Abstract
The fungal pathogen Fusarium verticillioides infects maize ears and produces fumonisins, known for their adverse effects on human and animal health. Basic questions remain unanswered regarding the kernel stage(s) associated with fumonisin biosynthesis and the kernel components involved in fumonisin regulation during F. verticillioides-maize interaction under field conditions. In this 2-year field study, the time course of F. verticillioides growth and fumonisin accumulation in developing maize kernels, along with the variations in kernel pH and amylopectin content, were monitored using relevant and accurate analytical tools. In all experiments, the most significant increase in fumonisin accumulation or in fumonisin productivity (i.e., fumonisin production per unit of fungus) was shown to occur within a very short period of time, between 22/32 and 42 days after inoculation and corresponding to the dent stage. This stage was also characterized by acidification in the kernel pH and a maximum level of amylopectin content. Our data clearly support published results based on in vitro experiments suggesting that the physiological stages of the maize kernel play a major role in regulating fumonisin production. Here we have validated this result for in planta and field conditions, and we demonstrate that under such conditions the dent stage is the most conducive for fumonisin accumulation.
INTRODUCTION
Maize ear rot caused by Fusarium species is one of the major diseases affecting maize production worldwide. Among Fusarium spp., F. verticillioides is the predominant species responsible for fusarium ear rot. This disease can also be caused by other members of the Gibberella fujikuroi species complex, including F. proliferatum. Both Fusarium spp. produce fumonisins, a group of polyketide-derived mycotoxins which have been classified as potent carcinogens (12). At least 60 distinct fumonisin molecules have been identified so far (2). Fumonisin B1 (FB1), FB2, and FB3 are encountered predominantly in maize kernels, with FB1 occurring at the highest level (22). To address this major food safety concern, the European Union has recently implemented a regulation that limits the FB1-plus-FB2 content in unprocessed maize to a maximum level of 4 mg kg−1 for human consumption (Commission Regulation [EC] no. 1126/2007). Fumonisins are heat-stable molecules and are not entirely eliminated during food processing (11). Control strategies therefore mainly consist of managing this disease in the field.
In the absence of other efficient disease control strategies, breeding maize for genetic resistance is currently the most promising way to control maize ear rot and fumonisin contamination. In countries where genetically modified organisms (GMO) are authorized, genetic engineering has been shown to potentially provide innovative and efficient solutions. Notably, the use of maize genetically engineered with Bacillus thuringiensis genes (BT maize) was reported to limit, under certain conditions, the levels of fumonisin contamination (23). In countries where GMO are not authorized, conventional breeding strategies are actively developed based on an increased knowledge of the mechanisms controlling resistance to disease and fumonisin accumulation.
So far, strategies for reducing fumonisin contamination have focused mainly on reducing fungal attacks, and plant breeders often select maize varieties based on visual disease symptoms. Given that symptomless maize ears may also be toxin contaminated (9, 22), limiting visual fungal infection may not be the sole effective method for reducing mycotoxin contamination in maize kernels. Developing varieties that accumulate less fumonisin is therefore a complementary approach to consider (9) and requires a better understanding of the conditions that regulate fumonisin biosynthesis during host colonization.
In the past decade, tremendous progress has been made in identifying the fungal genes required for fumonisin biosynthesis. Commonly, in toxigenic fungi, these genes are organized in a cluster designated the FUM gene cluster (27). Among the clustered genes, FUM1 plays a crucial role in fumonisin production since it encodes the polyketide synthase that catalyzes the first step of the biosynthetic pathway. One of the key challenges that research on F. verticillioides must deal with is elucidating the effects of environmental factors on the regulation of toxin biosynthesis. It is known that fumonisin production is modulated by components of the F. verticillioides-maize interaction, as recently reviewed by Picot et al. (26). Marín et al. (19) showed that fumonisin-producing strains were able to grow on kernels of various cereals. However, fumonisin production was higher on maize kernels, suggesting that this substrate provides specific conditions required for fumonisin production. Besides, fumonisin production strongly depends on the kernel stages, implying that it may also be regulated by physicochemical factors that vary during ear ripening (36). Recent in vitro studies have notably highlighted that sugar sources, especially amylopectin, along with the pH variations, are key factors that modulate the fumonisin biosynthetic pathway (3, 4, 10). Other factors, such as water activity (aw) and nitrogen sources, have also been identified as important regulators of fumonisin production (14, 16). However, these studies were conducted under laboratory conditions either on artificial media or on detached maize kernels from different stages of development. In such in vitro studies, the continuous process of maize kernel ripening is inaccurately reflected while interactions with environmental factors are ignored. Field experiments that describe kernel ripening as a whole are therefore needed to verify the validity of these in vitro results. To our knowledge, no field data are available concerning a possible link between the in planta physiological changes occurring over the course of kernel ripening and fumonisin accumulation.
In addition, the time course of F. verticillioides development and fumonisin accumulation in maize ears is still not well defined. Several field studies have monitored F. verticillioides growth and fumonisin accumulation in artificial or natural infections. In these studies, fumonisins were first detected from three to 5 weeks after silking (6, 28). However, the analytical methods used to assess fungal biomass (percentage of kernels infected and F. verticillioides DNA proportion quantified by band intensity of ethidium-bromide-stained agarose gels after PCRs) and fumonisins (immunodetection methods using enzyme-linked immunosorbent assay [ELISA]) are not sensitive enough to clearly establish the kinetics of fungal development and to precisely identify the kernel stage at which fumonisin production is initiated. In order to relate the fumonisin production to maize development, it is of primary importance to establish an accurate pattern of fungal development in the host plant.
In this work, F. verticillioides growth and fumonisin accumulation after silk inoculation were monitored at different stages of maize kernel development. The main objectives of this study are as follows: (i) to identify when fungal colonization and fumonisin production are initiated in maize ears under in planta conditions, (ii) to study how they evolve up to plant maturity, and (iii) to relate amylopectin content and pH variations to fumonisin production over the course of maize kernel colonization in order to verify in vitro results and test their validity under in planta and field conditions.
MATERIALS AND METHODS
Fungal inoculum.
The F. verticillioides INRA 63 strain used in this study was previously isolated from naturally infected maize kernels (UR 1264 MycSA; INRA Bordeaux, France).
A spore suspension was prepared by culturing the fungus in a fermenter (Bioflo III; New Brunswick Scientific, Edison, NJ) in modified Bilay's liquid medium supplemented with carboxymethyl cellulose (CMC) (30). After being cultivated for 7 days at 25°C, the medium was filtered through two layers of cheesecloth to harvest the spores, which were then enumerated with a hemocytometer. As observed microscopically, the spore suspension consisted exclusively of microconidia. It was then standardized to 108 spores ml−1 and stored at 4°C for a maximum of 1 month or at −20°C in a 1:10 mixture of glycerol and milk. Prior to inoculation, the spore suspension was diluted with distilled water to 4 × 106 spores ml−1. Spore viability was checked before and after inoculation.
Field experiments and inoculation procedure.
Two field maize varieties, PR38H20 and CRAZI (here referred to as V1 and V2, respectively), belonging to the semiearly precocity group, were sown in 2008 and 2009 in two locations in the southwest of France, 220 km apart (Montesquieu-Lauragais and Montardon) and at two sowing dates. Based on field assessments under natural contaminations, V1 was considered sensitive to F. verticillioides and V2 moderately resistant (1). Locations differ in their types of soil, with a calcareous-clay soil and a soil rich in organic matter in Montesquieu-Lauragais and Montardon, respectively. In addition, the soil pH is higher in Montesquieu-Lauragais (pH 8) than in Montardon. In the latter location, lime is applied each year (300 kg of Ca/ha) to correct the acidity and reach pH 6.5. The location-per-year experiments were referred to as Laur08, Laur09, Mont08, and Mont09 for the experiments performed in Montesquieu-Lauragais in 2008, in Montesquieu-Lauragais in 2009, in Montardon in 2008, and in Montardon in 2009, respectively. In this study, the dynamics of fungal colonization and toxin accumulation were related to the thermal time. Thermal time is defined as the sum of daily mean temperatures over the period considered. This measure has been shown to be an efficient way to compare experiments performed in different years or under different climatic conditions and is widely used in epidemiological experiments (18).
The first sowing was carried out at the beginning of May and the second one 15 to 30 days later (Table 1). However, the time interval between silking in both sowing date treatments was eventually reduced to 1 to 2 weeks. Consequently, inoculations in both sowing date treatments were always performed very close together (Table 1). For each sowing date-per-location experiment, a split-plot design was used with three replications. The varieties were randomized among the main plot units, each consisting of eight double-row subplot units corresponding to the eight sampling dates (Table 1). In 2009, an additional subplot unit was used, corresponding to a 32-day postinoculation sampling (Table 1). Kernel moisture content was estimated according to the thermal time from silking, based on the methods of Borrás et al. (5) and Sala et al. (31) (Table 2). Maize kernel stages were identified according to the number of days after silking, based on the work of Nielsen (24) (Table 2). Daily temperatures and precipitations were measured at a meteorological station located in each experimental station (Table 3).
Table 1.
Sowing dates, inoculation dates, and sampling dates for each location-per-year experimenta
| Yr | Location | Sowing date |
Inoculation date |
Sampling date(s) (days after inoculation) |
|||
|---|---|---|---|---|---|---|---|
| S1 | S2 | S 1 | S2 | S1 | S2 | ||
| 2008 | Montesquieu-Lauragais | 3 May | 22 May | 28 Jul | 6 Aug | 0, 3, 7, 10, 15, 22, 42 and 87 | 0, 2, 6, 9, 15, 22, 41 and 77 |
| Montardon | 8 May | 23 May | 29 Jul | 5 Aug | 0, 3, 7, 10, 15, 22, 41 and 77 | 0, 3, 6, 9, 15, 22, 41 and 91 | |
| 2009 | Montesquieu-Lauragais | 7 May | 2 June | 24 Jul | 12 Aug | 0, 4, 7, 9, 14, 21, 32, 41 and 81 | 0, 2, 6, 9, 14, 21, 30, 42 and 83 |
| Montardon | 7 May | 22 May | 20 Jul | 30 Jul | 0, 3, 7, 10, 14, 21, 32, 42 and 81 | 0, 4, 7, 11, 15, 22, 32, 43 and 92 | |
S1, first sowing; S2, second sowing.
Table 2.
Kernel characteristics (kernel moisture content and stages) for each sampling
| No. of sampling | Days after inoculation | Days after silking for V1, V2 | Thermal time from silking (°C day)a | Estimated kernel moisture contentb (%) | Kernel stage(s)c |
|---|---|---|---|---|---|
| 1 | 0 | 4, 7 | 51–140 | 90 | Silking |
| 2 | d2–d4 | 6–8, 9–11 | 136–224 | 85–90 | Silking |
| 3 | d6–d7 | 10–11, 13–14 | 182–312 | 85 | Blister |
| 4 | d9–d11 | 13–15, 16–18 | 275–384 | 85–80 | Blister, early milk |
| 5 | d14–d15 | 18–19, 21–22 | 371–506 | 70–80 | Milk |
| 6 | d21–d22 | 25–26, 28–29 | 505–654 | 60–70 | Dough |
| 7 | d30–d32 | 34–36, 37–39 | 774–893 | 45–50 | Early dent |
| 8 | d41–d43 | 45–47, 48–50 | 871–1,084 | 45–40 | Late dent |
| 9 | d77–d92 | 81–96, 84–99 | 1,465–1,843 | <25 | Harvest maturity |
See Materials and Methods.
Kernel moisture content was estimated according to the thermal time from silking, based on the methods of Sala et al. (31) and Borrás et al. (5).
Identification of the corresponding maize kernel stages for each sampling was determined according to the number of days after silking based on the work of Nielsen (24).
Table 3.
Meteorological data (mean monthly temperatures and total monthly precipitations) for each location-per-year experiment
| Parameter and yr | Location | Monthly value |
|||
|---|---|---|---|---|---|
| July | August | September | October | ||
| Mean monthly temp (°C) | |||||
| 2008 | Montesquieu-Lauragais | 20.5 | 20.7 | 17.5 | 13.9 |
| Montardon | 19.6 | 19.8 | 16.9 | 13.5 | |
| 2009 | Montesquieu-Lauragais | 21.7 | 23.0 | 18.9 | 14.9 |
| Montardon | 20.9 | 21.7 | 18.7 | 15.5 | |
| Total monthly precipitation (mm) | |||||
| 2008 | Montesquieu-Lauragais | 63 | 25.4 | 29.4 | 54 |
| Montardon | 50.6 | 82.2 | 46.6 | 112.6 | |
| 2009 | Montesquieu-Lauragais | 32.2 | 24 | 21.6 | 24.2 |
| Montardon | 53.2 | 34.6 | 64.6 | 98 | |
A spore suspension (0.5 ml) containing 4 × 106 spores ml−1 was injected into the silk channel with a self-refilling syringe equipped with an obtuse needle. Inoculations were performed the same day for both varieties, at 4 and 7 days after silking, for V1 and V2, respectively. The first days after silk emission have been shown to be the most sensitive period for F. verticillioides infection through the silk channel (29). Silking was assigned when silk emergence could be observed in 50% of the maize ears. In each subplot, the primary ears of 15 plants of one row having the same stage were inoculated. At each sampling date, 10 inoculated ears were randomly handpicked and kernels from the first third of each ear were quickly removed (within less than 5 min) using a scalpel and placed in a container maintained in dry ice. Lepidopteran damage represents an infection pathway for F. verticillioides and could indirectly bias our results. Therefore, ears showing lepidopteran damage were discarded. Once collected, the container was immediately placed in a Cryo Express dry shipper (CX 500; Taylor-Wharton) before storage at −80°C in the laboratory. Kernels were then ground to a fine powder in liquid nitrogen, lyophilized, and stored at −20°C before fumonisin and fungal DNA quantification.
Fumonisin analysis.
After lyophilization (Flexi-Dry; Oerlikon Leybold, Germany), fumonisins were extracted as originally described by Shephard et al. (34) with some modifications. Briefly, 2 g of lyophilized maize powder was agitated with 8 ml of methanol-water (3/1 [vol/vol]) for 15 min. After centrifugation, filtrates were adjusted at pH 6.5 and fumonisins were purified using Bond Elut Strong anion exchange (SAX) cartridges (Varian, Palo Alto, CA). Fumonisins were eluted with 8 ml of methanol-acetic acid (99/1 [vol/vol]), which was evaporated to dryness under a nitrogen stream. Dried samples were dissolved in 200 μl of methanol. The fumonisin concentration was determined using a high-performance liquid chromatograph, Agilent 1100 series (Agilent Technologies, Santa Clara, CA), equipped with an Equisorb ODS2 column and a fluorescence detector (excitation λ at 335 nm and emission λ at 440 nm). Ten microliters of the samples were derivatized with 90 μl of o-phthaldialdehyde reagent, and 20 μl of this solution was injected into the high-performance liquid chromatography (HPLC) system within 1 min after derivatization. Quantification was performed using external calibration with FB1, FB2, and FB3 standard solutions, ranging from 1 to 100 μg ml−1. The sum of FB1, FB2, and FB3 was used to quantify the amount of fumonisins, and results were converted into μg g−1 of dry maize powder. Limits of fumonisin detection and quantification were 5 and 20 ng g−1 of dry maize flour, respectively.
DNA extraction and quantification of fungal biomass.
Total DNA was extracted from 100 mg of each sample using the DNeasy plant minikit according to the manufacturer's instructions (Qiagen, Courtaboeuf, France). Grinding was performed with the TissueLyser system (Qiagen-Retsch, Courtaboeuf, France), using one stainless-steel bead in an Eppendorf tube containing 400 μl of AP1 Buffer (DNeasy plant minikit) for 90 s at 30 Hz. After total DNA quantification using a Nanodrop spectrophotometer (Nanodrop Technology, Cambridge, United Kingdom), each DNA sample was diluted to a final concentration of 20 ng μl−1. Quantification was performed by quantitative PCR (qPCR) using a LightCycler real-time detector (Roche Applied Science, Meylan, France).
For each analyzed sample, DNA was separately amplified with primers designed to track fumonisin-producing fungi or maize DNA. One primer pair (forward sequence, 5′-GGATTGGCTTGATCTTCACAG-3′; reverse sequence, 5′-GAAGATGGCATTGATTGCCTC-3′) was designed to amplify a 352-bp fragment (Tm, 65°C) from the polyketide synthase gene FUM1 (GenBank accession number AF155773) of fumonisin-producing fungi. The primer pairs used to track maize DNA were designed to amplify a fragment of the maize actin gene (accession number J01238): forward sequence, 5′-TCCTGACACTGAAGTACCCGATTG-3′; reverse sequence, 5′-CGTTGTAGAAGGTGTGATGCCAGTT-3′. The corresponding melting temperature was 60°C, and the amplified fragment length was 95 bp.
For the amplification of FUM1 and maize actin genes, the reaction mix contained 5 mM MgCl2, 0.5 μM (each) primer, and LightCycler FastStart DNA Master SYBR green I (1×; Roche Applied Science, Meylan, France) or QuantiTect SYBR green (1×; Qiagen, Courtaboeuf, France). For each assay, 9 μl of reaction mix and 1 μl of total DNA were mixed and transferred into capillaries. For the amplification of the FUM1 gene, the amplification conditions consisted of a first denaturation step for 15 min at 95°C, followed by 45 cycles of 15 s of denaturation at 95°C, 25 s of annealing at 65°C, and a 30-s extension at 72°C. For the amplification of the maize actin gene, experiments were performed under the following conditions: a first denaturation step for 15 min at 95°C and then 45 cycles of 15 s of denaturation at 95°C, 25 s of annealing at 57°C, and 30 s of polymerization at 72°C. Assays for each type of primer were performed in triplicate, with differences between triplicate threshold cycle (CT) values mainly being <0.1. No-template controls were included in each run, and no amplification was observed.
Quantifications were determined using standard curves of F. verticillioides DNA extracted from pure cultures and maize DNA extracted from a noncontaminated maize sample. Each standard curve was generated by serial dilutions ranging from 50 to 5 × 10−3 ng μl−1. PCR efficiency always ranged from 95 to 100%, and r2 values between the DNA concentration and CT were superior to 0.98. The lack of nonspecific PCR amplification and dimer formation was checked by including a melting-curve analysis in each run. The melting curve was generated using the following profile: 0 s at 95°C, 15 s at melting temperatures plus 10°C, and 0 s at 95°C with 0.1°C/s.
Relative quantification of F. verticillioides DNA was expressed as a log10 fungal DNA to log10 maize DNA ratio.
pH analysis.
Maize powder (100 to 300 mg) was suspended in 2 ml of deionized water, and the pH was measured with a pH meter, Inolab pH 720 (WTW, Germany), equipped with a surface pH combination electrode (Schott Instruments, Germany).
Amylopectin content.
Starch content was measured using simultaneous spectrophotometric detection of amylose and amylopectin by multiwavelength analysis according to the method of Séne et al. (32). Briefly, lipids were eliminated from maize powder (70 mg) using two consecutive acetone extractions (2 ml, 70% [vol/vol]). Samples were then solubilized in 5 ml of dimethyl sulfoxide (DMSO) (85% [vol/vol]) under agitation for 6 h. The spectrophotometric determination of the samples was done in a cuvette containing 0.03 ml of the sample, 0.92 ml of sodium phosphate buffer (0.05 M; pH 7), and 0.05 ml of iodine reagent (2 g liter−1 in DMSO). The molar extinction coefficients were determined using external calibration with pure commercial amylose and amylopectin powders purchased from Sigma-Aldrich (St. Louis, MO). The molar extinction coefficients were close to those measured by Séne et al. (32). Starch is composed of both amylose (AM) and amylopectin (AP). The basic absorbance relationships is the following: AAM+AP = AAM + AAP, as described by Sené et al. (32). To calculate the amylopectin concentration in starch, the absorbance of amylose was therefore subtracted from the total absorbance of the starch samples using a multiwavelength analysis.
Statistical analysis.
Statistical analysis was performed using the SAS software program (SAS Institute, Inc.). A mixed model was used in which the varieties and the sampling dates were considered fixed effects, while the sowing date, the location, the years, and the replications were considered random effects. The quantitative variables, fumonisin, F. verticillioides DNA, and fumonisin productivity (i.e., fumonisin production per unit of fungal DNA), were log transformed as log10(fumonisin + 0.1), log10(F. verticillioides DNA)/log10(maize DNA), and log10[(fumonisin + 0.1)/F. verticillioides DNA], respectively, to ensure normal distributions of residues and homogeneity of variance. The level of significance was set at α = 0.05.
RESULTS
Fungal growth and fumonisin production dynamics.
F. verticillioides growth and fumonisin accumulation were determined at different sampling times after silk inoculation, using qPCR and HPLC-fluorimetry, respectively (Fig. 1 and 2).
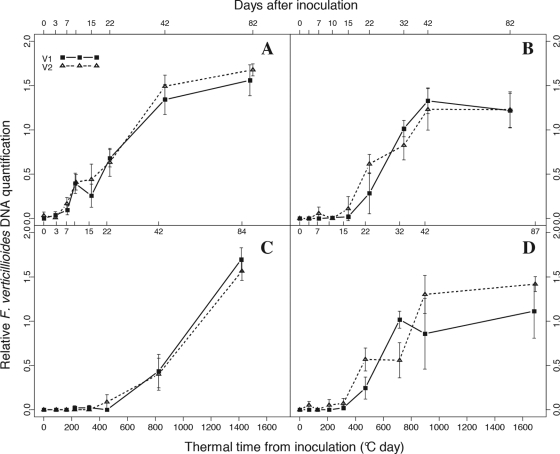
Fig. 1.
Relative quantification of fungal DNA in maize kernels, expressed as a log10 F. verticillioides DNA/log10 maize DNA ratio. Graphs A, B, C, and D correspond to samples collected in Laur08, in Laur09, in Mont08, and in Mont09, respectively. y values marked as 0 correspond to the background found below the quantification threshold (equal to −1.77). Vertical bars show standard deviations. Bottom x axis, thermal time from inoculation; top x axis, days after inoculation for each sampling. For each variety, data from the two sowing date treatments were pooled.
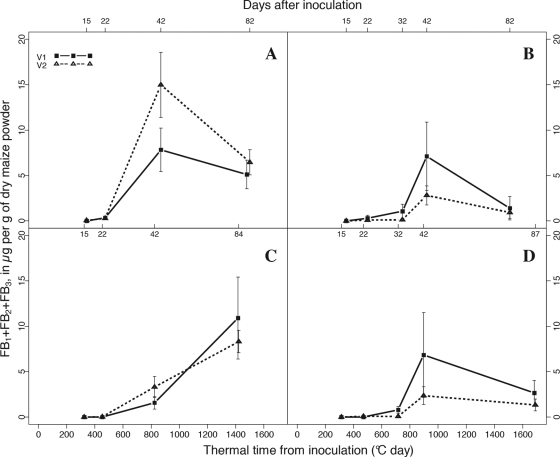
Fig. 2.
Levels of fumonisin accumulated in maize kernels after silk inoculation with F. verticillioides, sampled in Laur08, in Laur09, in Mont08, and in Mont09 (graphs A, B, C and D, respectively). Vertical bars show standard deviations. Bottom x axis, thermal time from inoculation; top x axis, days after inoculation for each sampling. For each variety, data from the two sowing date treatments were pooled.
Given that inoculations of maize ears sown at two different dates were always performed very close together, no significant differences in fumonisin accumulation or in fungal DNA contamination at any sampling time were observed between the two sowing date treatments, emphasizing the reliability of our data (data not shown). No significant differences in fumonisin content or in the levels of fungal DNA were observed between the V1 and V2 varieties (Fig. 1 and 2). Significant differences concerning fungal growth and fumonisin accumulation dynamics were nevertheless observed depending on the location and year.
First, the time of initial infection varied among location-per-year experiments. F. verticillioides reached a quantifiable level 7 days after inoculation (d7) in Laur08 (Fig. 1A), between d15 and d22 in Laur09 and Mont09 (Fig. 1B and D), and at d22 in Mont08 (Fig. 1C). The amount of F. verticillioides DNA steadily increased until d42, with the exception of Mont08, where it continued to increase until the last sampling. In the three other experiments, no significant differences were observed between the two last samplings, suggesting that F. verticillioides did not further colonize the maize ears after d42.
The first quantification of fumonisin occurred from d15 (15% of the samples collected at d15 were contaminated with fumonisin) to d22 (90%) in Laur08, from d22 (20%) to d32 (50%) in Laur09 and Mont09, and from d22 (10%) to d42 (100%) in Mont08 (data not shown). With the exception of Mont08, fumonisin levels reached a peak at approximately d42 (Fig. 2). Following this peak, there was an apparent decrease in the amount of fumonisin (Fig. 2), but this decrease was not significant (P = 0.0641, 0.0869, and 0.1806 for Laur08, Laur09, and Mont09, respectively). Moreover, it is worth noting that this observed decrease was reduced when calculations were corrected with the estimated moisture content of fresh collected kernels (results not shown). Note that the moisture content has not been measured in our study but is extrapolated based on the correlation between thermal time from silking and kernel moisture content established by Sala et al. (31) and Borrás et al. (5). In Mont08, fumonisin accumulation significantly increased from d42 until the last sampling, in agreement with the increased development of F. verticillioides after d42.
Fumonisin productivity.
Toxin productivity has been defined by Xu et al. (37) as “the toxin per unit of fungus.” Here, fumonisin productivity has been log10 transformed to ensure normal distributions of residues and homogeneity of variance. Even if fungal biomass and fumonisin continued to accumulate in maize kernels after d42 in Mont08, a significant increase in fumonisin productivity (P = 0.0278) was observed from d22 (1.26 ± 0.3) to d42 (1.96 ± 0.5), followed by a significant decrease (P = 0.0195) to maturity harvest (1.1 ± 0.3). A significant increase in fumonisin productivity was also observed in Laur08 (P = 0.0208) from d22 (0.89 ± 0.4) to d42 (1.38 ± 0.3), followed by a significant decrease (P = 0.0187) to maturity harvest (0.81 ± 0.3). In the two other experiments performed in 2009, there were no significant differences in fumonisin productivity between these three harvest dates. It can nevertheless be observed that the most significant increase in fumonisin accumulation occurred in a very short period of time, between d32 and d42, i.e., during the dent sage (Fig. 2B and D).
Variations in pH and amylopectin content.
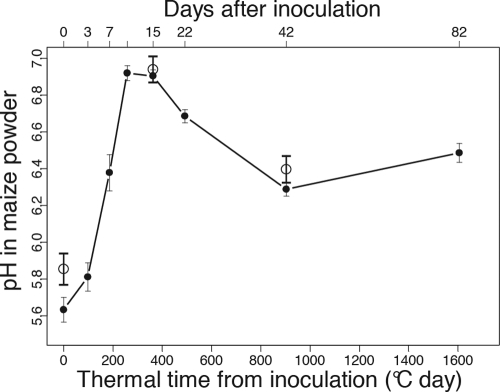
To test whether the amylopectin content and the kernel pH could interfere with fumonisin accumulation, variations in the kernel pH (Fig. 3) and in starch content (Fig. 4) were monitored for all the samplings in one sowing date treatment from Laur08. From d0 to d15, pH variation was first characterized by an increase from pH 5.6 and up to pH 6.9 (Fig. 3). Second, from the d15-to-d22 period to d42, acidification was observed, with a significant pH decrease of 0.6, with the pH then remaining stable until the last sampling (Fig. 3). No significant differences were found between varieties V1 and V2 (data not shown). pH was also measured on all the other samples but only at d0, d15, and d42, and the results suggest similar variations in kernel pH (Fig. 3). No significant differences were found between locations, suggesting that the soil pH did not influence the kernel pH. Additionally, similar pH variations were found in the control kernels compared to the findings for inoculated ones (Table 4).
Fig. 3.
pH values in maize kernels sampled in Laur08 in the first sowing date treatment and for both varieties after silk inoculation with F. verticillioides (filled circles). Vertical bars show standard deviations. The additional open circles show the mean pH ± standard deviations (n = 48) measured on three selected sampling dates (d0, d15, and d42) for all samples taken together, i.e., combined data for years, locations, sowing dates, and varieties.
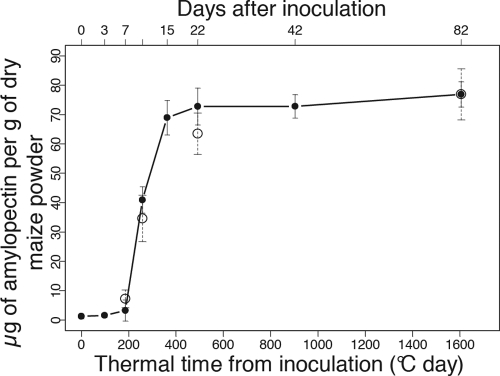
Fig. 4.
Amylopectin content in maize kernels sampled in Laur08 in the first sowing date treatment after silk inoculation with F. verticillioides (filled circles). Vertical bars show standard deviations. The additional open circles show the mean amylopectin content ± standard deviations (n = 24) measured on four selected sampling dates (d6, d10, d22, and at final harvest) for all samples from the first sowing date treatment taken together, i.e., combined data for years, locations, and varieties.
Table 4.
Comparisons of pH variations between inoculated and control samples
| Samples | Mean pH ± SDa |
pH variation |
|||
|---|---|---|---|---|---|
| d3 | d15 | d42 | d15-d3 | d15-d42 | |
| Inoculated | 5.89 ± 0.17 | 6.89 ± 0.09 | 6.32 ± 0.09 | 1 | 0.57 |
| Control | 5.97 ± 0.19 | 6.99 ± 0.18 | 6.46 ± 0.06 | 1.03 | 0.53 |
n = 9. pH values were determined for nine samples, corresponding to Montesquieu-Lauragais, sowing date 1 in 2008, for V1 and V2 and to Montesquieu-Lauragais, sowing date 2 in 2009, for V1.
Accumulation of amylopectin started in kernels from d6, with a maximum level at d15 to d22 (Fig. 4). From d15 to d22 to the final harvest, amylopectin continued to accumulate, but only slightly (Fig. 4). Amylopectin content was also analyzed in all the other samples but only at d6, d10, and d22 and at maturity harvest (dM) for the first sowing date treatment, confirming a similar evolution in amylopectin content (Fig. 4).
DISCUSSION
Initial stage of fungal development and fumonisin production.
In our experiments, F. verticillioides infection in kernels occurred between the blister stage (d7) and the dough stage (d22), depending on the location and year. These data are in accordance with previous reports that have described a first isolation of F. verticillioides from surface-sterilized kernels collected between 2 weeks after silking (17) and 4 to 5 weeks after pollination (defined as the milk stage by the authors) (6). Furthermore, in our experiments, initial contamination in fumonisin occurred between d15 and d42, showing that fumonisins were detected between 1 and 3 weeks after the initial detection of F. verticillioides (Fig. 2). Fumonisins could be quantified in most of our samples collected from d22 to d32, corresponding to the dough and early dent stages, respectively. In the study by Bush et al. (6), fumonisins did not appear before the dent stage. According to our results, fumonisin production can be initiated earlier, during the dough stage, which corresponds approximately to 60 to 70% of kernel moisture (Table 2). This result clearly illustrates the interest of using an accurate and sensitive method for fumonisin detection, which allows determining more precisely the initiation of in planta fumonisin production and therefore characterizing the substrate encountered by F. verticillioides at this crucial step.
The reasons for the delay in fungal infection and in fumonisin accumulation in Mont08 compared to findings in the three other experiments cannot be clearly explained but may be linked to different microenvironmental conditions during colonization of maize ears by F. verticillioides. Notably, in Mont08, the climatic conditions were characterized by rainier weather, especially during pollination time (i.e., in August), and colder weather over the growing season (Table 3), which are known as unfavorable climatic conditions for fusarium ear rot and fumonisin contamination (21, 25, 33, 35).
Enhancement of fumonisin production during the dent stage.
More interestingly, our results provide clear evidence that the kernel stage influences fumonisin biosynthesis in planta and under field conditions. Up to now, the potential involvement of components of the F. verticillioides-maize interaction in the regulation of fumonisin production was based only on in vitro experiments.
Our in planta observations provide a key confirmation from field data that the kernel environment in early stages is not appropriate for fumonisin production, as first suggested by Warfield and Gilchrist based on in vitro results (36). Kernels sampled in Laur08 at d10, d15, and d22 exhibited levels of fungal DNA that were not significantly different from those sampled in Mont08 at d42 (Fig. 1) (P = 0.0519, 0.1097, and 0.9718, respectively). However, the levels of fumonisin were significantly higher in the kernels sampled at d42 (dent stage) in Mont08 than in those sampled at d22 (dough stage) in Laur08 (P = 0.018), while almost no fumonisin was quantified at d10 and d15 (blister and early-milk stage) in Laur08 (Fig. 2). The absence of fumonisin in blister and early-milk stages and the lower levels of fumonisin in dough-stage kernels, relative to findings for the dent stage, can therefore not be accounted for by differences in fungal development. In vitro studies (3, 36) of detached kernels from different stages inoculated with F. verticillioides suggest that fumonisin production varies with kernel stages, with higher levels during dent stages and the lowest levels during the blister stage. Our results confirm and extend those findings to a more realistic range of conditions and establish that induction of fumonisin production is not always concomitant with fungal infection, in contrast to the first observations reported by Bush et al. (6), but also depends on the kernel stages at which fungal infection occurs. These results are also in agreement with the idea that fumonisin may not be required for disease development (7, 8) or at least for initial colonization of maize kernels.
In all our experiments, the most significant increase in fumonisin accumulation occurs between d22/d32 and d42, except in Mont08 (Fig. 2). However, in the latter experiment, even though fungal biomass and fumonisins continue to accumulate after d42, a significant peak in fumonisin productivity was observed at d42, suggesting that a stronger activation of fumonisin production occurred during the dent stage. Consequently, the four experiments led to a similar conclusion: fumonisin production was enhanced during the dent stage.
These results strongly support to the idea that physiological changes occurring during the dent stage may enhance fumonisin biosynthesis. Various physiological changes that may be involved in the regulation of fumonisin biosynthesis occur throughout maize kernel ripening. Field experiments always include a large variability which, to a large extent, cannot be controlled. In our experiments, however, a clear pattern emerged from this variability, in agreement with the findings of analytical in vitro studies suggesting that pH and amylopectin content variations seem to be associated with fumonisin regulation (26).
Role of amylopectin and pH variations in fumonisin regulation.
Multiple pieces of evidence, based on in vitro experiments, strongly suggest that amylopectin metabolism participates in fumonisin regulation during infection (3). At the molecular level, disruption of the gene FST1, a putative sugar transporter gene, has been demonstrated to induce repression of fumonisin production (4). In our field experiments, maize kernel development was characterized by a rapid accumulation of amylopectin during the first stages of kernel development from d6 to d15 (Fig. 4). The enhancement of fumonisin production appeared 3 weeks after completion of the kernel with amylopectin, suggesting that amylopectin is not a sufficient condition to favor fumonisin biosynthesis but that other mechanisms, such as pH variations, may be involved in fumonisin activation.
The PAC1 gene, a known regulator of pH, has been identified in F. verticillioides as a repressor of fumonisin biosynthesis under alkaline conditions (at pH 8.4) (10). It was later hypothesized that fumonisin repression in blister kernels may therefore be due to high-pH conditions (16). In our experiments, the kernel pH never reached alkaline conditions and was highest at d10 and d15 (pH 6.9 to 7) (Fig. 3). Each stage may therefore be potentially suitable for fumonisin production in terms of pH conditions. In other toxigenic species, including Aspergillus spp. (15) and F. graminearum (20), it has been demonstrated that even at neutral pH, PacC represses transcription of the toxin biosynthetic genes, inducing lower levels of toxin. Although it has not been demonstrated in F. verticillioides, it can be hypothesized that neutral-pH conditions may not be favorable to fumonisin production as well. In addition, it is noteworthy that the enhancement of fumonisin accumulation during the dent stage (from d22 to d42) was concomitant with a kernel acidification, observed in each location-per-year experiment. After inoculating F. verticillioides onto detached maize kernels, Bluhm and Woloshuk (3) showed that the highest level of fumonisin was reached on dent-stage kernels in which an acidification (pH variation of 1.5) was observed whereas blister kernels became alkalized and no fumonisin was produced. Our in vivo observations are therefore in agreement with their findings. Further in planta experiments are required, however, to verify the exact effect of kernel pH variations on fumonisin biosynthesis.
Our data revealed no significant differences in pH variations between inoculated and control ears with no visual symptoms or fungal DNA contamination (Table 4). This observation suggests that the fungal consumption of nitrogen and carbon sources during kernel ripening does not cause any pH variations, in contrast to what have been previously hypothesized (16). However, in our field experiments, the levels of fungal contamination may have been insufficient to trigger quantifiable pH variations. In addition, the method used here gives a global estimation of the kernel pH and not a specific value at the infection site.
Other physiological changes which have not been studied here, including the evolution of nitrogen sources and aw, may be involved in the regulation of fumonisin biosynthesis. First, it has been demonstrated that expression of AREA, a known regulator of nitrogen metabolism, induces a positive regulation of fumonisin biosynthesis (16). This gene may be activated when nitrogen sources become limited in the fungal environment to allow the utilization of other nitrogen sources. Amino acids and soluble nitrogen compounds reach their maximum levels around 30 days after pollination (i.e., during the early dent stage) before decreasing (13). Second, it has been shown that low aw values significantly increase FUM1 gene expression whereas fungal growth is reduced (14). During kernel ripening, the progressive decrease in water and nitrogen availability may therefore represent other physiological changes enhancing fumonisin biosynthesis.
In conclusion, our field results clearly support the previous hypothesis, based on in vitro experiments, that the physiological stages of maize kernel play a major role in regulating fumonisin production. In addition, our results underline that the passage from dough- to dent-stage kernels seems to represent a transition in signaling metabolites, which is perceived by the fungus and which, in turn, ultimately increases fumonisin production. Among the various physiological changes occurring during maize ear development, the changes in amylopectin and pH in kernels seem to be related to fumonisin production. It would be of interest to formally validate this relationship. A more detailed analysis of key stages in resistant varieties compared to susceptible ones may allow the identification of maize kernel components that may be potential inhibitors of fumonisin biosynthesis. This would be of particular interest for selecting maize varieties resistant to fumonisin accumulation.
ACKNOWLEDGMENTS
We are grateful to ARVALIS-Institut du Végétal and the ANRT (National Association for Research and Technology) for their financial support as part of a Ph.D. grant.
We thank Alain Boué-Laplace, Florian Gays, Gilles Marque, Sonia Elbelt, and Geania Gambarra for their help in the field experiments and Stephane François and Audrey Carrere for their help in the lab experiments.
Footnotes
Published ahead of print on 7 October 2011.
REFERENCES
- 1. ARVALIS Infos 2007. Qualité sanitaire du maïs grain: mobiliser tous les leviers pour preserver la qualité sanitaire. http://www.arvalisinstitutduvegetal.fr/fr/arvalis_infos/AI_mais_06I28/O6I28p17_20.pdf
- 2. Bartók T., Szecsi A., Szekeres A., Mesteráhzy A., Bartók M. 2006. Detection of new fumonisin mycotoxins and fumonisin-like compounds by reversed-phase high-performance liquid chromatography/electrospray ionization ion trap mass spectrometry. Rapid Commun. Mass Spectrom. 20: 2447–2462 [DOI] [PubMed] [Google Scholar]
- 3. Bluhm B. H., Woloshuk C. P. 2005. Amylopectin induces fumonisin B1 production by Fusarium verticillioides during colonization of maize kernels. Mol. Plant Microbe Interact. 18: 1333–1339 [DOI] [PubMed] [Google Scholar]
- 4. Bluhm B. H., Kim H., Butchko R. A., Woloshuk C. P. 2008. Involvement of ZFR1 of Fusarium verticillioides in kernel colonization and the regulation of FST1, a putative sugar transporter gene required for fumonisin biosynthesis on maize kernels. Mol. Plant Pathol. 9: 203–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Borrás L., Westgate M. E., Otegui M. E. 2003. Control of kernel weight and kernel water relations by post-flowering source-sink ratio in maize. Ann. Bot. 91: 857–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bush B. J., Carson M. L., Cubeta M. A., Hagler W. M., Payne G. A. 2004. Infection and fumonisin production by Fusarium verticillioides in developing maize kernels. Phytopathology 94: 88–93 [DOI] [PubMed] [Google Scholar]
- 7. Desjardins A., Plattner R. D. 1998. Distribution of fumonisins in maize ears infected with strains of Fusarium moniliforme that differ in fumonisin production. Plant Dis. 82: 953–958 [DOI] [PubMed] [Google Scholar]
- 8. Desjardins A. E., Munkvold G. P., Plattner R. D., Proctor R. H. 2002. FUM1—a gene required for fumonisin biosynthesis but not for maize ear rot and ear infection by Gibberella moniliformis in field tests. Mol. Plant Microbe Interact. 15: 1157–1164 [DOI] [PubMed] [Google Scholar]
- 9. Duvick J. 2001. Prospects for reducing fumonisin contamination of maize through genetic modification. Environ. Health Perspect. 109(Suppl. 2): 337–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Flaherty J. E., Pirttilä A. M., Bluhm B. H., Woloshuk C. P. 2003. PAC1, a pH-regulatory gene from Fusarium verticillioides. Appl. Environ. Microbiol. 69: 5222–5227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Humpf H. U., Voss K. A. 2004. Effects of thermal time food processing on the chemical structure and toxicity of fumonisin mycotoxins. Mol. Nutr. Food Res. 48: 225–269 [DOI] [PubMed] [Google Scholar]
- 12. IARC 2002. Fumonisin B1, p. 275–366 In IARC monographs on the evaluation of carcinogenic risk to humans, vol. 82 Some traditional herbal medicines, some mycotoxins, naphthalene and styrene International Agency for Research on Cancer, Lyons, France [Google Scholar]
- 13. Ingle J., Beitz D., Hageman R. H. 1965. Changes in composition during development and maturation of maize seeds. Plant Physiol. 40: 835–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jurado M., Marín P., Magan N., González-Jaén M. T. 2008. Relationship between solute and matric potential stress, temperature, growth, and FUM1 gene expression in two Fusarium verticillioides strains from Spain. Appl. Environ. Microbiol. 74: 2032–2036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Keller N. P., Nesbitt C., Sarr B., Phillips T. D., Burow G. B. 1997. pH regulation of sterigmatocystin and aflatoxin biosynthesis in Aspergillus spp. Phytopathology 87: 643–648 [DOI] [PubMed] [Google Scholar]
- 16. Kim H., Woloshuk C. P. 2008. Role of AREA, a regulator of nitrogen metabolism, during colonization of maize kernels and fumonisin biosynthesis in Fusarium verticillioides. Fungal Genet. Biol. 45: 947–953 [DOI] [PubMed] [Google Scholar]
- 17. King S. B. 1981. Time of infection of maize kernels by Fusarium moniliforme and Cephalosporium acremonium. Phytopathology 71: 796–799 [Google Scholar]
- 18. Lovell D. J., Powers S. J., Welham S. J., Parker S. R. 2004. A perspective on the measurement of time in plant disease epidemiology. Plant Pathol. 53: 705–712 [Google Scholar]
- 19. Marín S., et al. 1999. Fumonisin B1 production and growth of Fusarium moniliforme and Fusarium proliferatum on maize, wheat, and barley grain. J. Food Sci. 64: 921–924 [Google Scholar]
- 20. Merhej J., Richard-Forget F., Barreau C. 2011. The pH regulatory factor PacI regulates Tri gene expression and trichothecene production in Fusarium graminearum. Fungal Genet. Biol. 48: 275–284 [DOI] [PubMed] [Google Scholar]
- 21. Miller J. D., Savard M. E., Schaafsma A. W., Seifert K. A., Reid L. M. 1995. Mycotoxin production by Fusarium moniliforme and Fusarium proliferatum from Ontario and occurrence of fumonisin in the 1993 corn crop. Can. J. Plant Pathol. 17: 233–239 [Google Scholar]
- 22. Munkvold G. P., Desjardins A. E. 1997. Fumonisin: can we reduce their occurrence? Plant Dis. 81: 556–565 [DOI] [PubMed] [Google Scholar]
- 23. Munkvold G. P., Hellmich R. L., Rice L. G. 1999. Comparison of fumonisin concentrations in kernels of transgenic Bt maize hybrids and non-transgenic hybrids. Plant Dis. 83: 130–138 [DOI] [PubMed] [Google Scholar]
- 24. Nielsen R. L. B. 2001. Grain fill stages in corn. Department of Agronomy, Purdue University, West Lafayette, IN: http://www.kingcorn.org/news/timeless/GrainFill.html [Google Scholar]
- 25. Pascale M., Visconti A., Chelkowski J. 2002. Ear rot susceptibility and mycotoxin contamination of maize hybrids inoculated with Fusarium species under field conditions. Eur. J. Plant Pathol. 108: 645–651 [Google Scholar]
- 26. Picot A., et al. 2010. Factors of the Fusarium verticillioides-maize interaction modulating fumonisin production. Crit. Rev. Microbiol. 36: 221–231 [DOI] [PubMed] [Google Scholar]
- 27. Proctor R. H., Brown D. W., Plattner R. D., Desjardins A. E. 2003. Co-expression of 15 contiguous genes delineates a fumonisin biosynthetic gene cluster in Gibberella moniliformis. Fungal Genet. Biol. 38: 237–249 [DOI] [PubMed] [Google Scholar]
- 28. Reid L. M., et al. 1999. Interaction of Fusarium graminearum and Fusarium moniliforme in maize ears: disease progress, fungal biomass, and mycotoxin accumulation. Phytopathology 89: 1028–1037 [DOI] [PubMed] [Google Scholar]
- 29. Reid L. M., Woldemariam T., Zhu X., Stewart D. W., Schaafsma A. W. 2002. Effect of inoculation time and point of entry on disease severity in Fusarium graminearum, Fusarium verticillioides, or Fusarium subglutinans inoculated maize ears. Can. J. Plant Pathol. 24: 162–167 [Google Scholar]
- 30. Reid L. M., Zhu X. 2005. Screening corn for resistance to common diseases in Canada. Technical bulletin 2005-E. Agriculture and Agri-Food Canada, Ottawa, Canada [Google Scholar]
- 31. Sala R. G., Andrade F. H., Wesgate M. E. 2007. Maize kernel moisture at physiological maturity as affected by the source-sink relationship during grain filling. Crop Sci. 47: 711–716 [Google Scholar]
- 32. Séne M., Thévenot C., Prioul J. L. 1997. Simultaneous spectrophotometric determination of amylose and amylopectin in starch from maize kernel by multi-wavelength analysis. J. Cereal Sci. 26: 211–221 [Google Scholar]
- 33. Shelby R., White D. G., Burke E. M. 1994. Differential fumonisin production in maize hybrids. Plant Dis. 78: 582–584 [Google Scholar]
- 34. Shephard G. S., Sydenham E. W., Thiel P. G., Gelderblom W. C. 1990. Quantitative determination of fumonisins B1 and B2 by high-performance liquid chromatography with fluorescence detection. J. Liq. Chromatogr. 13: 2077–2080 [Google Scholar]
- 35. Vigier B., Reid L. M., Seifert K. A., Stewart D. W., Hamilton R. I. 1997. Distribution and prediction of Fusarium species associated with maize ear rot in Ontario. Can. J. Plant Pathol. 19: 60–65 [Google Scholar]
- 36. Warfield C. Y., Gilchrist D. G. 1999. Influence of kernel age on fumonisin B1 production in maize by Fusarium moniliforme. Appl. Environ. Microbiol. 65: 2853–2856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Xu X., Nicholson P., Ritieni A. 2007. Effects of fungal interactions among Fusarium head blight pathogens on disease development and mycotoxin accumulation. Int. J. Food Microbiol. 119: 67–71 [DOI] [PubMed] [Google Scholar]