Abstract
Bacteroides fragilis represents an early infant colonizer with important host interactions. Our knowledge about the diversity, transmission, and persistence of this bacterium, however, is limited. Here, we addressed these questions using a combination of multilocus sequence typing (MLST) and variable-number tandem repeat (VNTR) sequence analyses. We used both culture-dependent and -independent typing. We genotyped B. fragilis in fecal samples from a cohort of 93 mothers and their children, with samples taken from the mothers and from the children at the ages 1 to 10 days, 4 months, 1 year, and 2 years. By MLST we found two main B. fragilis groups, which we denoted clades A and B. Direct typing of stool samples using the icd gene revealed seven sequence types, five within clade A and two within clade B. A single clade A sequence type, however, represented 79% of all the sequences. This sequence type was further subtyped using VNTR. VNTR subtyping revealed 16 different VNTR types. Based on the distribution patterns of these, we show mother-to-child transmission and multiple-strain colonization. We argue that negative host selection promotes the coexistence of multiple strains. The significance of our findings is that we have started unraveling the transmission and persistence patterns of one of the most important human gut colonizers.
INTRODUCTION
Bacteroides fragilis is both an important pathogen and a commensal bacterium within the human gut. B. fragilis is highly prevalent in the human population and does not have other known niches than the gut of mammals (21) Despite its importance, our knowledge about the transmission and persistence of commensal B. fragilis within a healthy human population is very limited. Most of the research attention until now has been on disease-associated strains. These strains include enterotoxigenic strains (28) in addition to nonenterotoxigenic strains associated with anaerobic bacteremia (3) and a range of opportunistic diseases (23).
The aim of the present work was therefore to use a newly developed multilocus sequence typing (MLST) directly on feces (4) in combination with a variable-number tandem repeat (VNTR) sequence approach (35) to determine the B. fragilis transmission, persistence, and correlation to allergen-specific IgE in an unselected cohort of mothers and their children. This was done using material collected from the IM-PACT cohort, which is a part of the large prospective Prevention of Allergy among Children in Trondheim (PACT) study in Norway. PACT focuses on the impacts of systematic and structured interventions on childhood allergy, while IM-PACT is the immunology and microbiology (IM) part of PACT (20).
B. fragilis contacts the epithelial mucosa, and it demonstrates a closer host interaction than the other Bacteroides species (16). B. fragilis is able to modulate the surface structure, and 28 polysaccharide loci have been identified (22). The production of these is switched on and off by a reversible inversion of promoter-containing DNA segments (12). Recent data indicate that phase-variable surface structure is important for the persistence of B. fragilis within the gut (14). With respect to bacteria-bacteria competition, it has been shown that B. fragilis can produce a variety of bacteriocins which are important for suppressing closely related species (25).
There is relatively strong evidence that B. fragilis has immune-modulating properties, which is important for maturation of the human immune system (27). For the IM-PACT cohort, we have recently shown that, at the age of 1 and 2 years, the levels of B. fragilis are higher in children with high specific IgE (sIgE) (32). IgE plays a central role in asthma and allergy (8). Other studies have shown a correlation of B. fragilis with asthma (34) and pollen allergy (19).
The main findings presented demonstrate mother-to-child transmission of B. fragilis and indicate that multiple strains can colonize a single child. We argue that the multiple-strain coexistence is promoted by negative mucosal selective pressure.
MATERIALS AND METHODS
Test material.
In this study we analyzed 483 fecal samples from a total of 93 mothers and their children in connection with the IM-PACT study, which was started in Trondheim in 2001. Stool samples were taken from mothers around gestational weeks 13 and 38 and from their children at ages 1 to 10 days, 4 months, 1 year, and 2 years. The stool samples were immediately frozen in Cary-Blair transport medium (0.5% NaCl, 0.11% Na2HPO4, 0.15% HSCH2COONa, 0.09% CaCl2, pH 8.4) (18) at −20°C and transferred to −80°C for long-term storage. Venous blood samples from the children were taken at 2 years of age for specific IgE measurements to determine allergic sensitization, defined as an sIgE of >0.35 kU/ml for at least one allergen (32). IM-PACT is an extension of the PACT study of allergy in children in Trondheim. PACT began in 2000 with the objective of reducing the increase in the incidence of asthma and allergy and is described in detail elsewhere (31). Participants in the IM-PACT trial were recruited from the main study control cohort (20).
Cultivation of B. fragilis from stool samples.
Cultivation of B. fragilis was carried out using selective medium (Bacteroides bile esculin agar with amikacin; BD Diagnostic Systems, Heidelberg, Germany) with incubation at 37°C for 48 h. Incubation took place under anaerobic conditions created by an Oxoid AnaeroGen system (Oxoid Ltd., Hampshire, England). Single colonies were then picked and transferred to 200 μl of Cary-Blair transport medium and frozen at −80°C.
Isolation of DNA from stool samples.
For sequencing analyses, we used DNA purified with paramagnetic beads in accordance with an optimized and automated protocol (29). For VNTR sequence analyses, DNA was reisolated from stool samples using a QIAamp DNA Stool Mini Kit (Qiagen, Hilden, Germany). The protocol for isolation of DNA from stool samples for pathogen detection was followed.
Isolation of DNA from bacterial isolates.
Bacterial cell pellets from five strains of B. fragilis from the Deutsche Sammlung von Mikroorganismen und Zellkulturen (DSMZ; Braunschweig, Germany) were rehydrated in 500 μl of Tris-EDTA (TE) buffer. For DNA isolation we used a DNeasy Blood and Tissue Kit (Qiagen), following the protocols for pretreatment of Gram-negative bacteria. For isolation of DNA from growing colonies of B. fragilis, we used PrepMan Ultra Sample Preparation Reagent according to the manufacturer's recommendations (Applied Biosystems, Foster City, CA).
Primer design.
CLC Main Workbench (version 4.1.1) (CLCbio, Aarhus, Denmark) was used for the design of both the MLST and VNTR primers and analysis of sequenced fragments.
Construction of the MLST primers was based on the seven published MLST genes for Escherichia coli (24). These were identified in the sequenced genomes of B. fragilis strain NCTC 9343 and B. fragilis YHC46. Aligned sequences were used as templates for the design of forward and reverse primers in conserved regions using standard criteria for primer design. Subsequent to design, primer pairs for each of the housekeeping genes were examined for specificity using the Basic Local Alignment Search Tool (BLAST) in the NCBI database (blast.ncbi.nlm.nih.gov/Blast).
The five B. fragilis strains from DSMZ were used in the evaluation. Optimal annealing for PCR was chosen on the basis of visualization of PCR products by agarose gel electrophoresis. Only primers that gave consistent amplification were included in the final work (see Table S1 in the supplemental material).
To identify VNTR sequences, the mreps web server (bioinfo.lifl.fr/mreps/mreps.php) was used. mreps is a software program designed to identify tandem repeats in a DNA sequence. This program has no restrictions on the size of the repeated pattern and can therefore be used to detect all types of tandem repeats (11). The two sequenced and annotated genomes of B. fragilis strain NCTC 9343 (2) and B. fragilis YCH46 (13) were analyzed by the program with the error margin set to zero and no filtering for maximum or minimum length of the tandem repetitive sequence or the number of repetitions. The VNTR sequences found in these B. fragilis strains were further sorted using Excel to find matching regions. Sequences flanking the regions of matching VNTR sequences found in the two B. fragilis strains were used as templates for the design of VNTR primers using the same criteria and evaluation as for the MLST primers. PCR primer pairs for 12 VNTR sequences were constructed and evaluated, while only the primer pair that gave variation and consistent amplification for all the strains evaluated was used in the screening (see Table S2 in the supplemental material).
PCR amplification and DNA sequencing.
PCRs were performed in a 25-μl reaction volume, containing 1 μl of DNA solution as template, 1× Phusion HF buffer (providing 1.5 mM MgCl2) (Finnzymes, Espoo, Finland), 200 μM each deoxynucleoside triphosphate (dNTP) (Thermo Scientific, Waltham, MA) 0.2 μM each primer (forward and reverse) (Invitrogen, OR), and 0.02 U/μl Phusion Hot Start High-Fidelity DNA Polymerase (Finnzymes).
For MLST, the following amplification protocol was used: an initial denaturation at 98°C for 10 s, followed by 35 cycles at 98°C for 10 s, 54°C for 30 s, and 72°C for 30 s, with a subsequent extension at 72°C for 7 min. For the VNTR analyses the same protocol was used, changing the annealing temperature to 53°C and using 5′ 6-carboxyfluorescein (FAM)-labeled forward primers, while the cepA and cfiA PCR analyses were performed as previously published (9).
DNA sequencing was used to verify amplification products and for MLST genotyping of samples. To remove single-stranded, unincorporated oligonucleotides (DNA primers) before sequencing, 0.5 μl of PCR product (reaction) was treated with 0.4 U of exonuclease I ([Exo I] New England Biolabs, Ipswich, MA) in a volume of 5 μl containing 1× BigDye sequencing buffer (Applied Biosystems, Foster City, CA). The samples were incubated at 37°C for 30 min, followed by 80°C for 15 min to inactivate the exonuclease. For sequencing reactions, 0.5 μl of 5× sequencing buffer (Applied Biosystems, Foster City, CA), 1.0 μl of BigDye Terminator, version 1.1 (Applied Biosystems), and 0.32 μM forward or 0.32 μM reverse sequencing primer was added to the Exo I-treated PCR products in a total volume of 10 μl.
The cycle sequencing reaction took place under the following thermocycling conditions: 96°C for 1 min, followed by 25 cycles at 95°C for 15 s, 50°C for 5 s, and 60°C for 4 min. In order to purify and stabilize the sequencing reaction products, 45 μl of SAM Solution (Applied Biosystems) and 10 μl of XTerminator Solution (Applied Biosystems) were added, and the reaction mixtures were shaken (mini-shaker for immunology PSU-2T; BioSan, Riga, Latvia) at maximum speed (1,000 rpm) for 1 h in a microtiter plate. Detection of sequences was performed on an ABI 3130xl Genetic Analysis workstation (Applied Biosystems, Foster City, CA).
For the full MLST, complementary sequences from forward and reverse sequencing reactions were assembled into a consensus sequence for each sample. Discrepancies in the software selection and interpretation of the incorporated nucleotide were manually checked and corrected if necessary using the information from the electropherograms.
VNTR amplicon analyses by capillary gel electrophoresis.
One microliter of 6-FAM-labeled PCR product was added to 9.3 μl of deionized formamide (HiDi Formamide; Applied Biosystems) to lower the melting temperature, and 0.3 μl of Gene Scan Rox500 (Applied Biosystems, Warrington, United Kingdom) was added as an internal size standard. The samples were centrifuged for 10 s at 1,650 × g (IEC Centra CL3; Thermo Electron Corporation), denatured at 95°C for 2 min, and cooled on ice. Capillary gel electrophoresis was run on an ABI 3130xl Genetic Analysis workstation (Applied Biosystems).
The data from the VNTR capillary electrophoresis fragments were analyzed by using GeneMapper software, version 4.0 (Applied Biosystems). Allele sizes were determined by the size standard and the number of tandem repeating units (mono-, di-, tri-, and tetranucleotide repeats). Preset analysis parameters for tetranucleotide repeats were used. A bin window size of ±1.70 was employed for the detection of alleles, and homozygotes required a minimum peak of 200, while this cutoff was 100 for heterozygotes.
Phylogenetic and statistical analyses.
Sequences were aligned using the Muscle algorithm for alignment (CLC Main Workbench), and SplitsTree, version 4, was used for phylogenetic reconstruction (10). The basic principle of split networks is that incongruent phylogenetic relations are also visualized, as would be the case of recombination and horizontal gene transfer between the four genes in the full MLST.
Basic statistical analyses were performed using Tibco Spotfire S+, version 8.2, software (Palo Alto, CA). Cross-tables were analyzed for significance by using Fisher's exact test, while distributions were analyzed using the nonparametric Wilcoxon rank sum test.
Nucleotide sequence accession numbers.
Sequences generated in this work were deposited in GenBank with the accession numbers JF803602 to JF803629. Details about the sequences are given in Tables S3 and S5 in the supplemental material.
RESULTS
Diversity and transmission patterns determined by MLST.
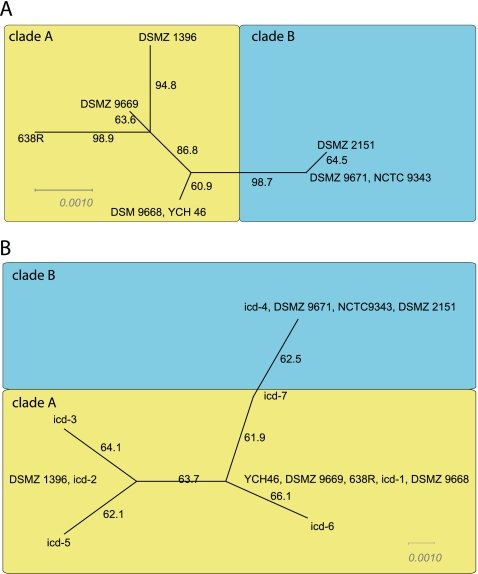
The first analyses performed evaluated whether consistent phylogenetic reconstruction for the strains analyzed could be derived for the four housekeeping genes included in this work. This was accomplished using SplitsTree (10) analysis for the full MLST, which showed congruent phylogenetic relations for all the strains evaluated. These analyses revealed two main clades, A (n = 4) and B (n = 3), with clade A being apparently more diverse than B (Fig. 1A).
Fig. 1.
SplitsTree for full MLST and icd genotype data. (A) The full MLST was determined for the pure strains included in this work, in addition to the genome-sequenced strains. (B) All identified icd genotypes were included in the tree. Numbering at the branches shows the bootstrap support in percentage. Distance bars represent uncorrected p-distances for neighbor-joining trees.
The icd gene was chosen for the direct culture-independent screening of the fecal samples. The rationale for this choice was that this gene gave the most robust amplification and contained the highest level of polymorphic sites. We obtained icd amplification products of the expected sizes from 160 out of 483 stool samples. Of these, 129 were confirmed as icd by DNA sequencing. These represented a total of seven sequence types. None of the direct sequences were mixed, indicating a single dominant sequence type. The sequence types from the direct sequencing formed the same clades as for the full MLST, with five clade A and two clade B sequence types (Fig. 1B).
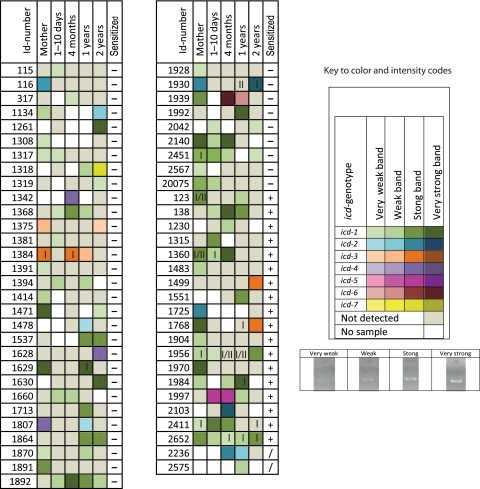
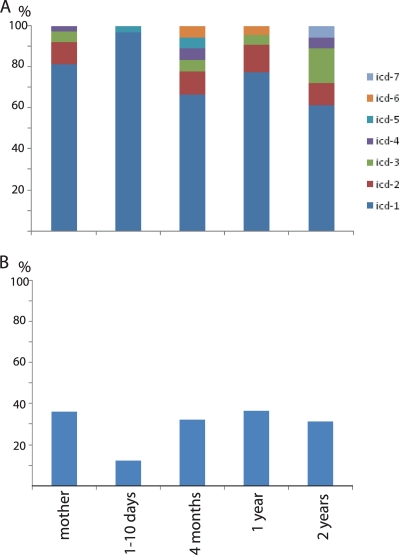
A semiquantitative investigation of the individual distribution of the identified sequence types was performed by combining the information from the band intensity of the PCR-amplified icd fragment with sequence type information (Fig. 2). These analyses revealed relatively large fluctuations in B. fragilis levels and a single dominant sequence type (icd-1). With respect to prevalence we found that icd-1 represented 79% of all the sequences. Interestingly, the age-related distributions of the sequence types showed an apparent reduced diversity at the age of 1 to 10 days, with a nearly complete dominance of icd-1 (Fig. 3A). The reduced diversity was also correlated to a decreased prevalence of B. fragilis at 1 to 10 days (Fig. 3B).
Fig. 2.
Distribution of icd genotypes among mothers and infants analyzed in this study. A color code and intensity scale are used to indicate genotype and signal intensity, respectively. The Roman numbers (I and II) indicate whether cepA- or cfiA-positive strains were isolated from the samples, respectively. IgE sensitization information is given for children 2 years of age: +, sensitized; −, nonsensitized; /, lack of information about sensitization. The 8-bit pixel values for the inverse band signal intensities were defined as follows: high, >200 (maximum, 256); medium, 200 to 170; weak, 170 to 150; and very weak, <150. The background signals were about 120. Id, identification.
Fig. 3.
Relative icd genotype composition (A) and proportion of B. fragilis-positive samples (B) for the different age categories investigated. The representations are summaries of the data presented in Fig. 2.
Transmission patterns were tested by Fisher's exact test. With respect to mother-to-child transmission, the icd-1 sequence type was at the border of significance at 1 to 10 days. Sequence type icd-3, on the other hand, showed a significant association with that of the mother at both 4 months and 1 year (Table 1).
Table 1.
The association between the dominant icd genotypes in mothers and their children
| Sequence type | Age of child | No. of instances of sequence type for the indicated mother/child conditiona |
P valueb | |||
|---|---|---|---|---|---|---|
| Present/present | Absent/present | Present/absent | Absent/absent | |||
| icd–1 | 1–10 days | 8 | 8 | 9 | 27 | 0.074 |
| 4 mos | 6 | 5 | 15 | 36 | 0.108 | |
| 1 yr | 5 | 9 | 8 | 31 | 0.217 | |
| 2 yr | 4 | 5 | 16 | 36 | 0.328 | |
| icd–2 | 1–10 days | 0 | 0 | 3 | 44 | 1 |
| 4 mos | 0 | 1 | 2 | 60 | 0.9999 | |
| 1 yr | 0 | 1 | 2 | 57 | 1 | |
| 2 yr | 1 | 1 | 2 | 59 | 0.094 | |
| icd–3 | 1–10 days | 0 | 0 | 2 | 54 | 1 |
| 4 mos | 1 | 0 | 0 | 61 | 0.016 | |
| 1 yr | 1 | 0 | 1 | 57 | 0.034 | |
| 2 yr | 1 | 2 | 1 | 60 | 0.092 | |
The presence or absence of the sequence type is given in respective order for mothers and children.
Fisher's exact test.
Subtyping by VNTR analyses.
Due to the very high prevalence of icd-1, we chose to search for the more polymorphic VNTR markers for resolving this sequence type. Potential hypervariable VNTR sequences were identified by comparison and analysis of the genome-sequenced strains using the web-based mreps program. We identified a set of 12 candidate VNTR regions while only one region (TRS1) showed size variation and consistent amplification of all five reference strains included in our work (see supplemental text for details).
VNTR genotyping was conducted for TRS1 using a selection of seven sensitized and six nonsensitized children. From the direct, culture-independent VNTR analyses, we routinely observed amplification as expected from the icd genotyping. Most samples yielded one allele per sample, but in some samples up to four alleles were detected, with the highest diversity at 2 years (Table 2). For the culture-dependent analyses, on the other hand, we observed the highest diversity in mothers (Table 3). In total, we detected 16 TRS1 alleles in this study, with 9 identified both by direct analyses and by cultivation (see Table S4 in the supplemental material). Three of the VNTR sequences were detected only in stool, while four were detected only by cultivation. Identity of the different TRS1 alleles was confirmed by DNA sequencing (see Table S5).
Table 2.
TRS1 genotypes identified directly from stool samples
| Identification no. | Sensitized | TRS1 genotype by sample groupa |
||||
|---|---|---|---|---|---|---|
| Mother | Child at: |
|||||
| 1-10 days | 4 mos | 1 yr | 2 yr | |||
| 1317 | − | 272 | 244 | 252 | ||
| 280 | 252 | 284 | ||||
| 1384 | − | 236 | 236 | 236 | NA | NA |
| 1629 | − | 268 | 252 | 252 | 244 | 244 |
| 1930 | − | 276 | 252 | NA | 252 | 196 |
| 276 | 236 | |||||
| 252 | ||||||
| 276 | ||||||
| 2451 | − | 224 | 240 | 240 | ||
| 240 | ||||||
| 20075 | − | 268 | 268 | NA | 268 | 252 |
| 268 | ||||||
| 272 | ||||||
| 284 | ||||||
| 123 | + | NA | 252 | 272 | 236 | 268 |
| 1360 | + | 252 | 252 | 252 | 252 | 252 |
| 1768 | + | 268 | 244 | 252 | 252 | |
| 252 | 252 | 196 | ||||
| 268 | ||||||
| 1956 | + | 244 | 244 | NA | NA | 236 |
| 1984 | + | 196 | 196 | 196 | ||
| 2411 | + | 272 | 272 | 272 | 240 | 256 |
| 272 | ||||||
| 2652 | + | 236 | NA | NA | 236 | 236 |
Dominant sequence types are in boldface. NA, not amplified; empty cell, no sample taken.
Table 3.
TRS1 genotypes identified from colonies
| Identification no. | Sensitized | TRS1 genotype by sample groupa |
||||
|---|---|---|---|---|---|---|
| Mother | Child at: |
|||||
| 1-10 days | 4 mos | 1 yr | 2 yr | |||
| 1384 | − | 220 | 288 | 236 | ||
| 236 | 236 | 272 | ||||
| 1629 | − | 232 | 244 | |||
| 1930 | − | NA | NA | |||
| 2451 | − | 240 | ||||
| 224 | ||||||
| 20075 | − | 236 | ||||
| 272 | ||||||
| 123 | + | NA | 236 | |||
| 1360 | + | 252 | 252 | |||
| 248 | ||||||
| 264 | ||||||
| 1768 | + | 272 | NA | |||
| 1956 | + | 252 | NA | |||
| 240 | ||||||
| 220 | ||||||
| 244 | ||||||
| 1984 | + | 196 | ||||
| 2411 | + | 256 | 272 | 272 | ||
| 272 | ||||||
| 280 | ||||||
| 2652 | + | 236 | 236 | 236 | ||
| 252 | ||||||
NA, not amplified; empty cell, no sample taken.
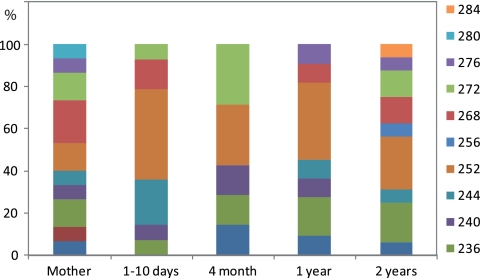
For the direct analyses of feces, we found that the TRS1 genotype diversity was highest in mothers and 2-year-old children. From 1 to 10 days to 1 year there was a relative increase in the prevalence of the 252 genotype (Fig. 4). As determined by Fisher's exact test, the dependencies between the mothers' and the children's TRS1 genotypes were highly significant for all age categories. There was, however, a decrease in significance with age from a P value of 10−6 at 1 to 10 days to 0.0005 at 2 years (see Table S6 in the supplemental material).
Fig. 4.
Relative TRS1 composition directly from the stool samples for the different age categories investigated. The percentages of the different TRS1 types identified are shown.
Typing of cultured isolates.
A total of 77 colonies were recovered from the samples analyzed. Of these, 55% (n = 42) were cepA positive, 8% (n = 6) were cfiA positive, 70% (n = 54) were icd positive, and 71% (n = 55) were VNTR positive (Table 4). The multiple VNTR genotypes for eight colonies indicate that these represented mixed strains. Generally, cepA- and cfiA-positive strains were mutually exclusive while icd-positive strains covered both groups.
Table 4.
Characteristics of colonies recovered from the stool samples
| Identification no. | Sample source (age) | Isolate profilea |
|||
|---|---|---|---|---|---|
| cepA | cfiA | icd sequence type | TRS1 type | ||
| 1384 | Mother | − | − | − | − |
| Mother | + | − | 3 | 236 | |
| Mother | + | − | 1 | 272 | |
| Mother | + | − | 3 | − | |
| Child (4 mos) | + | − | ND | 236 | |
| Child (1 yr) | − | − | − | 236/272 | |
| Child (1 yr) | − | − | − | 236 | |
| 1629 | Mother | − | − | − | 244 |
| Mother | − | − | − | − | |
| Child (1 yr) | + | − | 1 | 244 | |
| Child (1 yr) | + | − | 3 | − | |
| Child (1 yr) | + | − | 1 | 272 | |
| Child (1 yr) | + | − | 1 | 244 | |
| 1930 | Child (1 yr) | − | − | − | − |
| Child (1 yr) | − | − | − | − | |
| Child (1 yr) | − | + | 8 | 236 | |
| Child (1 yr) | − | − | 1 | 236/272 | |
| Child (2 yr) | − | − | − | − | |
| Child (2 yr) | − | − | − | − | |
| Child (2 yr) | + | − | ND | 252 | |
| 2451 | Mother | + | − | 1 | 240 |
| 20075 | Mother | − | − | − | − |
| Mother | − | − | 1 | 280 | |
| Mother | − | − | − | − | |
| 123 | Mother | − | − | − | − |
| Mother | − | − | ND | 272 | |
| Mother | − | + | 8 | − | |
| Child (1 yr) | + | − | 1 | 236 | |
| 1360 | Mother | + | + | ND | 252 |
| Mother | + | − | 1 | 272 | |
| Mother | − | − | 1 | − | |
| Mother | + | − | 1 | 248/264 | |
| Mother | + | − | 3 | 236 | |
| Mother | − | − | − | 236 | |
| Child (1–10 days) | + | − | 1 | 252 | |
| Child (1–10 days) | + | − | 1 | 236 | |
| Child (1–10 days) | + | − | 1 | 252 | |
| Child (1–10 days) | + | − | 1 | 252 | |
| Child (1–10 days) | + | − | 1 | 196 | |
| 1768 | Child (1 yr) | + | − | 8 | 272 |
| Child (1 yr) | − | − | ND | − | |
| Child (1 yr) | + | − | 1 | 252 | |
| Child (2 yr) | − | − | − | − | |
| Child (2 yr) | − | − | − | − | |
| Child (2 yr) | − | − | − | 236 | |
| 1956 | Mother | + | − | 1 | 220/244 |
| Child (4 mos) | − | + | 8 | 252 | |
| Child (4 mos) | + | − | 1 | 196 | |
| Child (4 mos) | + | − | 1 | 252 | |
| Child (1 yr) | − | + | 8 | − | |
| Child (1 yr) | − | + | 8 | − | |
| Child (1 yr) | + | − | 1 | 240 | |
| 1984 | Child (1 yr) | + | − | 1 | 196 |
| Child (1 yr) | − | − | − | 288 | |
| 2411 | Mother | + | − | 1 | 256/272 |
| Mother | − | − | 1 | 272 | |
| Mother | + | − | 1 | 252 | |
| Mother | − | − | − | − | |
| Child (1–10 days) | − | − | − | 252 | |
| Child (2 yr) | + | − | 1 | 272 | |
| Child (2 yr) | + | − | ND | 224/240 | |
| Child (2 yr) | − | − | − | − | |
| Child (2 yr) | − | − | 8 | − | |
| Child (2 yr) | + | − | 1 | 272 | |
| 2652 | Child (2 yr) | + | − | 8 | 220/236 |
| Child (2 yr) | + | − | 1 | 244 | |
| Child (2 yr) | + | − | 1 | 252 | |
| Child (2 yr) | − | − | − | − | |
| Child (2 yr) | + | − | ND | 272 | |
| Child (2 yr) | − | − | − | 272 | |
| Child (2 yr) | + | − | 1 | 272 | |
| Child (2 yr) | − | − | − | 236 | |
| Child (2 yr) | + | − | 1 | 236 | |
| Child (1 yr) | + | − | 1 | 236/252 | |
| Child (1 yr) | + | − | 1 | 244 | |
| Child (4 mos) | + | − | 1 | 236 | |
| Child (4 mos) | + | − | 1 | 244 | |
+, amplification; −, no amplification.
icd types are indicated by numbers, where 8 is a new sequence type detected only from colonies. ND, no readable sequence was recovered.
icd sequencing showed a dominance of icd-1. Interestingly, two out of four icd-3 strains were recovered from mother 1384, which also showed dominance for icd-3 in the direct stool analyses. A new sequence type, icd-8, dominated among cfiA-positive strains. This sequence type is distantly related to the other sequence types detected by the direct stool analyses, supporting the contention that it belongs to B. fragilis division II, with the other icd sequence types belonging to division I (9).
Correlation to IgE sensitization.
Using Fisher's exact test, we explored whether there was a significant association between the icd genotypes and allergic sensitization. When a Bonferroni correction was taken into account in these analyses, no significant associations were found. Likewise, we found no significant association between TRS1 genotypes and sensitization. With respect to TRS1 diversity, however, two out of four nonsensitized 2-year-old children with samples for that point had four different TRS1 alleles, while none of the six nonsensitized children harbored more than two alleles. We tested whether this represented a significant overrepresentation by use of a nonparametric Wilcoxon rank sum test with the null hypothesis that four alleles were equally distributed among sensitized and nonsensitized children. This test suggested that there was a significant overrepresentation of multiple alleles for the nonsensitized children (P = 0.03).
DISCUSSION
Our data support mother-to-child transmission of B. fragilis, followed by persistence of multiple closely related strains within a single child. We found the long-term persistence of multiple strains particularly intriguing. From ecological theory one can explain multiple-strain coexistence by (i) negative external selection pressure, (ii) mutualism, (iii) equilibrium interactions, (iv) physical separation, or (v) occupancy of different biochemical niches. The fact that B. fragilis has phase-variable surface epitopes (15) suggests that this bacterium could be under negative external selection pressure either by the host's adaptive immune system (6, 26), predation (36), or bacteriophage infections (22, 37). Thus, it is likely that negative external selection pressure (i) drives multiple-strain coexistence (30). With respect to physical separation (iv), it has been proposed that the mucosal microbiota has mosaic structure (5). Given that there is a flux of mucosa-associated bacteria to feces, this would lead to multiple-strain coexistence. Therefore, physical separation in conjunction with negative selection could be the underlying factor forcing diversity. We find mutualism, equilibrium interactions, and occupancy of different niches less likely explanations of the multiple-strain coexistence observed in this study. These mechanisms would imply structured interactions, which we did not observe.
Independently, we have found that high levels (32) and low diversity (this work) of B. fragilis were correlated to sensitization. Mucosal protection, however, is highly complex, relying on a balance between innate and adaptive responses (6, 17). The mechanisms (if any) for the correlations detected here cannot, therefore, easily be deduced.
Previous studies have indicated a relatively high global diversity for B. fragilis (1, 9). Our finding of the high prevalence of a single group of closely related phylotypes suggests that the strains colonizing the infants in the IM-PACT cohort represent a B. fragilis subpopulation. The ecological drivers for the subpopulation still remain unknown. However, since there was a relative increase in the dominant phylotype right after birth, this suggests an enrichment of this phylotype during the birth process.
On one hand, B. fragilis is an opportunistic pathogen (3, 28) while, on the other hand, this bacterium is important for human infant immune development and maturation (33). It will be very important in the future to determine whether the mother-to-child transmission is host selected, commensal, pathogenic, or symbiotic.
Supplementary Material
ACKNOWLEDGMENTS
Funding for the IM-PACT study was obtained from GlaxoSmithKline AS, Norway. The PACT study was funded by the Norwegian Department of Health and Social affairs from 1997 to 2003. A university scholarship from the Norwegian University of Science and Technology (NTNU) funded the research fellows. The IgE analyses were funded by Siemens Medical Solutions Diagnostics.
Footnotes
Supplemental material for this article may be found at http://aem.asm.org/.
Published ahead of print on 30 September 2011.
REFERENCES
- 1. Antunes E. N., et al. 2004. Non-toxigenic pattern II and III Bacteroides fragilis strains: coexistence in the same host. Res. Microbiol. 155: 522–524 [DOI] [PubMed] [Google Scholar]
- 2. Cerdeno-Tarraga A. M., et al. 2005. Extensive DNA inversions in the B. fragilis genome control variable gene expression. Science 307: 1463–1465 [DOI] [PubMed] [Google Scholar]
- 3. Cheng C. W., et al. 2009. Clinical significance of and outcomes for Bacteroides fragilis bacteremia. J. Microbiol. Immunol. Infect. 42: 243–250 [PubMed] [Google Scholar]
- 4. de Muinck E. J., Øien T., et al. 2011. Diversity, transmission and persistence of Escherichia coli in a cohort of mothers and their infants. Environ. Microbiol. Rep. 3: 352–359 [DOI] [PubMed] [Google Scholar]
- 5. Eckburg P. B., et al. 2005. Diversity of the human intestinal microbial flora. Science 308: 1635–1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Edelman S. M., Kasper D. L. 2008. Symbiotic commensal bacteria direct maturation of the host immune system. Curr. Opin. Gastroenterol. 24: 720–724 [DOI] [PubMed] [Google Scholar]
- 7. Reference deleted.
- 8. Gould H. J., Sutton B. J. 2008. IgE in allergy and asthma today. Nat. Rev. Immunol. 8: 205–217 [DOI] [PubMed] [Google Scholar]
- 9. Gutacker M., Valsangiacomo C., Bernasconi M. V., Piffaretti J. C. 2002. recA and glnA sequences separate the Bacteroides fragilis population into two genetic divisions associated with the antibiotic resistance genotypes cepA and cfiA. J. Med. Microbiol. 51: 123–130 [DOI] [PubMed] [Google Scholar]
- 10. Huson D. H., Bryant D. 2006. Application of phylogenetic networks in evolutionary studies. Mol. Biol. Evol. 23: 254–267 [DOI] [PubMed] [Google Scholar]
- 11. Kolpakov R., Bana G., Kucherov G. 2003. mreps: efficient and flexible detection of tandem repeats in DNA. Nucleic Acids Res. 31: 3672–3678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Krinos C. M., et al. 2001. Extensive surface diversity of a commensal microorganism by multiple DNA inversions. Nature 414: 555–558 [DOI] [PubMed] [Google Scholar]
- 13. Kuwahara T., et al. 2004. Genomic analysis of Bacteroides fragilis reveals extensive DNA inversions regulating cell surface adaptation. Proc. Natl. Acad. Sci. U. S. A. 101: 14919–14924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu C. H., Lee S. M., Vanlare J. M., Kasper D. L., Mazmanian S. K. 2008. Regulation of surface architecture by symbiotic bacteria mediates host colonization. Proc. Natl. Acad. Sci. U. S. A. 105: 3951–3956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nakayama-Imaohji H., et al. 2009. Identification of the site-specific DNA invertase responsible for the phase variation of SusC/SusD family outer membrane proteins in Bacteroides fragilis. J. Bacteriol. 191: 6003–6011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Namavar F., et al. 1989. Epidemiology of the Bacteroides fragilis group in the colonic flora in 10 patients with colonic cancer. J. Med. Microbiol. 29: 171–176 [DOI] [PubMed] [Google Scholar]
- 17. Neish A. S. 2009. Microbes in gastrointestinal health and disease. Gastroenterology 136: 65–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Neumann D. A., Benenson M. W., Hubster E., Thi Nhu Tuan N. 1972. Cary-Blair, a transport medium for Vibrio parahaemolyticus. Am. J. Clin. Pathol. 57: 33–34 [DOI] [PubMed] [Google Scholar]
- 19. Odamaki T., et al. 2007. Fluctuation of fecal microbiota in individuals with Japanese cedar pollinosis during the pollen season and influence of probiotic intake. J. Investig. Allergol. Clin. Immunol. 17: 92–100 [PubMed] [Google Scholar]
- 20. Oien T., Storro O., Johnsen R. 2006. Intestinal microbiota and its effect on the immune system—a nested case-cohort study on prevention of atopy among small children in Trondheim: the IMPACT study. Contemp. Clin. Trials 27: 389–395 [DOI] [PubMed] [Google Scholar]
- 21. Pamer E. G. 2007. Immune responses to commensal and environmental microbes. Nat. Immunol. 8: 1173–1178 [DOI] [PubMed] [Google Scholar]
- 22. Patrick S., et al. 2010. Twenty-eight divergent polysaccharide loci specifying within- and amongst-strain capsule diversity in three strains of Bacteroides fragilis. Microbiology 156: 3255–3269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Patrick S., Houston S., Thacker Z., Blakely G. W. 2009. Mutational analysis of genes implicated in LPS and capsular polysaccharide biosynthesis in the opportunistic pathogen Bacteroides fragilis. Microbiology 155: 1039–1049 [DOI] [PubMed] [Google Scholar]
- 24. Perez-Losada M., et al. 2006. Population genetics of microbial pathogens estimated from multilocus sequence typing (MLST) data. Infect. Genet. Evol. 6: 97–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pumbwe L., Skilbeck C. A., Wexler H. M. 2006. The Bacteroides fragilis cell envelope: quarterback, linebacker, coach—or all three? Anaerobe 12: 211–220 [DOI] [PubMed] [Google Scholar]
- 26. Rissing J. P., Buxton T. B., Edmondson H. T. 1979. Detection of specific IgG antibody in sera from patients infected with Bacteroides fragilis by enzyme-linked immunosorbent-assay. J. Infect. Dis. 140: 994–998 [DOI] [PubMed] [Google Scholar]
- 27. Round J. L., Mazmanian S. K. 2009. The gut microbiota shapes intestinal immune responses during health and disease. Nat. Rev. Immunol. 9: 313–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sears C. L. 2009. Enterotoxigenic Bacteroides fragilis: a rogue among symbiotes. Clin. Microbiol. Rev. 22: 349–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Skanseng B., Kaldhusdal M., Rudi K. 2006. Comparison of chicken gut colonisation by the pathogens Campylobacter jejuni and Clostridium perfringens by real-time quantitative PCR. Mol. Cell. Probes 20: 269–279 [DOI] [PubMed] [Google Scholar]
- 30. Skanseng B., et al. 2007. Co-infection dynamics of a major food-borne zoonotic pathogen in chicken. PLoS Pathog. 3: e175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Storro O., Oien T., Dotterud C. K., Jenssen J. A., Johnsen R. 2010. A primary health-care intervention on pre- and postnatal risk factor behavior to prevent childhood allergy. The Prevention of Allergy among Children in Trondheim (PACT) study. BMC Public Health 10: 443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Storro O., et al. 13 July 2011. Temporal variations in early gut microbial colonization are associated with allergen-specific immunoglobulin E but not atopic eczema at 2 years of age. Clin. Exp. Allergy. doi: 10.1111/j.1365-2222.2011.03817.x [DOI] [PubMed] [Google Scholar]
- 33. Troy E. B., Kasper D. L. 2010. Beneficial effects of Bacteroides fragilis polysaccharides on the immune system. Front. Biosci. 15: 25–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vael C., Nelen V., Verhulst S. L., Goossens H., Desager K. N. 2008. Early intestinal Bacteroides fragilis colonisation and development of asthma. BMC Pulm. Med. 8: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. van Belkum A., Scherer S., van Alphen L., Verbrugh H. 1998. Short-sequence DNA repeats in prokaryotic genomes. Microbiol. Mol. Biol. Rev. 62: 275–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wildschutte H., Wolfe D. M., Tamewitz A., Lawrence J. G. 2004. Protozoan predation, diversifying selection, and the evolution of antigenic diversity in Salmonella. Proc. Natl. Acad. Sci. U. S. A. 101: 10644–10649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zitomersky N. L., Coyne M. J., Comstock L. E. 2011. Longitudinal analysis of the prevalence, maintenance, and IgA response to species of the order Bacteroidales in the human gut. Infect. Immun. 79: 2012–2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.