alexa Fluor 488 secondary antibody (green). The pattern of immunostaining was analyzed with a laser-scanning confocal microscope (LSM 510 meta). Samples from which the primary antibody was omitted were used to control for the specificity of the immunofluorescence.
Pulldown assay.
Neuro2A cells were transfected with the indicated plasmid(s) for 48 h. Cells were lysed with ice-cold lysis buffer (20 mM Tris-HCl at pH 8.0, 1% [vol/vol] Nonidet P-40, 150 mM NaCl, 1 mM EDTA, 1× PhosSTOP phosphatase inhibitor cocktail, and 1× complete EDTA-free protease inhibitor cocktail [Roche]). Cell lysates (1 mg) were incubated with 10 μl of Dynabead protein G (Invitrogen) for 1 h at 4°C on a rolling wheel. After extensive washing, the resultant complexes were analyzed by SDS-PAGE and immunoblotting assays as described above.
Immunoprecipitation-AC assay.
The AC activity of the immunoprecipitated complex was assayed as described previously (9, 49). Briefly, brains were collected from the indicated mice and homogenized in lysis buffer (20 mM HEPES at pH 7.4, 1 mM EDTA, 2 mM MgCl2, 150 mM NaCl, 0.5% C12E9, and protease inhibitors) using a MagNA lyser (Roche) at a setting of 5,000 amplitude vibrations for 30 s. Samples of brain lysate (12 mg) were incubated with 4 μg of an anti-Snap25 antibody or 12 μg of a goat IgG antibody with or without competing peptides (0.5 μg) for 1 h at 4°C on a rolling wheel. Protein A agarose was added for 1 h. After extensive washing, the resultant immunoprecipitated complexes were suspended in 1 ml reaction buffer (50 mM HEPES at pH 7.4, 1 mM EDTA, 1 mM MgCl2, 0.04% C12E9, and protease inhibitors). Immunoprecipitated complexes (45 μl) were mixed with 400 μl of AC assay buffer (1 mM ATP, 100 mM NaCl, 50 mM HEPES, 0.5 mM IBMX, 6 mM MgCl2, 1 μM GTP, and 0.2 mM EGTA), and AC activity was assayed at 37°C for 10 min. The reactions were stopped by the addition of 600 μl of 10% trichloroacetic acid. The cAMP formed was isolated by Dowex chromatography (Sigma-Aldrich) and determined by a radioimmunoassay. The peptide sequence for Snapin33-51 was LRPAVQQLDSHVHAVRESQ, and that for the scrambled peptide was HLVRHPAAVVRQEQSLQDS.
RESULTS
We previously reported that AC6 is expressed predominantly in neurons in the central nervous system (35), and that AC6 and Snapin colocalize in the hippocampus and primary hippocampal cultures (12). Interestingly, AC6 expression levels markedly increased during the postnatal development of rat brain (Fig. 1A), suggesting that AC6 plays an important role in neuronal development. To assess the importance of this elevation in AC6 expression, we overexpressed AC6 in primary hippocampal neurons (at 4 DIV), using EGFP as the reporter, to identify neurons carrying transfected DNA. Intriguingly, neurons transfected with AC6, but not AC5, had shorter neurites (Fig. 1B). AC5 is the closest isozyme to AC6 in the AC superfamily. Unlike their N-terminal domains, the C1a and C2 domains of AC5 and AC6 are highly homologous (50, 74). A similar inhibitory effect of AC6 on the development of neuronal processes also was observed in a murine neuroblastoma cell line (Neuro2A cells) (Fig. 2A). These cells grow neurites after treatment with retinoic acid (RA) or after serum withdrawal (18) and can be readily transfected with high efficiency. Therefore, we used Neuro2A cells for subsequent experiments to test the suppressive effect of AC6 on the development of neuronal processes. This suppressive effect of AC6 was dependent on the amount of AC6 protein, because the higher the level of AC6 protein, the lower the percentage of neurite-bearing cells (Fig. 2B). No effect of AC5 was observed (Fig. 2A).
Fig. 1.

Elevated expression of type VI adenylyl cyclase (AC6) suppressed the development of neuronal processes in primary hippocampal neurons. (A) Plasma membrane fractions (50 μg) isolated from brains of postnatal day 1 (P1), P7, P15, and adult rats were subjected to Western blot analysis using an AC6 antibody (AC6D). (B) Primary hippocampal neurons at day in vitro (DIV) 4 were transfected with the indicated constructs (2 μg) along with 1/6 of the molar amount of an expression construct of enhanced green fluorescent protein (EGFP) for 72 h. Representative images of three independent experiments are shown. The scale bar is 20 μm. The length of the longest neurite of each neuron was measured in neurons expressing EGFP and then expressed as a percentage of the control group. Data are presented as the means ± standard errors from at least three independent experiments. ***, P < 0.001 compared to cells of the control group using one-way analysis of variance.
Fig. 2.
Elevated expression of type VI adenylyl cyclase (AC6) suppressed neurite outgrowth in Neuro2A cells. (A) Neuro2A cells were transfected with the indicated constructs (2 μg) along with 1/6 of the molar amount of an expression construct of enhanced green fluorescent protein (EGFP) and treated with retinoic acid (RA) for 48 h to induce differentiation. Representative images of three independent experiments are shown. The scale bar is 100 μm. For quantification, transfected cells were identified by EGFP expression. RA-evoked neurite-bearing cells were quantified using a fluorescence microscope. Data are presented as the means ± standard errors from at least three independent experiments. ***, P < 0.001 compared to cells of the control group using one-way analysis of variance (ANOVA). (B) Neuro2A cells were transfected with the indicated amount of an expression construct of AC6 and an empty vector to make up 2 μg of DNA, along with 1/6 of the molar amount of an expression construct of EGFP. Data from relative neurite-bearing cells are presented as the means ± standard errors from at least three independent experiments. **, P < 0.01, and ***, P < 0.001, compared to cells of the control group using one-way ANOVA. Expression levels of AC5 and AC6 were assessed by Western blot analyses using anti-AC5 and anti-AC6 antibodies, respectively. Representative Western blots are shown. Expression levels of AC5 and AC6 were assessed by Western blot analyses using anti-c-Myc and anti-AC6 antibodies, respectively. Representative Western blots are shown.
Consistently with the hypothesis that the level of AC6 is important for neurite outgrowth, we designed an shRNA construct directed against the mouse AC6 transcript sequence to knock down endogenous AC6 expression in Neuro2A cells and primary hippocampal neurons. As shown in Fig. 3A, the transient expression of an AC6-directed shRNA construct (designated siAC6-1) reduced the expression of both endogenous and transfected AC6 proteins, as well as forskolin-evoked cAMP accumulation in Neuro2A cells (Fig. 3A). Most importantly, the downregulation of AC6 using siAC6-1 significantly elevated RA-induced neurite outgrowth in Neuro2A cells, whereas elevated AC6 expression reduced neurite outgrowth (Fig. 3B; also see Fig. S1 in the supplemental material). To verify the importance of the AC6 level on neurite outgrowth, we designed another AC6-directed shRNA construct (designated siAC6-2) which also downregulated the endogenous level of mouse AC6 and enhanced the percentage of neurite-bearing cells in Neuro2A cells (Fig. 3C). Importantly, the exogenous expression of rat AC6, which resists the suppressive effect of siAC6-2 due to a sequence variation between the mouse and rat AC6 (see Table S1 in the supplemental material), retained the inhibitory effect on neurite outgrowth (Fig. 3C). Consistently with this finding, the downregulation of rat AC6 by siAC6-1 in primary hippocampal neurons promoted neurite elongation, whereas the overexpression of AC6 reduced the neurite length (Fig. 3D; also see Fig. S2 in the supplemental material). Furthermore, primary neurons purified from the hippocampi of AC6 knockout (KO) mice (11) had longer neuronal processes than WT neurons (Fig. 3E). Note that the maturation of the hippocampus, which was accompanied by increased dendritic length and decreased growth cones, was complete by postnatal stage P20 (20 days after birth) (34). Changes in the levels of the AC6 protein thus might contribute to the development and maturation of the hippocampus.
Fig. 3.
Downregulation of endogenous type VI adenylyl cyclase (AC6) enhanced neurite outgrowth. (A to C) Neuro2A cells were transfected with the indicated construct(s) for 72 h. Two short-hairpin RNA (shRNA) constructs for AC6 (designated siAC6-1 and siAC6-2) were tested for their ability to reduce AC6. (A) Cells were treated with forskolin (10 μM) for 20 min, and the accumulated cAMP was assessed. (A and C) Total lysates collected from cells transfected with the indicated construct(s) were subjected to Western blot analyses using AC6D. (B and C) Neuro2A cells were transiently transfected with the indicated construct(s) along with an expression construct of enhanced green fluorescent protein (EGFP) and treated with retinoic acid (RA) for 48 h to induce differentiation. Neurite-bearing cells were quantified as described in Materials and Methods. Data are presented as the means ± standard errors from three to six experiments. (D) Primary hippocampal neurons at day in vitro (DIV) 4 were transfected with the indicated construct(s) along with an expression construct of EGFP for 72 h. The length of the longest neurite was measured as described. Data are presented as the means ± standard errors from three experiments. (E) Primary hippocampal neurons (DIV 7) harvested from AC6-null (AC6−/−) or wild-type (AC6+/+) mice were immunostained with an anti-tuj1 antibody and visualized by an Alexa Fluor 568-conjugated secondary antibody (red). Representative images of three independent experiments are shown. The scale bar is 50 μm. The length of the longest neurite was measured as described. Data are presented as the means ± standard errors from three experiments. *, P < 0.05; **, P < 0.01; and ***, P < 0.001, each by one-way analysis of variance.
Because AC5 and AC6 are highly homologous except for their N termini (32), we next assessed whether the N-terminal domain of AC6 mediated the suppressive effect of AC6 described above using an AC6 variant (AC6-N5), the regulatory domain (i.e., the N terminus) of which was replaced by that of AC5. We previously showed that the enzymatic activity of AC6-N5 is similar to that of WT AC6 (30). To further verify the importance of the N terminus, we created another mutant (AC5-N6) in which the N terminus of AC5 (amino acids 1 to 215) was replaced with that of AC6 (amino acids 1 to 146). Accumulations of basal cAMP levels in Neuro2A cells expressing AC6, AC6-N5, or AC5-N6 were similar (Fig. 4A). Nevertheless, only AC variants that contained the N terminus of AC6 (e.g., AC6 and AC5-N6) suppressed neurite outgrowth in Neuro2A cells (Fig. 4B). AC6-N5 did not suppress neurite outgrowth in Neuro2A cells or primary hippocampal neurons in the way that AC6 did (Fig. 4B and C), further supporting our hypothesis that the N terminus of AC6 is critical for its action in suppressing neurite outgrowth. This observation also suggests that an N terminus-specific binding protein mediates the inhibitory effect of AC6.
Fig. 4.

N terminus of type VI adenylyl cyclase (AC6) mediates the suppression of neurite outgrowth. (A and B) Neuro2A cells were transfected with the indicated construct(s) along with an expression construct of enhanced green fluorescent protein (EGFP) and treated with retinoic acid (RA) for 48 h to induce differentiation. The accumulated cAMP content (A) and the percentage of neurite-bearing cells (B) were quantified as described in Materials and Methods. Data are presented as the means ± standard errors from three to six experiments. Expression levels of AC6, AC6N5, and AC5N6 were assessed by Western blot analyses using anti-AC6, anti-AC6, and anti-c-Myc antibodies, respectively. Representative Western blots are shown. (C) Primary hippocampal neurons purified from the hippocampi of AC6 knockout mice were transfected with the indicated construct(s) along with an expression construct of EGFP. Representative images of three independent experiments are shown. The scale bar is 50 μm. The length of the longest neurite of each neuron was measured, and results are presented as the means ± standard errors from three experiments. *, P < 0.05; **, P < 0.01; and ***, P < 0.001, compared to cells of the control group using one-way analysis of variance.
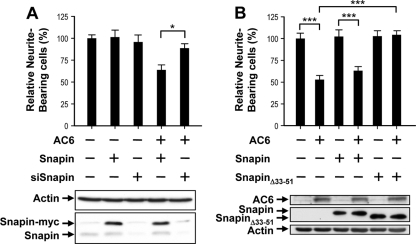
We previously reported that the N terminus of AC6 specifically binds to Snapin (12), a protein that binds Snap25 and the SNARE complex. Because the SNARE complex is implicated in neurite outgrowth (25, 31), we evaluated the role of Snapin in suppressing neurite outgrowth by AC6. As shown in Fig. 5A, the overexpression of Snapin in Neuro2A cells did not affect the extent of neurite outgrowth in the presence or absence of AC6 (Fig. 5A). However, when the endogenous level of Snapin was downregulated with an shRNA construct directed against the mouse Snapin transcript (designated siSnapin), the inhibitory effect of AC6 on neurite outgrowth was abolished (Fig. 5A), demonstrating that Snapin likely mediates the inhibitory effect of AC6. Immunoblotting analyses revealed that the transient expression of siSnapin markedly reduced the endogenous level of Snapin in Neuro2A cells. No effect on neurite outgrowth was observed in cells expressing siSnapin alone. Surprisingly, the overexpression of the SnapinΔ33-51 mutant, which is unable to bind AC6 (12), effectively reversed the suppressive effect of AC6 on neurite outgrowth (Fig. 5B), while the overexpression of the SnapinΔ33-51 mutant by itself did not affect neurite outgrowth. The observations described above further strengthen our hypothesis that Snapin mediates the action of AC6 in suppressing neurite outgrowth. Furthermore, the observation that the SnapinΔ33-51 mutant abrogated the inhibitory effect of AC6 on neurite outgrowth suggests that this mutant protein, acting in a dominant-negative fashion, is sequestering a third partner in the AC6-Snapin protein complex that, together with the other proteins, modulates neurite outgrowth.
Fig. 5.
Type VI adenylyl cyclase (AC6)-binding protein, Snapin, mediates AC6-induced suppression of neurite outgrowth. Neuro2A cells were transfected with the indicated construct(s) along with an expression construct of enhanced green fluorescent protein (EGFP) and treated with retinoic acid (RA) for 48 h to induce differentiation. Neurite-bearing cells were quantified as described in Materials and Methods. Data are presented as the means ± standard errors from three to six experiments. *, P < 0.05, and ***, P < 0.001, by one-way analysis of variance. Expression levels of AC6 and Snapin variants were assessed by Western blot analyses using anti-AC6 and anti-c-Myc antibodies, respectively. Representative Western blots are shown.
Snapin was initially identified as a protein that binds to Snap25, which plays key roles in exocytosis and several other processes, including neurite elongation and sprouting (19, 31, 55, 75). Therefore, we hypothesized that by interacting with Snapin, AC6 affects the neurite outgrowth process by interfering with the function of Snap25 in the membrane fusion machinery. While the detailed interacting domains between Snapin and Snap25 have not been mapped, it was reported that the interaction between Snapin and SNAP25 can be greatly increased by the PKA-dependent phosphorylation of Snapin at the Ser50 residue (10). Interestingly, two PKA inhibitors (H89 and KT5720) reversed the AC6-induced inhibition of neurite outgrowth (Fig. 6A; also see Fig. S3 in the supplemental material). H89 did not affect neurite outgrowth in the presence of an empty vector or AC6-N5 (Fig. 6A), demonstrating that PKA by itself, regardless of the cAMP level, does not affect neurite outgrowth. To further test this hypothesis, we used a Snapin mutant (SnapinS50A) that cannot be phosphorylated by PKA at Ser50 and therefore has a much-reduced capacity to interact with Snap25 relative to that of WT Snapin (10). The mutation of Ser50 in Snapin did not affect its interaction with AC6, because SnapinS50A was shown earlier to prevent the regulation of AC6 by PKC, as does WT Snapin (12). As shown in Fig. 6B, the overexpression of SnapinS50A reversed the AC6-mediated inhibition of neurite outgrowth, suggesting that a strong interaction between Snapin and Snap25 is required to inhibit neurite outgrowth by AC6.
Fig. 6.
Protein kinase A (PKA) activity is required for the Snapin-mediated inhibitory effect of type VI adenylyl cyclase (AC6) on neurite outgrowth. (A) Neuro2A cells were transfected with the indicated construct(s) along with an expression construct of enhanced green fluorescent protein (EGFP) and treated with retinoic acid (RA) for 48 h to induce differentiation in the presence of the desired concentration of a PKA inhibitor (H89). (B) Neuro2A cells were transfected with the indicated construct(s) along with an expression construct of EGFP and treated with RA for 48 h to induce differentiation. (C) Neuro2A cells were transfected with the indicated construct(s) along with an expression construct of EGFP and treated with RA for 48 h to induce differentiation in the presence of an inhibitor of adenylyl cyclase (SQ22536; 20 μM). (D) Neuro2A cells were transfected with the indicated construct(s) along with an expression construct of EGFP and treated with RA for 48 h to induce differentiation. Neurite-bearing cells were quantified as described in Materials and Methods. Data are presented as the means ± standard errors from three to six experiments. *, P < 0.05; **, P < 0.01; and ***, P < 0.001, each by one-way analysis of variance. Expression levels of AC6 and Snapin variants were assessed by Western blot analyses using anti-AC6 and anti-c-Myc antibodies, respectively. Representative Western blots are shown.
To evaluate the role of cAMP in the suppressive effect of AC6 on neurite outgrowth, we first employed a cell-permeable inhibitor of AC (SQ22536 [16]), which suppressed the basal and forskolin-evoked cAMP production by AC6 (see Fig. S4 in the supplemental material). Treating Neuro2A cells with SQ22536 reversed the suppressive effect of AC6 on neurite outgrowth (Fig. 6C). We next created a catalytically inactive AC6 mutant (AC6D426A [20]) which harbors a mutation in the C1 domain; it thus has no basal or forskolin-evoked AC activity (see Fig. S5 in the supplemental material). The expression of AC6D426A did not suppress neurite outgrowth in the presence of an empty vector or a Snapin variant (SnapinS50A) which cannot be phosphorylated by PKA (Fig. 6D). Importantly, the coexpression of AC6D426A (which contains an intact N terminus) and SnapinS50D (which is a phosphorylated mutant at Ser50 and binds to Snap25 with high affinity [10]) led to the suppression of neurite outgrowth (Fig. 6D). Collectively, the N-terminal domain and basal catalytic activity of AC6, which permitted the PKA-mediated phosphorylation of Snapin at Ser50 for a strong interaction between Snapin and Snap25, are required for the AC6-mediated inhibitory effect on neurite outgrowth.
To confirm that AC6, Snapin, and Snap25 form a complex, we produced an AC6 mutant (Fc-AC6) containing a fragment-crystallizable (Fc) region fused to the C terminus of AC6. The exogenous expression of Fc-AC6 in cells allowed the pulldown of protein G of protein complexes that contained AC6. In Neuro2A cells, Fc-AC6, but not Fc, successfully pulled down Snapin and Snap25 (Fig. 7A). Consistently with these findings, the expression of Fc-AC6-N5 could not pull down either Snapin or Snap25 (Fig. 7B). Interestingly, the expression of a nonphosphorylation-mimicking Snapin mutant (SnapinS50A), which had no interaction with Snap25, prevented the pulldown of Snap25 by Fc-AC6 (Fig. 7B). These data collectively demonstrated that Snapin and Snap25 form a complex with the AC6 protein. In addition, complex formation of AC6, Snapin, and Snap25 is dependent on the N terminus of AC6 and the phosphorylation of Snapin. To demonstrate that AC6, Snapin, and Snap25 form a complex in vivo, we first immunoprecipitated Snap25 from brains of WT and AC6-null mice and measured forskolin-stimulated AC activity in the Snap25 immunocomplex. As shown in Fig. 7C, marked basal and forskolin-evoked AC activities were detected in the Snap25 immunocomplex purified from brains of WT mice, but not from those of AC6-KO mice, demonstrating the formation of a complex between Snap25 and AC6. No significant AC activity was detected in the immunocomplex precipitated with normal goat serum. To evaluate whether the interaction between AC6 and Snapin is important for the formation of the complex between AC6 and Snap25, we used a peptide (Snapin33-51; LRPAVQQLDSHVHAVRESQ), comprising amino acids 33 to 51 of Snapin, to disrupt the interaction between AC6 and Snapin (12). As shown in Fig. 7D, Snapin33-51, but not a scrambled peptide, significantly reduced AC activity measured in the Snap25 complex, demonstrating that the interaction between AC6 and Snap25 is mediated by Snapin. Most importantly, the overexpression of Snap25 completely reversed the inhibitory effect of AC6 on neurite outgrowth in Neuro2A cells and in primary hippocampal neurons (Fig. 8A and C). Interestingly, the overexpression of Snap25 by itself did not increase neurite outgrowth in Neuro2A cells or in primary hippocampal neurons, probably due to a ceiling effect in our assay conditions. Conversely, the downregulation of Snap25 using two Snap25-directed shRNA constructs (designated siSnap25-3 and siSnap25-7) greatly reduced neurite outgrowth in Neuro2A cells and in primary hippocampal neurons (Fig. 8B and D), further supporting our hypothesis that Snap25 plays a critical role in regulating neurite outgrowth.
Fig. 7.
Type VI adenylyl cyclase (AC6) formed a complex with Snapin and Snap25. (A and B) Neuro2A cells were transfected with the indicated constructs for 48 h. Cell lysates were pulled down using protein G beads. The input fractions (1/40) and the resultant complexes were subjected to a Western blot analysis using anti-Flag, anti-Snapin, and anti-Snap25 antibodies. Representative Western blots of three independent experiments are shown. (C) Brain lysates of wild-type (WT) and AC6-KO mice were immunoprecipitated by an anti-Snap25 antibody or normal goat immunoglobulin G (IgG) as indicated. The resultant immunoprecipitation complexes then were assessed for AC activity upon stimulation with or without forskolin (10 μM). Data are presented as the means ± standard errors from three to five experiments. ***, P < 0.001 by one-way analysis of variance (ANOVA). (D) Brain lysates of WT mice were incubated with an anti-Snap25 antibody in the presence and absence of the indicated peptide (Snapin33-51 peptide or scrambled peptide; 0.5 μg). The AC activity of the resultant immunoprecipitation complexes was assessed upon stimulation with forskolin (10 μM). Data are presented as the means ± standard errors from three experiments. ***, P < 0.001 by one-way ANOVA.
Fig. 8.

Overexpression of the 25-kDa synaptosome-associated protein (Snap25) reversed the inhibitory effect of type VI adenylyl cyclase (AC6) on neurite outgrowth. (A) Neuro2A cells were transiently transfected with the indicated construct(s) along with an expression construct of enhanced green fluorescent protein (EGFP) and treated with retinoic acid (RA) for 48 h to induce differentiation. Neurite-bearing cells were quantified as described in Materials and Methods. Data are presented as the means ± standard errors from three to six experiments. (B) Neuro2A cells were transiently transfected with shRNA construct(s) for Snap25 (siSnap25-3 and siSnap25-7) along with an expression construct of EGFP and treated with RA for 48 h to induce differentiation. Neurite-bearing cells were quantified as described. Data are presented as the means ± standard errors from three to six experiments. Total lysates collected from cells transfected with the indicated construct(s) were subjected to Western blot analyses using an anti-Snap25 antibody. (C and D) Primary hippocampal neurons at day in vitro (DIV) 4 were transfected with the indicated construct(s) along with an expression construct of EGFP for 72 h. The length of the longest neurite of each neuron was measured in neurons expressing EGFP and then expressed as a percentage of the control group, which was transfected with the empty vector plus the EGFP reporter only. Data are presented as the means ± standard errors from three experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001, each by one-way analysis of variance.
To evaluate whether the expression of AC6 affects the localization of Snap25 and thereby interferes with its function of membrane fusion, we performed the immunohistochemical staining of primary hippocampal neurons expressing a reporter (YFP), a fusion protein of AC6-YFP, or an AC5 protein (AC5-c-Myc). As shown in Fig. 9, Snap25 and another SNARE component (Syntaxin 1) were located mainly on plasma membranes and throughout neuronal processes of primary hippocampal neurons. The expression of AC6, but not of AC5 or YFP, restricted the localization of Snap25 and Syntaxin 1 to soma and neurites in perisomatic regions. This AC6-mediated restriction of the localization of SNARE components likely limited SNARE-dependent membrane fusion and might account for the suppressive effect of AC6 on neurite outgrowth.
Fig. 9.
Expression of type VI adenylyl cyclase (AC6) altered the localization of the 25-kDa synaptosome-associated protein (Snap25) and Syntaxin 1. Primary hippocampal neurons on day in vitro (DIV) 4 were transfected with the indicated construct for 72 h. YFP- and YFP-AC6-transfected neurons were fixed and then stained with anti-Syntaxin 1 and anti-Snap25 antibodies. AC5-c-Myc-transfected neurons were stained with anti-c-Myc, anti-Syntaxin 1, and anti-Snap25 antibodies. Snap25 was visualized with an Alexa Fluor 568 secondary antibody (red). Syntaxin 1 was visualized with an Alexa Fluor 647-conjugated secondary antibody (blue). AC5-c-Myc was visualized with an Alexa Fluor 488-conjugated secondary antibody (green). Representative images of three independent experiments are shown. The arrows indicate the soma of transfected neurons.
DISCUSSION
cAMP has long been known to play critical roles in axon/dendrite formation and axon guidance and elongation in embryonic neuronal development (5, 27, 33, 53, 54, 64). The most important effectors of cAMP include PKA and Epac (47). These two molecules regulate axonal growth through distinct mechanisms (40, 41). Specifically, PKA induces growth cone repulsion at low cAMP levels, while Epac promotes growth cone attraction at high cAMP levels (5). The regulation of neurite outgrowth by cAMP thus is believed to be tightly controlled by multiple fine-tuned layers. In the present study, we demonstrated that AC6 suppressed neurite outgrowth by restricting the location of Snap25-containing SNARE machinery through a direct protein-protein interaction with the N terminus of AC6 and Snapin (Fig. 10). Although this interaction between AC6 and Snapin is independent of PKA (7), a tight association between Snapin and Snap25 requires PKA activity (33). Such modulation of neurite outgrowth by the formation of AC6/Snapin/Snap25 complexes likely is confined to locations where AC6 is enriched. While the relative levels and stoichiometry of the binding partners and functional consequence of increased AC6 levels during postnatal development are unknown, the dynamic regulation and trafficking of the AC6/Snapin/Snap25 complex during development may be important in the morphogenesis of neurons. Interestingly, Snapin was shown to regulate dendritic patterning in developing neurons by modulating cypin-promoted microtubule assembly (6). Future investigation of the subcellular localization of AC6 in neurons during development and its possible involvement in neuronal morphogenesis during development in vivo would be of great interest. In addition, the modulation of neurite outgrowth by AC6 likely is AC isoform specific, because AC6 exerts its effect via the N terminus, which is unique among all AC isozymes (7, 32). In fact, the N-terminal domains of all transmembrane ACs are distinct and act as specific regulatory domains (13, 23, 32, 67). Consistently with this hypothesis, we showed that the expression of AC5, the closest AC isozyme to AC6, had no effect (Fig. 1, 2, and 9). Our study is the first to demonstrate that an AC isoform modified neurite outgrowth through its interacting proteins, and it reveal a new layer of fine-tuning by the AC6/Snapin complex of neurite outgrowth during the maturation of the hippocampus.
Fig. 10.
Schematic representation of the inhibitory effect of type VI adenylyl cyclase (AC6) on neurite outgrowth. (A) Snap25 and Syntaxin 1 were located mainly in neuronal processes of primary hippocampal neurons. (B) Elevated expression of AC6 in primary hippocampal neurons suppressed neurite outgrowth by restricting the localization of the Snap25-containing SNARE machinery to soma and perisomatic neurites through direct protein-protein interactions with the N terminus of AC6 and Snapin.
Snapin was recognized initially for its involvement in regulating exocytosis (28, 63). Membrane vesicle trafficking and exocytosis play critical roles in the development of neuronal processes (69). In contrast to the well-characterized regulatory mechanisms underlying synaptic vesicle exocytosis in mature neurons, the regulatory mechanisms of membrane trafficking and the exact SNAREs that are required for membrane trafficking involved in neurite outgrowth are largely unknown and currently are under active investigation (69). The membrane fusion mechanism involved in neurite outgrowth is believed to differ from the classical synaptic core machinery, because treatment with tetanus neurotoxin (which cleaves VAMP2, a vesicular SNARE protein critical for Ca2+-mediated exocytosis) blocks neurotransmitter release but not the neurite outgrowth process (22, 43). Conversely, the suppression of Snap25 (a t-SNARE protein) by antisense oligonucleotides or a neurotoxin greatly reduced neurite outgrowth (39, 42, 43), whereas the overexpression of Snap25 induced neurite sprouting (31, 55). Consistently with its importance in neurite development, Snap25 was detected in nerve growth cones (39). Interestingly, although transcript levels of Snap25 were found in both excitatory and inhibitory neurons (2, 57), Snap25 exists in selective subpopulations of excitatory nerve terminals (19, 21, 36, 65). One of the mechanisms which might account for the selective localization of Snap25 in specific neuronal terminals is the differential axonal transport of Snap25 (38, 45). Altered expression of Snap25 was reported in mental diseases, including schizophrenia and bipolar I disorder (51, 62). In the present study, we reported that the expression of AC6 plays a critical role in controlling the subcellular localization of Snap25 in neurons (Fig. 9). The overexpression of AC6 in primary hippocampal neurons suppressed the length of neurites by approximately 40% (Fig. 1), which was comparable to the inhibition caused by the downregulation of Snap25 using an shRNA approach as reported in the present study (Fig. 8D) and that by the cleavage of Snap25 using botulinum neurotoxin A, as reported elsewhere (43).
We found that the AC6-mediated inhibition of neurite outgrowth was reversed by the overexpression of Snap25 or a Snapin mutant (SnapinS50A) that could not be phosphorylated by PKA and therefore had a low affinity for Snap25 (Fig. 6B and D and 7B). We also demonstrated the existence of a protein complex composed of AC6, Snapin, and Snap25 in brains of WT mice (Fig. 7C and D). Note that the percentages of Snapin and Snap25 that were pulled down by AC6 in Fig. 7A were 1.8 and 0.7%, respectively. These low percentages of apparent complex formation likely are underestimated, because interactions among membrane-bound AC6, Snapin, and Snap25 require membrane solubilization using detergents, a required step for the pulldown of these molecules from Neuro2A cells (Fig. 7A and B). Membrane solubilization using detergents is likely to cause the undesirable dissociation of assembled protein complexes, which is a common problem during the preparation of protein complexes (29, 56). In addition, Snapin is an important scaffold protein that interacts with more than 20 binding proteins and regulates a wide variety of cellular machineries with the vesicle-transporting system (68). It thus is reasonable that AC6 pulled down only a small proportion of the Snapin-mediated protein complexes. Our data suggest that the expression of AC6 in cell bodies and perisomatic neurites caused a redistribution of the Snap25-containing SNARE apparatuses (Fig. 9). The local, but not overall, concentrations of these molecules therefore are crucial for the suppressive effect of AC6 on neurite outgrowth. Such information currently is unavailable due to a lack of good antibodies with sufficiently high affinities to quantify endogenous and local levels of AC6, Snapin, and Snap25 in neurons. Nevertheless, primary hippocampal neurons isolated from AC6-null mice had much longer neurites (Fig. 3E), suggesting that the endogenous level of AC6 was sufficient to regulate neurite outgrowth in an N terminus-dependent manner (Fig. 3E and 4C). Further analyses of the localization and expression levels of AC6 in different neuronal populations and disease models might provide novel insights into the regulation of neuronal development and mental disease by Snap25.
The activation and regulation of ACs traditionally have been viewed in the context of serial signaling cascades linking protein G-coupled receptor activation to various physiological functions. In addition to its role as a cAMP-synthesizing enzyme, our study showed that AC6, by the binding of its N terminus to Snapin, negatively regulates neurite outgrowth (Fig. 1 and 2). Results obtained from cells treated with an AC inhibitor (SQ22536) or the expression of a catalytically inactive AC6 mutant suggested that the basal catalytic activity of AC6 is required for the suppression effect on neurite outgrowth (Fig. 6C and D). The slight increase in cellular accumulation of cAMP (Fig. 4A) appeared to be sufficient to activate PKA and cause the phosphorylation of its interacting protein (Snapin) to redistribute the cellular localization of the Snap25-containing SNARE complex (Fig. 6D and 9). We therefore reasoned that the further activation of AC6 through the stimulation of Gsα-coupled receptors would not have a significant effect on neurite outgrowth. In line with this hypothesis, the stimulation of the A2A adenosine receptor using CGS21680 significantly increased the intracellular cAMP content in AC6-expressing Neuro2A cells but failed to alter the AC6-mediated suppression of neurite outgrowth (see Fig. S6 in the supplemental material). It is important to point out that interaction with the Snapin and Snap25 complex did not affect the catalytic activity of AC6, as was reported earlier (12). Although the downregulation of Snapin or the overexpression of Snap25 reversed the inhibitory effect of AC6 on neurite outgrowth, the alteration of the level of Snapin or Snap25 did not affect intracellular cAMP accumulation evoked by AC6 (see Fig. S7 and S8 in the supplemental material). Our findings suggest that interactions of proteins from different pathways provide a spatial and temporal platform for signaling cross-talk. This is particularly important for neurons, because multiple signals must be selectively and precisely integrated. The binding of AC6 to Snapin leads to the efficient cross-regulation of a specific cAMP-producing enzyme and the SNARE apparatus.
The dynamic regulation of AC6 protein levels was reported beyond neuronal development, as demonstrated in the present study (Fig. 1A). For example, the level of AC6 is higher in thyroid tumors than normal thyroid tissues (70). AC6 also is actively regulated during circadian rhythms (24). It is important to note that AC6 is unique among members of the AC superfamily, in that it has very low basal enzymatic activity compared to that of other isoforms (48), and it can be inhibited by most of the signaling pathways examined, including those involving calcium, PKA, PKC, and nitric oxide (8, 33, 44, 74). Although the general presence of AC6 in neurons was reported more than a decade ago (35), its functional role in regulating neuronal activity still is largely unclear. Compared to the well-characterized roles of calcium-stimulated ACs (i.e., AC1 and AC8) (66, 72, 73), the contribution of AC6 to neuronal plasticity is particularly obscure, because cAMP elevation usually is associated with enhanced plasticity (15, 52, 64). A recent study reported that AC6 interacts with an A-kinase anchoring protein (AKAP79/150) (1, 17). The close association of AC6 with PKA is thought to allow the efficient phosphorylation of its binding protein (Snapin) at Ser50, promoting a Snapin/Snap25 interaction, and subsequently facilitating the ability of AC6 to control the localization of Snap25-containing SNARE complexes via Snapin. In summary, our findings suggest that the regulatory N-terminal domain of AC6 plays a specific role in the development of neuronal processes via its interaction with Snapin.
Supplementary Material
ACKNOWLEDGMENTS
We thank D. P. Chamberlin for reading and editing the manuscript and Ching-Lung Huang for technical support. We acknowledge technical support from the National RNAi Core Facility of Taiwan (http://rnai.genmed.sinica.edu.tw).
This work was supported by grants (NHRI-EX92-9203NI, NHRI-EX93-9203NI, and NHRI-EX94-9203NI) from the National Health Research Institutes and Academia Sinica, Taipei, Taiwan.
Footnotes
Supplemental material for this article may be found at http://mcb.asm.org/.
Published ahead of print on 10 October 2011.
REFERENCES
- 1. Bauman A. L., et al. 2006. Dynamic regulation of cAMP synthesis through anchored PKA-adenylyl cyclase V/VI complexes. Mol. Cell 23:925–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Boschert U., et al. 1996. Developmental and plasticity-related differential expression of two SNAP-25 isoforms in the rat brain. J. Comparative Neurol. 367:177–193 [DOI] [PubMed] [Google Scholar]
- 3. Bradke F., Dotti C. G. 1997. Neuronal polarity: vectorial cytoplasmic flow precedes axon formation. Neuron 19:1175–1186 [DOI] [PubMed] [Google Scholar]
- 4. Brewer G. J., Torricelli J. R., Evege E. K., Price P. J. 1993. Optimized survival of hippocampal neurons in B27-supplemented Neurobasal, a new serum-free medium combination. J. Neurosci. Res. 35:567–576 [DOI] [PubMed] [Google Scholar]
- 5. Cai D., et al. 2001. Neuronal cyclic AMP controls the developmental loss in ability of axons to regenerate. J. Neurosci. 21:4731–4739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen M., et al. 2005. A novel role for snapin in dendrite patterning: interaction with cypin. Mol. Biol. Cell 16:5103–5114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen Y., et al. 1997. Adenylyl cyclase 6 is selectively regulated by protein kinase A phosphorylation in a region involved in Galphas stimulation. Proc. Natl. Acad. Sci. U. S. A. 94:14100–14104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chern Y. 2000. Regulation of adenylyl cyclase in the central nervous system. Cell. Signal. 12:195–204 [DOI] [PubMed] [Google Scholar]
- 9. Chern Y., Lai H. L., Fong J. C., Liang Y. 1993. Multiple mechanisms for desensitization of A2a adenosine receptor-mediated cAMP elevation in rat pheochromocytoma PC12 cells. Mol. Pharmacol. 44:950–958 [PubMed] [Google Scholar]
- 10. Chheda M. G., Ashery U., Thakur P., Rettig J., Sheng Z. H. 2001. Phosphorylation of Snapin by PKA modulates its interaction with the SNARE complex. Nat. Cell Biol. 3:331–338 [DOI] [PubMed] [Google Scholar]
- 11. Chien C. L., et al. 2010. Impaired water reabsorption in mice deficient in the type VI adenylyl cyclase (AC6). FEBS Lett. 584:2883–2890 [DOI] [PubMed] [Google Scholar]
- 12. Chou J. L., et al. 2004. Regulation of type VI adenylyl cyclase by Snapin, a SNAP25-binding protein. J. Biol. Chem. 279:46271–46279 [DOI] [PubMed] [Google Scholar]
- 13. Crossthwaite A. J., Ciruela A., Rayner T. F., Cooper D. M. 2006. A direct interaction between the N terminus of adenylyl cyclase AC8 and the catalytic subunit of protein phosphatase 2A. Mol. Pharmacol. 69:608–617 [DOI] [PubMed] [Google Scholar]
- 14. Da Silva J. S., Dotti C. G. 2002. Breaking the neuronal sphere: Regulation of the actin cytoskeleton in neuritogenesis. Nat. Rev. Neurosci. 3:694–704 [DOI] [PubMed] [Google Scholar]
- 15. Dell'Acqua M. L., et al. 2006. Regulation of neuronal PKA signaling through AKAP targeting dynamics. Eur. J. Cell Biol. 85:627–633 [DOI] [PubMed] [Google Scholar]
- 16. Dixon D., Atwood H. L. 1989. Adenylate cyclase system is essential for long-term facilitation at the crayfish neuromuscular junction. J. Neurosci. 9:4246–4252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Efendiev R., et al. 2010. AKAP79 interacts with multiple adenylyl cyclase (AC) isoforms and scaffolds AC5 and -6 to alpha-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate (AMPA) receptors. J. Biol. Chem. 285:14450–14458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Evangelopoulos M. E., Weis J., Kruttgen A. 2005. Signalling pathways leading to neuroblastoma differentiation after serum withdrawal: HDL blocks neuroblastoma differentiation by inhibition of EGFR. Oncogene 24:3309–3318 [DOI] [PubMed] [Google Scholar]
- 19. Frassoni C., et al. 2005. Analysis of SNAP-25 immunoreactivity in hippocampal inhibitory neurons during development in culture and in situ. Neuroscience 131:813–823 [DOI] [PubMed] [Google Scholar]
- 20. Gao M. H., et al. 2011. Beneficial effects of adenylyl cyclase type 6 (AC6) expression persist using a catalytically inactive AC6 mutant. Mol. Pharmacol. 79:381–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Garbelli R., et al. 2008. Heterogeneous expression of SNAP-25 in rat and human brain. J. Comparative Neurol. 506:373–386 [DOI] [PubMed] [Google Scholar]
- 22. Grosse G., et al. 1999. SNAP-25 requirement for dendritic growth of hippocampal neurons. J. Neurosci. Res. 56:539–546 [DOI] [PubMed] [Google Scholar]
- 23. Gu C., Cooper D. M. 1999. Calmodulin-binding sites on adenylyl cyclase type VIII. J. Biol. Chem. 274:8012–8021 [DOI] [PubMed] [Google Scholar]
- 24. Han S., Kim T. D., Ha D. C., Kim K. T. 2005. Rhythmic expression of adenylyl cyclase VI contributes to the differential regulation of serotonin N-acetyltransferase by bradykinin in rat pineal glands. J. Biol. Chem. 280:38228–38234 [DOI] [PubMed] [Google Scholar]
- 25. Hirling H., et al. 2000. Syntaxin 13 is a developmentally regulated SNARE involved in neurite outgrowth and endosomal trafficking. Eur. J. Neurosci. 12:1913–1923 [DOI] [PubMed] [Google Scholar]
- 26. Horton R. M., Hunt H. D., Ho S. N., Pullen J. K., Pease L. R. 1989. Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene 77:61–68 [DOI] [PubMed] [Google Scholar]
- 27. Hutchins B. I. 2010. Competitive outgrowth of neural processes arising from long-distance cAMP signaling. Sci. Signal. 3:jc1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ilardi J. M., Mochida S., Sheng Z. H. 1999. Snapin: a SNARE-associated protein implicated in synaptic transmission. Nat. Neurosci. 2:119–124 [DOI] [PubMed] [Google Scholar]
- 29. Jones M. B., Garrison J. C. 1999. Instability of the G-protein beta5 subunit in detergent. Anal. Biochem. 268:126–133 [DOI] [PubMed] [Google Scholar]
- 30. Kao Y. Y., Lai H. L., Hwang M.-J., Chern Y. 2004. An important functional role of the N terminus domain of type VI adenylyl cyclase in Gαi-mediated inhibition. J. Biol. Chem. 279:34440–34448 [DOI] [PubMed] [Google Scholar]
- 31. Kimura K., Mizoguchi A., Ide C. 2003. Regulation of growth cone extension by SNARE proteins. J. Histochem. Cytochem. 51:429–433 [DOI] [PubMed] [Google Scholar]
- 32. Lai H. L., et al. 1999. The N terminus domain of type VI adenylyl cyclase mediates its inhibition by protein kinase C. Mol. Pharmacol. 56:644–650 [DOI] [PubMed] [Google Scholar]
- 33. Lai H. L., et al. 1997. Protein kinase C inhibits adenylyl cyclase type VI activity during desensitization of the A2a-adenosine receptor-mediated cAMP response. J. Biol. Chem. 272:4970–4977 [DOI] [PubMed] [Google Scholar]
- 34. Lang U., Frotscher M. 1990. Postnatal development of nonpyramidal neurons in the rat hippocampus (areas CA1 and CA3): a combined Golgi/electron microscope study. Anat. Embryol. (Berlin) 181:533–545 [DOI] [PubMed] [Google Scholar]
- 35. Liu F. C., et al. 1998. Expression of type VI adenylyl cyclase in the central nervous system: implication for a potential regulator of multiple signals in different neurotransmitter systems. FEBS Lett. 436:92–98 [DOI] [PubMed] [Google Scholar]
- 36. Mandolesi G., et al. 2009. Distribution of the SNAP25 and SNAP23 synaptosomal-associated protein isoforms in rat cerebellar cortex. Neuroscience 164:1084–1096 [DOI] [PubMed] [Google Scholar]
- 37. Martinez-Arca S., et al. 2001. A common exocytotic mechanism mediates axonal and dendritic outgrowth. J. Neurosci. 21:3830–3838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Matteoli M., et al. 2009. The synaptic split of SNAP-25: different roles in glutamatergic and GABAergic neurons? Neuroscience 158:223–230 [DOI] [PubMed] [Google Scholar]
- 39. Morihara T., et al. 1999. Distribution of synaptosomal-associated protein 25 in nerve growth cones and reduction of neurite outgrowth by botulinum neurotoxin A without altering growth cone morphology in dorsal root ganglion neurons and PC-12 cells. Neuroscience 91:695–706 [DOI] [PubMed] [Google Scholar]
- 40. Murray A. J., Shewan D. A. 2008. Epac mediates cyclic AMP-dependent axon growth, guidance and regeneration. Mol. Cell Neurosci. 38:578–588 [DOI] [PubMed] [Google Scholar]
- 41. Murray A. J., Tucker S. J., Shewan D. A. 2009. cAMP-dependent axon guidance is distinctly regulated by Epac and protein kinase A. J. Neurosci. 29:15434–15444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Osen-Sand A., et al. 1993. Inhibition of axonal growth by SNAP-25 antisense oligonucleotides in vitro and in vivo. Nature 364:445–448 [DOI] [PubMed] [Google Scholar]
- 43. Osen-Sand A., et al. 1996. Common and distinct fusion proteins in axonal growth and transmitter release. J. Comparative Neurol. 367:222–234 [DOI] [PubMed] [Google Scholar]
- 44. Ostrom R. S., Bundey R. A., Insel P. A. 2004. Nitric oxide inhibition of adenylyl cyclase type 6 activity is dependent upon lipid rafts and caveolin signaling complexes. J. Biol. Chem. 279:19846–19853 [DOI] [PubMed] [Google Scholar]
- 45. Oyler G. A., et al. 1989. The identification of a novel synaptosomal-associated protein, SNAP-25, differentially expressed by neuronal subpopulations. J. Cell Biol. 109:3039–3052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pan P. Y., Tian J. H., Sheng Z. H. 2009. Snapin facilitates the synchronization of synaptic vesicle fusion. Neuron 61:412–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Peace A. G., Shewan D. A. 2011. New perspectives in cyclic AMP-mediated axon growth and guidance: The emerging epoch of Epac. Brain Res. Bull. 84:280–288 [DOI] [PubMed] [Google Scholar]
- 48. Pieroni J. P., et al. 1995. Distinct characteristics of the basal activities of adenylyl cyclases 2 and 6. J. Biol. Chem. 270:21368–21373 [DOI] [PubMed] [Google Scholar]
- 49. Piggott L. A., Bauman A. L., Scott J. D., Dessauer C. W. 2008. The A-kinase anchoring protein Yotiao binds and regulates adenylyl cyclase in brain. Proc. Natl. Acad. Sci. U. S. A. 105:13835–13840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Premont R. T., Chen J., Ma H.-W., Ponnapalli M., Iyengar R. 1992. Two members of a widely expressed subfamily of hormone-stimulated adenylyl cyclases. Proc. Natl. Acad. Sci. U. S. A. 89:9809–9813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Scarr E., Gray L., Keriakous D., Robinson P. J., Dean B. 2006. Increased levels of SNAP-25 and synaptophysin in the dorsolateral prefrontal cortex in bipolar I disorder. Bipolar Disord. 8:133–143 [DOI] [PubMed] [Google Scholar]
- 52. Seino S., Shibasaki T. 2005. PKA-dependent and PKA-independent pathways for cAMP-regulated exocytosis. Physiol. Rev. 85:1303–1342 [DOI] [PubMed] [Google Scholar]
- 53. Shelly M., et al. 2010. Local and long-range reciprocal regulation of cAMP and cGMP in axon/dendrite formation. Science 327:547–552 [DOI] [PubMed] [Google Scholar]
- 54. Shewan D., Dwivedy A., Anderson R., Holt C. E. 2002. Age-related changes underlie switch in netrin-1 responsiveness as growth cones advance along visual pathway. Nat. Neurosci. 5:955–962 [DOI] [PubMed] [Google Scholar]
- 55. Shirasu M., et al. 2000. VAMP-2 promotes neurite elongation and SNAP-25A increases neurite sprouting in PC12 cells. Neurosci. Res. 37:265–275 [DOI] [PubMed] [Google Scholar]
- 56. Snow B. E., Betts L., Mangion J., Sondek J., Siderovski D. P. 1999. Fidelity of G protein beta-subunit association by the G protein gamma-subunit-like domains of RGS6, RGS7, and RGS11. Proc. Natl. Acad. Sci. U. S. A. 96:6489–6494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tafoya L. C., et al. 2006. Expression and function of SNAP-25 as a universal SNARE component in GABAergic neurons. J. Neurosci. 26:7826–7838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tan C. M., Kelvin D. J., Litchfield D. W., Ferguson S. S., Feldman R. D. 2001. Tyrosine kinase-mediated serine phosphorylation of adenylyl cyclase. Biochemistry 40:1702–1709 [DOI] [PubMed] [Google Scholar]
- 59. Tang B. L. 2001. Protein trafficking mechanisms associated with neurite outgrowth and polarized sorting in neurons. J. Neurochem. 79:923–930 [DOI] [PubMed] [Google Scholar]
- 60. Taussig R., Gilman A. G. 1995. Mammalian membrane-bound adenylyl cyclases. J. Biol. Chem. 270:1–4 [DOI] [PubMed] [Google Scholar]
- 61. Thakur P., Stevens D. R., Sheng Z. H., Rettig J. 2004. Effects of PKA-mediated phosphorylation of Snapin on synaptic transmission in cultured hippocampal neurons. J. Neurosci. 24:6476–6481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Thompson P. M., Egbufoama S., Vawter M. P. 2003. SNAP-25 reduction in the hippocampus of patients with schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 27:411–417 [DOI] [PubMed] [Google Scholar]
- 63. Tian J. H., et al. 2005. The role of Snapin in neurosecretion: snapin knock-out mice exhibit impaired calcium-dependent exocytosis of large dense-core vesicles in chromaffin cells. J. Neurosci. 25:10546–10555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Tojima T., Hines J. H., Henley J. R., Kamiguchi H. 2011. Second messengers and membrane trafficking direct and organize growth cone steering. Nat. Rev. Neurosci. 12:191–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Verderio C., et al. 2004. SNAP-25 modulation of calcium dynamics underlies differences in GABAergic and glutamatergic responsiveness to depolarization. Neuron 41:599–610 [DOI] [PubMed] [Google Scholar]
- 66. Villacres E. C., Wong S. T., Chavkin C., Storm D. R. 1998. Type I adenylyl cyclase mutant mice have impaired mossy fiber long-term potentiation. J. Neurosci. 18:3186–3194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wang S. C., et al. 2007. Regulation of type V adenylate cyclase by Ric8a, a guanine nucleotide exchange factor. Biochem. J. 406:383–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wang S. C., Lin J. T., Chern Y. 2009. Novel regulation of adenylyl cyclases by direct protein-protein interactions: insights from snapin and ric8a. Neuro-Signals 17:169–180 [DOI] [PubMed] [Google Scholar]
- 69. Wang Y., Tang B. L. 2006. SNAREs in neurons—beyond synaptic vesicle exocytosis. Mol. Membrane Biol. 23:377–384 [DOI] [PubMed] [Google Scholar]
- 70. Wicker R., et al. 2000. Cloning and expression of human adenylyl cyclase type VI in normal thyroid tissues. Biochim. Biophys. Acta 1493:279–283 [DOI] [PubMed] [Google Scholar]
- 71. Willoughby D., Cooper D. M. 2007. Organization and Ca2+ regulation of adenylyl cyclases in cAMP microdomains. Physiol. Rev. 87:965–1010 [DOI] [PubMed] [Google Scholar]
- 72. Wong S. T., et al. 1999. Calcium-stimulated adenylyl cyclase activity is critical for hippocampus-dependent long-term memory and late phase LTP. Neuron 23:787–798 [DOI] [PubMed] [Google Scholar]
- 73. Wu Z. L., et al. 1995. Altered behavior and long-term potentiation in type I adenylyl cyclase mutant mice. Proc. Natl. Acad. Sci. U. S. A. 92:220–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Yoshimura M., Cooper D. M. 1992. Cloning and expression of a Ca(2+)-inhibitable adenylyl cyclase from NCB-20 cells. Proc. Natl. Acad. Sci. U. S. A. 89:6716–6720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Zhou Q., Xiao J., Liu Y. 2000. Participation of syntaxin 1A in membrane trafficking involving neurite elongation and membrane expansion. J. Neurosci. Res. 61:321–328 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.