Abstract
Expression of vascular endothelial growth factor (VEGF) increases in cancer cells during hypoxia. Herein, we report that the MDM2 oncoprotein plays a role in hypoxia-mediated VEGF upregulation. In studying the characteristics of MDM2 and VEGF expression in neuroblastoma cells, we found that hypoxia induced significantly higher upregulation of both VEGF mRNA and protein in MDM2-positive cells than in the MDM2-negative cells, even in cells without wild-type (wt) p53. We found that hypoxia induced translocation of MDM2 from the nucleus to the cytoplasm, which was associated with increased VEGF expression. Enforcing overexpression of cytoplasmic MDM2 by transfection of the mutant MDM2/166A enhanced expression of VEGF mRNA and protein production, even without hypoxia. The results of mechanistic studies demonstrated that the C-terminal RING domain of the MDM2 protein bound to the AU-rich sequence within the 3′ untranslated region (3′UTR) of VEGF mRNA; this binding increased VEGF mRNA stability and translation. In addition, knockdown of MDM2 by small interfering RNA (siRNA) in MDM2-overexpressing cancer cells resulted in inhibition of VEGF protein production, cancer cell survival, and angiogenesis. Our results suggest that MDM2 plays a p53-independent role in the regulation of VEGF, which may promote tumor growth and metastasis.
INTRODUCTION
The MDM2 protein is a multifunctional oncoprotein that has been shown to play both p53-dependent and -independent roles in oncogenesis. Normally, MDM2 is a nuclear phosphoprotein that contains several conserved functional regions. The MDM2 NH2 terminus is well characterized; it binds to p53 and represses p53-mediated transcription (30). Thus, it suppresses tumor suppressor activity. The COOH terminus contains a RING finger domain that is an E3 ligase that can regulate ubiquitination of p53 and MDM2 itself (16). Early studies show that in addition to its activity as an E3 ligase, the COOH-terminal RING finger domain of MDM2 is able to directly bind specific RNA sequences or structures, at least in vitro (11, 22). Recently published studies, including ours, show that the MDM2 RING finger domain binds to many cellular mRNAs, such as p53 and X-linked inhibitor of apoptosis (XIAP) mRNAs, in vivo, regulating mRNA translation and protein synthesis (5, 15).
MDM2 gene amplification occurs in diverse human malignancies that include soft tissue sarcomas, neuroblastoma, and cancers of the brain, breast, ovary, cervix, lung, colon, prostate, and bone (9, 23, 29). In addition, high-level MDM2 expression can occur even in cancers without MDM2 gene amplification, sometimes associated with a single nucleotide polymorphism in the MDM2 gene promoter (3). Thus, overexpression of MDM2 appears to be a favorable state for cancer cells. In fact, in the clinic, overexpression of MDM2 in tumors does correlate with a poor prognosis for cancer patients (34). In addition, previous studies show that MDM2 overexpression occurs more frequently in metastatic and recurrent cancers than in primary tumors (10, 21). One study of 100 tumor samples taken from patients with esophageal squamous cell carcinoma revealed that MDM2 expression is the most significant risk factor for distant metastases (26). A recent series of studies indicate that MDM2 overexpression is associated with cancer metastases and predicts a poor prognosis for developing metastatic, but not localized, cancer (14, 19, 32, 41). It has been reported that MDM2 expression is correlated with increased levels of the angiogenic factor vascular endothelial growth factor (VEGF) (31, 42), which may facilitate vascularization, as well as intravasation and seeding of tumor cells in distant places, thus actively contributing to tumor metastasis.
VEGF is a potent angiogenic factor that plays a crucial role in the regulation of both normal physiologic and pathological angiogenesis (12). Appropriately timed expression of VEGF at normal levels is essential for normal vascular development and homeostasis (6). However, VEGF-induced angiogenesis is also essential for the growth of solid tumors (20). In solid tumors, VEGF is highly expressed, and moreover, it is required for the development and maintenance of the tumor blood vessels that are a prerequisite for successful tumor growth and metastasis (33). Inhibition of VEGF expression, by specific antibodies or by overexpression of a dominant-negative mutant of the VEGF receptor, results in a clear inhibitory effect on tumor growth, thus demonstrating the truly pivotal role of VEGF in the promotion of tumor growth and metastasis (17).
Hypoxia is an important stimulator of new blood vessel formation that is seen during tumor angiogenesis, progression, and metastasis (2). This state of reduced oxygen tension is a characteristic of virtually all circumstances in which new blood vessel growth is observed. In cancer, an insufficient vascular supply and the resultant reduction in tissue oxygen tension within solid tumors often increase compensatory neovascularization, in an attempt to satisfy the ever-hungry metabolic needs of growing tumor tissue. Hypoxia is a crucial stimulator for VEGF expression (36, 37), which leads to vascular growth. Although hypoxia can stimulate VEGF transcription through induction of hypoxia-inducible factor 1α (HIF-1α) (13), this mechanism does not seem to be important for hypoxia-mediated increases in VEGF mRNA. Instead, regulation at the posttranscriptional level represents the major point of control for VEGF expression. A previous study shows that hypoxia can induce VEGF steady-state mRNA 25-fold, whereas the hypoxia-mediated VEGF transcription rate increases only 3.1-fold (in rat cardiac myocytes) (24).
Posttranscriptional regulatory mechanisms, especially those that modulate mRNA stability, have already been shown to play a major role in gene expression (18). The turnover rate of a given mRNA can be determined by the interaction between trans-acting factors and specific cis elements that are located within the 3′ untranslated region (3′UTR) (1). The presence of AU-rich elements (AREs) in the 3′UTR is associated with a rapid mRNA turnover and attenuation of translation (4, 7). In fact, the 3′UTR of VEGF contains a single 126-bp ARE that is critical for the stabilization of VEGF mRNA under hypoxia (8, 35). This region is able to form at least seven hypoxia-inducible mRNA-protein complexes (25). The RNA-binding proteins interacting with this VEGF 3′UTR element that have been identified so far include hnRNP L, HuR, and DRBP76/NF90. All of these proteins are able to regulate VEGF mRNA stabilization, according to reports in the literature (25, 35, 39).
Because MDM2 has been identified as another RNA-binding protein (11, 15), we chose to investigate whether specific binding and MDM2-induced VEGF expression occurred at the posttranscriptional level, regulating VEGF mRNA stability. Our study results clearly showed that MDM2 was able to bind to the VEGF 3′UTR and then regulate VEGF mRNA stabilization. Of particular interest, we found that during hypoxia, MDM2 was translocated from the nucleus to the cytoplasm, where it was able to induce high levels of expression of VEGF in cancer cells.
MATERIALS AND METHODS
Cell lines and culture conditions.
Four human neuroblastoma cell lines (NB-1691, SHEP, SK-N-SH, and LA1-55N) were used in this study. The first three of these four lines have wild-type (wt) p53, while the LA1-55N cell line is p53-null. While NB-1691, SHEP, and LA1-55N cells overexpress MDM2, SK-N-SH cells have a very low level of MDM2 expression, as previously characterized (15). All four cell lines were obtained from H. Findley (Emory University). In addition, the MDM2+/+ p53−/− and MDM2−/− p53−/− mouse embryonic fibroblast (MEF) cells (derived from double-knockout mice) were kindly provided by Guillermine Lozano (University of Texas, M.D. Anderson Cancer Center). All cell lines were cultured in standard medium (RPMI 1640 medium containing 10% fetal bovine serum [FBS], 2 mmol/liter of l-glutamine, 50 units/ml penicillin, and 50 μg/ml streptomycin) at 37°C, in a humidified atmosphere. In order to study the effects of hypoxia, the same cells were cultured under either normoxic conditions (5% CO2, 21% O2, and the remainder was N2) or hypoxic conditions (5% CO2, 1% O2, and the remainder was N2).
Plasmid constructions.
The wt MDM2 expression plasmid pCMV-MDM2 (CMV stands for cytomegalovirus) was provided by B. Vogelstein (Johns Hopkins University). Also, the wt MDM2 cDNA was cloned into a pDsRed1-C1 vector, to generate the plasmid pDsRed1-C1/MDM2. The QuikChange site-directed mutagenesis kit was used to mutate the Akt phosphorylation site from serine 166 to either alanine or glutamic acid (MDM2/166A or MDM2/166E, respectively) in pCMV-MDM2 and pDsRed1-C1/MDM2. In addition, the HuR expression plasmid was generated by cloning the HuR cDNA into the pcDNA3.1 vector. The wt and various C-terminal truncated glutathione S-transferase (GST)-tagged MDM2 and HuR constructs were generated by PCR and cloned into the bacterial pGEX expression vector. Also, the pSUPER MDM2 (siMDM2) plasmid was constructed by inserting the specific 19-nucleotide (nt) MDM2 sequence (GAAGTTATTAAAGTCTGTT) into an expression plasmid, pSUPER-neo vector, purchased from OligoEngine (Seattle, WA). For a control, a 19-nt scrambled sequence (GAGGCTATTATACTGTGAT) was inserted into another pSUPER-neo.
To generate the pGL3-VEGF 3′-UTR reporter plasmid, a 834-base-pair (bp) segment of the VEGF 3′UTR, corresponding to nucleotides 2731 to 3564 of the human VEGF mRNA (GenBank accession no. NM_001025366.2), was amplified by reverse transcription-PCR (RT-PCR) and subcloned into a pGL3-Promoter (pGL3P) plasmid (Promega) at the XbaI site. The sense and antisense orientations of the VEGF 3′UTR in the luciferase reporter were identified by both enzyme digestion and DNA sequencing. A pGL3-VEGF 3′UTR plasmid containing a mutated ARE within the 3′UTR was constructed as a control.
Gene transfection and reporter assay.
To enforce MDM2 expression in cells with low levels of MDM2, we stably transfected wt MDM2, MDM2/166A, MDM2-166E, and the control plasmids into SK-N-SH or MDM2−/− p53−/− MEF cells by electroporation at settings of 300 V and 950 μF, using a Gene Pulser II System (Bio-Rad, Hercules, CA). At 48 h posttransfection, the cells were seeded into culture dishes to select for the G418-resistant colonies that would be carrying the desired plasmids, which were then grown in medium containing G418 (300 μg/ml) for 2 to 3 weeks. The resulting clone-carrying colonies were picked and grown in RPMI 1640 medium, with or without G418. For stable siMDM2 transfection, LA1-55N cells that were in an exponential growth stage were transfected with pSUPER/MDM2 small interfering RNA (siRNA) (wt) or pSUPER/control siRNA plasmids by electroporation.
Transient transfection was performed to examine the effects of MDM2 on the 3′UTR activity of VEGF. SK-N-SH cells were cotransfected with various MDM2 expression plasmids and pGL3-VEGF 3′UTR reporter plasmids. The pRL-CMV vector was cotransfected into each transfection to provide an internal control. The subcellular distribution of transfected MDM2 in the pDsRed1-C1 vector was detected by confocal microscopy. The live transfected cells were resuspended in 10 ml of RPMI 1640 medium containing 10% FBS and incubated for 24 to 36 h. Cell extracts were prepared with 1× lysis buffer. A 20-μl aliquot of supernatant from each different cell/plasmid combination was mixed first with 100 μl of luciferase assay reagent II (Promega) to measure the firefly luciferase (FL) activity, and next, the Renilla luciferase (RL) activity of each was determined after adding Stop & Glo reagent to the same sample. Their luciferase activities were analyzed via Microplate Instrumentation (BioTek).
Western blot analyses.
Cellular proteins were prepared by lysing cells for 30 min at 4°C in a lysis buffer composed of 150 mM NaCl, 50 mM Tris (pH 8.0), 5 mM EDTA, 1% (vol/vol) Nonidet P-40, 1 mM phenylmethylsulfonyl fluoride (PMSF), 20 μg/ml aprotinin, and 25 μg/ml leupeptin. Equal amounts of protein extracts were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a nitrocellulose filter. After the filter was blocked with buffer containing 5% nonfat milk, 20 mM Tris-HCl (pH 7.5), and 500 mM NaCl for 1 h at room temperature, it was incubated with specific antibodies for 3 h at room temperature, then washed, and incubated with horseradish peroxidase (HRP)-labeled secondary antibody, and finally it was developed using a chemiluminescence detection system (ECL kit; Amersham Life Science, Buckinghamshire, England).
VEGF ELISA.
SK-N-SH cells that were transfected with MDM2 and LA1-55N cells that were transfected with siMDM2, in normoxic or hypoxic conditions of culture, were collected by centrifugation. The level of VEGF found in the supernatant of each tube was quantified, using an enzyme-linked immunosorbent assay (ELISA) kit for human VEGF as instructed by the manufacturer (R&D Systems).
RT-PCR.
Quantitative reverse transcription-PCR (qRT-PCR) was used to measure the levels of VEGF mRNA expression and turnover, as well as its association with polyribosomes. To assess mRNA turnover, RNA synthesis was terminated by the addition of 5 μg/ml of actinomycin D to the culture medium. At different time points after the actinomycin D addition, the cells were harvested, and their total RNA was isolated using the RNeasy minikit (Qiagen, Hilden, Germany). The first-strand cDNA synthesis was performed with a mixture of random nonamers and oligo(dT) as primers (Qiagen). Amplification of VEGF was performed with a 7500 real-time RT-PCR system (Applied Biosystems, Foster City, CA), using a QuantiFast SYBR green RT-PCR kit (Qiagen), according to the manufacturer's instructions. The primers for VEGF and the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were purchased from Qiagen.
Polysome preparation and analysis.
Polysome profiling was carried out as described previously (15), with only slight modifications. Briefly, MDM2−/− p53−/− MEF cells that were transfected with MDM2/166A or MDM2/166E were incubated with 100 μg/ml cycloheximide (CHX) for 15 min in order to arrest polyribosome migration. To isolate the cytoplasmic extracts, the cells were lysed in a buffer containing 20 mM Tris-HCl (pH 8.0), 100 mM NaCl, 5 mM MgCl2, 0.5% Triton X-100, 500 U/ml RNAsin, and a cocktail of protease inhibitors. The cell lysates were fractionated on a 15 to 45% (wt/vol) sucrose gradient and then centrifuged in a SW41Ti rotor at 39,000 rpm for 2 h. Fractions from each gradient tube were collected by upward replacement, while absorption at an optical density at 254 nm (OD254) was monitored using a fractionator (Isco, Lincoln, NE). The RNA in each fraction was extracted and subjected to qRT-PCR as described above.
Expression of GST-tagged proteins.
The expression and purification of GST-fused MDM2 and HuR proteins were performed as described previously (15). Briefly, after transformation of various GST-fused MDM2 and HuR plasmids into Escherichia coli BL21, the cells were incubated in LB medium at 30°C. After incubation with 0.1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) for 2 h, the cells were harvested. The GST-fused proteins produced were purified by lysing the induced cells with sonication, followed by isolation with glutathione-agarose beads (Pharmacia). The purity and correct expression of each of the GST-fused proteins were analyzed by gel electrophoresis and Coomassie brilliant blue G250 staining, as well as by a Western blot assay using anti-GST antibody.
UV cross-linking and RNA binding assays.
UV cross-linking and immunoprecipitation assays were performed as described previously (15) with minor modifications. Briefly, we chemically synthesized the DNA fragment corresponding to the VEGF 3′UTR RNA probe with the following sequence: 5′-TAATACGAGTCACTATAGGGAAATTCTACATACTAAATCTCTCTCCTTTTTTAA TTTTAATATTTG-3′. This DNA fragment incorporated the T7 promoter sequence (underlined) and contained the ARE of the VEGF 3′UTR (remainder). Similar DNA fragments with mutations in ARE, plus a DNA fragment from the VEGF 3′UTR that lacked an ARE were also synthesized for controls. Internally labeled RNA probes were synthesized by in vitro transcription with T7 polymerase (MAXIScript T7 RNA polymerase kit; Ambion) in the presence of [α-32P]UTP (Amersham). The cell extracts, recombinant human MDM2 (rhMDM2) (EMD Chemicals Inc.), and various GST-fused MDM2 and HuR proteins were mixed with 32P-labeled RNA probes. Then UV cross-linking of the RNA-protein complexes formed was done using a 254-nm UV light source, set at 400,000 μJ/cm2. Finally, these UV-irradiated RNA-protein complexes were treated with RNase T1, resolved by being run through a 10% SDS-polyacrylamide gel, and visualized by autoradiography.
For the in vivo protein-RNA binding assay, VEGF mRNA was coimmunoprecipitated from whole-cell extracts, using previously described methods with minor modifications (15). Briefly, cells were exposed to hypoxia for 4 h, then UV cross-linked, and harvested. Low-speed centrifugation at 4°C was used to collect the cell pellets, and the pellets were resuspended in 100 μl of RNA binding buffer (20 mM Tris [pH 7.5], 150 mM NaCl, 5 mM MgCl2, 0.1% NP-40, 50 μM ZnCl2, 2% glycerol, 1 mM dithiothreitol [DTT]) that was supplemented with 10 U of RNase inhibitor (5 Prime-3 Prime). These cell extracts were further prepared by the freeze-thaw method to lyse cells. Whole-cell extracts were treated with 5 μl of monoclonal anti-MDM2, anti-HuR, or anti-GAPDH antibodies, along with 20 μl of protein A plus protein G agarose beads and 5 U of RNase inhibitor, and the resulting mixture was incubated for 60 min at room temperature. Next, the beads were washed extensively with RNA binding buffer supplemented with RNase inhibitor. The RNA that became associated with the antibody-antigen complexes was isolated by repeated phenol-chloroform extractions and then precipitated with 2 M ammonium acetate and 3 volumes of cold ethanol. All resulting RNA was analyzed by RT-PCR, using VEGF-specific primers.
Filter binding assay.
The 32P-labeled VEGF RNA probes as described above were incubated with rhMDM2 protein at 4°C for 40 min in 100 μl buffer containing 10 mM HEPES (pH 7.4), 100 mM KCl, 2.5 mM MgCl2, 5% glycerol, 1 mM DTT, 50 μg/ml yeast tRNA, and 50 μg/ml bovine serum albumin (BSA). The reaction mixtures were diluted 1:10 with 900 μl cold buffer and immediately filtered through nitrocellulose membranes (0.45 μm; Millipore) at a flow rate of 0.5 ml/min and rinsed with 5 ml cold buffer. The membranes were dried at 60°C for 1 h, and the amount of bound RNA was measured using a liquid scintillation counter. The 0% (baseline) and 100% (maximum binding) values were determined by spotting the probe with filtering with and without washing, respectively. Kd (dissociation constant) was calculated as the protein concentration required for 50% RNA binding. Each experiment was repeated at least three times.
Cell survival analysis.
Cells were cultured in 96-well microtiter plates, with or without hypoxia and cisplatin treatment, for a 20-hour period. Next, water-soluble tetrazolium salt (WST) (25 μg/well) was added, and incubation continued for an additional 4 h. The optical density of all wells was obtained with a microplate reader set at a test wavelength of 450 nm and a reference wavelength of 620 nm. Controls lacking cells were included to determine the background absorbance.
Human LA1-55N xenograft model.
SCID mice (C.B-17/IcrHsd; female; 4 to 5 weeks of age) were used for human cancer cell engraftment to comparatively assess the cells' ability to induce tumorigenesis and angiogenesis in vivo. Our LA1-55N cells, transfected either with siMDM2 or siRNA controls, were injected subcutaneously into both flanks of the mice. Any growth of local tumors was followed by twice-weekly caliper measurements, as soon as the tumors were palpable. Confirmation of MDM2 expression and of angiogenesis, as determined by CD34 expression, was done for each of these xenografted tumors by employing standard immunohistochemistry techniques.
RESULTS
MDM2 is involved in hypoxia-regulated VEGF mRNA expression.
A previously published study demonstrates that there is a correlation between overexpression of MDM2 and upregulation of VEGF in angiosarcomas (42). When we studied human neuroblastomas, we also found that overexpression of MDM2 was closely associated with increased VEGF expression. As is shown in Fig. 1A, three neuroblastoma cell lines (NB-1691, SHEP, and LA1-55N) that overexpress MDM2 also expressed relatively high levels of VEGF. To further confirm this association, we examined the levels of VEGF protein expression in MDM2+/+ p53−/− and MDM2−/− p53−/− MEF cells that were derived from double-knockout (KO) mice. As expected, VEGF expression was higher in the MDM2+/+ p53−/− cells than in the MDM2−/− p53−/− cells (Fig. 1A).
Fig. 1.
Effect of hypoxia on MDM2 modification and induction of VEGF. (A) Expression of MDM2, p53, and VEGF proteins in four cultured neuroblastoma cell lines indicated above the blots, plus the pair of MDM2+/+ p53−/− and MDM2−/− p53−/− MEF cells, all detected by Western blotting. The positions of molecular mass markers (in kilodaltons [kd]) are indicated to the left of the blots. (B) SHEP cells exposed to hypoxia for different times (in hours) as indicated above the top blot. Cell extracts were tested by Western blot assay for MDM2, phosphorylated MDM2 (p166), and VEGF expression in whole-cell extract (WCE), as well as for MDM2 in nucleus (Nuc) and cytoplasm (Cyt) fractions. The values under protein bands in the blots represent expression levels after normalization for controls (GAPDH or nucleolin and tubulin), compared with untreated (0) samples (defined as 1 unit). (C and D) Comparison of VEGF mRNA induction during hypoxia in MDM2-positive versus MDM2-negative cells. SHEP (MDM2-positive) and SK-N-SH (MDM2-negative) cells that have wt p53 (C), and both MDM2+/+ p53−/− and MDM2−/− p53−/− MEF cells (D) were exposed to hypoxia for different times, as indicated, and VEGF mRNA expression was detected by qRT-PCR.
Because hypoxia is an important regulator of VEGF, we then investigated whether MDM2 is involved in the regulation of VEGF during hypoxia. First, we examined the possible effects of hypoxia on MDM2 expression. Consistent with the effect of hypoxia on MDM2 upregulation that was previously described for murine KHT fibrosarcoma cells (40), hypoxia induced MDM2 expression in human neuroblastoma cells. By examining a whole-cell extract (WCE) of SHEP cells, we found that not only was total MDM2 expression increased, but the phosphorylation of MDM2 at serine 166 was decreased following hypoxia, which was accompanied by an increased expression of VEGF (Fig. 1B). One interesting finding was that hypoxia regulated the translocation of MDM2 from the nucleus to the cytoplasm. When we examined the cellular redistribution of MDM2 following hypoxia exposure in SHEP cells, we found that hypoxic treatment decreased nuclear MDM2 and increased cytoplasmic MDM2 expression, as is shown in Fig. 1B.
The fact that hypoxia regulates VEGF mRNA expression has been well studied. We evaluated whether MDM2 influences hypoxia-mediated VEGF mRNA expression. We compared VEGF mRNA expression following hypoxia in four specific cell lines: SHEP (MDM2 overexpression) versus SK-N-SH (no MDM2 overexpression), plus MDM2+/+ p53−/− versus MDM2−/− p53−/− MEF cells. In these experiments, quantitative RT-PCR results indicated that VEGF mRNA expression became greatly induced by hypoxia in SHEP cells, and less VEGF mRNA induction was observed in the SK-N-SH cells when given a similar hypoxia exposure (Fig. 1C). Similarly, a higher level of VEGF mRNA was induced by hypoxia in MDM2+/+ p53−/− MEF cells than in MDM2−/− p53−/− MEF cells (Fig. 1D).
Cytoplasmic MDM2 stabilizes VEGF mRNA.
It is well-known that hypoxia regulates VEGF mRNA stabilization by way of RNA-binding proteins. We investigated whether MDM2, when located in the cytoplasm, can serve as a RNA-binding protein to regulate VEGF mRNA stability. First, we examined the half-life (t1/2) of VEGF mRNA in the paired MDM2+/+ p53−/− and MDM2−/− p53−/− MEF cells by qRT-PCR, following treatment of the mRNA synthesis inhibitor actinomycin D. The half-life of VEGF mRNA was indeed longer in MDM2+/+ p53−/− cells (t1/2 = 55 ± 6 min) than in MDM2−/− p53−/− cells (t1/2 = 28 ± 4 min), even when the cells were in normoxia (Fig. 2A). When the MDM2+/+ p53−/− cells were exposed to hypoxia, the half-life of VEGF mRNA was further increased (t1/2 = 77 ± 8 min) (P < 0.05) (Fig. 2B), suggesting that there is a role for MDM2 in hypoxia-regulated VEGF mRNA stabilization.
Fig. 2.
Effect of MDM2 modification on VEGF mRNA stability. (A) MDM2+/+ p53−/− and MDM2−/− p53−/− MEF cells were treated with 5 mg/ml actinomycin D. After the times (in minutes) indicated for the addition of actinomycin D, the cells were harvested, and total RNA was isolated. The amount of VEGF mRNA remaining in the cells was determined by qRT-PCR. The RT-PCR products that were run in an agarose gel are shown. The numbers under bands in the top blot represent the percentages of VEGF mRNA after normalization to the GAPDH mRNA value compared with samples (value at 0 min defined as 100). (B) Graphic representation of the relative amount of VEGF mRNA, as detected by qRT-PCR in MDM2+/+ p53−/− cells in normoxia and hypoxia after actinomycin D treatment. Data shown are from a representative experiment with each time point performed in triplicate. (C) Subcellular distribution in SK-N-SH cells of transfected MDM2/166A and MDM2/166E, as demonstrated by the red fluorophore tags, seen by confocal microscopy. Bars, 10 μm. (D) The relative amount of VEGF mRNA in SK-N-SH cells that were transfected either with MDM2/166A or MDM2/166E, following actinomycin D treatment, as detected by qRT-PCR.
To further confirm that it was specifically the cytoplasmic MDM2 that regulated the VEGF mRNA stabilization, we performed transfections of MDM2 expression plasmids carrying mutations at site 166: MDM2/166A and MDM2/166E. MDM2/166A, in which serine 166 is mutated to alanine, cannot be phosphorylated by phosphatidylinositol 3-kinase (PI3K)/Akt, whereas MDM2/166E, in which serine 166 is substituted with glutamic acid, mimics phosphorylated MDM2 (27). When we checked the subcellular distribution of the mutant MDM2 proteins in the otherwise negative SK-N-SH cells that were transfected with these mutant plasmids tagged by red fluorescence protein (pDsRed1-C1), we found that transfected MDM2/166A, as indicated by the red fluorophores, was mainly expressed in the cytoplasm, whereas MDM2/166E had predominately localized in the nucleus (Fig. 2C). The half-life of VEGF mRNA was longer in those SK-N-SH cells with the transfected MDM2/166A (t1/2 = 74 ± 7 min) than in those transfected with MDM2/166E (t1/2 = 47 ± 5 min) (P < 0.05) (Fig. 2D). This suggests that cytoplasmic MDM2 regulates VEGF mRNA stabilization.
MDM2 binds to the VEGF 3′UTR.
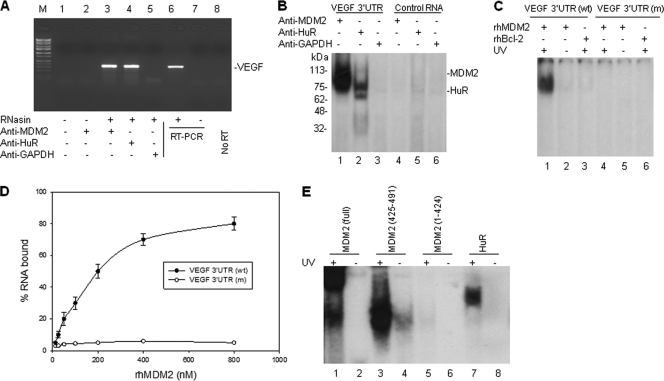
We investigated whether MDM2 can bind to the VEGF mRNA and stabilize it. First, we performed a protein-RNA binding assay. Results from immunoprecipitation (IP) and RT-PCR analyses showed that the MDM2 protein was able to bind VEGF mRNA (Fig. 3A). To investigate whether MDM2, like HuR, binds specifically to the VEGF 3′UTR, we performed UV cross-linking of a 32P-labeled VEGF 3′UTR probe, using cell extracts from MDM2-overexpressing NB-1691 cells that were immunoprecipitated with both MDM2 and HuR antibodies. A RNA of the same size, but without an AU-rich sequence, was also labeled with 32P, to serve as a control. We found that MDM2 strongly bound to the VEGF 3′UTR probe, but not to the nonspecific control RNA probe (Fig. 3B).
Fig. 3.
MDM2 protein binds to VEGF mRNA in vivo and in vitro. (A) Cell extracts from NB-1691 cells were prepared in RNA binding buffer in the presence of RNase inhibitor (RNasin). Following coimmunoprecipitation (co-IP) with anti-MDM2, anti-HuR (positive control), and anti-GAPDH (negative control), VEGF mRNA was detected by RT-PCR analysis. The positive control (NB-1691 total RNA as the template) (lane 6), negative control (no template) (lane 7), and no RT (lane 8) controls for RT-PCR are also shown. Lane M contains molecular size markers. (B) Cellular extracts from NB-1691 were incubated with 32P-labeled RNA probes corresponding to the VEGF 3′UTR and a control RNA, next UV cross-linked, and then immunoprecipitated with the indicated antibodies. These protein-RNA probe complexes were run on an SDS-polyacrylamide gel and imaged by autoradiography. The positions of molecular mass markers (in kilodaltons) are shown to the left of the gel. (C) The rhMDM2 and rhBcl-2 (control) proteins were incubated with 32P-labeled normal VEGF 3′UTR and ARE-mutated (m) VEGF 3′UTR, respectively. The protein-RNA complexes were detected as described above for panel B. (D) Filter binding assay showing the binding of rhMDM2 to VEGF 3′UTR. The means ± standard deviations (error bars) (SD) of the results from three independent experiments are shown. (E) GST-fused full-length MDM2, different GST-fused MDM2 fragments of the C-terminal RING domain (positions 425 to 491), a MDM2 fragment with a deletion of the C-terminal RING domain (positions 1 to 424), and the GST-fused HuR control were incubated with 32P-labeled VEGF 3′UTR. Formation of any MDM2-VEGF RNA complexes were detected as described above for panel B.
To further confirm that the MDM2 protein binds to the VEGF 3′UTR, we performed UV cross-linking and gel electrophoresis in vitro, using a recombinant human MDM2 (rhMDM2) and the 32P-labeled VEGF 3′UTR probe. Results showed that the MDM2 protein strongly and specifically bound to the VEGF 3′UTR RNA in vitro, without other cellular elements present (Fig. 3C). The filter binding assay results revealed that MDM2 bound VEGF 3′UTR mRNA with a Kd of approximately 200 nM (Fig. 3D) and that hypoxia increased the binding of MDM2 to VEGF mRNA in NB-1691 cells (data not shown). We also performed similar UV cross-linking and gel electrophoresis experiments to screen for the domain of MDM2 that is required for its binding to the VEGF 3′UTR. The results of the last RNA binding assays revealed that the C-terminal RING domain (positions 425 to 491), but not any other regions of the MDM2 protein (positions 1 to 424), bound to the VEGF 3′UTR (Fig. 3E). These results were consistent with other previous characterizations of the RING domain of MDM2, which is responsible for RNA binding (5, 11, 15).
Cytoplasmic MDM2 increases VEGF translation.
Because MDM2, when localized in the cytoplasm, stabilized VEGF mRNA, we evaluated whether there also was an increase in VEGF protein synthesis because of the MDM2-mediated mRNA stabilization. First, we measured the effects of both wild-type (wt) MDM2 and MDM2/166A on the VEGF 3′UTR-mediated translation of a luciferase (FL)-tagged mRNA. Before the experiment, we constructed a FL reporter plasmid in a pGL3-Promoter containing the wild-type VEGF 3′UTR (pGL3-VEGF 3′UTR) and a control plasmid containing mutated ARE within the VEGF 3′UTR. These plasmids were transiently transfected into SK-N-SH cells, together with a wt MDM2 or MDM2/166A and HuR expression plasmids. We found that cotransfection of MDM2/166A and HuR significantly increased the FL activity in SK-N-SH cells transfected with the pGL3-VEGF 3′UTR in the sense, but not antisense, orientation (Fig. 4A). In addition, the wt MDM2 induced less synthesis of the FL protein than the MDM2/166A did.
Fig. 4.
Effect of MDM2 and its cytoplasmic location on VEGF translation. (A) SK-N-SH cells were cotransfected with 5 μg pGL3-VEGF 3′UTR (wt or mutant [mut]) plasmids and with increasing amounts (5 and 10 μg) (indicated by the height of the black triangle) of the wt MDM2 plasmid (MDM2/wt), MDM2/166A plasmid, or pcDNA 3.1-HuR plasmid. After 24 h, cell extracts were prepared, and their quantitative FL (pGL3-MYCN 3′UTR) was detected using the Dual-Luciferase reporter system. Only the FL activities in the transfection of pGL3-MYCN 3′UTR were set at 1. Bars represent the means plus SD (error bars) of FL in three independent experiments, after being normalized to the RL activity (cotransfected pRL-SV40 vector was internal control). (B) Comparison of VEGF protein production during hypoxia in SK-N-SH cells that were transfected with wt MDM2, MDM2/166A, or control vector. Equal numbers of transfected cells were exposed to hypoxia, and then at the indicated time point, cell supernatants were harvested to detect VEGF levels by ELISA. Values that are significantly different are indicated by asterisks as follows: *, P < 0.01; **, P > 0.05. (Inset) The expression levels of transfected MDM2s were detected by Western blotting. (C) Representative polyribosomal profiles from MDM2/166E and MDM2/166A-transfected MDM2−/− p53−/− MEF cells. The traces obtained during fraction collection at 254 nm are shown. (D and E) Relative distribution of VEGF (D) and GAPDH (E) mRNA in MDM2−/− p53−/− MEF cells that were transfected with either MDM2/166E or MDM2/166A. Their cytoplasmic lysates were fractionated on a sucrose gradient. RNA extracted from each fraction was subjected to qRT-PCR. Data represent the percentage of the total amount of corresponding mRNA for each fraction.
Next, we directly measured the levels of VEGF protein that were secreted in the culture supernatant by ELISA to assess SK-N-SH cells transfected with wt MDM2 and MDM2/166A with and without exposure to hypoxia. We found that in normoxia, the VEGF level was significantly higher in the supernatant of the cells transfected with MDM2/166A than in those transfected with wt MDM2 (Fig. 4B). However, during hypoxia, in particular when cells were exposed to hypoxia for 8 h, the VEGF level became significantly increased in the SK-N-SH cells transfected with the wt MDM2, which was comparable to the level of VEGF in MDM2/166A-transfected cells that had a similar exposure to hypoxia. This suggests that the hypoxia-mediated dephosphorylation and translocation of MDM2 from the nucleus to the cytoplasm play a critical role in the production of VEGF protein.
In addition, we performed linear sucrose gradient fractionation to assess the association between polyribosomes and VEGF mRNA to further confirm that the cytoplasmic MDM2 regulates VEGF mRNA translation. For this assay, we used MDM2−/− p53−/− MEF cells transfected with the MDM2/166A and MDM2/166E plasmids (Fig. 4C). We found that VEGF mRNA in MDM2/166A-transfected cells, but not MDM2/166E-transfected cells, was clearly shifted from the fractions containing translation-dormant complexes (Fig. 4D, fractions 1 to 4) to fractions that were enriched with translating polyribosomes (Fig. 4D, fractions 5 to 11), which is indicative of enhanced translation. This observation was likely specific, because MDM2/166A expression had no effect on the polyribosome profile of a control GAPDH mRNA (Fig. 4E). Thus, induction of VEGF expression by cytoplasmic MDM2 appeared to occur at the translational level.
Inhibition of MDM2-mediated VEGF suppresses cancer cell growth and angiogenesis.
We evaluated the possible impact of the MDM2-mediated upregulation of VEGF in hypoxia on tumor cell growth and in vivo angiogenesis. We evaluated whether the knockdown of MDM2 by siRNA in MDM2-overexpressing cancer cells could reduce VEGF production and consequently inhibit cell growth and angiogenesis. In order to rule out the p53-dependent role of MDM2 in regulating these activities, we used the p53-null neuroblastoma cell line LA1-55N. First, we generated a pSUPER/MDM2 plasmid containing a 19-nt siRNA sequence that was made to be specific for targeting MDM2 (siMDM2) to determine whether silencing MDM2 would inhibit VEGF production during hypoxia and reduce the tumorigenic and/or angiogenic potential. Transfection of this plasmid into LA1-55N cells suppressed over 90% of the endogenous MDM2 protein (Fig. 5A). Following hypoxia in LA1-55N cells transfected with either the control siRNA or siMDM2, we indeed detected kinetic induction of VEGF protein: hypoxia induced greater production of VEGF in the LA1-55N control cells than in LA1-55N cells that were transfected with siMDM2 (Fig. 5B).
Fig. 5.
Knockdown of MDM2 inhibits cancer cell growth and angiogenesis by reducing VEGF production. (A) Stable suppression of MDM2 by transfection of a pSUPER MDM2 siRNA plasmid. MDM2 expression in LA1-55N cells transfected with the pSUPER vector containing a scrambled 19-nt control (siRNA control) and in four clones transfected with pSUPER MDM2 (siMDM2), as seen by Western blotting. (B) VEGF protein levels as detected by ELISA in culture supernatant of LA1-55N cells transfected with either the siRNA control or siMDM2 during hypoxia. (C) Hypoxia-induced drug resistance in MDM2-overexpressing LA1-55N cells (siRNA control), but not in the same cells following MDM2 inhibition with siMDM2. The cells were cultured for 24 h in hypoxia or normoxia and then were treated with 10 μM cisplatin or without cisplatin, under normoxia, for 24 more hours. Cell viability was detected by the WST assay. Data represent the mean percentages (bars) plus SD (error bars) of cell survival relative to the control (normoxia without cisplatin was defined as 100). Values that are significantly different (P < 0.05) are indicated by an asterisk. (D) Effect of anti-VEGF antibody on hypoxia-mediated cisplatin resistance in LA1-55N cells. Cells were exposed to hypoxia and treated with cisplatin as described above for panel C in the absence (−) or presence (+) of increasing concentrations (25, 50, and 100 ng/ml) (indicated by the height of the black triangle) of anti-VEGF antibody or increasing concentrations (50 and 100 ng/ml) of control IgG. Cell survival was detected by the WST assay. (E) Representative histological comparison of tumor vessel content in xenografts of SCID mice that were inoculated with LA1-55N cancer cells containing siRNA control or LA1-55N siMDM2-transfected cells, after 4 weeks, as stained by CD34 immunohistochemistry.
Next, we evaluated the role of MDM2 inhibition-mediated VEGF reduction during hypoxia in tumor cell survival, in response to the chemotherapeutic drug cisplatin. We treated LA1-55N cells that were either transfected with siMDM2 or the control siRNA with cisplatin, under normoxia and hypoxia. Interestingly, we found that hypoxia treatment reduced cisplatin (10 μM) cytotoxicity of the MDM2-overexpressing LA1-55N cancer cells by 20% (P < 0.05), while hypoxia appeared to have no protective effect on cisplatin cytotoxicity if the MDM2-silenced LA1-55N cells were cultured under similar hypoxic conditions (compared to normoxia) (Fig. 5C). To further confirm that MDM2 was involved in the regulation of VEGF during hypoxia, enhancing tumor cell survival, the parental LA1-55N cells that have MDM2 naturally overexpressed were treated with a neutralizing anti-VEGF antibody and hypoxia, followed by exposure to cisplatin. Thus, even though MDM2 was present and would increase VEGF production, the VEGF produced would be neutralized, and the effect of MDM2-regulated VEGF on cell viability would be detected. This antibody treatment not only completely reversed hypoxia-mediated cisplatin resistance, it was also able to significantly increase cisplatin's cytotoxicity as the concentration of anti-VEGF antibody increased (Fig. 5D).
In addition to affecting tumor cell survival in response to chemotherapy, MDM2 overexpression is also involved in angiogenesis. We established xenografts in SCID mice by inoculating the LA1-55N cells that were transfected either with siMDM2 or control siRNA. We observed a larger tumor size in mice with MDM2-overexpressing LA1-55N cells than in those having MDM2-silenced cells (data not shown). Upon processing the tumor tissue, we found that the microvessel density (MVD), as stained for using CD34 in immunohistochemistry, was significantly increased in the xenografts from MDM2-overexpressing cancer cells (i.e., the negative siRNA control), compared with those cells in which MDM2 was inhibited (siMDM2) (Fig. 5E).
DISCUSSION
Clinical studies indicate that MDM2 expression is associated with tumor growth and metastases in a variety of cancers. Evidence demonstrating that MDM2 regulates VEGF expression has been provided, and MDM2 regulation of VEGF expression may contribute to various MDM2 functions in promoting cancer growth and metastases. Still, the mechanisms by which MDM2 regulates VEGF expression remain obscure, although studies show that MDM2 interacts with HIF-1 to induce VEGF transcription (38). So far, the expression of VEGF appeared to be mainly regulated at the posttranscriptional level during hypoxia, which induces VEGF mRNA stabilization (24), suggesting that any MDM2-regulated VEGF promoter activity is not a major mechanism for induction of VEGF expression. In the present study, we demonstrated that MDM2 was able to bind directly to the VEGF 3′UTR, regulating VEGF translation. Our results indicated that MDM2 bound to the VEGF mRNA in a manner similar to the way it was previously shown to bind XIAP and p53 mRNA: the COOH-terminal RING domain, but not other parts of MDM2, bound to the AU-rich elements within the 3′UTR of the VEGF mRNA. This binding to the VEGF 3′UTR increased VEGF mRNA stabilization and subsequently its translation, which enhanced its production significantly particularly in hypoxia, as MDM2 was found to be translocated from the nucleus to the cytoplasm under that “environmental” condition, one which often prevails in cancer-stricken areas of the body.
We previously found that redistribution of MDM2 into the cytoplasm is required for it to bind to the XIAP mRNA to regulate its translation (15). This translocation of MDM2 from the nucleus to the cytoplasm can occur when cells undergo stress. For example, under genotoxic stress, such as exposure to irradiation, MDM2 becomes dephosphorylated. Dephosphorylation of MDM2 at S166, which lies within close proximity of the nuclear localization sequence (NLS) and nuclear export sequence (NES), results in inhibition of entry into the nucleus for MDM2, so it is left in the cytoplasm (28). In the present study, we found that the stress condition of hypoxia can also mediate a migration or translocation of MDM2 from the nucleus to the cytoplasm. Our results showed that during hypoxia, the phosphorylation of MDM2 at S166 was reduced. This indicated that the cytoplasmic redistribution of MDM2 is simply associated with hypophosphorylation of the protein. When the MDM2 protein is translocated from the nucleus to the cytoplasm, it becomes physically possible to bind to VEGF mRNA and thus, to regulate its stability and translation. This concept was validated by the results we obtained in experiments using gene transfection of a mutant MDM2 (at site 166), where we showed that cytoplasmic, but not nuclear, MDM2 stabilized VEGF mRNA.
It has been well established that hypoxia stimulates VEGF transcription through HIF-1-dependent pathways. It has also been established that hypoxia stabilizes VEGF mRNA and increases its translation via attachment of a number of mRNA-binding proteins such as HuR, hnRNP L, and DRBP76/NF90 (25, 35, 39). However, the biological and clinical significance of these RNA-binding proteins acting to regulate VEGF expression during hypoxia is not known. For example, to date, it is not known what the status of expression of these proteins in cancer patients is and whether their regulation of VEGF influences tumor growth and metastasis. In the present study, we demonstrated clearly that VEGF mRNA stability and translation were affected by MDM2, because the MDM2 protein was significantly increased during hypoxia in MDM2-overexpressing cancer cells than in MDM2-negative cells. Moreover, enforced overexpression of MDM2/166A in SK-N-SH cells remarkably stimulated colony formation of these cancer cells (data not shown). We believe that in addition to the known function of MDM2-regulated inhibition of p53 in these cells, the previously unknown upregulation of VEGF that can be achieved by the cytoplasmically located MDM2 is also an active contributor to increased cancer cell growth.
The notion that VEGF upregulation by hypoxia-modulated MDM2 stimulates tumor cell growth was more firmly ascertained by our experimental design using siRNA to knock down MDM2, specifically in an MDM2-overexpressing neuroblastoma cell line, LA1-55N. We found that inhibition of MDM2 by RNA silencing resulted in not only reduced VEGF secretion but also decreased survival of LA1-55N cancer cells in response to treatment by the chemotherapeutic drug cisplatin during induced hypoxia. A similarly decreased cell survival in response to cisplatin during hypoxia can also be obtained solely by treatment with an anti-VEGF antibody as well in LA1-55N cells without any MDM2 inhibition. Furthermore, we proved that in vivo, in a xenograft mouse model, the silencing of MDM2 reduced LA1-55N tumor growth and angiogenesis. Because LA1-55N cells lack wt p53, we believe that there is a p53-independent role for MDM2 in the regulation of VEGF during hypoxia and that it plays a critical role in tumor growth and angiogenesis. Thus, our present study has provided important insight into the conditions that promote cancer by strongly suggesting that the regulation of VEGF mRNA stability and translation by MDM2 could likely be a significant mechanism contributing to the promotion of tumor growth and metastasis in cancer patients whose tumor cells overexpress MDM2. An understanding of this mechanism may lead to more-targeted ways to avoid tumor growth and metastasis.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (grant 81072168) and the National Institutes of Health of the United States (R01 CA123490).
We have no conflicts of interest.
We thank Guillermine Lozano for providing us with the MDM2+/+ p53−/− and MDM2−/− p53−/− MEF cell lines.
Footnotes
Published ahead of print on 10 October 2011.
REFERENCES
- 1. Benjamin D., Moroni C. 2007. mRNA stability and cancer: an emerging link? Expert Opin. Biol. Ther. 7:1515–1529 [DOI] [PubMed] [Google Scholar]
- 2. Bertout J. A., Patel S. A., Simon M. C. 2008. The impact of O2 availability on human cancer. Nat. Rev. Cancer 8:967–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bond G. L., et al. 2004. A single nucleotide polymorphism in the MDM2 promoter attenuates the p53 tumor suppressor pathway and accelerates tumor formation in humans. Cell 119:591–602 [DOI] [PubMed] [Google Scholar]
- 4. Cairrao F., Halees A. S., Khabar K. S., Morello D., Vanzo N. 2009. AU-rich elements regulate Drosophila gene expression. Mol. Cell. Biol. 29:2636–2643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Candeias M. M., et al. 2008. p53 mRNA controls p53 activity by managing Mdm2 functions. Nat. Cell Biol. 10:1098–1105 [DOI] [PubMed] [Google Scholar]
- 6. Carmeliet P., Collen D. 1999. Role of vascular endothelial growth factor and vascular endothelial growth factor receptors in vascular development. Curr. Top. Microbiol. Immunol. 237:133–158 [DOI] [PubMed] [Google Scholar]
- 7. Chen C. Y., Shyu A. B. 1995. AU-rich elements: characterization and importance in mRNA degradation. Trends Biochem. Sci. 20:465–470 [DOI] [PubMed] [Google Scholar]
- 8. Claffey K. P., et al. 1998. Identification of a human VPF/VEGF 3′ untranslated region mediating hypoxia-induced mRNA stability. Mol. Biol. Cell 9:469–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Corvi R., et al. 1995. Non-syntenic amplification of MDM2 and MYCN in human neuroblastoma. Oncogene 10:1081–1086 [PubMed] [Google Scholar]
- 10. Datta M. W., Macri E., Signoretti S., Renshaw A. A., Loda M. 2001. Transition from in situ to invasive testicular germ cell neoplasia is associated with the loss of p21 and gain of mdm-2 expression. Mod. Pathol. 14:437–442 [DOI] [PubMed] [Google Scholar]
- 11. Elenbaas B., Dobbelstein M., Roth J., Shenk T., Levine A. J. 1996. The MDM2 oncoprotein binds specifically to RNA through its RING finger domain. Mol. Med. 2:439–451 [PMC free article] [PubMed] [Google Scholar]
- 12. Ferrara N., Davis-Smyth T. 1997. The biology of vascular endothelial growth factor. Endocr. Rev. 18:4–25 [DOI] [PubMed] [Google Scholar]
- 13. Forsythe J. A., et al. 1996. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol. Cell. Biol. 16:4604–4613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ganly I., et al. 2007. Identification of angiogenesis/metastases genes predicting chemoradiotherapy response in patients with laryngopharyngeal carcinoma. J. Clin. Oncol. 25:1369–1376 [DOI] [PubMed] [Google Scholar]
- 15. Gu L., et al. 2009. Regulation of XIAP translation and induction by MDM2 following irradiation. Cancer Cell 15:363–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Haupt Y., Maya R., Kazaz A., Oren M. 1997. Mdm2 promotes the rapid degradation of p53. Nature 387:296–299 [DOI] [PubMed] [Google Scholar]
- 17. Ho Q. T., Kuo C. J. 2007. Vascular endothelial growth factor: biology and therapeutic applications. Int. J. Biochem. Cell Biol. 39:1349–1357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Keene J. D. 2007. RNA regulons: coordination of post-transcriptional events. Nat. Rev. Genet. 8:533–543 [DOI] [PubMed] [Google Scholar]
- 19. Khor L. Y., et al. 2009. MDM2 and Ki-67 predict for distant metastasis and mortality in men treated with radiotherapy and androgen deprivation for prostate cancer: RTOG 92-02. J. Clin. Oncol. 27:3177–3184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kim K. J., et al. 1993. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature 362:841–844 [DOI] [PubMed] [Google Scholar]
- 21. Ladanyi M., et al. 1993. MDM2 gene amplification in metastatic osteosarcoma. Cancer Res. 53:16–18 [PubMed] [Google Scholar]
- 22. Lai Z., Freedman D. A., Levine A. J., McLendon G. L. 1998. Metal and RNA binding properties of the hdm2 RING finger domain. Biochemistry 37:7005–7015 [DOI] [PubMed] [Google Scholar]
- 23. Leach F. S., et al. 1993. p53 mutation and MDM2 amplification in human soft tissue sarcomas. Cancer Res. 53:2231–2234 [PubMed] [Google Scholar]
- 24. Levy A. P., Levy N. S., Goldberg M. A. 1996. Post-transcriptional regulation of vascular endothelial growth factor by hypoxia. J. Biol. Chem. 271:2746–2753 [DOI] [PubMed] [Google Scholar]
- 25. Levy N. S., Chung S., Furneaux H., Levy A. P. 1998. Hypoxic stabilization of vascular endothelial growth factor mRNA by the RNA-binding protein HuR. J. Biol. Chem. 273:6417–6423 [DOI] [PubMed] [Google Scholar]
- 26. Mathew R., et al. 2002. Alterations in p53 and pRb pathways and their prognostic significance in oesophageal cancer. Eur. J. Cancer 38:832–841 [DOI] [PubMed] [Google Scholar]
- 27. Mayo L. D., Donner D. B. 2001. A phosphatidylinositol 3-kinase/Akt pathway promotes translocation of Mdm2 from the cytoplasm to the nucleus. Proc. Natl. Acad. Sci. U. S. A. 98:11598–11603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Meek D. W., Knippschild U. U. 2003. Posttranslational modification of MDM2. Mol. Cancer Res. 1:1017–1026 [PubMed] [Google Scholar]
- 29. Momand J., Jung D., Wilczynski S., Niland J. 1998. The MDM2 gene amplification database. Nucleic Acids Res. 26:3453–3459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Momand J., Zambetti G. P., Olson D., George D., Levine A. J. 1992. The MDM-2 oncogene product forms a complex with the p53 protein and inhibits p53-mediated transactivation. Cell 69:1237–1245 [DOI] [PubMed] [Google Scholar]
- 31. Narasimhan M., Rose R., Ramakrishnan R., Zell J. A., Rathinavelu A. 2008. Identification of HDM2 as a regulator of VEGF expression in cancer cells. Life Sci. 82:1231–1241 [DOI] [PubMed] [Google Scholar]
- 32. Perfumo C., et al. 2009. MDM2 SNP309 genotype influences survival of metastatic but not of localized neuroblastoma. Pediatr. Blood Cancer 53:576–583 [DOI] [PubMed] [Google Scholar]
- 33. Plate K. H., Breier G., Weich H. A., Risau W. 1992. Vascular endothelial growth factor is a potential tumour angiogenesis factor in human gliomas in vivo. Nature 359:845–848 [DOI] [PubMed] [Google Scholar]
- 34. Rayburn E., Zhang R., He J., Wang H. 2005. MDM2 and human malignancies: expression, clinical pathology, prognostic markers, and implications for chemotherapy. Curr. Cancer Drug Targets 5:27–41 [DOI] [PubMed] [Google Scholar]
- 35. Shih S. C., Claffey K. P. 1999. Regulation of human vascular endothelial growth factor mRNA stability in hypoxia by heterogeneous nuclear ribonucleoprotein L. J. Biol. Chem. 274:1359–1365 [DOI] [PubMed] [Google Scholar]
- 36. Shweiki D., Itin A., Soffer D., Keshet E. 1992. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature 359:843–845 [DOI] [PubMed] [Google Scholar]
- 37. Shweiki D., Neeman M., Itin A., Keshet E. 1995. Induction of vascular endothelial growth factor expression by hypoxia and by glucose deficiency in multicell spheroids: implications for tumor angiogenesis. Proc. Natl. Acad. Sci. U. S. A. 92:768–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Skinner H. D., Zheng J. Z., Fang J., Agani F., Jiang B. H. 2004. Vascular endothelial growth factor transcriptional activation is mediated by hypoxia-inducible factor 1alpha, HDM2, and p70S6K1 in response to phosphatidylinositol 3-kinase/AKT signaling. J. Biol. Chem. 279:45643–45651 [DOI] [PubMed] [Google Scholar]
- 39. Vumbaca F., Phoenix K. N., Rodriguez-Pinto D., Han D. K., Claffey K. P. 2008. Double-stranded RNA-binding protein regulates vascular endothelial growth factor mRNA stability, translation, and breast cancer angiogenesis. Mol. Cell. Biol. 28:772–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhang L., Hill R. P. 2004. Hypoxia enhances metastatic efficiency by up-regulating Mdm2 in KHT cells and increasing resistance to apoptosis. Cancer Res. 64:4180–4189 [DOI] [PubMed] [Google Scholar]
- 41. Zhou G., et al. 2007. MDM2 promoter SNP309 is associated with risk of occurrence and advanced lymph node metastasis of nasopharyngeal carcinoma in Chinese population. Clin. Cancer Res. 13:2627–2633 [DOI] [PubMed] [Google Scholar]
- 42. Zietz C., et al. 1998. MDM-2 oncoprotein overexpression, p53 gene mutation, and VEGF up-regulation in angiosarcomas. Am. J. Pathol. 153:1425–1433 [DOI] [PMC free article] [PubMed] [Google Scholar]