Abstract
We demonstrated the production of poly-β-1,6-N-acetylglucosamine (PNAG) polysaccharide in the biofilms of Burkholderia multivorans, Burkholderia vietnamiensis, Burkholderia ambifaria, Burkholderia cepacia, and Burkholderia cenocepacia using an immunoblot assay for PNAG. These results were confirmed by further studies, which showed that the PNAG hydrolase, dispersin B, eliminated immunoreactivity of extracts from the species that were tested (B. cenocepacia and B. multivorans). Dispersin B also inhibited biofilm formation and dispersed preformed biofilms of Burkholderia species. These results imply a role for PNAG in the maintenance of Burkholderia biofilm integrity. While PNAG was present in biofilms of all of the wild-type test organisms, a ΔpgaBC mutant of B. multivorans (Mu5) produced no detectable PNAG, indicating that these genes are needed for Burkholderia PNAG formation. Furthermore, restoration of PNAG production in PNAG negative E. coli TRXWMGΔC (ΔpgaC) by complementation with B. multivorans pgaBCD confirmed the involvement of these genes in Burkholderia PNAG production. While the confocal scanning laser microscopy of untreated wild-type B. multivorans showed thick, multilayered biofilm, Mu5 and dispersin B-treated wild-type biofilms were thin, poorly developed, and disrupted, confirming the involvement of PNAG in B. multivorans biofilm formation. Thus, PNAG appears to be an important component of Burkholderia biofilms, potentially contributing to its resistance to multiple antibiotics and persistence during chronic infections, including cystic fibrosis-associated infection.
INTRODUCTION
Bacteria of the Burkholderia cepacia complex (Bcc) have emerged as opportunistic pathogens in patients with cystic fibrosis (CF) and immunocompromised individuals (20). Approximately 80% of the Bcc isolates recovered from the sputum of CF patients produce large amounts of exopolysaccharides (EPS), suggesting a possible role for this EPS in Bcc pathogenesis (5). Most of the Bcc strains investigated produced only one type of EPS, consisting of a highly branched heptasaccharide repeating unit called cepacian (27). Several studies have pointed to cepacian as a virulence factor contributing to the overall pathogenicity of Bcc members and thus to their success as pathogens. In addition, Bcc species are capable of forming biofilms in vitro and in vivo, which plays an important role in virulence and significantly increases antibiotic resistance (26). Studies with cepacian-defective mutants have demonstrated that, although not required for the initiation of biofilm formation, cepacian is required for the formation of thick and mature biofilms (6). Although cepacian is often produced along with other EPS molecules, only a small percentage of Bcc strains examined produced EPS completely different from cepacian (18).
In addition to cepacian, Burkholderia spp. are believed to produce other types of EPS, such as poly-β-1,6-N-acetylglucosamine (PNAG; also called polysaccharide intercellular adhesin [PIA] and poly-β-1,6-N-acetyl-d-glucosamine [PGA]), which is a major component of biofilms in staphylococci (18, 19, 21). PNAG is an important virulence factor, as it protects bacteria against innate host defenses (17, 28). In staphylococci, the icaADBC operon encodes the proteins involved in the synthesis of PNAG (10). Furthermore, the pgaABCD operon of Escherichia coli was shown to promote the synthesis of PNAG (29). Functionally and genetically related loci occur in other Gram-negative bacteria, including Klebsiella pneumoniae, Yersinia spp., Bordetella spp., Pseudomonas fluorescens, Actinobacillus pleuropneumoniae, Burkholderia cepacia, and Aggregatibacter actinomycetemcomitans (12, 13, 22, 29). The production of PNAG in E. coli (29), A. pleuropneumoniae (13), Acinetobacter baumannii (4), Bordetella spp. (22), A. actinomycetemcomitans (14), and Yersinia pestis (2) has been biochemically and/or immunologically confirmed. A BLASTP search in the NCBI nonredundant protein database using pgaABCD, which encodes proteins involved in PNAG biosynthesis and export in E. coli, revealed the presence of a four-gene locus in Burkholderia spp. that shares a high degree of similarity with the genetic locus encoding PNAG-biosynthetic proteins in other Gram-negative bacteria.
The objectives of this study were to (i) test for the production of PNAG and its dependence upon the pgaABCD locus in Burkholderia spp., (ii) probe for the PNAG polysaccharide component in the biofilms of Burkholderia species such as Burkholderia multivorans, Burkholderia vietnamiensis, Burkholderia ambifaria, Burkholderia cepacia, and Burkholderia cenocepacia, and (iii) examine the role of PNAG in Burkholderia sp. biofilm formation using biochemical, genetic methods, and confocal microscopy.
MATERIALS AND METHODS
Chemicals, bacteria, and culture conditions.
All chemicals (including media ingredients) were of analytical grade. Proteinase K, DNase I, RNase A, α-amylase, Western blocker solution, horseradish peroxidase-conjugated goat anti-mouse immunoglobulin M (IgM), and 3,3′,5,5′-tetramethylbenzidine (TMB) were purchased from Sigma-Aldrich (St. Louis, MO). Immun-Blot polyvinylidene difluoride (PVDF) membranes were from Bio-Rad Laboratories (Hercules, CA). Restriction endonucleases and Taq DNA polymerases were purchased from Fermentas (Burlington, ON, Canada). T4 DNA ligase and shrimp alkaline phosphatase were from New England BioLabs (Mississauga, ON, Canada) and Roche Diagnostics (Laval, QC, Canada), respectively. Synthetic oligonucleotides were obtained from Sigma Genosys (Oakville, ON, Canada). The enzyme dispersin B was purified from a recombinant E. coli strain as previously described (16). The enzyme had a specific activity of ∼103 units mg−1 of protein. Bacterial strains used in this study are shown in Table 1. All strains were maintained at −80°C in 15% glycerol, recovered in tryptic soy broth (TSB) or brain heart infusion broth (BHI), incubated at 37°C for 48 h. For exopolysaccharide extraction, Burkholderia and E. coli strains were cultured in 50 ml BHI and Luria Bertani (LB) broth, respectively, at 37°C for 3 days under static conditions (i.e., conditions which did not change). E. coli XL1-Blue was used for cloning and plasmid maintenance.
Table 1.
Bacterial stains and plasmids used in this study
| Strain or plasmid | Relevant characteristic(s) | Source or reference |
|---|---|---|
| Strains | ||
| B. multivorans C5393 | Clinical isolate, wild type | S. T. Cardona |
| B. vietnamiensis LM618835 | Wild type | S. T. Cardona |
| B. cepacia ATCC25416 | Wild type | ATCCa |
| B. cenocepacia J2315 | Wild type | S. T. Cardona |
| B. ambifaria LM619467 | Wild type, cystic fibrosis isolate | S. T. Cardona |
| B. multivorans MU5 | B. multivorans C5393 ΔpgaBC | This study |
| E. coli XL1-Blue | recA1 endA1 gyrA96 thi-1 hsdR17 supE44 relA1 lac [F′ proAB lacIqZΔM15 Tn10 (Tetr)] | Stratagene |
| E. coli TRMG1655 | E. coli MG1655 csrA::kan | 23 |
| E. coli TRXWMGΔC | E. coli TRMG ΔpgaC | 29 |
| Plasmids | ||
| pwFRT-Tprc | Tpr Apr; Tpr cassette flanked by wild-type FRTb | 1 |
| pBSKII(−) | Apr | Stratagene |
| pMCS5 | Apr | Mo Bi Tec |
| pBCMu | Apr Tpr; gene replacement vector | This study |
| pMCSpgaBCD | Apr; B. multivoranspgaBCD | This study |
ATCC, American Type Culture Collection.
FRT, Flp recombination target.
Immunoblot analysis.
Burkholderia biofilm-associated PNAG was extracted by following the method for the isolation of polysaccharide intercellular adhesin (PIA) from Staphylococcus epidermidis with some modifications (28). Cells were harvested by centrifugation, resuspended in 0.5 M EDTA, and incubated at 100°C for 5 min and at 85°C for 30 min. After centrifugation, the clarified supernatant was first dialyzed against deionized water and then against 50 mM Tris-HCl (pH 8.0) and 20 mM MgCl2. The crude polysaccharide preparation was treated with 100 μg/ml α-amylase, 500 μg/ml lysozyme, 250 μg/ml DNase I, and 100 μg/ml RNase A at 37°C for 2 h followed by 2 mg/ml proteinase K for 16 h at 55°C in the presence of 1 mM CaCl2 and 0.5% sodium dodecyl sulfate. The samples were incubated at 85°C for 1 h to inactivate proteinase K and dialyzed against deionized water. The polysaccharide preparations were lyophilized and dissolved in 50 μl phosphate-buffered saline (PBS), and a 15-μl aliquot was spotted onto an Immun-Blot PVDF membrane. The blot was blocked with Western blocker solution and probed with a 1:2,000 dilution of murine IgM monoclonal antibody (MAb) raised against E. coli PGA (11) as the primary antibody. Horseradish peroxidase-conjugated goat anti-mouse IgM at a dilution of 1:10,000 was used as the secondary antibody and detected with TMB.
DNA manipulations.
All enzymatic reactions were performed according to the manufacturers' instructions. E. coli cells were transformed by heat shock using frozen competent cells prepared by using the calcium chloride method described in Molecular Cloning (24). Burkholderia strains were transformed by the electrotransformation method (7). Plasmid construction and plasmid DNA extraction were carried out according to standard molecular biology techniques (25). Genomic DNA was extracted following the phenol-chloroform method (8). PCR was carried out using a Px2 thermal cycler (Thermo Electron Corporation, Milford, MA).
pBCMu construction.
The genomic sequence containing putative genes responsible for PNAG synthesis, deacetylation, and export were isolated from B. multivorans C5393 genomic DNA by PCR using primers based on the sequence information of the genomic region containing the BMULJ_04028, BMULJ_04027, BMULJ_04026, and BMULJ_04025 genes of B. multivorans ATCC 17616, which are homologous to E. coli pgaA, pgaB, pgaC, and pgaD, respectively. For construction of pBCMu, the 826-bp fragment (F1) that includes 778 bp of the 3′ coding region of pgaA and 33 bp of the 5′ coding region of pgaB and an 851-bp fragment (F2) spanning 24 bp of the 3′ coding region of pgaC, the complete pgaD gene, and 329 bp of the downstream region were isolated using the primer pairs P1F (5′ TAATATTCTAGAAGCTTGCAACCGTTCGGCGACAACTG 3′)-P1R (5′ TAATATGGATCCGCATCCGCACATGAAGGTCCGTC 3′) and P2F (5′ TAATATGGATCCATGAAGAACGCGCCGATTATTG 3′)-P2R (5′ TAATATCTCGAGAAGCTTGGCGCGCCGTGCGTCGCCCAG 3′), respectively. The PCR mixture contained 0.25 μM (each) forward and reverse primers, 200 μM (each) deoxynucleoside triphosphates, 100 ng template DNA, 75 mM Tris-HCl (pH 8.8), 20 mM (NH4)2SO4, 2.5 mM MgCl2, 0.01% Triton X-100, 5% dimethyl sulfoxide, and 1.5 units of Taq DNA polymerase. The thermal program consisted of 1 cycle of 94°C for 2 min, 25 cycles of 94°C for 30 s, 58°C for 30 s, and 72°C for 2 min followed by 5 min at 72°C. First, the PCR fragments F1 and F2 were cloned between the XbaI and HindIII sites of pBSKII(−), and then the trimethoprim-resistant cassette excised from pwFRT-Tprc was introduced into the BamHI site to flank F1 to F2 and yield the mutagenesis vector pBCMu.
pMCSpgaBCD construction.
A 558-bp DNA fragment containing the PC-s12 promoter of pwFRT-Tprc and a 669-bp genomic DNA fragment containing a 5′ segment of pgaB of B. multivorans C5393 were amplified by PCR, as described above, using the primer pairs PCS12-F (5′ TATAATCTCGAGTCTAGATGATTCCCTTTGTCAACAGCAATGG 3′)-PCS12-R (5′ TATAATAAGCTTGTCGAATCCTTCTTGTGAATCTATTATGGCG 3′) and P4F (5′ TATAATAAGCTTCCATGCAATCCAGACGGACCTTC 3′)-P4R (5′ GTTGCGCACGCGTGCGTGGAATTCCTCGTC 3′), respectively, and cloned between the XhoI and EcoRI sites of pBSKII(−) to produce the vector pPCS12pgaB5′. A 3,351-bp genomic fragment containing the 1,419 bp at the 3′ end of pgaB and pgaCD of B. multivorans C5393 was amplified by PCR, as described above, as three fragments using the primer pairs P5F (5′ GACGAGGAATTCCACGCACGCGTGCGCAAC 3′)-P5R (5′ TCAGATCCTCGTAGATCTCCTC 3′), P6F (5′ TCGAGGAGATCTACGAGGATCTG 3′)-P6R (5′ CGACGGGGTACCAGACGGTGTCAAG 3′), and P7F (5′ ACACCGTCTGGTACCCCGTCGC 3′)-P7R (5′ TAATATAAGCTTAGTCGTAGCCGCGATACTCGAG 3′) and assembled between the EcoRI and XhoI sites of pBSKII(−) with several subcloning steps to produce pPgaBCD. The EcoRI-XbaI fragment of plasmid pPCS12pgaB5 containing PC-s12 promoter and 5′ region of pgaB gene and EcoRI-HindIII fragment of plasmid pPgaBCD containing 3′ region of pgaB gene and pgaCD was cloned in between XbaI-HindIII sites of the vector pMCS5 to produce the final complementation plasmid, pMCSpgaBCD.
Construction of the pgaBC mutant strain.
Burkholderia multivorans C5393 was transformed with pBCMu by electroporation, and the transformants were plated on solid TSB medium containing 100 mg/liter trimethoprim. Genomic DNA was extracted from the trimethoprim-resistant colonies and analyzed by PCR to confirm replacement of pgaBC by the trimethoprim resistance marker carried on the plasmid. The PCR was carried out with the primers P3F (5′ GCTGGCCGACAACAGCTTC 3′), which anneals with the pgaA genomic sequence upstream of the pgaA sequence carried on the transformed plasmid, and P3R (5′ TGAAGCTAATTCGAGCTCGGTAC 3′), which anneals with the trimethoprim resistance cassette.
Genetic complementation.
The PNAG-negative E. coli strain TRXWMGΔC (ΔpgaC) was transformed with plasmid pMCSpgaBCD. Crude exopolysaccharide was extracted form both E. coli TRMGΔCpgaBCD and E. coli TRXWMGΔC, which had been cultured under the same conditions, and examined for the presence of PNAG by immunoblot analysis.
Biofilm assay.
Biofilms were assayed by crystal violet staining, as described previously (15). The overnight-grown cultures were diluted 100 times in TSB or BHI medium in the presence and absence of dispersin B at 100 μg/ml concentrations. Aliquots of cells were transferred to the wells of a 96-well microtiter plate (Corning Inc., New York, NY), and the plate was incubated at 37°C for 48 h. Biofilms were washed with water and then stained for 15 min with 200 μl crystal violet. Stained biofilms were rinsed with water, and the amount of biomass was quantified by destaining the biofilm with 200 μl of 33% acetic acid and then measuring the absorbance at 630 nm. To determine the effect of dispersin B on biofilm dispersal, 200 μl of diluted overnight culture was added to each well of a 96-well microtiter plate, and the plates were incubated at 37°C for 18 h. After incubation, the planktonic cells were removed, and 200 μl of dispersin B (100 μg/ml) or water (controls) was added to the wells. The plates were further incubated at 37°C for 6 h. The biofilm dispersal was determined by measuring the absorbance of crystal violet-stained biofilm at 630 nm. At least six replicates were carried out for each sample, and each experiment was performed at least three times. The results are presented as averages and standard deviations from three or more experiments. Statistical analysis was performed using Student's t test. P values of ≤0.01 were considered statistically significant.
Biofilm killing assay.
Biofilms were grown in 1.5-ml polypropylene microcentrifuge tubes as described previously (13). Tubes were filled with 200 μl of inoculum (diluted 100 times in fresh TSB). After 16 h of incubation at 37°C, the broth was aspirated and replaced with fresh broth containing 0 and 250 μg/ml tobramycin (4 times the MIC). After 3 h, the cells were vortexed, pelleted, and rinsed with sterile saline three times to remove tobramycin. Cell pellets were resuspended in saline by vortexing, and the number of CFU/ml was determined by serial dilution plating.
Confocal microscopy.
Biofilm staining and confocal scanning laser microscopy (CSLM) was performed as described previously (3). For biofilm growth, plastic coverslips were individually placed in each well of a 12-well tissue culture plate, 2 ml inoculated broth was added to each well, and the plate was incubated at 37°C for 16 h. Biofilms were grown in the presence and absence of 200 μg/ml dispersin B. Biofilm growth on plastic coverslips was stained with wheat germ agglutinin-Alexa Fluor 488 conjugate, which selectively binds to N-acetylglucosamine and gives green fluorescence. The biofilm was observed by using an Olympus IX-70 with an argon laser for excitation at 488 nm (green fluorescence). Images were captured and thickness was determined by using Fluoview software and Image Pro Plus software.
RESULTS
pgaABCD locus and PNAG polysaccharide production.
By performing a BLAST search, we identified four open reading frames in B. cenocepacia J2315 and B. multivorans ATCC 17616 that encode proteins with high levels of similarity to the E. coli proteins PgaA, PgaB, PgaC, and PgaD. Percents similarity and identity were calculated by using EMBOSS, an online pairwise sequence alignment tool from EMBL-EBI (http://www.ebi.ac.uk). Based on the similarity and identity of this locus with E. coli pgaABCD and the genetic as well as biochemical data below, we believe that this locus is pgaABCD and is responsible for the synthesis of PNAG in Burkholderia spp. We constructed B. multivorans Mu5 ΔpgaBC, an isogenic mutant in which pgaBC is replaced with a trimethoprim resistance cassette, in order to confirm that the production of PNAG polysaccharide requires these genes in B. multivorans. pgaB encodes a predicted 695-amino-acid protein with a putative polysaccharide deacetylase domain. B. multivorans PgaB shares 56% similarity and 38% identity with E. coli PgaB. E. coli PgaB is predicted to be a periplasmic lipoprotein that, along with PgaA, is necessary for PNAG export (11). pgaC is predicted to encode a 423-amino-acid N-glycosyltransferase, which is thought to catalyze the first committed step in the synthesis of PNAG. B. multivorans PgaC shares 64% similarity and 45% identity with E. coli PgaC. While the immunoblot analysis of polysaccharide extract from wild-type B. multivorans biofilm showed the presence of PNAG, there was no detectable PNAG in the polysaccharide extract of Mu5 (Fig. 1a and b). The involvement of Burkholderia pgaABCD genes in PNAG production was further confirmed by the observation that the restoration of PNAG production after genetic complementation of PNAG-negative E. coli TRXWMGΔC (ΔpgaC) with plasmid-carrying genes under the expression signals of PC-s12 promoter (Fig. 1c). Our attempts to complement B. multivorans Mu5 (ΔpgaBC) with a plasmid expressing B. multivorans C5393 pgaBCD were hampered due to the very high levels of antibiotic resistance of Burkholderia strains (data not shown). When we compared the biofilm dispersal effect of dispersin B on wild-type B. multivorans biofilm with that on Mu5 using a 96-well biofilm dispersal assay, it was found that dispersin B dispersed biofilm only in the wild-type strain (Fig. 2). In addition, the sensitivity of biofilm in wild-type B. multivorans and Mu5 to 250 μg/ml of tobramycin was determined in a biofilm killing assay (Table 2). Tobramycin showed 3.25 and 5.1 log10 reductions in biofilm-embedded cells of the wild type and Mu5, respectively. The 2-log10-greater reduction in the biofilm-embedded cells of Mu5 than that of the wild type indicates an increased sensitivity of the Mu5 biofilm to antibiotics. This indirectly suggests that the pgaBC mutant did not produce PNAG and that PNAG is involved in forming normal biofilm, which confers antibiotic resistance.
Fig. 1.
Immunological detection of cell-bound PNAG. (a) Crude exopolysaccharide extracted from Burkholderia spp. (b) Effect of dispersin B (25 μg) on PNAG from crude extracts of B. cenocepacia and B. multivorans. Crude exopolysaccharide extracted from E. coli TRMG1655 was used a as positive control. (c) Effect of complementation of E. coli TRXWMGΔC(ΔpgaC) with pMCSpgaBCD on PNAG in exopolysaccharide extract.
Fig. 2.
Comparison of the biofilm dispersal effect of dispersin B on wild-type B. multivorans with that on the pgaBC mutant. Values are means ± standard deviations from two experiments with six replicates per sample. *, P < 0.01 compared with untreated control biofilm.
Table 2.
Sensitivity of B. multivorans wild-type and PNAG-negative Mu5 (ΔpgaBC) biofilms to 250 μg/ml tobramycin
| Strain | Mean log10 CFU of biofilm-embedded cells/ml ± SD with: |
Log reduction due to tobramycin treatmenta | Pb | |
|---|---|---|---|---|
| No treatment (control) | Tobramycin | |||
| Wild type | 9.95 ± 0.27 | 6.7 ± 0.08 | 3.25 | 0.001 |
| Mu5 | 8.87 ± 0.13 | 3.77 ± 0.16 | 5.1 | <0.0001 |
Mean log density for untreated control minus the mean log density for corresponding tobramycin treatment.
Determined using two-tailed Student's t test with unequal variance.
Detection of PNAG polysaccharide component.
To detect the PNAG that is synthesized by the pgaABCD-encoded proteins in Burkholderia spp., we used E. coli PNAG-specific MAb (11) for immunoblot analysis of purified polysaccharides from five Burkholderia spp. In this experiment, a known quantity of highly purified PNAG from E. coli TRMG 1655 (csrA::kan) was used as a positive control (11). The immunoblot signals showed the presence of PNAG in the polysaccharide extracts of all five Burkholderia spp. tested (Fig. 1a). The presence of PNAG was further confirmed by the observation that the positive immunoblot signals of B. cenocepacia and B. multivorans disappeared when the polysaccharide extracts were treated with dispersin B (Fig. 1b), which specifically hydrolyzes PNAG (11). However, in all five species of Burkholderia, detection of PNAG was possible only from highly concentrated preparations of polysaccharide.
Role of PNAG in Burkholderia biofilm formation.
We tested both the inhibitory and dispersal effects of the PNAG-hydrolyzing enzyme dispersin B on biofilm formation and preformed biofilms, respectively, in B. multivorans, B. vietnamiensis, B. cepacia, and B. cenocepacia. All of these bacteria formed substantial biofilms in the control wells of a 96-well microtiter plate under the assay conditions. Dispersin B at 100 μg/ml showed a significant inhibitory effect (P < 0.01) on the biofilm formation of all Burkholderia spp. (Fig. 3a). Additionally, there were no discernible differences in the planktonic growth of Burkholderia spp. tested in the presence or absence of dispersin B (data not shown). When dispersin B was tested against preformed biofilms in these organisms, the enzyme dispersed significantly more biofilm (P < 0.01) than the control at 37°C in all the test organisms (Fig. 3b).
Fig. 3.
Effect of untreated control (▪) and dispersin B (□) on biofilm formation (a) and biofilm dispersal (b) in B. multivorans (Bm), B. vietnamiensis (Bv), B. cepacia (Bc), and B. cenocepacia (Bcn). The values are means ± standard deviations. *, P < 0.01 compared with untreated control biofilm.
CSLM analysis of wild-type B. multivorans and Mu5 biofilms.
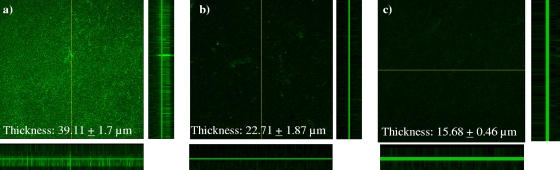
CSLM was used to examine the morphological features of biofilms formed by wild-type B. multivorans and Mu5 after 16 h of growth. In addition, the wild-type biofilm grown in the presence of 200 μg/ml dispersin B was analyzed by CSLM. As shown in Fig. 4a and b, wild-type B. multivorans formed a multilayered biofilm which was inhibited by 200 μg/ml dispersin B. Conversely, Mu5 biofilm adhered poorly to the coverslip and showed loosely attached as well as scattered cells across the coverslip (Fig. 4c). To confirm the CLSM observations of biofilm structure, Fluoview image analysis software was used to evaluate the thickness of the biofilm. The biofilm produced by wild-type B. multivorans was thicker (39.11 ± 1.7 μm) than dispersin B-treated biofilm (22.71 ± 1.87 μm) and the biofilm produced by Mu5 (15.68 ± 0.46 μm). Note that there was no apparent difference in the growth of the wild-type and mutant strains (data not shown).
Fig. 4.
Confocal image of B. multivorans biofilm formation on plastic coverslip. (a) Wild-type biofilm in the absence of dispersin B; (b) wild-type biofilm in the presence of 200 μg/ml dispersin B; (c) B. multivorans Mu5 (ΔpgaBC) biofilm in the absence of dispersin B. The images are maximum projections or reconstructed confocal stacks consisting of a series of xy (center), yz (left), and xz (bottom) sections. A representative CSLM image for each sample is shown.
DISCUSSION
We confirmed that pgaABCD locus in Burkholderia spp. shares sequence similarity with and is functionally related to the pga locus in E. coli, which is required for the synthesis of PNAG polysaccharide and biofilm formation. The replacement of pgaB and pgaC genes with the Tpr cassette in wild-type B. multivorans resulted in a B. multivorans mutant, Mu5, defective for PNAG production, and PNAG production was restored in PNAG-negative E. coli TRXWMGΔC (ΔpgaC) after complementation with B. multivorans pgaBCD, as determined by immunoblotting. Compared to the strong immunoblot signal from polysaccharide extract of wild-type B. multivorans biofilm, Mu5 biofilm polysaccharide extract had an undetectable signal, indicating that the mutant did not produce PNAG. This confirmed that the pgaABCD locus is needed for the biosynthesis of PNAG polysaccharide component of Burkholderia biofilm. Additional confirmation that Mu5 does not produce PNAG because of the replacement of the pgaBC locus with the Tpr cassette was obtained by showing that the PNAG-hydrolyzing enzyme dispersin B does not disperse Mu5 biofilm. Furthermore, because it lacks PNAG, Mu5 produced a weaker biofilm and was therefore significantly more sensitive to tobramycin than the wild type (P < 0.0001), suggesting a role for PNAG in antibiotic resistance of Burkholderia. Furthermore, confocal microscopy of the Mu5 biofilm showed that it was poorly developed and thin compared to the multilayered thick biofilm of the wild type, suggesting that the pgaABCD locus is required for the synthesis of a PNAG polysaccharide component, which may play a critical role in the development of Burkholderia biofilms.
An immunoblot analysis of polysaccharide extracts from Burkholderia spp. biofilms using a PNAG-specific MAb confirmed the presence of PNAG polysaccharide, which appears to be a minor component compared to the major polysaccharide cepacian in the Burkholderia cepacia complex (6). The presence of PNAG in Burkholderia biofilms was further confirmed by treating the polysaccharide extracts with the PNAG-hydrolyzing enzyme dispersin B, which led to the disappearance of immunoblot signals. Further evidence for the presence of PNAG in Burkholderia biofilms was obtained by showing that dispersin B inhibited and dispersed biofilms in Burkholderia spp. More importantly, the inhibition of Burkholderia biofilm formation by the PNAG substrate-specific enzyme dispersin B provides evidence that PNAG is needed for biofilm formation in Burkholderia spp. Interestingly, studies performed on cepacian-defective mutants have demonstrated that, although not required for the initiation of biofilm formation, cepacian is required for the formation of thick and mature biofilms (6). Our studies seem to suggest that PNAG in Burkholderia is needed for initiating biofilm formation. The confocal microscopy of untreated and dispersin B-treated B. multivorans biofilms showed multilayered and disrupted biofilms, respectively, further demonstrating that PNAG, though a minor polysaccharide component, plays an important role in forming and maintaining the integrity of Burkholderia biofilms.
The ability of many pathogens to adhere to human tissues and medical devices and form biofilms is a major virulence factor that correlates with an increase in antibiotic resistance, reduced phagocytosis, and overall persistence of the bacterial population. Of all the different molecules identified as biofilm components, PNAG is perhaps the most important and widely conserved factor (17, 28). As this surface polysaccharide is a known virulence factor in various staphylococcal infections and is a target for developing vaccines (9), PNAG production may have a role in the pathogenesis of Burkholderia infection. Burkholderia infection has been shown to be an important threat for CF patients, owing to its association with a dramatic increase in symptoms and decline in pulmonary function. This is in addition to causing a potentially fatal necrotizing pneumonitis with bacteremia called “cepacia syndrome” in infected individuals. Multidrug resistance (MDR) and patient-to-patient transmission are other possible consequences of Burkholderia infection, and they contribute to increased morbidity and mortality in CF patients. PNAG-specific immunotherapies or PNAG-hydrolyzing dispersin B-antibiotic combinations could provide effective approaches and tools for combating CF-associated Burkholderia infections, which pose a significant challenge for current antibiotic therapies.
ACKNOWLEDGMENTS
We are grateful to T. Hong (University of Hawaii, Honolulu, HI) for providing pwFRT-Tprc. We also acknowledge funding from the Manitoba Health Research Council (MHRC), operating grant 309342, received by Silvia T. Cardona.
Footnotes
Published ahead of print on 7 October 2011.
REFERENCES
- 1. Barrett A. R., et al. 2008. Genetic tools for allelic replacement in Burkholderia species. Appl. Environ. Microbiol. 74: 4498–4508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bobrov A. G., Kirillina O., Forman S., Mack D., Perry R. D. 2008. Insights into Yersinia pestis biofilm development: topology and co-interaction of Hms inner membrane proteins involved in exopolysaccharide production. Environ. Microbiol. 10: 1419–1432 [DOI] [PubMed] [Google Scholar]
- 3. Chandra J., Kuhn D. M., Mukhrjee P. K., Hoyer L. L., Ghannoum M. A. 2001. Biofilm formation by the fungal pathogen Candida albicans: development, architecture, and drug resistance. J. Bacteriol. 183: 5385–5394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Choi A. H. K., Slamti L., Avci F. Y., Pier G. B., Maira-Litran T. 2009. The pgaABCD locus of Acinetobacter baumannii encodes the production of poly-β-1-6-N-acetylglucosamine, which is critical for biofilm formation J. Bacteriol. 191: 5953–5963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cunha M. V., et al. 2003. Molecular analysis of Burkholderia cepacia complex isolates from a Portuguese cystic fibrosis center: a 7-year study. J. Clin. Microbiol. 41: 4113–4120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cunha M. V., et al. 2004. Studies on the involvement of the exopolysaccharide produced by cystic fibrosis-associated isolates of the Burkholderia cepacia complex in biofilm formation and persistence of respiratory infections. J. Clin. Microbiol. 42: 3052–3058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dubarry N., Du W., Lane D., Pasta F. 2010. Improved electrotransformation and decreased antibiotic resistance of the cystic fibrosis pathogen Burkholderia cenocepacia strain J2315. Appl. Environ. Microbiol. 76: 1095–1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ergunay E., et al. 2008. Comparison of extraction methods for PCR detection of Burkholderia cepacia complex (BCC) from cystic fibrosis patients. Cen. Eur. J. Med. 3: 157–162 [Google Scholar]
- 9. Gening M. L., et al. 2010. Synthetic β-(1-6)-linked N-acetylated and nonacetylated oligoglucosamines used to produce conjugate vaccines for bacterial pathogens. Infect. Immun. 78: 764–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Heilmann C., et al. 1996. Molecular basis of intercellular adhesion in the biofilm-forming Staphylococcus epidermidis. Mol. Microbiol. 20: 1083–1091 [DOI] [PubMed] [Google Scholar]
- 11. Itoh Y., et al. 2008. Roles of pgaABCD genes in synthesis, modification, and export of the Escherichia coli biofilm adhesin poly-beta-1, 6-N-acetyl-d-glucosamine. J. Bacteriol. 190: 3670–3680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Itoh Y., Wang X., Hinnebusch B. J., Preston J. F., III, Romeo T. 2005. Depolymerization of beta-1,6-N-acetyl-d-glucosamine disrupts the integrity of diverse bacterial biofilms. J. Bacteriol. 187: 382–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Izano E. A., et al. 2007. Poly-N-acetylglucosamine mediates biofilm formation and antibiotic resistance in Actinobacillus pleuropneumoniae. Microb. Pathog. 43: 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Izano E. A., et al. 2008. Poly-N-acetylglucosamine mediates biofilm formation and detergent resistance in Aggregatibacter actinomycetemcomitans. Microb. Pathog. 44: 52–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jackson D. W., et al. 2002. Biofilm formation and dispersal under the influence of the global regulator CsrA of Escherichia coli. J. Bacteriol. 184: 290–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kaplan J. B., Ragunath C., Ramasubbu N., Fine D. H. 2003. Detachment of Actinobacillus actinomycetemcomitans biofilm cells by an endogenous β-hexosaminidase activity. J. Bacteriol. 185: 4693–4698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kropec A., et al. 2005. Poly-N-acetylglucosamine production in Staphylococcus aureus is essential for virulence in murine models of systemic infection. Infect. Immun. 73: 6868–6876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lagatolla C., et al. 2002. Microbiological characterization of Burkholderia cepacia isolates from cystic fibrosis patients: investigation of the exopolysaccharides produced. FEMS Microbiol. Lett. 209: 89–94 [DOI] [PubMed] [Google Scholar]
- 19. Mack D., et al. 1996. The intercellular adhesin involved in biofilm accumulation of Staphylococcus epidermidis is a linear beta-1,6-linked glucosaminoglycan: purification and structural analysis. J. Bacteriol. 178: 175–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mahenthiralingam E., Vandamme P. 2005. Taxonomy and pathogenesis of the Burkholderia cepacia complex. Chron. Respir. Dis. 2: 209–217 [DOI] [PubMed] [Google Scholar]
- 21. Maira-Litran T., et al. 2002. Immunochemical properties of the staphylococcal poly-N-acetylglucosamine surface polysaccharide. Infect. Immun. 70: 4433–4440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Parise G., Mishra M., Itoh Y., Romeo T., Deora R. 2007. Role of a putative polysaccharide locus in Bordetella biofilm development. J. Bacteriol. 189: 750–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Romeo T., Gong M., Liu M. Y., Brun-Zinkernagel A. M. 1993. Identification and molecular characterization of csrA, a pleiotropic gene from Escherichia coli that affects glycogen biosynthesis, gluconeogenesis, cell size, and surface properties. J. Bacteriol. 175: 4744–4755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sambrook J., Russell D. W. 2001. Molecular cloning, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 25. Sambrook J., Fritsch E. F., Maniatis T. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 26. Savoia D., Zucca M. 2007. Clinical and environmental Burkholderia strains: biofilm production and intracellular survival. Curr. Microbiol. 54: 440–444 [DOI] [PubMed] [Google Scholar]
- 27. Sist P., et al. 2003. Macromolecular and solution properties of Cepacian: the exopolysaccharide produced by a strain of Burkholderia cepacia isolated from a cystic fibrosis patient. Carbohydr Res. 338: 1861–1867 [DOI] [PubMed] [Google Scholar]
- 28. Vuong C., et al. 2004. Polysaccharide intercellular adhesin (PIA) protects Staphylococcus epidermidis against major components of the human innate immune system. Cell Microbiol. 6: 269–275 [DOI] [PubMed] [Google Scholar]
- 29. Wang X., Preston J. F., III, Romeo T. 2004. The pgaABCD locus of Escherichia coli promotes the synthesis of a polysaccharide adhesin required for biofilm formation. J. Bacteriol. 186: 2724–2734 [DOI] [PMC free article] [PubMed] [Google Scholar]