Abstract
Apical constriction of epithelial cells is a widely used morphogenetic mechanism. In the Drosophila embryo, the apical constrictions that internalize the mesoderm are controlled by the transcription factor Twist and require intact adherens junctions and a contractile acto-myosin network. We find that adherens junctions in constricting mesodermal cells undergo extensive remodeling. A Twist target gene encoding a member of the tumor necrosis factor (TNF) receptor-associated factor (TRAF) family, Traf4, is involved in this process. While TRAFs are best known for their functions in inflammatory responses, Traf4 appears to have a different role, and its mechanism of action is poorly understood. We show that Traf4 is required for efficient apical constriction during ventral furrow formation and for proper localization of Armadillo to the apical position in constricting cells. Traf4 and Armadillo interact with each other physically and functionally. Traf4 acts in a TNF receptor- and Jun N-terminal protein kinase (JNK)-independent manner to fine-tune the assembly of adherens junctions in the invaginating mesodermal cells.
INTRODUCTION
The modulation of cadherin-based cell adhesion plays a major role in morphogenetic mechanisms during development and pathology (reviewed in reference 41). The downregulation of adherens junctions is a prerequisite for epithelial-mesenchymal transitions, for example, during gastrulation, neurulation, or metastatic behavior of tumor cells. In the developing Drosophila embryo, the first dramatic defect resulting from failure in the proper formation of junctions is seen during the apical constrictions of the ventral cells of the blastoderm epithelium that lead to the invagination of the mesoderm (17, 71).
The cell shape changes associated with the invagination of the prospective mesoderm have served as a paradigm for elucidating the complete genetic program that controls apical constriction. They occur over a short period of time, in the absence of cell division or other tissue movements (reviewed in references 44 and 66). A set of factors that are present in the egg before fertilization are specifically modulated in the mesoderm under the control of genes expressed in the embryo at the time when apical constriction is due to occur. These factors include the components of adherens junctions (such as E-cadherin and the catenins), a heterotrimeric G protein, and the cytoskeletal regulators RhoGEF2, Abl, and Ena (5, 19, 28, 37, 58). RhoGEF2 accumulates apically in mesodermal cells prior to constriction and is necessary for the apical accumulation of myosin and apical constriction (5, 26, 28, 54). The heterotrimeric G protein, defined by its alpha-subunit Concertina, contributes to the localization and activation of RhoGEF2 (37).
Interfering with the formation of adherens junctions impairs ventral furrow formation (15, 17, 53, 71). In the absence of properly assembled junctions, the contracting cytoskeleton is unable to constrict the apical part of the cells but rather detaches from the periphery and collapses in a large aggregate under the apical surface. In addition to the junctions being assembled properly, the underlying cortical domain also has to be patterned or polarized correctly for apical constriction to occur. Thus, F-actin, which is normally concentrated apically in constricting cells, is distributed over a much wider membrane domain in embryos lacking Abl, and this correlates with apical constrictions being irregular and too slow (19).
We previously found that the apical positioning of the adherens junction component beta-catenin/Armadillo in the cells that form the ventral furrow occurs in a two-step process: the loss of Armadillo from its subapical position, controlled by the transcription factor Snail, which is followed by its reassembly at the most apical point of the lateral membranes of ventral cells (37), mediated by two other Twist targets, the secreted peptide Fog and the membrane protein T48, acting via RhoGEF2 and myosin. We have recently found a fourth Twist target, which encodes a homolog of the mammalian cytoplasmic protein Traf4, that is involved in the formation of the ventral furrow (50).
Traf4 is a member of the family of tumor necrosis factor (TNF) receptor-associated factors (TRAFs) (reviewed in references 8 and 14), a group of cytoplasmic proteins, of which there are three in Drosophila, named Traf3, -4, and -6 (23, 43, 50). Unlike the other members of this family, which interact with the TNF receptor and are involved in transmitting signals leading to immune, inflammatory, or apoptotic responses, Traf4 has not been found to bind to the TNF receptor, and its participation in TNF-mediated events may be indirect. Instead, it seems to be involved in morphogenetic and developmental processes (49).
The subcellular mechanism by which Traf4 acts is not yet understood, and localization of Traf4 varies in different cell types. Cytoplasmic and nuclear staining have been reported, and it has also been observed at sites of cell-cell contact and at tight junctions (21, 35). Searches for interaction partners have identified, among others, beta-catenin and several cytoskeleton-associated proteins (64, 73). One role for Traf4 may be in determining the subcellular localization of proteins, as for example in targeting NADPH oxidase to the tips of membrane ruffles (73).
The functions of Drosophila Traf4 and Traf6 appear to resemble those of their vertebrate counterparts. Transmission of the signal from the Drosophila TNF-like ligand Eiger requires Traf6 but not Traf4 (75). Traf6 can act both on NF-κB and on Jun N-terminal protein kinase (JNK) (11, 75), while Traf4 can signal via the JNK pathway and interacts physically with the Ste20 kinase Msn (11, 46, 51). Traf4 has an accessory function in asymmetric cell divisions in the nervous system, where it participates in localizing the cell fate determinants Prospero and Miranda (72). Traf4 itself is localized apically in the asymmetrically dividing cell, and this localization depends on the Drosophila Par3 homolog Bazooka (72). From these results and the work reported here, a role emerges for Traf4 in organizing the fine patterning of the cortical domain of cells undergoing morphogenetic processes.
MATERIALS AND METHODS
Fly stocks and crosses.
The upstream activating sequence (UAS)-Traf1HA stock was from Masayuki Miura (40), the UAS-Eiger stock was from Konrad Basler (51), the UAS-Puckered stock was from Alfonso Martinez-Arias (48), the UAS-DN Misshapen stock was from Yong Rao (33), and the UAS-Misshapen, UAS-Basket, and UAS-Hemipterous stocks were from Marek Mlodzik (7). Traf4ex1 and Traf6ex1 stocks were from Jongkyeong Chung (11), and the Traf4L2 stock was from Yang Xiaohang (72); the Traf43 stock was described previously (50). The p38a1 stock was from Ross Cagan (16), and the UAS-p38bIR stock was from Takashi Adachi-Yamada (2). The bifR47 stock was provided by William Chia (3). The Twist-Gal4 stock was from Michael Akam (24), and the matGal4 [P(w+ mat α-tub Gal VP16)] stock from Daniel St. Johnston. The UAS-armover and UAS-armunder stocks were from Jean-Paul Vincent (22). The Armadillo-green fluorescent protein (GFP) stock was P[arm-GFP.P]83 and was obtained from the Bloomington stock collection. ctaR10/CyO, twiEY53/CyO, and the snail deficiency [Df(2L)TE116GW11] stocks were from the Tübingen stock collection, and the t48 deletion stock [Df(3R)CC1.2] was from David Strutt. All other stocks were from the Bloomington, Szeged, or Exelixis stock centers.
Germ line clones were generated using standard methods, as described previously (13).
Constructs.
The full-length Traf4 cDNA for the Traf4-RA transcript, LD20987 (BDGP), was used as a template for all the constructs described below. To construct full-length and truncated UAS-Traf4 constructs for transgenic Drosophila stock generation, oligonucleotides with a 5′ BamHI overhang and a 3′ XhoI overhang, respectively, were used to amplify the required product and cloned into the same sites in the pUAST vector (9). The full-length Traf4 coding sequence (CDS) was cloned into the pCaSpeR 2× proximal enhancer (PE) β-galactosidase (β-Gal) vector (34) using BglII and XbaI overhangs to generate the 2× PETraf4 transgene.
The full-length Traf4 coding sequence was cloned into the S2 cell vector pDeGFP (10) using the BglII and Sbf1 sites, using oligonucleotides with the same overhangs. Full-length Traf4 was generated for S2 cell transfections by cloning them into the copper-inducible vector pRmHa-3 with hemagglutinin (HA) or Flag tags using BamHI and XbaI overhangs.
The pUAST-wengen construct was generated from the Wengen cDNA clone RE29502 by amplifying the coding sequence with oligonucleotides having EcoRI and BglII overhangs and cloning into the same sites in pUAST.
The Armadillo cDNA clone LD23131 was used to amplify the coding sequence with BglII and XbaI overhangs and cloned into pRmHa-3 with HA or Flag tags.
To construct glutathione S-transferase (GST)-tagged Armadillo, the coding sequence lacking the ATG (start codon) was amplified with BamHI and XhoI overhangs and cloned into the corresponding sites of pGEX-4T-3. A template for in vitro translation of Traf4 was generated by amplification of the CDS of Traf4 (LD20987) with BglII overhangs and cloning into the BglII site of pSP64T carrying the β-globin 5′ and 3′ untranslated regions (UTR) (39).
Production of Traf4 antibody.
To generate antisera against the Traf4 protein, two synthetic peptides (RILRGRMTLHKSKD [amino acids 123 to 136] and TWENFQRPSNEPD [amino acids 360 to 372]) were synthesized (Biogenes, Germany), and 2 individual rabbits each were immunized with each of the peptides. Bleeds were obtained after 30 days and 60 days. The antisera were tested for specificity against the Traf4 protein on embryos and S2 cells overexpressing GFP-Traf4 constructs by immunofluorescence as well as by Western blotting. The results were compared to background signals from preimmune serum. The sera from the animals showing the best immunoreactivity were preabsorbed using Traf4 mutant embryos and used further.
Embryo fixation, immunofluorescence, immunohistochemistry, and in situ hybridization.
Embryos were fixed and processed according to standard protocols (60). After dechorionation using bleach, they were fixed using either 4% formaldehyde in phosphate-buffered saline (PBS) and equal volumes of heptane (for Traf4, Twist, Eve, P-tyrosine, myosin, Stardust, Par6, atypical protein kinase C [aPKC], Dlg) or using heat fixation (for Canoe and Armadillo). For E-cadherin staining using the rat antibody, a modified buffer was used instead of PBS (55 mM NaCl, 40 mM KCl, 15 mM MgSO4, 1 mM CaCl2, 10 mM tricine [pH 6.9], 20 mM glucose, 50 mM sucrose) (55). Devitellinization of embryos was done by vortexing in methanol for 30 s, except in the case of E-cadherin and phalloidin, where 80% ethanol was used instead. For Armadillo and E-cadherin (rabbit antibody) as well as neurotactin and E-cadherin costaining, the embryos were heat fixed.
Immunofluorescence was carried out using standard methods. For immunohistochemical detection, a biotinylated secondary antibody (anti-rabbit biotinylated antibody from Dianova, Germany) was used, and detection was carried out using the Vectastain ABC Elite kit (Vector Laboratories) using diamindoenzidine (DAB) as a substrate. Cobalt chloride (1%) and nickel chloride (1%) were added to obtain a blue color for one of the antibodies in cases where 2 antigens were detected.
For in situ hybridization, embryos were fixed in 4% formaldehyde in PBS with equal volumes of heptane, devitellinized in methanol, and processed as previously described (69).
Antibodies.
The following antibodies were used (dilutions used for immunofluorescence or immunohistochemistry are indicated): rabbit anti-Traf4 antibody, 1:200; mouse anti-Traf4 antibody, 1:500 (72); mouse anti-Armadillo antibody, 1:1,000 (DSHB) (61); rat anti-DE-cadherin (DCAD2) antibody, 1:40 (DSHB) (57); rabbit anti-E-cadherin antibody (Santa Cruz; catalog no. sc-33743); mouse antineurotactin antibody, 1:5,000 (from C. Goodman [32]); rabbit anti-Canoe antibody, 1:500 (Ana Carmena); rabbit anti-Par-6 antibody, 1:500 (59); rabbit anti-ζPKC antibody, 1:1,000 (C20, Santa Cruz); chicken anti-zipper antibody, 1:250 (Eric Wieschaus), and rabbit antimyosin antibody, 1:500 (36); mouse antiphosphotyrosine antibody, 1:20 (Deborah Morrison); mouse anti-Discs-large (4F3) antibody, 1:100 (DSHB); rabbit anti-Twist antibody, 1:3,000 (63); rabbit anti-Eve antibody, 1:5,000 (20); rat anti-Stardust antibody, 1:500 (Elisabeth Knust); and sheep antidigoxigenin antibody, 1:500 (Roche).
Secondary antibodies used were mouse-, rat-, and rabbit-specific antibodies conjugated to Alexa dyes 488, 568, and 647 at 1:500 (Invitrogen). Hoechst for nuclear staining was from Sigma. Tetramethyl rhodamine isocyanate (TRITC)-phalloidin (Sigma) was used at 1:250.
Dilutions of the above-described antibodies for Western blots were as follows mouse anti-Armadillo antibodies, 1:500; rabbit anti-Traf4 antibodies, 1:400. In addition, we used rabbit anti-GFP antibody (1:5,000; Torrey Pines Biolabs), mouse anti-Flag antibody (1:1,000; Sigma), rat anti-HA antibody (1:1,000; Roche), anti-mouse IRDye 700; anti-mouse IRDye 800; anti-rabbit IRDye 700; anti-rabbit IRDye 800; anti-rat IRDye 700; and anti-rat IRDye 800 (all from Rockland, used at 1:5,000).
Mounting, sectioning, and imaging of embryos.
For whole-mount DAB-stained embryos, embryos were washed in PBS-Tween after staining and dehydrated in an ethanol gradient, followed by incubation in water-free acetone. After this step, the embryos were incubated overnight in an acetone-araldite (1:1) solution at −20°C. Embryos were either stored in this form or mounted on plastic cups (2-cm diameter), where they were sorted, staged, and statistically analyzed. Individual embryos were mounted on a drop of araldite on glass slides and photographed. A similar procedure was also used in the case of embryos derived from in situ hybridization.
To obtain cross sections of fluorescently stained embryos, embryos after the antibody-staining protocol were washed in PBS-Tween. About 50 to 100 embryos were mounted in PBS-Tween on a coverslip and were staged. Embryos at the right stage were then transferred to ProLong Gold antifade reagent (Invitrogen) and hand cut using a 26-gauge needle. The cut middle portion of the embryo was stood on its end before the medium hardened. The head and tail sections of the cut embryos were kept close by for staging purposes later on. The medium was allowed to harden for 16 to 24 h at room temperature, and after hardening, the coverslips were inverted onto a drop of the same medium on a glass slide. The medium was allowed to harden further for 24 h before imaging.
Imaging was done using the Zeiss Axioplan (immunohistochemical stainings) microscope and a Zeiss Apotome, Leica SP2 confocal (HCX PL APO 63×, numerical aperture [NA] of 1.32) or Olympus Fluoview FV1000 (UPLS APO 60×, NA of 1.35). Image processing was done using Adobe Photoshop CS4.
Measurement of line profiles.
Line profiles were measured on the original, unedited confocal images using Volocity three-dimensional (3D) image analysis software (PerkinElmer). A 1-pixel-width line was drawn along the cell membrane of mesodermal cells from apical to basal, and the pixel intensities were measured along the first 20 μm (approximately 50% of the total cell length). For the comparison of E-cadherin and neurotactin distribution, the intensity values for 39 lines measured on 7 embryos were averaged; the significant difference between the two means was determined with Student's t test in Microsoft Excel. For the comparison of E-cadherin and Armadillo distribution, the fluorescence intensity values for 5 lines were averaged and subsequently calculated as the percentage of the brightest average pixel value for each channel.
Statistic evaluation of gastrulation defects.
A total of 200 to 500 fixed and stained embryos were embedded in approximately 1 ml araldite without allowing the araldite to polymerize. Under a dissecting microscope, all embryos between the cellular blastoderm stage and early germ band extension were then manually collected without regard to phenotype and lined up in four age groups. The first group included all embryos up to a stage where the head fold had constricted only on the outer, apical side, but cells had not moved basally below the surface of the blastoderm epithelium. The staging of these embryos is very difficult, and the group contains many embryos that have not yet begun to form a furrow even in collections of wild-type embryos. It was therefore not used for statistical evaluations. The second group included embryos up to the point where the basal end of the cells in the head fold had begun to invaginate, i.e., the Even-skipped (Eve) staining in the first stripe began to appear partly below the level of staining in the other Eve stripes. In the third and fourth groups, Eve stripe 1 had invaginated so deeply that it was fully below the level of the other stripes. Within this group, we differentiated between embryos showing no or almost no folds in the dorsal epithelium (group 3) and those showing clear folds in the dorsal epithelium (group 4). A criterion used in previous analyses of this type, the degree of movement of the posterior midgut invagination, was not taken into consideration, as many of the mutants we analyzed appeared to have defects in this process (see Fig. 4H for an example).
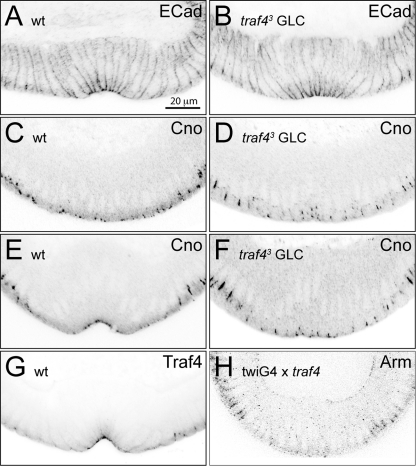
Fig. 4.
Subcellular defects in the traf4 mutant and overexpressing embryos. Embryos stained with antibodies against the indicated junction-associated proteins. (A, B) E-cadherin (ECad) localization is unaffected in traf43 mutant embryos (B) compared to that of wild-type embryos (A). (C to F) Canoe (Cno) shows a slight mislocalization in the mesoderm of traf43 mutant germ line clone-derived embryos at the onset of gastrulation (D) and at stage 6, when wild-type embryos have formed a furrow (F). (C, E) In wild-type embryos, Canoe localizes apically at both stages. (G) Traf4 localizes apically in the mesoderm. (H) Overexpression of Traf4 in the mesoderm using a twist-Gal4 driver displaces Armadillo (Arm) from the junctions. Scale bar, 20 μm.
We then analyzed wild-type embryos in each age group for a criterion to determine which stage of mesodermal morphogenesis had been completed by the majority of embryos at each stage. For the first group, this was the formation of visible, parallel ridges in the ventral epithelium; for the second group, the formation of a visible furrow to the point where cells from the two sides touch each other along the midline; and for the third and fourth group, the complete closure of the ventral furrow. In the evaluation, we considered embryos in each group as normal if the criterion was fulfilled for the whole length of the furrow. Partial furrows or partial closure was considered defective. In the second group, very abnormal-looking furrows were also considered defective, even if ridges were visible along the length of the mesoderm.
S2 cells: transfections, immunofluorescence, and coimmunoprecipitation.
S2 cells were maintained using standard protocols (62). For transfections, cells were plated out in 6-well plates. After 24 h, DNA was transfected using the Fugene reagent (Roche) following the protocol provided by the supplier. The transfected constructs were induced 24 h later with 0.1% copper sulfate solution, and the cells were harvested 24 h after induction. Cell lysis was carried out using radioimmunoprecipitation assay (RIPA) buffer without SDS (150 mM NaCl, 20 mM Tris [pH 7.4], 1% NP-40, 0.05% Triton X-100, 0.5% sodium deoxycholate, 2.5 mM EDTA). Coimmunoprecipitations were carried out on cell lysates which were diluted 5 times using the immunoprecipitation (IP) dilution buffer (190 mM NaCl, 60 mM Tris [pH 7.6], 6 mM EDTA, 1.25% Triton X-100). GFP or Flag antibodies at a dilution of 1:400 were used for the coimmunoprecipitation. Protein G agarose beads (Amersham) were used to bind the antibodies along with immunoprecipitated proteins. The proteins were separated using SDS-PAGE, and Western blotting was carried out using standard protocols on a nitrocellulose membrane. The blots were processed for detection using the Odyssey system (LI-COR Biosciences).
GST pulldown and coimmunoprecipitation.
GST fusion proteins were expressed in Escherichia coli BL21 Star (DE3) (Invitrogen). pGEX-4T-3 was used to prepare GST as a control. Cultures were induced at an optical density at 600 nm (OD600) of ∼0.6 with 0.1 mM IPTG (isopropyl-β-d-thiogalactopyranoside) and incubated for 4 h at 37°C. Purification of GST fusion proteins was performed as described in reference 37 with glutathione Sepharose 4B beads (GE Healthcare). The beads were resuspended in IP buffer (10% glycerol, 50 mM HEPES [pH 7.5], 150 mM NaCl, 1% Triton X-100, 1.5 mM MgCl2, 1 mM EGTA).
Apart from GST-Armadillo, the GST-tagged constructs used were the same as those described in reference 37.
In vitro translation to generate 35S-labeled proteins was done using the TNT SP6 quick-coupled transcription/translation system (Promega) according to the manufacturer's instructions. pSP64T-traf4 and pSP64T-t48intra (37) were used as templates.
For coimmunoprecipitation, the in vitro translated Traf4 or T48intra was incubated with the different GST fusion proteins or GST coupled to glutathione Sepharose beads in IP buffer (10% glycerol, 50 mM HEPES [pH 7.5], 150 mM NaCl, 1% Triton X-100, 1.5 mM MgCl2, 1 mM EGTA) for 3 h at 4°C. The beads were washed with IP wash buffer (10% glycerol, 20 mM HEPES [pH 7.5], 0.1% Triton X-100, 150 mM NaCl), resuspended in Laemmli buffer, and boiled for 2 min. The proteins were separated by SDS-PAGE and analyzed by Coomassie staining and autoradiography.
RESULTS
Traf4 is required for efficient apical constrictions of ventral furrow cells.
Embryos with a traf4 mutation show defects in the formation of the ventral furrow during gastrulation (see Fig. 10F) (50). As in embryos homozygous for fog or t48, the mutant embryos did not fail to make appropriate cell shape changes per se, but apical constrictions in ventral cells were less pronounced, less coordinated, and appeared later than in wild-type embryos.
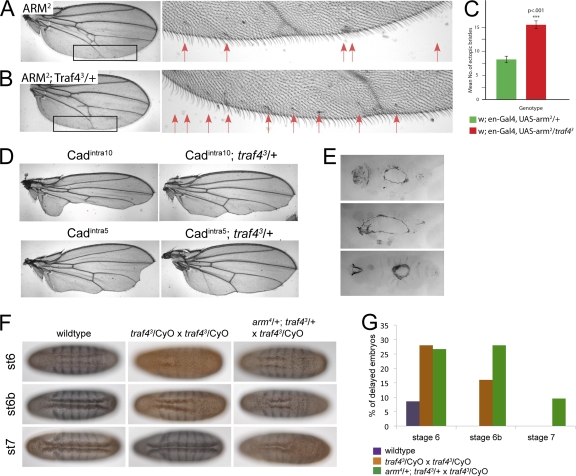
Fig. 10.
(A to D) Genetic interaction between Armadillo and Traf4 in the adult wing. An Armadillo transgene (UAS-arm2) or the intracellular domain of E-cadherin (Cadintra10 and Cadintra5) were expressed in the engrailed domain of the wing of wild-type or heterozygous traf43/+ flies. Reduction of Traf4 enhances the effects of Armadillo overexpression as shown by ectopic bristles (A, B, C) and suppresses the effects of Cadintra overexpression as shown by rescue of the wing blade area (D). (E) Cuticle defects in embryos derived from traf43 homozygous mutant germ line clones: dorsal holes, head open, and ventral holes. (F, G) Genetic interaction between Armadillo and Traf4 in the embryo during gastrulation. (F) Wild-type, homozygous traf43 embryos and traf43 mutant embryos from a mother in which the dose of Armadillo was reduced by half (from an arm4/+; traf43 × arm+/Y; traf43 mating) stained for Even-skipped (blue) and Twist (brown). The ventral surfaces of 3 embryos at successive developmental stages are shown for each genotype. (G) Statistical analysis of the mutant phenotype shown in panel F. Embryos were grouped according to their developmental stage judged from the depth of the head fold and the appearance of dorsal folds. In each group, the percentage of embryos that showed a delay in ventral furrow formation was scored. The reduction of Armadillo levels in traf43 mutant embryos results in a strong enhancement of the mutant phenotype at later stages of gastrulation. Number of delayed embryos and total number of embryos counted: wild type, st6, 3 and 35, st6b, 0 and 34, st7, 0 and 30; traf43/CyO × traf43/CyO, st6, 7 and 25, st6b, 4 and 25, st7 0 and 22; and arm4/+; traf43/+ × traf43/CyO, st6, 8 and 30, st6b, 7 and 25, st7, 2 and 21.
We also surveyed the requirements of traf4 during other stages of the Drosophila life cycle. Homozygous traf43 embryos died during late embryonic or early larval stages without any evident morphological defects. Embryos from traf43 mutant germ line clones showed the same phenotype during gastrulation as zygotic homozygous mutants, but with stronger penetrance. In addition, while zygotic mutants completed embryonic development without visible defects, 29.4% of the fertilized embryos from traf43 germ line clones (n = 500) failed to hatch. They developed variable cuticle defects (43% ventral holes, 29% dorsal closure defects, 19% head defects, and 9% nonspecific defects), which reveal a role for the maternally supplied Traf4 in ectodermal morphogenesis (see Fig. 10E and Fig. 3C and F). Embryos receiving a wild-type chromosome from the father are viable, showing that zygotic expression of Traf4 is sufficient to support normal development.
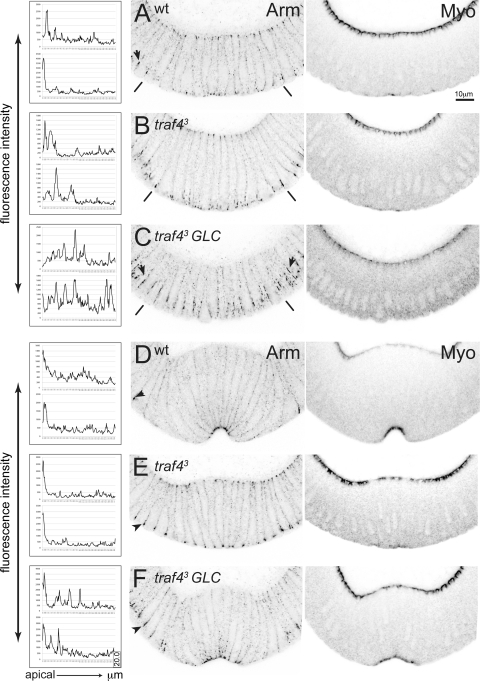
Fig. 3.
Subcellular defects in gastrulating traf4 mutant embryos. All images are confocal photographs of cross sections of gastrulating wild-type and traf4 mutant embryos stained with the indicated antibodies. The images were inverted in Photoshop. (A to F) Armadillo (left; Arm) and myosin (right; Myo) localization in wild-type (A, D) and traf43 (B, E) embryos and embryos from traf43 germ line clones (GLCs) (C, F) at the beginning of and during ventral furrow formation. The graphs in the left panel show fluorescent intensity measurements of Armadillo staining along the lateral membranes measured on the confocal image shown. Single-pixel intensity values (y axis, 1 pixel = 102.899 nm) are plotted for the first 20 μm of the cell membrane, with the total cell length being approximately 40 μm. y axis, intensity values; x axis, distance in micrometers from apical (0 μm) to basal (20 μm). (A to C) Onset of gastrulation. (A) In wild-type embryos, Armadillo is lost from a subapical position in mesodermal cells and starts to relocalize apically. Armadillo fails to relocalize properly in the mesoderm in traf43 zygotic mutant (B) and in traf43 germ line clone-derived mutant (C) embryos, covering a broader domain than normal. Apical localization of myosin is observed in traf43 mutant embryos. Embryos derived from traf43 mutant germ line clones show a delay in myosin accumulation and a broader domain of Armadillo localization in the ectoderm (arrows, compare panels C and A). The lines mark the border of the mesoderm. (D to F) Ventral furrow formation. (D) Armadillo is localized to tight apical spots in wild-type constricting mesodermal cells. Apical localization of Armadillo occurs in traf43 mutant embryos (E) although it is less efficient and sometimes covers a broader domain than wild-type embryos (F). Apical myosin accumulation is seen in constricting traf43 mutant cells, although it is less pronounced (E, F). Armadillo in the ectoderm continues to show a broader localization in traf43 GLC mutant embryos (arrowhead in panel F, compare to panels D and E). Magnification, ×60; scale bar, 10 μm.
Expression of Traf4 RNA and protein.
Traf4 is expressed throughout development. The first zygotic expression is seen in the mesodermal primordium and in a dynamic pattern in the ectoderm (e.g., a strong domain in the region that will give rise to the ectodermal hindgut) (Fig. 1A). We investigated, by RNA in situ hybridization on twist and snail mutant embryos, which of the mesodermal transcriptional regulators controlled Traf4 expression. Mesodermal Traf4 expression is lost in twist mutant embryos and is not affected in snail mutants (Fig. 1B and C), showing that the traf4 gene is controlled by Twist and, given the presence of Twist-binding sites in its promoter (65), probably a direct Twist target.
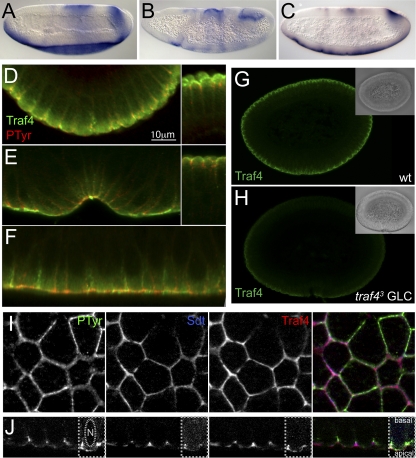
Fig. 1.
Expression of Traf4 mRNA and protein. (A to C) In situ hybridization showing the expression pattern of traf4 in wild-type (A), twist (B), and snail (C) mutant embryos. The ventral expression is lost in twist but not in snail embryos. (D to F) Cross sections (D, E) and a longitudinal section through the mesoderm (F) of gastrulating embryos stained with antibodies against Traf4 (green) and a phosphotyrosine epitope (red). In panels D and E, the left panels show the mesoderm and the right panels the ectoderm in the same embryos. Traf4 localizes at a more apical position than the phosphotyrosine epitope. In the mesoderm (F), additional, lower levels of Traf4 are seen in a broader domain radiating away from the apical end. (G, H) Specificity of the Traf4 antiserum. Wild-type embryos (G) and traf43 germ line clone-derived mutant embryos devoid of maternal and zygotic Traf4 (H) were stained with the Traf4 antiserum. The insets show differential interference contrast (DIC) images of the same embryos. (I, J) Surface view (I) and optical cross section (J) of the ectoderm of two stage 7 embryos stained for phosphotyrosine (green), Stardust (blue), and Traf4 (red). Traf4 colocalizes with Stardust at the apical point of the lateral cell membranes, partly overlapping the domain of phosphotyrosine, which extends more basally. The right part of each of the images in panel J was contrast enhanced to visualize the outlines of the cell and the nucleus.
To understand the function of Traf4 in regulating cell shape changes in the mesoderm, it is necessary to know where in the cell it acts. Most of the Traf proteins in mammals interact with the TNF receptor, but this has not been found to be the case for Traf4. To determine the subcellular localization of Traf4, we generated antisera against peptides from two regions of the protein.
In cross sections of cellular blastoderms, Traf4 shows a broad apical distribution, partially overlapping with the phosphotyrosine epitope (Fig. 1D). This localization sharpens in the ectoderm (Fig. 1D and E, right). In the mesoderm, Traf4 remains tightly apical during ventral furrow formation (Fig. 1E). Additional, weaker staining for Traf4 can be seen extending away basally from the apical high concentration in the mesoderm during gastrulation (Fig. 1F). Unlike the mammalian Traf4, which was also seen in the nucleus in transfected cells, Drosophila Traf4 is not seen in the nucleus in embryos. In surface and longitudinal views of the ectoderm of stage 7 embryos, Traf4 is seen at the outlines of cells, where it colocalizes with Stardust at the apical end of cell membranes, above the slightly more basally localized unidentified phosphotyrosine epitope (Fig. 1I and J). The staining we observe is specific, as it is not seen in embryos devoid of Traf4 (Fig. 1G and H).
Traf4 signaling pathway.
We wished to know by which pathway Traf4 affects ventral furrow formation. Briefly, the results reported below show that it does not act through any of the known pathways through which other proteins of the Traf family act. The major mediators of Traf signaling in vertebrates are the JNK cascade and NF-κB, but conflicting observations from overexpression and loss-of-function studies for the participation of Traf4 in these pathways in flies have been published (11, 40, 46, 51, 75). It seemed possible that Traf4 in the mesoderm might act through the Drosophila NF-κB-homolog Dorsal, which is responsible for fate determination in the mesoderm. However, we found no evidence for an impairment of Dorsal activity in traf4 mutants, as judged by the expression patterns of genes that are sensitive to levels of Dorsal, such as single-minded (not shown).
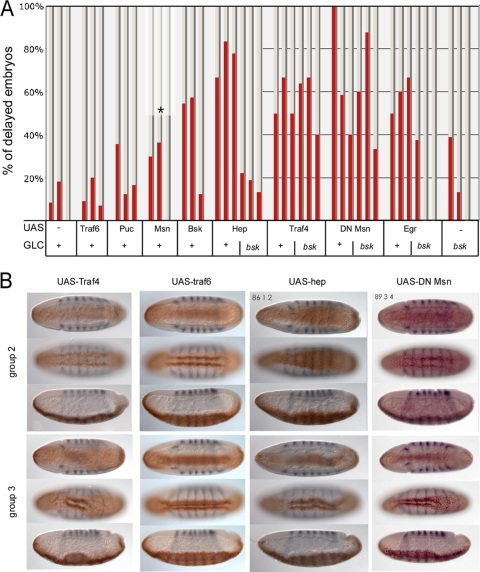
To test whether components of the TNF or JNK signaling pathways might be involved in the same pathway in the mesoderm as Traf4, we exploited the fact that overexpression of Traf4 in the mesoderm caused gastrulation defects which were morphologically similar to those resulting from loss of Traf4 (Fig. 2B). The MATH domain of Traf4 is both sufficient and necessary for this effect (data not shown), which would be consistent with an involvement in JNK signaling (46). We therefore tested whether the overexpression of components of the TNF and JNK pathways had the same effects. Expressing Eiger, Basket, or Hemipterous in the mesoderm (under the control of Twist-Gal4) caused defects very similar to those caused by Traf4 overexpression (Fig. 2A and B), while expression of the JNK antagonist Puckered had no effect. Thus, while high expression levels of TNF or JNK pathway components interfere with mesodermal cell shape changes, blocking the pathway does not seem to have this effect. These findings were confirmed by genetic loss-of-function studies. Homozygous (eiger) or hemizygous [wengen, Df(1)ED447] mutant embryos or embryos from basket or hemipterous mutant germ line clones showed no defects in the early mesoderm. This shows that whereas the ectopic activation of the TNF receptor and the JNK pathway interferes with mesodermal cell shape changes, these pathways, in contrast to Traf4 itself, are not required for the cell shape changes in the early mesoderm.
Fig. 2.
Overexpression of Trafs and components of the TNF and JNK pathways. (A) The JNK pathway and Traf proteins were overexpressed in the mesoderm of wild-type embryos or embryos derived from homozygous mutant bsk germ line clones using a Twist-Gal4 driver line. Embryos were fixed and stained with antibodies against Twist and Even-skipped and sorted under a dissecting microscope into groups at three different stages of gastrulation (shown as three columns for each genotype) judged on the depth of the head fold and the appearance of dorsal folds (see Materials and Methods). In each group, the embryos were sorted into those without any apparent defects and those with delayed or defective ventral furrows. The percentage of the latter is shown as red bars. With the exception of the UAS-Msn construct, all constructs were derived from homozygous fathers, such that all embryos had both the Twist-Gal4 construct and the UAS construct. In the case of UAS-Msn, only half of the embryos carry both constructs. (B) Examples of the phenotypes scored in panel A. Each set of three images depicts the same embryo in a ventral midsagittal section, a ventral surface view, and a lateral midsagittal section. An early stage (top photographs, representative of column 2 in the graph above) and a late stage (bottom photographs, representative of column 3) are shown for each genotype.
The Ste20-like kinase Misshapen (Msn) can activate the JNK cascade and has been shown to interact physically with Traf4. Since Msn is needed during oogenesis, it is not possible to generate embryos lacking Msn (70), but the expression of a dominant negative version of Msn (33, 38) produces defects in the ventral furrow, indicating that Msn might be required for furrow formation (Fig. 2A and B).
The fact that embryos lacking JNK are phenotypically normal during early development allowed us to conduct epistasis experiments to determine which of the putative upstream components act through JNK. To test this, we expressed Eiger, Hemipterous, Traf4, and dominant negative Msn in embryos from bsk homozygous mutant germ line clones. The effects of Eiger and Hemipterous are fully suppressed by bsk, confirming the well-established signaling cascade. In contrast, the effects of Traf4 and Msn are not changed by the lack of JNK (Fig. 2A). Therefore, Traf4 and Msn do not act through the JNK cascade in this instance. Together these results indicate that the JNK pathway is not required for the cell shape changes and that Msn and Traf4 must act through a different pathway.
One candidate for an alternative downstream mediator was the p38 mitogen-activated protein kinase (MAPK), which in mammals can be activated by TRAFs (31). Drosophila has two p38 genes, p38a and p38b. We examined the phenotypes of embryos homozygous for a null mutant for p38a (16), embryos overexpressing a p38b antisense construct (2), and a combination of the two. In none of these stocks did we find any significant defects in ventral furrow formation, suggesting that Traf4 does not function via the p38 cascade during ventral furrow formation.
In summary, Traf4 does not use any of the known Traf signal transduction pathways to mediate its function in mesodermal cells.
Subcellular defects in Traf4 mutants.
Successful apical constriction in the mesoderm depends on intact adhesive junctions between cells and on the correct assembly of a functional contractile acto-myosin network. We therefore examined the distributions of the components of these systems and their known regulators in traf4 mutant embryos.
The functionality of the acto-myosin network that is responsible for the apical constriction of mesodermal cells depends on the proper distribution of actin regulators on the lateral membrane domains. The Abl kinase prevents the actin-stabilizing Ena, and thereby actin, from spreading over a wide region of the lateral membranes (19). We examined the distribution of actin in traf4 mutants but found no abnormalities (not shown), and specifically, we do not see abnormally high actin levels along the apical part of the lateral membranes, which indicates that Traf4 is not required for the actin-regulating pathway downstream of or in parallel to Abl and Ena. Myosin (Fig. 3) and RhoGEF2 (not shown) are able to relocalize apically in traf4 mutants, though possibly less efficiently. The effect of loss of traf4 is not enhanced by concomitant loss of fog or t48 (not shown), both of which act through RhoGEF2 and therefore through myosin. Thus, although subtle effects on the acto-myosin network cannot be excluded, there appears to be no essential requirement for Traf4 in its regulation.
Adherens junctions are necessary as anchoring points for the contractile acto-myosin network. We have previously shown that the movement of the adherens junction component Armadillo from its original, subapical position to the extreme apical position occurs in two steps (37). First, in a Snail-dependent process, Armadillo dissociates from the subapical point, and subsequently, under the control of T48 and Concertina signaling, it reassembles apically. We tested whether either of these steps was affected in traf4 mutants. In pregastrulation traf43 mutants, Armadillo is seen in its usual subapical position. In late traf43 mutant embryos, in which a furrow has formed, Armadillo is localized apically, similar to wild-type embryos. However, the transition between these states shows defects: it is delayed and inefficient. Thus, Armadillo is often seen spread over a much wider region in traf43 mutants in early stages (Fig. 3B), with only a minor enrichment at the apical end at later stages (Fig. 3E), and sometimes no apical enrichment is seen. Embryos from the traf43 mutant germ line clones show similar defects in Armadillo relocalization in the mesoderm (Fig. 3C and F). We quantified these Armadillo relocalization defects by measuring the Armadillo fluorescence intensity on the lateral membranes of mesodermal cells prior to and during gastrulation in wild-type, traf43 zygotic, and traf43 germ line clone-derived embryos and verified that the distribution of Armadillo was different in the traf43 mutant embryos than in the wild-type embryos (Fig. 3, left). In addition, ectodermal adherens junctions are affected and show a broader distribution of Armadillo, consistent with a role of maternal Traf4 in ectodermal morphogenesis (Fig. 3C and F).
We also investigated the distribution of Armadillo in embryos in which Traf4 had been overexpressed in the mesoderm. In these embryos, we found a strong reduction or loss of Armadillo from the adherens junctions (Fig. 4H). Thus, both loss and overexpression of Traf4 affect Armadillo localization. The loss of Armadillo in the Traf4-overexpressing embryos is consistent with the more pronounced later morphological defects in these embryos (data not shown). These results indicate that Traf4 is involved in fine-tuning Armadillo reassembly.
A specialized membrane domain in constricting cells.
The distribution of Armadillo along a broad domain of the lateral membranes in the traf4 mutant mesodermal cells raised the question of what distinguishes this region.
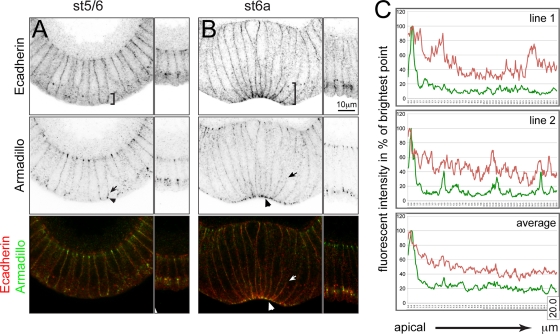
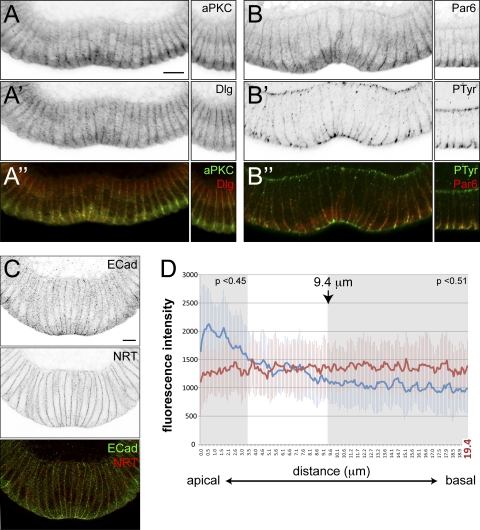
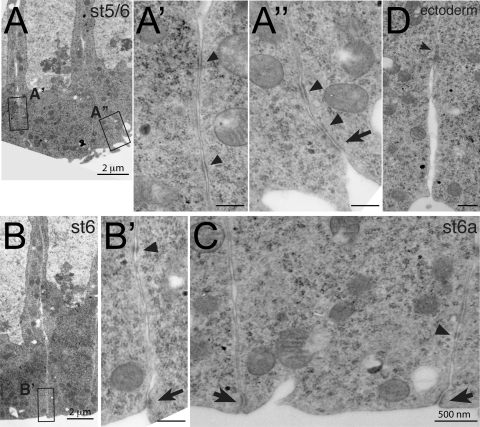
We had previously assumed that when Armadillo reassembles at its apical position, it follows E-cadherin, which is present at high concentrations at the apical ends of constricting mesodermal cells. We were therefore surprised to find that E-cadherin does not show the same distribution as Armadillo in constricting mesodermal cells (Fig. 5). Although E-cadherin is enriched at the most apical point of the membrane, we also see significant staining in a broader domain covering about one quarter of the lateral plasma membrane, with lower levels stretching throughout the remaining lateral plasma membrane (Fig. 5B and C). This contrasts with the distribution of another membrane protein, neurotactin, which is present at uniform levels throughout the lateral membrane (Fig. 6C and D). The fluorescent intensity of E-cadherin is significantly higher than neurotactin up to 3.4 μm (P < 0.45) and significantly lower beyond 9.4 μm (P < 0.51) from the apical end (shaded areas in Fig. 6D). Thus, in addition to the apical adherens junction proper, with its demonstrable electron-dense structure (56), mesodermal cells also have a broader, specialized membrane domain that is not seen in ectodermal cells. The presence of E-cadherin throughout the more apical quarter is also reflected in the presence of electron-dense structures with the appearance of adherens junctions at a distance from the apical end (Fig. 7A to C).
Fig. 5.
Distribution of E-cadherin and Armadillo in the mesoderm of gastrulating wild-type embryos. (A) Armadillo (green) relocalizes from its subapical to an apical position at the onset of gastrulation (arrowhead). E-cadherin (red) covers a broader domain (brackets), partly colocalizing with Armadillo (arrow). (B) In apically constricted cells, Armadillo colocalizes with E-cadherin in tight apical spots (arrowhead). A gradient of E-cadherin can be seen over the apical quarter of the membrane (brackets). Low-level staining for Armadillo is observed within that domain (arrow). Magnification, ×60; scale bar, 10 μm. (C) Line profile (1 pixel, 68.9 nm) of fluorescence intensities along the cell membrane for E-cadherin (red) and Armadillo (green) measured in the stage 6a embryo shown in panel B plotted against distance (20 μm, x axis). The upper panels show examples for two individual lines given as percentage values of the brightest point (y axis). In the lower graph, the fluorescent intensity values were averaged (5 lines) and are presented as percentages of the brightest average signal (y axis).
Fig. 6.
A specialized membrane domain in mesodermal cells. (A, B) Confocal images of the ventral furrows in hand-sectioned wild-type embryos stained for aPKC (green), Dlg (red), Par6 (red), and Ptyr (green) proteins. The panels on the right show a set of ectodermal cells from the same embryo. aPKC (A), Dlg (A′), and Par6 (B) localize to a broad apical region in mesodermal cells, while an unidentified phosphotyrosine epitope (B′) is concentrated apically in a similar manner as Armadillo. A′′ and B′′ are merged images of panels A and A′ and B and B′, respectively. Magnification, ×63; scale bar, 12 μm. (C, D) Distribution of E-cadherin (ECad) (green) in comparison to the evenly distributed membrane protein neurotactin (NRT) (red). (C) Confocal image in a hand-sectioned embryo costained for E-cadherin and neurotactin used for the measurement of line profiles shown in panel D. The images were inverted in Photoshop, and the bottom panel shows a merged image. (D) Thirty-nine fluorescence intensity line profiles for E-cadherin (blue) and neurotactin (red) were measured along the membrane of mesodermal cells on confocal sections of 7 different embryos from apical to basal (measured length = 20 μm, total cell length = 40 μm). The average of single-pixel intensities (y axis, 1 pixel = 102.899 nm) is plotted against distance (x axis, μm). Magnification, ×60; scale bar, 12 μm.
Fig. 7.
Electron micrographs of mesodermal and ectodermal adherens junctions from gastrulating wild-type embryos. (A to D) Electron micrographs of mesodermal cells (A to C) and ectodermal cells (D). Panels A′, A′′, and B′ show higher magnifications (×20,000) of the boxed areas indicated in the overview (panel A, ×7,000; panel B, ×4,400). The apical sides of mesodermal cells are flattened (A) and slightly constricted (B). In addition to apical spot junctions (A′′ and B′, arrows), electron-dense structures are found along the apical quarter of the lateral membrane (arrowheads in A′, A′′, B′). (C) In apically constricted cells, the apical junction has developed further (arrows), yet some electron-dense spots are still seen further basally (arrowhead). Magnification, ×12,000. (D) Lateral adherens junction in ectodermal cells (from the same embryo as those shown in panel C). Magnification, ×12,000.
The analysis of further junctional proteins reveals that this domain is not exclusively defined by the presence of E-cadherin but also contains aPKC, Par6, and Dlg (Fig. 6A and B). In the ectoderm, aPKC and Par6 concentrate apically during the period of gastrulation, while Dlg is present throughout the membrane but becomes more enriched in the basal region (29, 30) (Fig. 6A and B, right). In contrast, in the mesoderm their distribution again covers the apical quarter of the lateral plasma membrane (Fig. 6A and B).
The broad domain in which these proteins reside resembles that which is occupied by Armadillo in traf4 mutants, indicating that this is also the default assembly domain for Armadillo in the absence of Traf4. However, not all apical or junction-associated proteins localize to the broad domain, since Traf4 itself (Fig. 4G), Canoe (Fig. 4E), and the phosphotyrosine epitope (Fig. 6B) localize only to the most apical part of the lateral membrane.
Junctional remodeling in the mesoderm.
The finding that many junctional proteins of the blastoderm epithelium associate in a broad domain in mesodermal cells rather than apically like Armadillo prompted us to test whether their dynamics followed the same two-step relocalization mechanism observed for Armadillo (Snail-dependent disassembly followed by T48/Concertina-dependent reassembly).
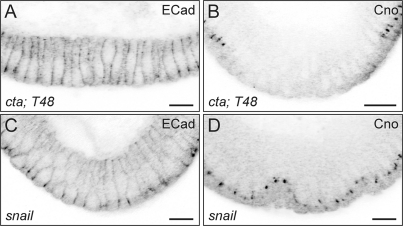
We first tested whether all junctional proteins are disassembled during the course of apical constriction. Disassembly of junctional proteins is difficult to see in wild-type embryos, because the time window in which the proteins have disappeared from their original location but not reaccumulated apically is very short, and indeed the two processes probably overlap temporally. However, the loss of Armadillo from its original position is easily seen if the T48- and Cta-dependent reassembly mechanism is impaired. We therefore used t48; cta double mutant embryos to test the behavior of other apically localized proteins and markers (E-cadherin, aPKC, and Canoe) and found that none of them reassemble at the plasma membrane in these mutants, as we illustrate for E-cadherin and Canoe in Fig. 8A and B. This shows that a general disassembly of the junctions occurs in the mesoderm and that the pathway downstream of T48/Cta signaling is necessary for the reassembly of all junctional proteins.
Fig. 8.
Dynamics of E-cadherin (ECad) and Canoe (Cno) in cta; t48 and snail mutant embryos. (A, B) In embryos lacking the G protein Concertina and the transmembrane protein T48, the junctional proteins E-cadherin and Canoe are disassembled from their subapical positions and fail to be reassembled. Their positions in the ectodermal epithelium are unaffected and can be seen in the cells at the right and left of the mesodermal region. (C, D) E-cadherin and Canoe are not cleared from their ectoderm-specific positions in snail mutant embryos. The mesodermal region in mutants is identified by the morphology of the whole embryo. cta; t48 embryos were from ctaR10; Df(3R)CC1.2 homozygous mothers mated to ctaR10/CyO; Df(3R)CC1.2/Df(3R)CC1.2 males. Homozygous snail mutant embryos were Df(2L)TE116GW11. Magnification, ×63; scale bar, 12 μm.
We next asked whether Snail controls the disassembly of all junctional proteins. In snail mutant embryos, we find that, like Armadillo, all of the proteins we tested remain at their original positions in or adjacent to the adherens junctions (shown for E-cadherin and Canoe in Fig. 8C and D). Thus, Snail controls a process by which proteins are cleared from the lateral junctional complexes.
Finally, we tested whether the proteins that require T48/Cta for their assembly at the junction also need Traf4 for their precise location, as we have found to be the case for Armadillo. We found no significant difference in the localization of E-cadherin in embryos mutant for traf4 (not shown) or derived from traf43 germ line clones (Fig. 4A and B). Canoe (Fig. 4D and F), which accumulates apically in mesodermal cells, shows less efficient apical reassembly in traf4 mutant embryos, but it does not show the same broad distribution as Armadillo. Since T48 and the Concertina signaling pathway act through RhoGEF2, we tested whether loss of RhoGEF2 affected these relocalizations in the same way as loss of T48 and Cta and found that this was the case (not shown).
In summary, at least two factors contribute to the apical localization of Armadillo. A T48/Cta- and RhoGEF2-dependent process targets Armadillo to E-cadherin, whereas Traf4 is responsible for its enrichment in the most apical part of the E-cadherin domain. Traf4 could either contribute to the efficient disassembly of junctions or to the localized reassembly of junction components in mesodermal cells.
Interaction of Traf4 with Armadillo.
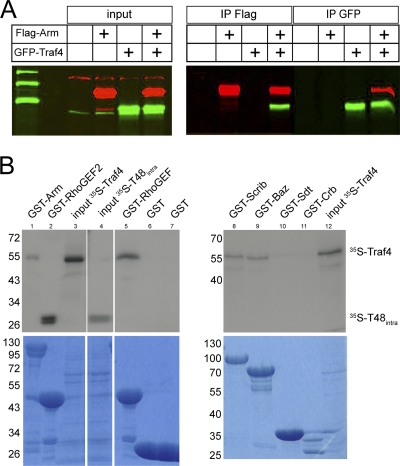
In view of suggestions that mammalian Traf4 may bind to beta-catenin (64), we tested whether the effect of Traf4 on Armadillo might involve a direct physical interaction. When we immunoprecipitated Armadillo (or Traf4) from detergent lysates of Schneider S2 cells expressing tagged versions of the two molecules, we found that Traf4 (or Armadillo) was coprecipitated (Fig. 9A). This could mean that the two proteins interact indirectly, forming part of a larger multiprotein complex, or that they directly bind to each other. To distinguish between these possibilities, we conducted the coprecipitations with proteins made in vitro. We produced GST-tagged Armadillo, as well as a number of control proteins, and 35S-labeled Traf4 by in vitro translation. GST-Armadillo was able to pull down Traf4 (Fig. 9B). 35S-labeled Traf4 was also able to associate with several different PDZ domains. It was pulled down by the PDZ domains of RhoGEF2, Bazooka/Par3, and Scribbled but not Stardust or control constructs (Fig. 9B).
Fig. 9.
Physical interaction between Traf4 and Armadillo. (A) Coimmunoprecipitation of GFP-tagged Traf4 (green) and Flag-tagged Armadillo (red) from Schneider S2 cell lysates. (B) Coimmunoprecipitation of 35S-Traf4 with GST-tagged Armadillo, RhoGEF-PDZ, Baz-PDZ, and Scrib-PDZ. Traf4 does not coimmunoprecipitate with Sdt-PDZ or the intracellular domain of Crb. The interaction of 35S-T48intra with RhoGEF-PDZ serves as a positive control.
We also sought in vivo evidence for a functional interaction between Traf4 and Armadillo. The results described above suggest that Traf4 is necessary for the accumulation of high levels of Armadillo at the most apical position in the lateral membrane and that this in turn is necessary for efficient apical constriction. However, in traf4 mutants, constriction is not completely abolished but only delayed, suggesting that the residual Armadillo is still able to mediate some of its normal function. We reasoned that a reduction of the amount of Armadillo in traf4 mutants might exacerbate the traf4 mutant phenotype. We therefore examined homozygous mutant traf4 embryos derived from heterozygous armadillo mothers (using three different alleles, arm1, arm2, and arm4), i.e., embryos containing only half the normal dose of Armadillo and no Traf4. Embryos from such crosses had more severe delays in furrow formation than homozygous traf4 mutant embryos (Fig. 10F and G), indicating that the amount of Armadillo in the junctions is indeed a limiting factor in this situation. Embryos from arm−/+ mothers fertilized by wild-type males showed no gastrulation defects (data not shown).
The interaction between Armadillo and Traf4 is not restricted to mesodermal cells. We also employed an assay system that has been successfully used to identify genetic interactors for Armadillo in the wing (22) and that uses phenotypic defects resulting from faulty wingless signaling as a readout. Modulating wingless signaling by overexpression of Armadillo (“Armadillo over”) or reduction in free Armadillo by expression of the cytoplasmic domain of E-cadherin (“Armadillo under”) results in easily scorable morphological defects in the wing (22) (Fig. 10A to D). These defects are sensitive to levels of components of the wingless signaling pathway, and intriguingly, also to those of cell adhesion molecules, such as E-cadherin, Crumbs, Stardust, Discs-large, and others (22). We tested whether Traf4 behaved in the same manner in such assays by expressing Armadillo or the cytoplasmic domain of E-cadherin in the engrailed domain of the wing disc of wild-type and traf43 heterozygous flies. Figures 10A to D show that reduction of Traf4, like that of adhesion complex molecules, also reduces the defects caused by the presence of intracellular E-cadherin and enhances the defects caused by overexpression of Armadillo. These findings support the notion that Traf4 interacts with Armadillo and suggest that this interaction is not restricted to embryos during gastrulation but is of a more general nature.
DISCUSSION
The results described here are relevant for the understanding of the morphogenetic program controlled by the transcription factor Twist, of the function of the Traf4 protein, and of the regulation of the dynamics of junctional complexes.
Morphogenesis controlled by Twist.
The transcription factor Twist is a conserved regulator of mesodermal development (6). In Drosophila, in addition to its role in mesodermal differentiation, it is also responsible for controlling the morphogenesis of the mesodermal primordium, beginning with the formation of the ventral furrow.
Twist acts through six zygotically active genes that are needed for the early cell shape changes (52, 67). Only one target, the transcriptional repressor Snail, is essential. Snail is necessary to allow the disassembly (or prevent the full assembly) of the apical and subapical junctional complexes, again a highly conserved function (4). Two Twist targets, Tribbles and Frühstart, have a purely permissive role in that they prevent cell division from interfering with the orderly progression of epithelial cell shape change (25, 27, 67). The three remaining genes contribute directly to apical constrictions. fog and t48 cooperate in the localization of the essential cytoskeletal regulator RhoGEF2, and finally traf4 is involved in the fine-tuning of the apical assembly of the junctional complex. It will be interesting, but technically challenging, to determine whether the expression of these three genes together with Snail is sufficient to induce furrow formation, i.e., whether they represent the full transcriptional cassette that mediates early Twist function. It would be necessary to test whether they can fully reconstitute a furrow in twist mutant embryos, as has been tested for Fog and Snail previously (68).
Cell biological function for Traf4.
Very little is known about the mechanism of action of Traf4. Vertebrate Traf4 resembles the other TNF receptor-associated factors in structure but unlike these does not appear to have a major role in inflammation, although it is able to signal to NF-κB and JNK (1, 18, 74). Specific inactivation of Traf4 in cells of the immune system reveals a subtle defect in cell migration, but again the subcellular basis for this defect remains obscure (12). A common theme emerging from studies in cultured cells and biochemical interactions is a role for Traf4 in the organization of the cell cortex (reviewed in reference 49). Traf4 is localized at sites of cell contact (21) or tight junctions in epithelia (35) and is required for the polarized targeting of NADPH oxidase to filopodia in migrating cells (45, 73). It can interact with Par3/bazooka and Misshapen in Drosophila (46, 72) and with Hic-5 and MEKK4 in mammalian cells (1, 73). We now show that the previously documented interaction with Par3 most likely occurs via binding to the PDZ domain and that Traf4 can also interact with PDZ domains of other proteins. Together these findings strengthen the notion of a general role for Traf4 in the organization and patterning of the cell cortex.
Modulation of junctions in the invaginating mesoderm.
The Traf4 mutant phenotype reveals the importance of partitioning proteins to specialized subregions of the cell cortex. It was already known that fully functional apical junctions were a prerequisite for apical constriction (17), and our results now show that an important aspect of the functionality is that the junctional region be patterned properly. This fits well with observations that a broadening of the cortical region in which F-actin is present also correlates with defects in apical constriction (19).
The reestablishment of adherens junctions in the mesoderm after the Snail-mediated disassembly occurs in an unexpected way. It might have been expected that all components of the junction assemble at the apical point of the lateral plasma membranes where Armadillo is seen. Instead, there seems to be a broad region of about one quarter of the lateral membrane in which an enrichment of E-cadherin and other components of adhesion complexes can be seen. This suggests that the presence of higher levels of E-cadherin mark a specialized cortical region which can recruit Armadillo and other proteins and which becomes patterned further to create a functional specialization of this membrane domain at its most apical point.
We do not know whether E-cadherin itself is the initial defining molecule for the domain or whether its presence in this position requires a further underlying molecular asymmetry. It is conceivable that the process of cellularization, which occurs in two qualitatively different phases, has left remnants in the lateral domain that distinguish the membrane inserted during the first, slow phase from the membrane inserted in the later, fast phase (42).
Conclusion.
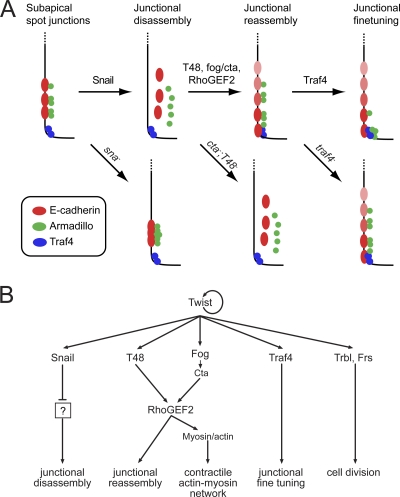
In summary, we propose the following model (Fig. 11) for the establishment of the junctional architecture in mesodermal cells. First, the presence of Snail in the mesoderm blocks the mechanisms that set up the adherens junctions of the blastoderm epithelium. Components already located at the subapical and apical regions in mesodermal cells are stripped. We know nothing about the hierarchy of these events nor do we know the genes that need to be repressed by Snail to allow the disassembly of junctions.
Fig. 11.
Model for the behavior of junction-associated proteins and their genetic regulation during ventral furrow formation. (A) Diagrams of the apical ends of late blastoderm to early gastrulating mesodermal cells with their associated proteins (apical is down). A prejunctional complex established during cellularization is completely remodeled under the control of the Twist targets Snail, T48, Fog, and Traf4 in the mesoderm. Junctional disassembly is Snail dependent, junctional reassembly is T48, Fog/Cta, and RhoGEF2 mediated, and junctional fine-tuning is Traf4 dependent. For further explanations, see the text. (B) Genetic hierarchy controlling ventral furrow formation. The mesodermal transcription factor Twist activates the expression of six target genes that are necessary for the proper formation of the ventral furrow. Snail, a transcriptional repressor, is responsible for the disassembly of junctions via regulation of unknown target genes. Fog (via the heterotrimeric G protein subunit Concertina) and T48 mediate the assembly of junctions and apical concentration of RhoGEF2, which in turn is necessary for the accumulation and activation of myosin. Traf4 controls apical concentration of Armadillo. Frühstart and Tribbles are needed to prevent cell division from interfering with the cell shape changes during ventral furrow formation but do not contribute to the shape changes per se. Arrows denote transcriptional or posttranscriptional regulation. This simplified diagram ignores the input of Snail into the proper transcriptional regulation of Fog, Frühstart, and Tribbles and the input of Dorsal into the activation of Snail.
Once the junctions have been dismantled, E-cadherin becomes distributed in a gradient over a wider apical domain of the membranes between mesodermal cells. It is unlikely that the E-cadherin we see in the mesoderm is newly synthesized, because there is no zygotic expression of E-cadherin in the mesoderm, and the maternal mRNA has decayed at this stage.
We suggest that the presence of E-cadherin then defines a platform for the assembly of further proteins. The delivery of all junctional components to the lateral membrane requires the activity of T48 and the Fog/Cta signaling pathways. The fact that we also do not see E-cadherin in these embryos may suggest that it is normally delivered actively to the region rather than being left behind to diffuse from the original junction, just as the de novo establishment of adherens junctions in other systems depends on RhoGEF and myosin (47). Apart from assembling junctional proteins, this region can apparently also recruit Ena and therefore nucleate or anchor F-actin, unless this is suppressed by the activity of Abl (19).
Finally, a specialized domain at which the junction will be concentrated must be defined at the apical point of the cortical domain. We propose that several parallel mechanisms operate to restrict a functional actin-capturing junction only at the most apical point of the membrane. Abl ensures that Ena and therefore F-actin is depleted from all but the most apical point. Armadillo, and perhaps also other proteins which we have not studied, are localized apically by Traf4. Finally, Traf4 must rely on other as-yet-unknown cues, as must a number of proteins, such as RhoGEF2, myosin, or Canoe, since they still concentrate apically in traf4 mutants.
ACKNOWLEDGMENTS
We thank K. Basler, W. Chia, K. Johnson, E. Knust, M. Mlodzik, M. Miura, A. Martinez Arias, Y. Rao, J. Chung, Y. Xiaohang, R. Cagan, T. Adachi-Yamada, J.P. Vincent, A. Carmena, D. Kiehart, J. Knoblich, S. Roth, D. Morrison, M. Frasch, E. Wieschaus, the Bloomington, Szeged, and Exelixis stock centers, and the DSHB for kindly providing fly stocks and antibodies used in this study. We also thank C. Niessen, V. Riechmann, A. Müller, and T. Lecuit for discussions and critical comments on the manuscript and Lisa Vogelsang, Heidi Thelen, and Juliane Hancke for technical assistance. We are particularly grateful to Ferdi Grawe (Düsseldorf) and Lisa Vogelsang for the electron micrographs. FlyBase was used as a reference source throughout this work.
The work was funded by the DFG (LE546/4-1), an EMBO long-term fellowship (to M.R.), and an NRW Graduate School fellowship (to S.J.M.).
S.J.M. and M.R. performed all experiments and prepared the manuscript. M.L. was involved in the supervision and discussion of the work, as well as some of the phenotypic analysis and the writing.
Footnotes
Published ahead of print on 10 October 2011.
REFERENCES
- 1. Abell A. N., Johnson G. L. 2005. MEKK4 is an effector of the embryonic TRAF4 for JNK activation. J. Biol. Chem. 280:35793–35796 [DOI] [PubMed] [Google Scholar]
- 2. Adachi-Yamada T., et al. 1999. p38 mitogen-activated protein kinase can be involved in transforming growth factor beta superfamily signal transduction in Drosophila wing morphogenesis. Mol. Cell. Biol. 19:2322–2329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bahri S. M., Yang X., Chia W. 1997. The Drosophila bifocal gene encodes a novel protein which colocalizes with actin and is necessary for photoreceptor morphogenesis. Mol. Cell. Biol. 17:5521–5529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Barrallo-Gimeno A., Nieto M. A. 2005. The Snail genes as inducers of cell movement and survival: implications in development and cancer. Development 132:3151–3161 [DOI] [PubMed] [Google Scholar]
- 5. Barrett K., Leptin M., Settleman J. 1997. The Rho GTPase and a putative RhoGEF mediate a signaling pathway for the cell shape changes in Drosophila gastrulation. Cell 91:905–915 [DOI] [PubMed] [Google Scholar]
- 6. Baylies M. K., Bate M. 1996. twist: a myogenic switch in Drosophila. Science 272:1481–1484 [DOI] [PubMed] [Google Scholar]
- 7. Boutros M., Paricio N., Strutt D. I., Mlodzik M. 1998. Dishevelled activates JNK and discriminates between JNK pathways in planar polarity and wingless signaling. Cell 94:109–118 [DOI] [PubMed] [Google Scholar]
- 8. Bradley J. R., Pober J. S. 2001. Tumor necrosis factor receptor-associated factors (TRAFs). Oncogene 20:6482–6491 [DOI] [PubMed] [Google Scholar]
- 9. Brand A. H., Perrimon N. 1993. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118:401–415 [DOI] [PubMed] [Google Scholar]
- 10. Bunch T. A., Grinblat Y., Goldstein L. S. 1988. Characterization and use of the Drosophila metallothionein promoter in cultured Drosophila melanogaster cells. Nucleic Acids Res. 16:1043–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cha G. H., et al. 2003. Discrete functions of TRAF1 and TRAF2 in Drosophila melanogaster mediated by c-Jun N-terminal kinase and NF-kappaB-dependent signaling pathways. Mol. Cell. Biol. 23:7982–7991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cherfils-Vicini J., et al. 2008. Characterization of immune functions in TRAF4-deficient mice. Immunology 124:562–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chou T. B., Perrimon N. 1996. The autosomal FLP-DFS technique for generating germline mosaics in Drosophila melanogaster. Genetics 144:1673–1679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chung J. Y., Park Y. C., Ye H., Wu H. 2002. All TRAFs are not created equal: common and distinct molecular mechanisms of TRAF-mediated signal transduction. J. Cell Sci. 115:679–688 [DOI] [PubMed] [Google Scholar]
- 15. Cox R. T., Kirkpatrick C., Peifer M. 1996. Armadillo is required for adherens junction assembly, cell polarity, and morphogenesis during Drosophila embryogenesis. J. Cell Biol. 134:133–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Craig C. R., Fink J. L., Yagi Y., Ip Y. T., Cagan R. L. 2004. A Drosophila p38 orthologue is required for environmental stress responses. EMBO Rep. 5:1058–1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dawes-Hoang R. E., et al. 2005. Folded gastrulation, cell shape change and the control of myosin localization. Development 132:4165–4178 [DOI] [PubMed] [Google Scholar]
- 18. Esparza E. M., Arch R. H. 2004. TRAF4 functions as an intermediate of GITR-induced NF-kappaB activation. Cell. Mol. Life Sci. 61:3087–3092 [DOI] [PubMed] [Google Scholar]
- 19. Fox D. T., Peifer M. 2007. Abelson kinase (Abl) and RhoGEF2 regulate actin organization during cell constriction in Drosophila. Development 134:567–578 [DOI] [PubMed] [Google Scholar]
- 20. Frasch M., Levine M. 1987. Complementary patterns of even-skipped and fushi tarazu expression involve their differential regulation by a common set of segmentation genes in Drosophila. Genes Dev. 1:981–995 [DOI] [PubMed] [Google Scholar]
- 21. Glauner H., et al. 2002. Intracellular localization and transcriptional regulation of tumor necrosis factor (TNF) receptor-associated factor 4 (TRAF4). Eur. J. Biochem. 269:4819–4829 [DOI] [PubMed] [Google Scholar]
- 22. Greaves S., Sanson B., White P., Vincent J. P. 1999. A screen for identifying genes interacting with Armadillo, the Drosophila homolog of beta-catenin. Genetics 153:1753–1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Grech A., Quinn R., Srinivasan D., Badoux X., Brink R. 2000. Complete structural characterisation of the mammalian and Drosophila TRAF genes: implications for TRAF evolution and the role of RING finger splice variants. Mol. Immunol. 37:721–734 [DOI] [PubMed] [Google Scholar]
- 24. Greig S., Akam M. 1993. Homeotic genes autonomously specify one aspect of pattern in the Drosophila mesoderm. Nature 362:630–632 [DOI] [PubMed] [Google Scholar]
- 25. Grosshans J., Müller H. A., Wieschaus E. 2003. Control of cleavage cycles in Drosophila embryos by Frühstart. Dev. Cell 5:285–294 [DOI] [PubMed] [Google Scholar]
- 26. Grosshans J., et al. 2005. RhoGEF2 and the formin Dia control the formation of the furrow canal by directed actin assembly during Drosophila cellularisation. Development 132:1009–1020 [DOI] [PubMed] [Google Scholar]
- 27. Grosshans J., Wieschaus E. 2000. A genetic link between morphogenesis and cell division during formation of the ventral furrow in Drosophila. Cell 101:523–531 [DOI] [PubMed] [Google Scholar]
- 28. Häcker U., Perrimon N. 1998. DRhoGEF2 encodes a member of the Dbl family of oncogenes and controls cell shape changes during gastrulation in Drosophila. Genes Dev. 12:274–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Harris T. J., Peifer M. 2004. Adherens junction-dependent and -independent steps in the establishment of epithelial cell polarity in Drosophila. J. Cell Biol. 167:135–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Harris T. J., Peifer M. 2005. The positioning and segregation of apical cues during epithelial polarity establishment in Drosophila. J. Cell Biol. 170:813–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Horie R., et al. 2007. TRAF activation of C/EBPbeta (NF-IL6) via p38 MAPK induces HIV-1 gene expression in monocytes/macrophages. Microbes Infect. 9:721–728 [DOI] [PubMed] [Google Scholar]
- 32. Hortsch M., Patel N. H., Bieber A. J., Traquina Z. R., Goodman C. S. 1990. Drosophila neurotactin, a surface glycoprotein with homology to serine esterases, is dynamically expressed during embryogenesis. Development 110:1327–1340 [DOI] [PubMed] [Google Scholar]
- 33. Houalla T., Hien Vuong D., Ruan W., Suter B., Rao Y. 2005. The Ste20-like kinase Misshapen functions together with Bicaudal-D and dynein in driving nuclear migration in the developing Drosophila eye. Mech. Dev. 122:97–108 [DOI] [PubMed] [Google Scholar]
- 34. Jiang J., Kosman D., Ip Y. T., Levine M. 1991. The dorsal morphogen gradient regulates the mesoderm determinant Twist in early Drosophila embryos. Genes Dev. 5:1881–1891 [DOI] [PubMed] [Google Scholar]
- 35. Kedinger V., et al. 2008. Tumor necrosis factor receptor-associated factor 4 is a dynamic tight junction-related shuttle protein involved in epithelium homeostasis. PLoS One 3:e3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kiehart D. P., Feghali R. 1986. Cytoplasmic myosin from Drosophila melanogaster. J. Cell Biol. 103:1517–1525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kölsch V., Seher T., Fernandez-Ballester G. J., Serrano L., Leptin M. 2007. Control of Drosophila gastrulation by apical localization of adherens junctions and RhoGEF2. Science 315:384–386 [DOI] [PubMed] [Google Scholar]
- 38. Koppen M., Fernandez B. G., Carvalho L., Jacinto A., Heisenberg C. P. 2006. Coordinated cell-shape changes control epithelial movement in zebrafish and Drosophila. Development 133:2671–2681 [DOI] [PubMed] [Google Scholar]
- 39. Krieg P. A., Melton D. A. 1984. Functional messenger RNAs are produced by SP6 in vitro transcription of cloned cDNAs. Nucleic Acids Res. 12:7057–7070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kuranaga E., et al. 2002. Reaper-mediated inhibition of DIAP1-induced DTRAF1 degradation results in activation of JNK in Drosophila. Nat. Cell Biol. 4:705–710 [DOI] [PubMed] [Google Scholar]
- 41. Lecuit T., Lenne P. F. 2007. Cell surface mechanics and the control of cell shape, tissue patterns and morphogenesis. Nat. Rev. Mol. Cell Biol. 8:633–644 [DOI] [PubMed] [Google Scholar]
- 42. Lecuit T., Wieschaus E. 2000. Polarized insertion of new membrane from a cytoplasmic reservoir during cleavage of the Drosophila embryo. J. Cell Biol. 150:849–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Leptin M. 2008. FBrf0203183 = Leptin et al., 2008.2.11, Renaming of the genes Traf1, Traf2 and Traf3. Flybase. (http://flybase.bio.indiana.edu/).
- 44. Leptin M. 1999. Gastrulation in Drosophila: the logic and the cellular mechanisms. EMBO J. 18:3187–3192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Li J. M., Fan L. M., Christie M. R., Shah A. M. 2005. Acute tumor necrosis factor alpha signaling via NADPH oxidase in microvascular endothelial cells: role of p47phox phosphorylation and binding to TRAF4. Mol. Cell. Biol. 25:2320–2330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Liu H., Su Y. C., Becker E., Treisman J., Skolnik E. Y. 1999. A Drosophila TNF-receptor-associated factor (TRAF) binds the ste20 kinase Misshapen and activates Jun kinase. Curr. Biol. 9:101–104 [DOI] [PubMed] [Google Scholar]
- 47. Maddugoda M. P., Crampton M. S., Shewan A. M., Yap A. S. 2007. Myosin VI and vinculin cooperate during the morphogenesis of cadherin cell-cell contacts in mammalian epithelial cells. J. Cell Biol. 178:529–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Martin-Blanco E., et al. 1998. puckered encodes a phosphatase that mediates a feedback loop regulating JNK activity during dorsal closure in Drosophila. Genes Dev. 12:557–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mathew S. J., Haubert D., Kronke M., Leptin M. 2009. Looking beyond death: a morphogenetic role for the TNF signalling pathway. J. Cell Sci. 122:1939–1946 [DOI] [PubMed] [Google Scholar]
- 50. Mathew S. J., Kerridge S., Leptin M. 2009. A small genomic region containing several loci required for gastrulation in Drosophila. PLoS One 4:e7437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Moreno E., Yan M., Basler K. 2002. Evolution of TNF signaling mechanisms: JNK-dependent apoptosis triggered by Eiger, the Drosophila homolog of the TNF superfamily. Curr. Biol. 12:1263–1268 [DOI] [PubMed] [Google Scholar]
- 52. Müller H. A., Samanta R., Wieschaus E. 1999. Wingless signaling in the Drosophila embryo: zygotic requirements and the role of the frizzled genes. Development 126:577–586 [DOI] [PubMed] [Google Scholar]
- 53. Müller H. A., Wieschaus E. 1996. armadillo, bazooka, and stardust are critical for early stages in formation of the zonula adherens and maintenance of the polarized blastoderm epithelium in Drosophila. J. Cell Biol. 134:149–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Nikolaidou K. K., Barrett K. 2004. A Rho GTPase signaling pathway is used reiteratively in epithelial folding and potentially selects the outcome of Rho activation. Curr. Biol. 14:1822–1826 [DOI] [PubMed] [Google Scholar]
- 55. Oda H., Tsukita S. 1999. Nonchordate classic cadherins have a structurally and functionally unique domain that is absent from chordate classic cadherins. Dev. Biol. 216:406–422 [DOI] [PubMed] [Google Scholar]
- 56. Oda H., Tsukita S., Takeichi M. 1998. Dynamic behavior of the cadherin-based cell-cell adhesion system during Drosophila gastrulation. Dev. Biol. 203:435–450 [DOI] [PubMed] [Google Scholar]
- 57. Oda H., Uemura T., Harada Y., Iwai Y., Takeichi M. 1994. A Drosophila homolog of cadherin associated with armadillo and essential for embryonic cell-cell adhesion. Dev. Biol. 165:716–726 [DOI] [PubMed] [Google Scholar]
- 58. Parks S., Wieschaus E. 1991. The Drosophila gastrulation gene concertina encodes a G alpha-like protein. Cell 64:447–458 [DOI] [PubMed] [Google Scholar]
- 59. Petronczki M., Knoblich J. A. 2001. DmPAR-6 directs epithelial polarity and asymmetric cell division of neuroblasts in Drosophila. Nat. Cell Biol. 3:43–49 [DOI] [PubMed] [Google Scholar]
- 60. Reuter R., Panganiban G. E., Hoffmann F. M., Scott M. P. 1990. Homeotic genes regulate the spatial expression of putative growth factors in the visceral mesoderm of Drosophila embryos. Development 110:1031–1040 [DOI] [PubMed] [Google Scholar]
- 61. Riggleman B., Schedl P., Wieschaus E. 1990. Spatial expression of the Drosophila segment polarity gene armadillo is posttranscriptionally regulated by wingless. Cell 63:549–560 [DOI] [PubMed] [Google Scholar]
- 62. Rolli V., et al. 2002. Amplification of B cell antigen receptor signaling by a Syk/ITAM positive feedback loop. Mol. Cell 10:1057–1069 [DOI] [PubMed] [Google Scholar]
- 63. Roth S., Stein D., Nusslein-Volhard C. 1989. A gradient of nuclear localization of the dorsal protein determines dorsoventral pattern in the Drosophila embryo. Cell 59:1189–1202 [DOI] [PubMed] [Google Scholar]
- 64. Rozan L. M., El-Deiry W. S. 2006. Identification and characterization of proteins interacting with Traf4, an enigmatic p53 target. Cancer Biol. Ther. 5:1228–1235 [DOI] [PubMed] [Google Scholar]
- 65. Sandmann T., et al. 2007. A core transcriptional network for early mesoderm development in Drosophila melanogaster. Genes Dev. 21:436–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Schejter E. D., Wieschaus E. 1993. Functional elements of the cytoskeleton in the early Drosophila embryo. Annu. Rev. Cell Biol. 9:67–99 [DOI] [PubMed] [Google Scholar]
- 67. Seher T. C., Leptin M. 2000. Tribbles, a cell-cycle brake that coordinates proliferation and morphogenesis during Drosophila gastrulation. Curr. Biol. 10:623–629 [DOI] [PubMed] [Google Scholar]
- 68. Seher T. C., Narasimha M., Vogelsang E., Leptin M. 2007. Analysis and reconstitution of the genetic cascade controlling early mesoderm morphogenesis in the Drosophila embryo. Mech. Dev. 124:167–179 [DOI] [PubMed] [Google Scholar]
- 69. Tautz D., Pfeifle C. 1989. A non-radioactive in situ hybridization method for the localization of specific RNAs in Drosophila embryos reveals translational control of the segmentation gene hunchback. Chromosoma 98:81–85 [DOI] [PubMed] [Google Scholar]
- 70. Treisman J. E., Ito N., Rubin G. M. 1997. misshapen encodes a protein kinase involved in cell shape control in Drosophila. Gene 186:119–125 [DOI] [PubMed] [Google Scholar]
- 71. Wang F., Dumstrei K., Haag T., Hartenstein V. 2004. The role of DE-cadherin during cellularization, germ layer formation and early neurogenesis in the Drosophila embryo. Dev. Biol. 270:350–363 [DOI] [PubMed] [Google Scholar]
- 72. Wang H., Cai Y., Chia W., Yang X. 2006. Drosophila homologs of mammalian TNF/TNFR-related molecules regulate segregation of Miranda/Prospero in neuroblasts. EMBO J. 25:5783–5793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Wu R. F., et al. 2005. Subcellular targeting of oxidants during endothelial cell migration. J. Cell Biol. 171:893–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Xu Y. C., et al. 2002. Involvement of TRAF4 in oxidative activation of c-Jun N-terminal kinase. J. Biol. Chem. 277:28051–28057 [DOI] [PubMed] [Google Scholar]
- 75. Xue L., et al. 2007. Tumor suppressor CYLD regulates JNK-induced cell death in Drosophila. Dev. Cell 13:446–454 [DOI] [PubMed] [Google Scholar]