Abstract
Escherichia coli O157:H7 continues to be an important human pathogen and has been increasingly linked to food-borne illness associated with fresh produce, particularly leafy greens. The aim of this work was to investigate the fate of E. coli O157:H7 on the phyllosphere of lettuce under low temperature and to evaluate the potential hazard of viable but nonculturable (VBNC) cells induced under such stressful conditions. First, we studied the survival of six bacterial strains following prolonged storage in water at low temperature (4°C) and selected two strains with different nonculturable responses for the construction of E. coli O157:H7 Tn7gfp transformants in order to quantitatively assess the occurrence of human pathogens on the plant surface. Under a suboptimal growth temperature (16°C), both E. coli O157:H7 strains maintained culturability on lettuce leaves, but under more stressful conditions (8°C), the bacterial populations evolved toward the VBNC state. The strain-dependent nonculturable response was more evident in the experiments with different inoculum doses (109 and 106 E. coli O157:H7 bacteria per g of leaf) when strain BRMSID 188 lost culturability after 15 days and strain ATCC 43895 lost culturability within 7 days, regardless of the inoculum dose. However, the number of cells entering the VBNC state in high-cell-density inoculum (approximately 55%) was lower than in low-cell-density inoculum (approximately 70%). We recorded the presence of verotoxin for 3 days in samples that contained a VBNC population of 4 to 5 log10 cells but did not detect culturable cells. These findings indicate that E. coli O157:H7 VBNC cells are induced on lettuce plants, and this may have implications regarding food safety.
INTRODUCTION
Contamination of fresh horticultural products with Shiga toxin-producing Escherichia coli (STEC) O157:H7 has become a concern worldwide, with a contributing factor being the ability of the pathogen to survive for extended periods of time in agricultural and food environments (7, 16, 28, 38). Moreover, activation of efficient stress resistance mechanisms that enable survival and persistence in suboptimal environments seems to be correlated with the high virulence of E. coli O157:H7 and the low infectious dose (18). E. coli O157:H7 has proved to be a robust and versatile pathogen that can readily adapt to a wide range of sublethal stresses, including starvation, shifts in temperature, UV radiation, low pH, and osmotic shock (4, 13, 25, 32, 40). Such stress-provoking environmental conditions have been reported to induce a dormancy state, referred to as the viable but nonculturable (VBNC) state, in which bacteria can remain for long periods of time (30, 37). The VBNC state is thought to be a transient physiological phase in which cells switch off activities typical for growing organisms but maintain a low level of metabolic activity without remaining culturable on routine microbiological media (26, 31, 39). There is compelling evidence that the nonculturable response occurs in different environmental and food matrices, but the significance of the passage into a dormancy stage and recovery is controversial (14).
The induction of the VBNC state has been well investigated for E. coli O157:H7 in laboratory studies, but only a few researchers have focused on detection in food and agricultural systems (2, 13, 25, 34, 38, 41). The possibility that the VBNC state of E. coli O157:H7 contributed to a food-borne outbreak was suggested by Makino et al. in a study on strains isolated from salted salmon roe (25). Moreover, the expression of the Shiga-toxin genes in VBNC cells (5, 24, 40) and resuscitation by bacterial growth inducer, e.g., H2O2-degrading compounds such as sodium pyruvate and the antioxidant Oxyrase (4, 33), have been demonstrated. Also, pathogenic bacteria linked with the consumption of the fresh produce, including Salmonella spp. and Listeria monocytogenes, have been shown to evolve toward the VBNC state in the environment and the food chain (3, 15, 29, 30). Recently, the first report was published that provides strong evidence of the induction of the VBNC state for the human pathogen L. monocytogenes when it is exposed to low relative humidity on the plant surface (15). Dreux et al. investigated the survival of L. monocytogenes on parsley leaves under dry conditions and evaluated the ability of the bacteria to recover culturability under saturated humidity (15, 16). The authors noted the difference between enumeration of L. monocytogenes populations by standard direct plate counts from vegetables exposed to a high relative humidity and under stress conditions, where both culture and enrichment methods underestimated the total number of viable cells.
Little is known about the effects of the VBNC state on food safety, which may have relevance given the potentially low infectious dose for human enteropathogens such as E. coli O157:H7. The presence of enteric pathogens in the VBNC state underestimates the actual number of viable bacteria that generate a real health risk when viable but nonculturable cells retain the potential for virulence or recover culturability and pathogenicity. Therefore, detailed knowledge of the magnitude of the VBNC phenomenon and of the food safety risk associated with the nonculturable response, in addition to improved detection methods, is of the utmost importance in order to develop effective intervention strategies. A better understanding of the ecology of the organism and its impact on food safety may allow for the nonculturability of pathogenic organisms to be considered in food-borne outbreak investigations.
The objective of this study was to investigate the fate of E. coli O157:H7 under stress (starvation and low temperature) on the leaf surface of lettuce and to evaluate the potential hazard of VBNC cells induced during the response to this challenge. We also assessed the strain specificity and the effect of inoculum level on the bacterial nonculturable response on the aerial habitat of leaves colonized by microbes (phyllosphere).
MATERIALS AND METHODS
Bacterial strains, plasmid vectors, and growth conditions.
A total of six E. coli O157:H7 strains isolated from bovine (BRMSID 187, 188, and 259), cider (BRMSID 38), and humans (BRMSID 40 and ATCC 43895) were used in the study. E. coli O157:H7 wild-type and gfp-tagged strains were cultured in Bacto tryptic soy broth (TSB; Becton Dickinson and Co., Sparks, MD) at 37 °C until late-log phase was reached. Cells were harvested by centrifugation (3,200 × g for 10 min), washed three times with sterile distilled water, and resuspended to a final concentration of 106, 107, or 109 CFU ml−1, confirmed by serial dilution plating on Trypticase soy agar (TSA; Becton Dickinson and Co.). The bacterial inoculum was washed and prepared by resuspending the bacterial pellet in sterile distilled water in order to completely remove the medium and was subsequently vortexed for 60 s prior to inoculation to break up bacterial aggregates.
Plasmid vectors pBK-miniTn7-gfp3 and pGRG36 were maintained in Luria-Bertani (LB; Invitrogen Co., Carlsbad, CA) medium supplemented with 50 μg ml−1 kanamycin (Invitrogen Co.) and 100 μg ml−1 ampicillin (Amresco Inc., Solon, OH), respectively.
Construction of E. coli O157:H7 attTn7::Tn7gfp strains.
For construction of vector pGRG-gfp, the 2-kb NotI fragment from pBK-miniTn7-gfp3 was cloned into the unique NotI site of donor vector pGRG36. Transposon Tn7-based delivery into E. coli O157:H7 ATCC 43895 and BRMSID 188 and screening by PCR for the presence of transgene in a single copy across a chromosomal site (attTn7) were performed as previously described by McKenzie et al. (27). Plasmids were prepared using a QIAPrep Spin Miniprep kit (Qiagen Science, Mississauga, Ontario, Canada) with a very-low-copy-number plasmid protocol, and the restriction enzyme digest, ligation, and electroporation were carried out using standard methods (36).
Plant growth conditions.
Romaine lettuce (Lactuca sativa cv. Parris Island) was grown in a plant growth chamber with a 14-h photoperiod at 19 ± 2°C for 35 days. The plants were fertilized weekly after germination with a 15-15-18 (NPK) fertilizer (Plant Products Co. Ltd., Brampton, Ontario, Canada). Lettuce plants were then placed in a humid growth chamber with a 14-h photoperiod and exposed to temperatures of 8°C (8 ± 1.5°C) and 16°C (16.5 ± 1.5°C) for a maximum of 17 days. Relative humidity ([RH] >70%) and temperature were recorded with a HOBO H8 Pro Series data logger (Anset Computer Co., Bourne, MA), and light was recorded with an IL 400A photometer (International Light Inc., Newburyport, MA).
Viable and total cell counts.
Viability was evaluated based on fluorescence of gfp-tagged strains, membrane integrity using Live/Dead BacLight Bacterial Viability Kits (Molecular Probes Inc., Eugene, OR), and esterase activity using 6-carboxyfluorescein diacetate (6-CFDA; Sigma-Aldrich Inc., St. Louis, MO).
Viable counts of E. coli O157:H7 BRMSID 188 attTn7::Tn7gfp (BRMSID 188 Tn7gfp) and E. coli O157:H7 ATCC 43895 attTn7::Tn7gfp (ATCC 43895 Tn7gfp) were determined after the aliquots of samples (1 ml) were filtered through black polycarbonate membrane Nuclepore filters (pore size, 0.2 μm; diameter, 25 mm; Whatman Inc., Clifton, NJ), and fluorescent cells were enumerated.
Membrane integrity is a well-accepted criterion for distinguishing viable cells from dead cells. Live/Dead BacLight Bacterial Viability Kits detect live cells with an intact membrane stained green by SYTO9 and dead cells with a damaged membrane stained red by propidium iodide (PI). A 1-ml aliquot of each sample was stained as described by the manufacturer, and bacteria were captured by microfiltration through a 0.2-μm-pore-size black polycarbonate membrane filter. Total bacteria were represented as the sum of green (viable) cells and red (nonviable) cells.
Assessment of esterase activity was performed using the fluorescein diacetate method that allows for the enumeration of live cells with an intact membrane using intracellular esterase activity (28). One milliliter of sample was incubated in the dark with 10 μl of a stock solution (10 mM in anhydrous dimethyl sulfoxide) of 6-CFDA for 1 h at 37°C; then the mixture was filtered onto black membrane, and green fluorescent cells that accumulated fluorescein (viable) were counted.
The total number of bacterial cells was determined after 1 ml of a sample was fixed with 30 μl of formalin and stained with 4′,6-diamidino-2-phenylindole (DAPI; Molecular Probes, Eugene, OR) at a final concentration of 10 μg ml−1. Samples were incubated for 10 min at room temperature in the dark before they were filtered, and the number of blue-stained cells (viable and dead) was recorded (35).
All microscopic counts were determined with a Zeiss Axiophot fluorescence microscope (Carl Zeiss, Oberkochen, Germany). The detection limit of microscopic examination was 104 cells per ml.
Stability of GFP expression during the VBNC response.
To assess the stability in expression of green fluorescent protein (GFP) during the VBNC response, E. coli O157:H7 attTn7::Tn7gfp strains were stored for 2 weeks in a plant growth chamber under conditions that were used to induce the VBNC state on the plant surface (8 ± 1.5°C), as described above. Viable enterobacteria that expressed fluorescence due to GFP were enumerated, and results were compared with standard plate counts on TSA and TSA supplemented with 50 μg ml−1 kanamycin (TSAKm); counts were obtained using Live/Dead BacLight Bacterial Viability Kits and the esterase activity assay. The total number of bacteria was determined by the BacLight and DAPI staining methods described above. Duplicate analyses of viable and culturable cells and the total number of cells were performed on each sample. A minimum of 20 randomly chosen microscope fields was observed per sample, and at least 100 cells per filter were counted. Two independent experiments were conducted.
Fate of E. coli O157:H7 in water microcosms.
Sterile distilled deionized water (SDW) with a resistivity of 18 MΩ (Milli-Q water system; Millipore Corp., Bedford, MA) was aliquoted (99 ml) into screw-top flasks and autoclaved (at 121°C for 15 min). Microcosms were inoculated with E. coli O157:H7 suspensions to achieve a final concentration of 107 CFU ml−1 and were held static in the dark at 3.9 ± 0.5°C. Samples (1 ml) were diluted in peptone water and plated on TSA and sorbitol-MacConkey agar (SMAC; Oxoid Nepean, Ontario, Canada). Plates were incubated at 37°C for 24 h prior to the determination of bacterial populations. When the number of colonies recovered was less than 10 CFU ml−1, samples of 10 ml of SDW were centrifuged, and the resulting pellet was plated onto TSA medium. Viability and total cell count assays were performed by staining with 6-CFDA and DAPI, respectively, as described above. All experiments were performed in duplicate, and two independent experiments were conducted.
Fate of E. coli O157:H7 Tn7gfp on lettuce.
Cell suspensions of E. coli O157:H7 transformants containing final concentrations of 106, 107, or 109 culturable bacteria were inoculated by the spot method on green and healthy lettuce leaves that were part of the open rosette. Each leaf was inoculated individually with 50 μl of inoculum applied in volumes of 12.5 μl at four equidistant locations on the plant surface. Leaves inoculated with SDW and incubated under the same conditions were used as controls. At each sampling time, three samples of two leaves each were aseptically removed from different plants and collected in a sterile stomacher bag. Samples were weighed and then homogenized in 10 ml of peptone water (0.1% [wt/vol] Bacto peptone) with a Stomacher 400 laboratory blender (Seward Medical, London, United Kingdom) at high speed for 1 min. Leaf homogenates were serially diluted in peptone water, and 100 μl from appropriate dilutions was spread plated onto TSAKm and SMAC media in triplicate to determine the number of culturable bacteria. Plates were incubated at 37°C for 24 h, and green-fluorescing colonies were counted. Viable gfp-tagged enterobacteria that expressed fluorescence were enumerated, and results were compared with standard plate counts on TSAKm and SMAC media. Two independent replicates of the experiment were performed.
CSLM.
Lettuce leaves were inoculated as previously described and collected when no CFU were detected on TSAKm medium for two consecutive days. Lettuce pieces (2 by 2 cm) cut with a sterile surgical knife were mounted with sterile water onto a glass-bottom FluoroDish (World Precision Instruments, Sarasota, FL). The presence of VBNC cells of BRMSID 188 Tn7gfp on the intact leaf surface was determined using a confocal scanning laser microscope (CSLM) Leica TCS SP2 AOBS Ar laser (Leica Microsystems, Wetzlar, Germany). The fluorescence was detected at an excitation wavelength of 488 nm, and the emitted light was collected at 493/566 nm for GFP fluorescence and 650/729 nm for autofluorescence of the chloroplasts.
Verotoxin detection.
Supernatants from leaf homogenate samples were tested for verotoxin production with a commercially available Ridascreen verotoxin enzyme immunoassay kit (R-Biopharm AG, Darmstadt, Germany). The assay was performed following the manufacturer's instructions, without enrichment, on medium supplemented with mitomycin C in order to obtain more accurate results of the toxin presence in samples that contain only VBNC cells. The results were recorded photometrically (optical density at 450 nm [OD450]) and reported as positive (+), equivocal (0), and negative (−) based on cutoff values as recommended by the manufacturer.
Statistical analysis.
Statistical calculations were performed using SAS, version 9.1 (SAS Institute Inc., Cary, NC). Culturable, viable, and total cell counts were log transformed, and data represent the average of two independent trials with a minimum of two replicates each. Coefficient of variation (CV) shows the biological variation within a treatment and was calculated from the ratio of standard deviation and arithmetic mean. The culturable population decline (PD) that occurred during different time intervals after inoculation was determined by dividing the arithmetic mean of the population size at the beginning by that of end of each interval. Analysis of variance (ANOVA) at 95% significance (P = 0.05) was used to determine statistical significance by treatment (culturable on TSA and TSAKm medium) and between viable/total cell count methods in the experiment that assessed the stability in expression of GFP during VBNC response. The trends of all data sets in experiments on the fate of bacteria at low temperature in SDW and on plants were appropriately described by linear regression. The slopes of the lines were obtained by plotting the log of population size over time using the PROC REG function in SAS, and the slopes obtained were compared with Tukey's test at a confidence level of 95%. The difference in frequencies of positive samples for verotoxin detected by the Ridascreen verotoxin enzyme immunoassay kit was tested using a chi-square test.
RESULTS
Fate of E. coli O 157:H7 strains in water microcosms at low temperature.
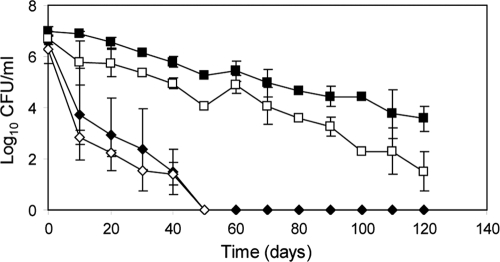
To investigate the behavior of E. coli O157:H7 strains isolated from different sources (bovine, cider, and human) under starvation and low-temperature conditions in water microcosms, we used plating on nonselective TSA medium and selective SMAC medium, where sublethally injured bacterial populations cannot grow (Fig. 1). The culturability of five E coli O 157:H7 strains (BRMSID 38, 40, 187, 188, and 259) following prolonged storage in water at 4°C declined slowly during the first 120 days, but cells remained culturable until day 210 (data not shown). At 120 days postinoculation, the average population decline for these strains on SMAC medium (PD of 3.93 ± 0.83) was 1.82 times higher than that on TSA medium, suggesting that a large subpopulation of sublethally injured bacteria was induced. However, a comparison of curves and slopes of the regression lines on TSA medium proved that survival curves for all five strains are not different (P > 0.05). E. coli O157:H7 ATCC 43895 was undetectable, as determined by plate count, on both TSA and SMAC media by day 50, but a biological variation within the experiment higher than 10% was observed (CV of 22.41%). The culturability curves on TSA and SMAC media were similar (P > 0.05), with slope values of −0.11 and −0.10, respectively (Fig. 1). At day 50, staining with 6-CFDA showed that 55.94% ± 3.29% of the initial population retained viability, and a mixed cell population with distinct morphology was observed, one having a typical rod-shaped morphology and one with coccoid morphology, a shape frequently observed for VBNC forms (data not shown). Strains ATCC 43895 and BRMSID 188 were selected for the experiments on lettuce plants as differential strain survival was observed.
Fig. 1.
Culturability of E. coli O157:H7 plated on TSA (filled symbols) and SMAC (open symbols) media. E. coli O157:H7 ATCC 43895 (diamonds) and BRMSID 188 (squares) were incubated at 4°C in sterile deionized distilled water for up to 120 days. The data are mean values (n = 4) from two independent experiments with errors bars representing the standard deviations.
Stability of GFP expression during VBNC response.
Different methods for assessing viability were compared in order to avoid potential pitfalls in the detection of green fluorescent protein (GFP) fluorescence in a mixed population containing culturable and VBNC forms. Cultures of both E. coli O157:H7 Tn7gfp transformants were stored under conditions of low temperature and light for 14 days, and the nonculturable response was evaluated as a difference between viable (8 log10) and culturable bacteria on TSA or TSAKm (6 log10) medium. Viable bacteria were enumerated based on GFP fluorescence, membrane integrity with the Live/Dead BacLight Bacterial Viability Kit, and esterase activity using staining with 6-CFDA. There was no significant difference between the counts of gfp-tagged cells and the viable counts given by the BacLight test or fluorescein diacetate method (P > 0.05; F = 2.87 for ATCC 43895 Tn7gfp and F = 0.13 for BRMSID 188 Tn7gfp). Similarly, total counts enumerated with the BacLight and the DAPI methods were not significantly different (P > 0.05; F = 0.66 for ATCC 43895 Tn7gfp and F = 0.80 for BRMSID 188 Tn7gfp). The results provided evidence that E. coli O157:H7 gfp-tagged transformants can be used as a marker of viable cells in experiments on plants.
Fate of E. coli O157:H7 Tn7gfp on lettuce under low temperature.
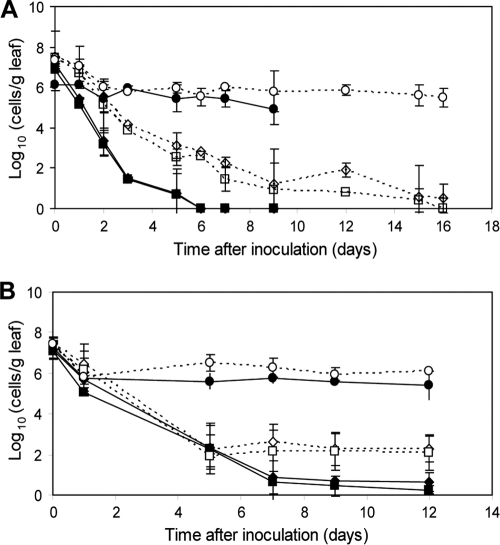
In order to analyze the population dynamics of E. coli O157:H7 Tn7gfp on lettuce leaves at low temperatures, viability and culturability were assessed. The difference between viable and standard plate counts represented the number of VBNC cells in a population at a given time. The experiments were conducted at 8°C (near the minimum temperature of growth of 7°C) and 16°C (suboptimal growth temperature), with an initial inoculum of 107 E. coli O157:H7 bacteria per g of leaf.
At 8°C there was a steady decline in the culturability of both strains, with BRMSID 188 Tn7gfp remaining culturable longer than ATCC 43895 Tn7gfp (Fig. 2A). For the latter bacterial strain, plate counts decreased to an undetectable level within 6 days, and slope values on TSAKm (−1.02) and SMAC (−1.07) media were similar (P > 0.05). Furthermore, when no colonies developed on media between day 6 and day 9 after inoculation, viable counts dropped by approximately 0.6 log10 E. coli O157:H7 bacteria per g of leaf; but due to the limit of microscopic detection, VBNC cells could not be enumerated at 12 days postinoculation. When results from both E. coli O157:H7 transformants were compared, the culturability curves for strain BRMSID 188 Tn7gfp were different (strain-specific pattern) (P < 0.05), but the patterns of decline in viability were similar (P > 0.05) (Fig. 2A).
Fig. 2.
Fate of E. coli O157:H7 ATCC 43895 Tn7gfp (filled symbols) and BRMSID 188 Tn7gfp (open symbols) on lettuce plants incubated at 8°C (A) and 16°C (B). Culturable populations on TSA (diamonds) and SMAC (squares) media and viable counts (circles) are indicated. The data are mean values (n = 6) from two independent experiments with different bacterial cultures and different sets of plants. Error bars represent the standard deviations.
Although the population size of both E. coli O157:H7 Tn7gfp strains decreased over time on lettuce incubated at 16°C, the bacteria remained culturable on both TSAKm and SMAC media at 12 days postinoculation (Fig. 2B). The behavior of strain BRMSID 188 Tn7gfp was similar to that of ATCC 43895 Tn7gfp, with the slope of the regression lines on both media slightly lower (−1.55) for bacterial strain ATCC 43895 Tn7gfp than for BRMSID 188 Tn7gfp (−1.69) and with culturability curves that were not significantly different (P > 0.05). However, the population decline for ATCC 43895 Tn7gfp (PD of 17.87) was 5.4-fold higher on TSAKm medium than for strain BRMSID 188 Tn7gfp (PD of 3.30) (Fig. 2B). Significant differences in the viable and culturable population dynamics and the occurrence of VBNC subpopulations have been recorded. Moreover, a biphasic pattern of decline was found for the viable population that decreased by approximately 2 log10 E. coli O157:H7 bacteria per g of leaf in the first 2 days and then remained relatively constant for the next 10 days. At the end of the experiment, the viable counts were 2.78-fold and 8.27-fold higher than the plate counts on nonselective TSAKm medium enumerated for BRMSID 188 Tn7gfp and ATCC 43895 Tn7gfp, respectively (Fig. 2B).
During the experiment the number of VBNC cells increased because a stable population of approximately 5 log10 viable fluorescent bacteria per leaf was maintained over the next 7 and 14 days for ATCC 43895 Tn7gfp and BRMSID 188 Tn7gfp, respectively, while the standard plate counts decreased. Under low temperature (8°C) on lettuce leaves, a large fraction of the initial population remained viable, and both gfp-tagged strains entered a complete nonculturable state.
Effect of inoculum size on the VBNC response of E. coli O157:H7 Tn7gfp.
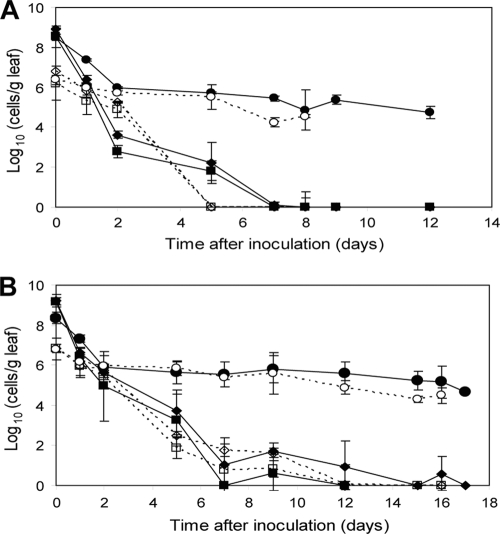
In order to determine the effect of inoculum concentration on the VBNC induction of E. coli O157:H7 Tn7gfp on plants, two different inoculum concentrations were assessed, 106 and 109 culturable bacteria per g of leaf. At 8°C, regardless of the inoculum size, counts of culturable bacteria decreased to an undetectable level by days 7 and 15 for strains ATCC 43895 Tn7gfp (Fig. 3A) and BRMSID 188 Tn7gfp (Fig. 3B), respectively. With an initial inoculum of 109 E. coli O157:H7 bacteria per g of leaf, the culturable population of BRMSID 188 Tn7gfp (slope values of −1.70 on TSAKm and −1.96 on SMAC medium) survived better than ATCC 43895 Tn7gfp (slope values of −1.05 on TSAKm and −1.08 on SMAC medium). Similar results were obtained when the values of the slopes (P < 0.05) were compared for the low-cell-density inocula (Fig. 3A and B). Viable cell counts decreased in 2 days by 2.5 log10 for the high-density inoculum and by a maximum of 1 log10 for the low-density inoculum and then slowly declined, reaching a constant level, regardless of strain and inoculum dose. At the end of experiment with 109 bacteria per g of leaf inoculum, viability counts represented 55.7% ± 2.7% and 56.0% ± 1.2% of the initial viable population for ATCC 43895 Tn7gfp (Fig. 3A) and BRMSID 188 Tn7gfp (Fig. 3B), respectively. When an inoculum of 106 bacteria per g of leaf was applied, 71.2% ± 3.9% and 66.0% ± 10.8% of cells remained viable for ATCC 43895 Tn7gfp (Fig. 3A) and BRMSID 188 Tn7gfp (Fig. 3B), respectively. A large VBNC population of 4 to 5 log10 was induced and remained constant for 3 to 5 days, regardless of the initial inoculum size (109 or 106 E. coli O157:H7 bacteria per g of leaf). Moreover, it appears that the initial concentration of inoculum does not significantly affect the time required by an E. coli O157:H7 strain to induce the VBNC response and to become completely nonculturable.
Fig. 3.
Effect of inoculum size on the VBNC response of E. coli O157:H7 ATCC 43895 Tn7gfp (A) and BRMSID 188 Tn7gfp (B). Lettuce leaves were inoculated with 109 (filled symbols) and 106 (open symbols) E. coli O157:H7 bacteria per g of leaf and incubated at 8°C. Culturable populations plated on TSA (diamonds) and SMAC (squares) media and viable counts (circles) are indicated. The data are mean values (n = 6) from two independent experiments with different bacterial cultures and different sets of plants. Error bars represent the standard deviations.
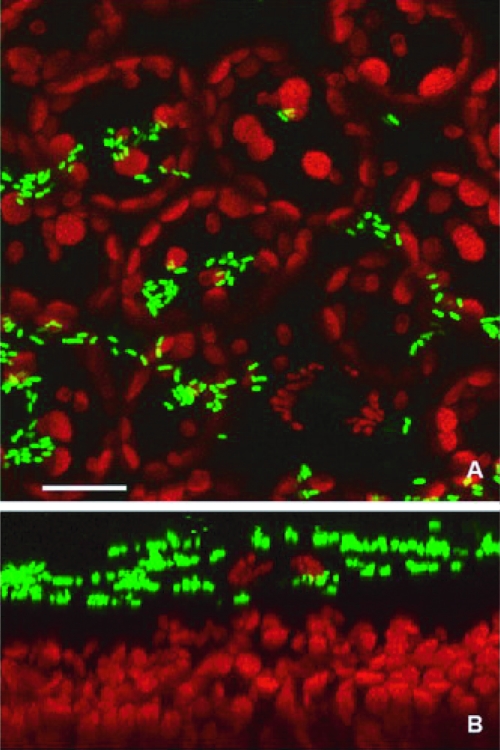
The presence of BRMSID 188 Tn7gfp VBNC forms on the surface of leaves was visualized using CSLM on samples with undetectable culturable cells and a VBNC population of approximately 4.5 log10 cells that were collected 16 days after inoculation. Bacterial VBNC cells associated in large clusters were often observed, and an optical cross-section of the same area showed that most of the viable but nonculturable bacteria were detected above plant epidermal cells (Fig. 4). To our knowledge, this study is the first report that shows evidence that E. coli O157:H7 evolves toward a VBNC state under stress conditions on the aerial leaf surface of lettuce plants.
Fig. 4.
(A) Confocal microscopy showing the presence of VBNC forms of E. coli O157:H7 BRMSID 188 Tn7gfp in large clusters at 16 days postinoculation on the surface of lettuce leaves. (B) Optical cross-section of the same image that indicates the localization of E. coli O157:H7 cells on the surface of plant tissue. Scale bar, 20 μm.
Verotoxin detection.
Strain ATCC 43895 Tn7gfp was inoculated with 106 and 107 bacteria per g of leaf on lettuce plants exposed to low temperature (8°C). In order to determine the presence of Vero (Shiga) toxins 1 (Stx1) and 2 (Stx2) in samples that did not contain any detectable culturable bacteria, leaf homogenate samples were tested with a Ridascreen verotoxin enzyme immunoassay kit. It is important that the Ridascreen test detects strains producing Stx1 and variants, as well as Stx2 and variants, when they are produced in sufficient amounts (6). Therefore, false-negative results may occur when the level of toxin in the leaf homogenate samples has dropped below the detection threshold. In the case of inoculation with 107 E. coli O157:H7 bacteria per g of leaf, the number of samples positive for enterotoxin was 8 of 12 samples analyzed at 7 days postinoculation, and this number remained consistent for 2 days. There was no significant difference (P > 0.05) in the proportions of positive samples at day 0 and day 9 after inoculation. Similar results were obtained with the lowest inoculum (106 CFU E. coli O157:H7 bacteria per g of leaf) for 7 and 8 days postinoculation.
DISCUSSION
Knowledge about the occurrence and potential hazard of nonculturable forms of enteropathogens in plant-associated environments is essential to obtain a full understanding of bacterial behavior under stress with implications for food safety. Therefore, the possibility that stress-provoking conditions in the plant-associated environment may have the potential to induce VBNC cell forms in enteric pathogens such as E. coli O157:H7 was studied.
First, two strains of E. coli O157:H7 were selected and gfp tagged based on their ability to survive under low-temperature and starvation conditions for subsequent inoculation onto the phyllosphere of lettuce. Numerous studies showed that low temperature in aquatic microcosms, which are generally low energy and extremely poor in nutrients, induces nonculturability in E. coli O157:H7 (21, 34, 38). Moreover, a significant variation has been observed regarding the time required for enteropathogens to enter the VBNC state, but comparisons are difficult due to variations in temperatures, water sources, and the strains used (34, 41). In the present study, differences among strains were found, and only in the microcosm containing E. coli O157:H7 ATCC 43895 was the entire population of viable bacteria induced to become VBNC cells. For this strain, which failed to grow on medium at 50 days postinoculation, the entry into the VBNC state was confirmed by staining with 6-CFDA and microscopic investigations that detected the cell size reduction. Our results are in agreement with those of Kolling et al. (21), who reported that under similar conditions strain E. coli O157:H7 ATCC 43895-1 decreased to an undetectable level within 60 to 65 days (21). The authors recorded a high biological variation in culturability within the experiment, as observed in our study. Other research has shown that the morphological change of E. coli O157:H7 cells entering the VBNC state has been considered a survival advantage that enhances the resistance of the cell to stress (9, 41). In our study, E. coli O157:H7 BRMSID 188 survived for a longer period of time in water, remaining culturable for more than 120 days, and induced a large subpopulation of sublethally injured cells. Both VBNC cells and sublethally injured enterobacteria represent a potential hazard to food safety as they are viable pathogens which are not detected by routine analysis.
The Tn7 transposition system seems to be a very useful tool for tagging Gram-negative bacteria, mainly because the Tn7 transposon can insert in a manner that is site and orientation specific at a high frequency into bacterial chromosomes and at a single attTn7 site located downstream of the glmS gene. A panel of Tn7-based vectors intended for tagging with fluorescent proteins or bacterial luciferase (lux) has been constructed (12, 20, 22, 27). However, there are only a few reports on the monitoring of VBNC forms using fluorescent/luminescent bacteria (1, 10, 11, 17, 20). In the present study, we constructed E. coli O157:H7 Tn7gfp transformants to study the transition to the VBNC state under stress conditions on the plant surface. In VBNC cells that have low metabolic activity, green fluorescent protein needs to be at the detectable level for all or most individual cells in order to be applicable for microscopic quantification on fresh produce. In addition, because GFP is normally a very stable protein and persists in the cell for long periods of time, there is the possibility that the fluorescent marker is not suitable for differentiation of viable pathogens. Therefore, the stability of GFP expression was evaluated in a mixed population that contained 2 log10 VBNC cells, and no significant difference was noted between counts of gfp-tagged cells and viable counts obtained using two different methods (BacLight and the fluorescein diacetate method). We concluded that Tn7gfp-tagged transformants are particularly well suited for detection of VBNC cells, and the insertion was stably maintained under the conditions evaluated without any known adverse effects on the host.
Little is known about the ability of animal enteropathogens to persist on the phyllosphere of agricultural crops under optimal or stress conditions. Recently, Brandl et al. (7, 8) demonstrated that E. coli O157:H7 and Salmonella enterica can multiply on the leaf surface at incubation temperatures of between 16 and 30°C (7, 8). Dreux et al. (15) studied the fate of Listeria monocytogenes on parsley leaves under high and low relative humidity (47 to 69%) and reported induction of the VBNC state due to the stress caused by changes in water availability (15). In our study, exposure of E. coli O157:H7 to low temperatures of 8°C and 16°C on the surface of a cold-season plant was considered comparable for natural conditions. Since splashing irrigation water is a suspect vehicle for the transfer of pathogens onto the plant surface, we selected spot inoculation which would allow for a more accurate determination in the reduction of the initial concentration of inoculum applied to the plant surface (19, 23). Although we used higher-density inocula than are likely to contaminate plants in the field, results suggest that VBNC forms are induced by stress regardless of the initial concentration of bacteria on the plant surface and remain for long periods of time on the plant leaves, retaining their potential for virulence. Under the suboptimal growth temperature (16°C) both ATCC 43895 Tn7gfp and BRMSID 188 Tn7gfp maintained culturability on the lettuce leaves but induced VBNC forms. The significant difference between culturable and viable counts indicates that a subpopulation of cells entered into the VBNC state. Viability was reduced by a 2 log10 scale within the first 2 days and then reached a level that remained stable until the end of experiment (12 days postinoculation) for both strains. Similar results were reported by Dreux et al. (15) when parsley plants inoculated with 109 culturable L. monocytogenes bacteria per leaf were exposed to low relative humidity (47 to 69%), and the viable population detected decreased based on the same biphasic pattern (15). In a more stressful challenge (8°C) the entire E. coli population evolved toward a VBNC state, but the time required to induce the VBNC response was different for selected strains. This strain-dependent VBNC response was more evident in the experiments with different inoculum sizes (109 and 106 E. coli O157:H7 bacteria per g of leaf) when strain BRMSID 188 lost culturability after 15 days and strain ATCC 43895 lost culturability within 7 days, regardless of the inoculum dose. For both strains the number of cells that remained viable from the initial population was dependent on the inoculum size, and the survival in high-cell-density inocula (approximately 55%) was lower than in low-cell-density inocula (approximately 70%). However, our work suggests that inoculum size does not affect the VBNC response since at the end of experiment both strains induced a large (4 to 5 log10) and stable VBNC population over the next 3 to 5 days, regardless of the initial concentration of bacteria and the level of stress. This finding is particularly relevant to the monitoring of bacteria under stress, providing evidence that samples with low or undetectable levels of culturable cells might contain large viable populations that are reduced slowly over time and are not detected by conventional plating. However, in our work the culturability curves for TSA and SMAC media were similar, suggesting that significant sublethally injured subpopulations were not induced, regardless of the strain tested. These results differ from those observed in aquatic microcosms maintained in the dark at 4°C, when during the experiment a sublethally injured subpopulation of approximately 1 to 2 log10 E. coli O157:H7 bacteria was recorded. Dreux et al. reported the occurrence of sublethally injured L. monocytogenes under low relative humidity on parsley (15). To date, there have been no other reports that offer more information about the presence of these cells on the plant surface, and at this point, we can only speculate about the factors that generate this result. The severity of stress may account for the differences as well as the bacterial species tested and ecological factors specific for each vegetable.
Studies on the localization of E. coli O157:H7 showed the presence of large aggregates of culturable bacteria at some sites on middle lettuce leaves (8). In the present study, spot inoculation was considered more accurate in the measurement of the reduction in pathogens but might facilitate the formation of microcolonies and aggregates observed on the surface of the cuticle of leaf tissue. Conversely, it is possible that E. coli O157:H7 VBNC forms are better protected when large clusters form on the surfaces of leaves. Therefore, further investigations are required to draw clear conclusions on the distribution of cells and localization of VBNC forms of E. coli O157:H7 on the leaf surface.
To be considered a risk factor for food safety, VBNC cells should continue to harbor the potential for virulence. The constitutive and stable expression of the virulence genes hly and stx1 and stx2 and enterotoxin production in VBNC cells have been reported (5, 24, 32, 40). In our work we recorded the presence of verotoxin for 3 days in samples that contained a VBNC population of approximately 4.5 log10 bacteria and no detectable culturable cells. If, however, few metabolically active cells were present in the samples, they could not produce enough enterotoxin to be detected by the Ridascreen test without an enrichment procedure. Therefore, our data support the interpretation that metabolically active VBNC cells induced by low temperature on the phyllosphere of lettuce are capable of producing small amounts of verotoxin, thereby explaining the constant level of toxin for 3 days. These findings highlight the importance of monitoring viable but nonculturable cells of human pathogens that may be infectious and pose a potential health risk.
Overall, our data indicate that under the stressful conditions of starvation and low temperature, E. coli O157:H7 induces a large population of viable but nonculturable cells that produce verotoxins on raw foods that are to be consumed without further heat treatment. The presence of stressed highly infective pathogens may be a major health concern since they cannot be detected by culturing techniques and resuscitation methods but are nonetheless ubiquitous and harbor the potential for virulence. Further investigations are required in order to better understand the risk associated with VBNC cells that would provide a basis for risk assessment in food safety.
ACKNOWLEDGMENTS
Support for L.-D. Dinu was provided through the NSERC-VF program.
We thank Ole Nybroe and Gregory J. McKenzie for providing plasmids pBK-miniTn7-gfp3 and pGRG36, respectively. We thank Colleen Harlton, Michael Weiss, and Pascal Delaquis for their technical expertise.
Footnotes
Published ahead of print on 30 September 2011.
REFERENCES
- 1. Arana I., Seco C., Muela A., Fernandez-Astorga A., Barcina I. 2003. gfp-tagged cells as a useful tool to study the survival of Escherichia coli in the presence of the river microbial community. Microb. Ecol. 45: 29–38 [DOI] [PubMed] [Google Scholar]
- 2. Artz R. R. E., Avery L. M., Jones D. L., Killham K. 2006. Potential pitfalls in the quantitative molecular detection of Escherichia coli O157:H7 in environmental matrices. Can. J. Microbiol. 52: 482–488 [DOI] [PubMed] [Google Scholar]
- 3. Asakura H., et al. 2002. Passage in mice causes a change in the ability of Salmonella enterica serovar Oranienburg to survive NaCl osmotic stress: resuscitation from the viable but non-culturable state. FEMS Microbiol. Lett. 212: 87–93 [DOI] [PubMed] [Google Scholar]
- 4. Asakura H., Igimi S., Kawamoto K., Yamamoto S., Makino S. 2005. Role of in vivo passage on the environmental adaptation of enterohemorrhagic Escherichia coli O157:H7: cross-induction of the viable but nonculturable state by osmotic and oxidative stresses. FEMS Microbiol. Lett. 253: 243–249 [DOI] [PubMed] [Google Scholar]
- 5. Asakura H., et al. 2007. Proteomic characterization of enterohemorrhagic Escherichia coli O157:H7 in the oxidation-induced viable but non-culturable state. Microbiol. Immunol. 51: 875–881 [DOI] [PubMed] [Google Scholar]
- 6. Beutin L., et al. 2007. Comparative evaluation of the Ridascreen verotoxin enzyme immunoassay for detection of Shiga-toxin producing strains of Escherichia coli (STEC) from food and other sources. J. Appl. Microbiol. 102: 630–639 [DOI] [PubMed] [Google Scholar]
- 7. Brandl M. T., Mandrell R. E. 2002. Fitness of Salmonella enterica serovar Thompson in the cilantro phyllosphere. Appl. Environ. Microbiol. 68: 3614–3621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brandl M. T., Amundson R. 2008. Leaf age as a risk factor in contamination of lettuce with Escherichia coli O157:H7 and Salmonella enterica. Appl. Environ. Microbiol. 74: 2298–2306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Byrd J. J. 2000. Morphological changes leading to the nonculturable state, p. 7–18 In Colwell R. R., Grimes D. J. (ed.), Nonculturable microorganisms in the environment. ASM Press, Washington, DC [Google Scholar]
- 10. Cho J. C., Kim S. J. 1999. Viable, but non-culturable, state of a green fluorescence protein-tagged environmental isolate of Salmonella typhi in groundwater and pond water. FEMS Microbiol. Lett. 170: 257–264 [DOI] [PubMed] [Google Scholar]
- 11. Cho J. C., Kim S. J. 1999. Green fluorescent protein-based direct viable count to verify a viable but non-culturable state of Salmonella typhi in environmental samples. J. Microbiol. Methods 36: 227–235 [DOI] [PubMed] [Google Scholar]
- 12. Choi K. H., et al. 2005. A Tn7-based broad-range bacterial cloning and expression system. Nat. Methods 2: 443–448 [DOI] [PubMed] [Google Scholar]
- 13. Davies C. M., Evison L. M. 1991. Sunlight and the survival of enteric bacteria in natural waters. J. Appl. Bacteriol. 70: 265–274 [DOI] [PubMed] [Google Scholar]
- 14. Dinu L.-D., Delaquis P., Bach S. 2009. Nonculturable response of animal enteropathogens in the agricultural environment and implications for food safety. J. Food Prot. 72: 1342–1354 [DOI] [PubMed] [Google Scholar]
- 15. Dreux N., et al. 2007. Viable but non-culturable Listeria monocytogenes on parsley leaves and absence of recovery to a culturable state. J. Appl. Microbiol. 103: 1272–1281 [DOI] [PubMed] [Google Scholar]
- 16. Dreux N., Albagnac C., Carlin F., Morris C. E., Nguyen-The C. 2007. Fate of Listeria spp. on parsley leaves grown in laboratory and field cultures. J. Appl. Microbiol. 103: 1821–1827 [DOI] [PubMed] [Google Scholar]
- 17. Duncan S., Glover L. A., Killham K., Prosser J. I. 1994. Luminescence-based detection of activity of starved and viable but nonculturable bacteria. Appl. Environ. Microbiol. 60: 1308–1316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Franz E., van Bruggen A. H. C. 2008. Ecology of E. coli O157:H7 and Salmonella enterica in the primary vegetable production chain. Crit. Rev. Microbiol. 34: 143–161 [DOI] [PubMed] [Google Scholar]
- 19. Heaton J. C., Jones K. 2008. Microbial contamination of fruit and vegetables and the behaviour of enteropathogens in the phyllosphere: a review. J. Appl. Microbiol. 104: 613–626 [DOI] [PubMed] [Google Scholar]
- 20. Koch B., Jensen L. E., Nybroe O. 2001. A panel of Tn7-based vectors for insertion of the gfp marker gene or for delivery of cloned DNA into Gram-negative bacteria at a neutral chromosomal site. J. Microbiol. Methods 45: 187–195 [DOI] [PubMed] [Google Scholar]
- 21. Kolling G. L., Matthews K. R. 2001. Examination of recovery in vitro and in vivo of nonculturable Escherichia coli O157:H7. Appl. Environ. Microbiol. 67: 3928–3933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lambertsen L., Sternberg C., Molin S. 2004. Mini-Tn7 transposons for site-specific tagging of bacteria with fluorescent proteins. Environ. Microbiol. 6: 726–732 [DOI] [PubMed] [Google Scholar]
- 23. Lang M. M., Harris L. J., Beuchat L. R. 2004. Survival and recovery of Escherichia coli O157:H7, Salmonella, and Listeria monocytogenes on lettuce and parsley as affected by method of inoculation, time between inoculation and analysis, and treatment with chlorinated water. J. Food Prot. 67: 1092–1103 [DOI] [PubMed] [Google Scholar]
- 24. Liu Y., Wang C., Tyrrell G., Li X.-F. 2010. Production of Shiga-like toxins in viable but nonculturable Escherichia coli O157:H7. Water Res. 44: 711–718 [DOI] [PubMed] [Google Scholar]
- 25. Makino S. I., et al. 2000. Does enterohemorrhagic Escherichia coli enter the viable but nonculturable state in salted salmon roe? Appl. Environ. Microbiol. 66: 5536–5539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McDougald D., Rice S. A., Weichart D., Kjelleberg S. 1998. Nonculturability: adaptation or debilitation? FEMS Microbiol. Ecol. 25: 1–9 [Google Scholar]
- 27. McKenzie G. J., Craig N. L. 2006. Fast, easy and efficient: site-specific insertion of transgenes into enterobacterial chromosomes using Tn7 without need for selection of the insertion event. BMC Microbiol. 6: 39–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Na S. H., Miyanaga K., Unno H., Tanji Y. 2006. The survival response of Escherichia coli K12 in a natural environment. Appl. Microbiol. Biotechnol. 72: 386–392 [DOI] [PubMed] [Google Scholar]
- 29. Oliver J. D. 2000. The public health significance of viable but nonculturable bacteria, p. 277–300 In Colwell R. R., Grimes D. J. (ed.), Nonculturable microorganisms in the environment. ASM Press, Washington, DC [Google Scholar]
- 30. Oliver J. D. 2005. Viable but nonculturable bacteria in food environments, p. 99–112 In Fratamico P. M., Bhunia A. K., Smith J. L. (ed.), Food borne pathogens: microbiology and molecular biology . Horizon Scientific Press, Norfolk, United Kingdom [Google Scholar]
- 31. Oliver J. D. 2005. The viable but non-culturable state in bacteria. J. Microbiol. 43: 93–100 [PubMed] [Google Scholar]
- 32. Pommepuy M., et al. 1996. Retention of enteropathogenicity by viable but nonculturable Escherichia coli exposed to seawater and sunlight. Appl. Environ. Microbiol. 62: 4621–4626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Reissbrodt R., et al. 2002. Resuscitation of Salmonella enterica serovar Typhimurium and enterohemorrhagic Escherichia coli from the viable but nonculturable state by heat-stable enterobacterial autoinducer. Appl. Environ. Microbiol. 68: 4788–4794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rigsbee W., Simpson L. M., Oliver J. D. 1997. Detection of the viable but nonculturable state in Escherichia coli O157:H7. J. Food Saf. 16: 255–262 [Google Scholar]
- 35. Rodriguez G. G., Phipps D., Ishiguro K., Ridgway H. F. 1992. Use of fluorescent redox probe for direct visualization of actively respiring bacteria. Appl. Environ. Microbiol. 58: 1801–1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sambrook J., Russell D. W. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 37. Sylvester D. M, Taylor R., LaHann T. R. 2001. Viable but nonculturable bacteria: a public health threat? Infect. Dis. Rev. 3: 70–82 [Google Scholar]
- 38. Wang G., Doyle M. P. 1998. Survival of enterohemorrhagic Escherichia coli O157:H7 in water. J. Food Prot. 61: 662–667 [DOI] [PubMed] [Google Scholar]
- 39. Yamamoto H. 2000. Viable but nonculturable state as a general phenomenon of non-spore-forming bacteria, and its modeling. J. Infect. Chemother. 6: 112–114 [DOI] [PubMed] [Google Scholar]
- 40. Yaron S., Matthews K. R. 2002. A reverse transcriptase-polymerase chain reaction assay for detection of viable Escherichia coli O157:H7: investigation of specific target genes. J. Appl. Microbiol. 92: 633–640 [DOI] [PubMed] [Google Scholar]
- 41. Zhao L., Matthews K. R. 2000. Influence of starvation, temperature, and pH on culturability of enterohemorrhagic Escherichia coli O157:H7. J. Food Saf. 20: 193–208 [Google Scholar]