Abstract
Pyritic mine tailings (mineral waste generated by metal mining) pose significant risk to the environment as point sources of acidic, metal-rich effluents (acid mine drainage [AMD]). While the accelerated oxidative dissolution of pyrite and other sulfide minerals in tailings by acidophilic chemolithotrophic prokaryotes has been widely reported, other acidophiles (heterotrophic bacteria that catalyze the dissimilatory reduction of iron and sulfur) can reverse the reactions involved in AMD genesis, and these have been implicated in the “natural attenuation” of mine waters. We have investigated whether by manipulating microbial communities in tailings (inoculating with iron- and sulfur-reducing acidophilic bacteria and phototrophic acidophilic microalgae) it is possible to mitigate the impact of the acid-generating and metal-mobilizing chemolithotrophic prokaryotes that are indigenous to tailing deposits. Sixty tailings mesocosms were set up, using five different microbial inoculation variants, and analyzed at regular intervals for changes in physicochemical and microbiological parameters for up to 1 year. Differences between treatment protocols were most apparent between tailings that had been inoculated with acidophilic algae in addition to aerobic and anaerobic heterotrophic bacteria and those that had been inoculated with only pyrite-oxidizing chemolithotrophs; these differences included higher pH values, lower redox potentials, and smaller concentrations of soluble copper and zinc. The results suggest that empirical ecological engineering of tailing lagoons to promote the growth and activities of iron- and sulfate-reducing bacteria could minimize their risk of AMD production and that the heterotrophic populations could be sustained by facilitating the growth of microalgae to provide continuous inputs of organic carbon.
INTRODUCTION
Mining of metals has been integral to the development of human civilization. As higher-grade ores become depleted, the primary ores that are processed by mining companies are increasingly of lower grade (metal content) and the amount of waste material produced by mining operations is consequently greater. In many cases, metal ores are crushed and ground, and the target metal minerals are concentrated by a process known as froth flotation (9). This involves the addition of chemicals (“collectors”) to ground ore suspensions which attach to the target minerals, causing their surfaces to become hydrophobic. Controlled aeration of the treated suspension allows air bubbles to attach to the modified minerals, causing them to float and facilitating their separation from hydrophilic minerals which settle or remain in suspension. Other chemicals, such as lime, can enhance the separation of target and nontarget minerals. The fine-grain mineral waste which results from froth flotation is referred to as “tailings,” and in copper mining, these can account for 95 to 99% of the crushed and ground ores (9).
Many base metal ores are sulfidic, and they frequently contain large concentrations of the most abundant of all metal sulfides, pyrite (FeS2), which, with other nontarget minerals, ends up mostly in the tailing wastes. Pyrite and other sulfide minerals are potentially highly reactive minerals. In the presence of both air (oxygen) and water, pyrite oxidizes ultimately to sulfate and ferric iron with the concomitant production of proton acidity, as illustrated in the following equation, where the oxidized iron mineral phase generated is shown as schwertmannite (a commonly encountered ferric iron mineral in ferruginous waters with pH values of ∼3 to 5): 8 FeS2 + 30 O2 + 18 H2O → Fe8O8(SO4)(OH)6 + 15 SO42− + 30 H+. Pyritic mine tailings therefore have the potential to become extremely acidic and enriched with soluble sulfate and, because of their greater solubilities in acidic liquors, transition metals and aluminum. Surface waters percolating through tailings and other mine wastes (low-grade waste rocks etc.) can transfer these potentially noxious solutes to the wider environment, where they can have a severe detrimental impact on affected stream and river ecosystems as “acid mine drainage” (AMD) (20). The mechanisms by which the oxidation of pyrite and other sulfide minerals occurs have been the subject of a considerable body of research (reviewed in reference 23). Acidophilic iron-oxidizing prokaryotes can accelerate the rate of pyrite oxidation in acidic liquors by several orders of magnitude, and therefore these microorganisms are perceived to have a crucial role in generating AMD (14, 24).
To reduce the risk of potentially reactive mine tailings generating acidic, metal-rich effluents, they are generally stored under water to minimize their exposure to oxygen. However, this is only a partial solution to the problem, as oxygen diffusion will facilitate ferrous iron oxidation in the tailings/water surface zones, and migration of the ferric iron produced to lower zones can cause oxidation of pyrite and other sulfide minerals even in anoxic zones. In situations where tailing impoundments are allowed to drain, distinct zonation has been reported, with a surface “oxidation zone” overlying (in sequence) a “neutralization zone” and an unaltered “primary zone.” Mineral-oxidizing and acidophilic heterotrophic bacteria have been found in largest numbers in the “oxidation front,” the junction between the oxidation and neutralization zones (7).
Besides their importance in AMD genesis, microorganisms can also play a role in mitigating the environmental effects of mine water pollution. There have been several reports of natural attenuation of AMD in which microbially catalyzed changes in water chemistry have caused streams to become less toxic as they flow from their points of discharge (see, e.g., references 3, 21, and 22). Dissimilatory microbial processes, such as iron oxidation (inducing precipitation of ferric iron minerals) and sulfate reduction, have been highlighted as contributing to the mitigation of acidic, metal-rich water bodies. Dissimilatory reduction of ferric iron can be either a proton-generating reaction, in the case of soluble ferric iron, or a proton-consuming reaction, in the case of amorphous and crystalline ferric minerals as illustrated in the following equation, where schwertmannite is shown as the mineral phase subjected to bacterial reductive dissolution and CH2O represents a generic organic electron donor: Fe8O8(SO4)(OH)6 + 2 CH2O + 2 H2O → 8 Fe2+ + 14 OH− + SO42− + 2 CO2. The ferrous iron solubilized will, however, reoxidize either in situ or downstream unless it is otherwise immobilized (e.g., as a sulfide mineral), and the ferric iron produced will hydrolyze, with concomitant net production of protons. Bacterial iron reduction does, however, have a role in mitigating AMD production at its source, since it removes, at least in part, the chemical (ferric iron) which is the prime oxidant of sulfide minerals at low pH.
Dissimilatory sulfate reduction at low pH is a proton-consuming process, as illustrated in the following equation: SO42− + 2 CH2O + 2 H+ → H2S + 2 CO2 + 2 H2O. The hydrogen sulfide produced reacts with a variety of metals, generating highly insoluble metal sulfides, and the selective immobilization of different transition metals by acidophilic sulfate-reducing bacteria (SRB) has been demonstrated (19).
Given the conflicting roles of acidophilic microorganisms in generating or mitigating AMD pollution, we have sought to test the hypothesis that by promoting the growth and activities of acidophilic communities that can counter the processes of acid generation and metal mobilization, it would be possible to mitigate the potential environmental impact of reactive mine tailings.
MATERIALS AND METHODS
Mine tailings.
Pyritic tailings were obtained from the Aguablanca nickel-copper mine, located in southeast Spain. This mining operation produces a nickel-copper concentrate from a sulfidic ore body by conventional crushing, grinding, and froth flotation. The waste ground ore is stored underwater at the mine site in a tailings lagoon. Tailings obtained from the mine were ground, washed with 3 M sulfuric acid to remove residual alkalinity, rinsed repeatedly with reverse-osmosis (RO)-grade water, and dried at 50°C. The pyrite content of the tailings was determined using the method described by Dacey and Colbourn (5). Mineralogical analysis was carried by X-ray diffraction (XRD) using a Philips PW3040/60 X′ Pert PRO and data analyzed using the PANalytical search-match program “Highscore.”
Microorganisms.
Several cultures of acidophilic bacteria and algae, sourced from the acidophile culture collection maintained at Bangor University, were used to inoculate the tailing mesocosms, as described below. These were as follows: (i) the iron- and pyrite-oxidizing autotrophic bacteria Acidithiobacillus ferrooxidansT (ATCC 23270), Acidithiobacillus ferrivoransT (strain NO37) (12) and Leptospirillum ferrooxidans strain CF12 (13) (in addition to being able to couple the oxidation of ferrous iron and reduced sulfur to the reduction of molecular oxygen, both Acidithiobacillus spp. are able to catalyze the dissimilatory reduction of ferric iron in anaerobic environments); (ii) the iron-reducing heterotrophic bacteria Acidiphilium strain SJH, Acidocella strain PFBC, and Acidobacterium strain Thars1 (2, 4, 21); (iii) an acidophilic SRB consortium taken from two laboratory bioreactors used to selectively remove transition metals from mine waters at low pH (2–4, 19) (acidophilic SRB identified in this consortium included Desulfosporosinus strain M1, “Desulfobacillus” strain CL4, and Desulfitobacterium strain CEB3); and (iv) two pure cultures of unicellular algae (Chlorella protothecoides var. acidicola and Euglena mutabilis (I. N̆ancucheo and D. B. Johnson, unpublished data). The acidophilic microorganisms were all grown in appropriate liquid media (15, 26). These were (i) 1% (wt/vol) pyrite-basal salts (pH 2.0) for the iron oxidizers, (ii) 5 mM fructose-0.02% (wt/vol) yeast extract (pH 3.5) for the iron-reducing heterotrophs, (iii) 3 mM glycerol-0.01% (wt/vol) yeast extract (pH 3.0) for the bioreactor SRB consortium, and (iv) basal salts-trace elements supplemented with 100 μM ferrous sulfate (pH 2.5) for the acidophilic algae. Each microorganism (except the anaerobic consortium) was grown as a separate pure culture, and combined inocula of each physiological group (iron-oxidizing autotrophs, iron-reducing heterotrophs, sulfate reducers, and algae) were used to inoculate the mesocosms, as described below.
Mesocosm setup.
Stainless steel cylinders (5 cm high, 5 cm in diameter; 100-cm3 capacity) designed for sampling soil cores (Eikelkamp Agrisearch Equipment bv, The Netherlands) were filled with 100 g of dry, acid-washed tailings. Plastic covers were pushed onto the base of each cylinder, and silicon sealant was used to prevent water seepage. The amount of water required to produce 100% saturation of the tailings mesocosms was determined. Subsequently, sterile RO-grade water (with or without a bacterial/algal inoculum) was added initially to produce 75% saturation of each mesocosm. At 5 weeks into the experiment it was noted that this amount of water was insufficient to allow adequate growth of the acidophilic algae on the tailing surface, and therefore additional water was added to bring the tailings mesocosms to 95% saturation.
Five tailings mesocosm variants were set up. These were as follows: (i) treatment I (TI) control mesocosms, to which only water was added; (ii) treatment II (TII) mesocosms, which were inoculated with iron-oxidizing autotrophs (∼7.4 × 108 cells/mesocosm; similar numbers of At. ferrooxidans, At. ferrivorans, and L. ferrooxidans); treatment III (TIII) mesocosms, which were the same as TII but also inoculated with iron-reducing heterotrophs (∼1.6 ×109 cells/mesocosm; similar numbers of Acidiphilium, Acidocella, and Acidobacterium); treatment IV (TIV) mesocosms, which were the same as TIII but also inoculated with SRB bioreactor liquor (∼3.3 × 108 cells/mesocosm); and treatment V (TV) mesocosms, which were the same as TIV but also inoculated with acidophilic algae (∼4.1 ×108 cells/mesocosm). All bacterial inocula were included with the bulk water added to the tailings, but the algae were added after this, in an attempt to concentrate the phototrophs at the surfaces of the mesocosms.
A total of 60 tailing mesocosms were prepared (12 for each treatment). These were weighed individually, covered loosely with sterile plastic petri plate lids, and placed in a plant growth room, which was maintained at 22°C and illuminated with 70 μmol of photons m−2 s−1. Each mesocosm was reweighed on a weekly basis and water loss corrected for by adding the equivalent amount of RO water.
Sampling of mesocosms and analysis of tailings.
The experiment was set up during March 2010, and samples were removed for analysis at 3-month intervals until April 2011. On each sampling occasion, three mesocosms for each treatment variant were removed and sampled destructively. The tailings cores were removed carefully from their stainless steel containers, sectioned with a knife, and inspected visually for signs of sulfide mineral oxidation (presence of rust-colored ferric iron precipitates) and sulfate reduction (evolution of hydrogen sulfide following addition of hydrochloric acid to tailings samples). Vertical sections (∼10 g) from each core were removed and mixed to give a homogeneous representative tailings sample, and subsamples of this were analyzed for physicochemical and microbiological parameters, as described below. Samples removed from the surfaces of alga-inoculated mesocosms were examined with a phase-contrast microscope (Leitz Labolux; magnification, ×400).
Physicochemical analysis.
One gram (dry weight equivalent) of homogenized tailings sample was mixed with 2.5 ml of RO water, vortexed, and then left to settle at room temperature for 30 min. The pH and redox potential (Eh values, relative to a standard hydrogen reference cell) of the suspension were measured using a pHase combination glass electrode and a platinum combination redox electrode (VWR, United Kingdom), both coupled to an Accumet pH/redox meter. Concentrations of total soluble iron, ferrous iron, copper, zinc, nickel, and sulfate were also determined in 2.5:1 water extracts of homogenized tailings filtered through 0.2-μm-pore-size nitrocellulose membranes. Acid (5 M HCl)-extractable iron in tailing samples was determined using the method described by Dopson and Lindstrom (10). Metal concentrations in water extracts and acid extracts were determined using a Dionex-320 ion chromatograph fitted with an IonPAC CS5A column and an AD absorbance detector. Sulfate concentrations in water extracts were measured using a Dionex IC25 ion chromatograph with an Ion Pac AS-11 column equipped with a conductivity detector. Concentrations of ferrous iron were determined using the ferrozine assay (18).
Microbiological and molecular analyses.
To enumerate aerobic iron-oxidizing and heterotrophic bacteria, 1 g of a homogenized tailings sample from each mesocosm was added to 2.5 ml of basal salts solution (pH 3.5) and mixed by vortexing. The samples were serially diluted and spread onto ferrous iron overlay solid medium (15) to enumerate iron-oxidizing autotrophic bacteria and onto 5 mM fructose-0.02% (wt/vol) yeast extract solid medium (pH 3.5) to enumerate heterotrophic iron-reducing heterotrophic bacteria. These plates were incubated aerobically at 30°C for 20 days, and CFU were counted. To enumerate anaerobic acidophiles, tailings suspensions (prepared as described above) were serially diluted and spread onto an overlay medium (pH ∼3.7) containing 4 mM glycerol, 0.02% (wt/vol) yeast extract, 7 mM zinc, and 0.5 mM ferrous iron (19). Plates were incubated anaerobically (using the AnaeroGen system [Oxoid, United Kingdom]) at 30°C for 30 days and numbers of CFU recorded.
Bacterial colonies were enumerated and physiological groups identified from colony morphologies, as described elsewhere (15). In order to confirm the identities of isolates (and to identify colonies corresponding to bacteria that were not present in the inocula), colonies were picked off plates, resuspended in basal salts, and DNA extracted and 16S rRNA genes were amplified, sequenced, and compared to those in public databases, as described previously (21).
Dissimilatory ferric iron reduction.
Dissimilatory reduction of ferric iron by a Curtobacterium sp. isolated from the tailings was tested in anaerobic liquid medium. Duplicate universal bottles containing 100 mg of amorphous ferric hydroxide (2) were filled with a liquid medium containing 5 mM glucose and 0.02% (wt/vol) yeast extract (adjusted to pH 4.0 with dilute sulfuric acid) and inoculated with an aerobically grown culture of the isolate. The bottles, together with a noninoculated control, were incubated at 30°C and sampled at regular intervals to determine concentrations of ferrous iron (using the ferrozine assay) and cell numbers (using a Thoma counting chamber). The pH values of inoculated and noninoculated media were recorded at the end of the experiment.
Statistical analysis.
Data were analyzed using SPSS version 17.0 (SPSS, Chicago, IL). One-way analysis of variance (ANOVA) was carried out to compare means for different treatments, and pairwise comparisons were done using the Tukey test at a 0.05 significance level.
Nucleotide sequence accession numbers.
The gene sequences of the At. ferrooxidans, C. ammoniagenes, and Alicyclobacillus isolates identified in this study have been deposited in GenBank and assigned accession numbers JN224813, JN224814, and JN224815, respectively.
RESULTS
Chemistry and mineralogy of the tailing material.
Chemical analysis of the tailings from the Aguablanca mine showed that it had an equivalent pyrite (FeS2) content of 6.5% (by weight). XRD analysis confirmed that silicates were the dominant minerals in the tailings. Peaks corresponding to pyrite and arsenopyrite, but not to any other sulfide minerals, were apparent. Acid washing was required to remove residual alkalinity in the fresh tailings. Lime is often added to suppress pyrite flotation during the production of metal concentrates, but it tends to be readily washed out of tailing impoundments and has only a short-term effect on pH buffering of pyritic wastes (9). Analysis of the acid-washed tailings showed that they contained very small concentrations of soluble iron (∼1 μg/g dry tailings) and that concentrations of soluble copper, zinc, and nickel were below detectable levels. The pH of the tailings slurry (1:2.5 water suspension) was 2.5 following acid treatment, and the grain size was between 61 and 200 μm.
Physicochemical changes in incubated mesocosms.
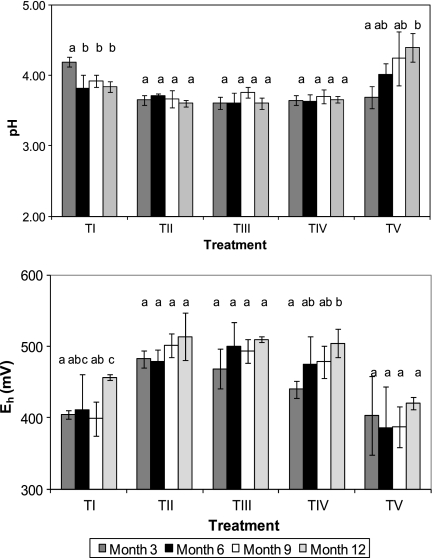
Time course changes in both physicochemical and microbiological properties of the tailings mesocosms, on a treatment-by-treatment basis, are shown in Fig. 1 to 6. Three months after the start of the experiment, the pH values of all of the inoculated mesocosms were significantly lower than those of the noninoculated controls (Fig. 1, top). During the course of the experiment, the pH of the noninoculated tailings declined a little, though it tended to remain slightly higher than those of the inoculated mesocosms. The exception to this was the tailings that had been inoculated with the acidophilic algae, where mean pH values increased progressively during the course of the experiment (Fig. 1, top) and were significantly higher by month 12 than in all other mesocosms. Redox potentials were more positive in most of the inoculated mesocosms than in control (noninoculated) mesocosms after 3 months and tended to remain so, though there was a notable increase in the noninoculated tailings by month 12 (Fig. 1, bottom). Again, the alga-inoculated tailings did not follow this trend. Eh values tended to remain at around +400 mV throughout (Fig. 1, bottom) and were significantly less positive on all sampling occasions (except at month 6) than in all other inoculated mesocosms.
Fig. 1.
Time course changes in pH values (top) and redox potentials (bottom) in mine tailings mesocosms inoculated with different populations of acidophilic microorganisms. TI, no inoculum (control); TII, TI plus Fe(II) oxidizers; TIII, TII plus Fe(III) reducers; TIV, TIII plus SRB; TV, TIV plus algae. For each treatment data set, bars not annotated with the same letter are significantly different (95% confidence limit). Error bars represent standard deviations.
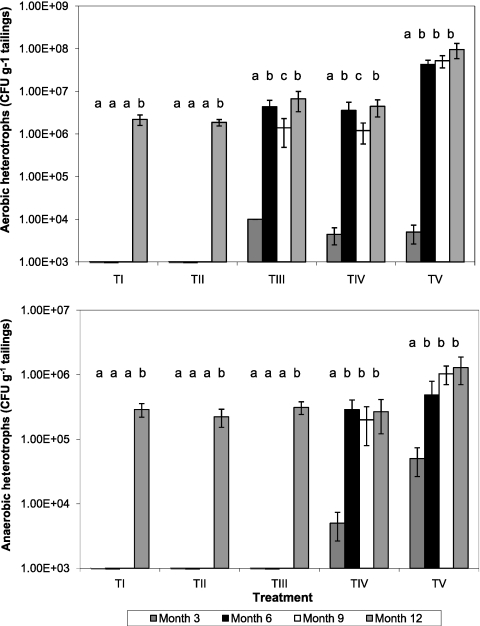
Fig. 6.
Time course changes in CFU of aerobic (top) and anaerobic (bottom) heterotrophic acidophiles in mine tailings inoculated with different populations of acidophilic microorganisms. TI, no inoculum (control); TII, TI plus Fe(II) oxidizers; TIII, TII plus Fe(III) reducers; TIV, TIII plus SRB; TV, TIV plus algae. For each treatment data set, bars not annotated with the same letter are significantly different (95% confidence limit). Error bars represent standard deviations.
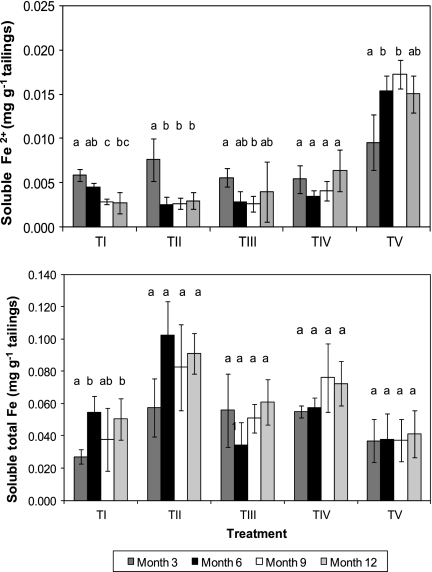
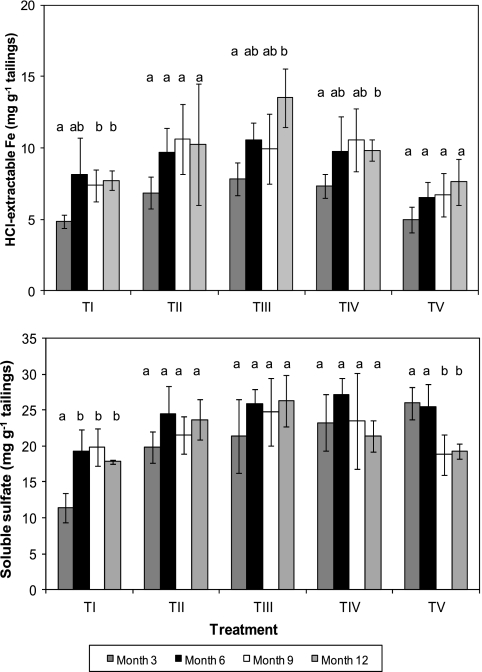
Concentrations of soluble ferrous iron tended to decline with time in both control and inoculated mesocosms, with the exception of those that were inoculated with acidophilic algae, where they increased significantly from month 3 to month 6 (Fig. 2, top). Comparison of different treatments on each sampling time showed that while there were no significant differences at month 3, ferrous iron contents were significantly greater in alga-inoculated tailings on all subsequent sampling occasions. In contrast, total soluble iron tended to be greatest in tailings mesocosms that had been inoculated only with pyrite-oxidizing bacteria (Fig. 2, bottom), though differences were not always significant. Acid-extractable iron, which would have included secondary ferric iron precipitates as well as soluble iron (10), showed a general increase with time in all tailings mesocosms, but differences were often not significant (Fig. 3, top). Visual inspection of sectioned cores showed that secondary ferric iron precipitates tended to accumulate in a distinct layer below the surface algal growths in TV mesocosms but were more randomly distributed in other mesocosms (see Fig. S1 in the supplemental material). It was also noted that hydrogen sulfide was released from acidified TV tailing mesocosms on month 12, though no odor was detected for other treatments on any sampling occasion. Concentrations of soluble sulfate decreased from month 6 in tailings mesocosms that had been inoculated with the mixed culture of SRB but did so significantly only in those tailings that had also been inoculated with algae (Fig. 3, bottom). The greater sulfate concentration in inoculated compared to control mesocosms at month 3 was probably due to sulfate added with the inoculating liquors.
Fig. 2.
Time course changes in ferrous iron (top) and total iron (bottom) in water extracts of mine tailings inoculated with different populations of acidophilic microorganisms. TI, no inoculum (control); TII, TI plus Fe(II) oxidizers; TIII, TII plus Fe(III) reducers; TIV, TIII plus SRB; TV, TIV plus algae. For each treatment data set, bars not annotated with the same letter are significantly different (95% confidence limit). Error bars represent standard deviations.
Fig. 3.
Time course changes in concentrations of HCl-extractable iron (top) and soluble sulfate (bottom) in mine tailings inoculated with different populations of acidophilic microorganisms. TI, no inoculum (control); TII, TI plus Fe(II) oxidizers; TIII, TII plus Fe(III) reducers; TIV, TIII plus SRB; TV, TIV plus algae. For each treatment data set, bars not annotated with the same letter are significantly different (95% confidence limit). Error bars represent standard deviations.
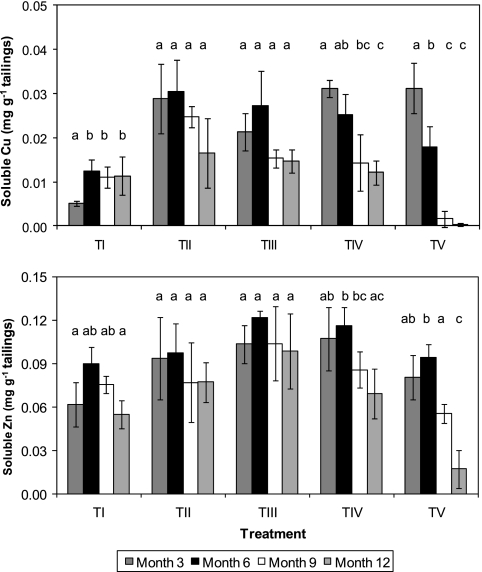
Several transition metals, in addition to iron, were detected in water extracts of the tailing mesocosms. Copper concentrations increased from month 3 to month 6 in control (TI) mesocosms and remained relatively stable thereafter, and all time-related changes in TII and TIII mesocosms were not significant. This contrasted with mesocosms that had been inoculated with sulfate-reducing acidophiles (TIV and TV), where copper concentrations declined with time and were significantly less in both cases at month 12 than at months 3 to 6 (Fig. 4, top). Changes in soluble copper concentrations with time were more accentuated in the alga-inoculated tailings (TV) and decreased significantly throughout the greater part of the experimental period. Zinc concentrations in the tailings mesocosms showed trends similar to those for copper (Fig. 4, bottom), though the lowest concentrations measured were much greater than those of copper (mean values of 17 and 0.05 μg/g tailings, respectively, recorded in TV mesocosms at month 12). Concentrations of soluble nickel in the tailings, which varied from ca. 20 to 40 μg/g tailings, did not display any obvious time-related or treatment-related effects (data not shown).
Fig. 4.
Time course changes in concentrations of copper (top) and zinc (bottom) in water extracts of mine tailings inoculated with different populations of acidophilic microorganisms. TI, no inoculum (control); TII, TI plus Fe(II) oxidizers; TIII, TII plus Fe(III) reducers; TIV, TIII plus SRB; TV, TIV plus algae. For each treatment data set, bars not annotated with the same letter are significantly different (95% confidence limit). Error bars represent standard deviations.
Microbiological changes in incubated mesocosms.
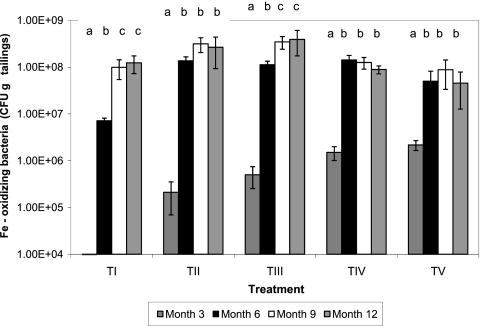
Although no iron-oxidizing autotrophs were detected in control (TI) mesocosms at month 3 of the experiment, CFU of these bacteria, equivalent to ca. 0.1 to 10% of those in inoculated mesocosms, were found on subsequent sampling occasions (Fig. 5). All tailing mesocosms displayed significantly increased numbers of CFU of iron-oxidizing acidophiles from month 3 to month 6, though these tended not to change greatly beyond this time, except in TIII mesocosms. However, when compared on different sampling occasions, the different inoculation regimens used at the outset of the experiment appeared to have little impact on the numbers of iron/pyrite-oxidizing bacteria that were detected in the incubated tailings.
Fig. 5.
Time course changes in CFU of acidophilic iron- and pyrite-oxidizing bacteria in mine tailings inoculated with different populations of acidophilic microorganisms. TI, no inoculum (control); TII, TI plus Fe(II) oxidizers; TIII, TII plus Fe(III) reducers; TIV, TIII plus SRB; TV, TIV plus algae. For each treatment data set, bars not annotated with the same letter are significantly different (95% confidence limit). Error bars represent standard deviations.
Numbers of aerobic heterotrophic bacteria increased in those mesocosms inoculated with these bacteria (TIII, TIV, and TV) from month 3 to month 6 and followed the same trends as for iron-oxidizing acidophiles (Fig. 6, top). After month 6, changes in numbers of aerobic heterotrophs isolated did not appear to be time related. No aerobic heterotrophs were detected in TI and TII mesocosms at months 3, 6, and 9, which was not surprising as these tailings had not been inoculated with these bacteria. However, as with the iron oxidizers, aerobic heterotrophs were detected in all tailings samples at month 12. The dominant CFU (small crescent-shaped, yellow colonies) in TI and TII mesocosms (76% and 63% of total CFU) were distinct from those of the three aerobic heterotrophs added in the original inocula. The same colony form corresponded to only 14% of total CFU in TIII mesocosms and ∼1% in TIV and TV mesocosms at the same times. Total numbers of aerobic heterotrophs were significantly greater on all sampling occasions (except at month 3 for TIV and TV mesocosms) in tailings that had been inoculated with algae.
Anaerobic heterotrophs followed trends similar to those for aerobic heterotrophs, in that their numbers increased significantly from month 3 to 6 in those mesocosms where they had been inoculated but were not apparent in tailings that had not been inoculated until month 12 (Fig. 6, bottom). On three of the four sampling occasions, numbers of anaerobic heterotrophs were found to be significantly greater in tailings that had been inoculated with acidophilic algae (TV) than in those that has been inoculated with the same suite of bacteria but no algae (TIV). Microscopic examination of algal growths on the tailing surface (see Fig. S1 in the supplemental material) found that Euglena-like cells but no Chlorella cells were present on all sampling occasions.
Identification of bacterial isolates.
Several of the bacteria isolated from the tailings mesocosms on solid media were identified from sequence analysis of their 16S rRNA genes. A representative of the single morphological type of ferric iron-stained colony obtained from the noninoculated (TI) mesocosms was identified as At. ferrooxidans (100% 16S rRNA gene similarity to the type strain; 1,305-nucleotide [nt] PCR product length). The dominant iron oxidizers isolated from inoculated tailings were also Acidithiobacillus-like bacteria, though whether these were At. ferrooxidans or At. ferrivorans was not ascertained. The closest cultivated relative of the dominant and distinct aerobic heterotrophic colony variant isolated from control and TII mesocosms at month 12 was Curtobacterium ammoniigenes, with which it shared 99.0% 16S rRNA gene similarity (1,182-nt PCR product length). In contrast, the most numerous heterotrophic bacterium in inoculated tailings was Acidiphilium strain SJH (100% gene similarity to the bacterium included in the inoculum). The most abundant colony morphology on anaerobic plates inoculated with TIII and TV mesocosm tailings, and also with the noninoculated controls, was related (98.0% gene similarity; 1,291-nt PCR product length) to Alicyclobacillus sp. strain AGC-2. The Curtobacterium isolate was shown to cause the reductive dissolution of amorphous ferric hydroxide, as evidenced by greater concentrations of ferrous iron (Fig. 7) and higher pH values (4.26 ± 0.3) in inoculated anaerobic cultures than in a sterile control (final pH 4.07). Concomitant increases in cell numbers suggested that this isolate could also grow via ferric iron respiration (Fig. 7).
Fig. 7.
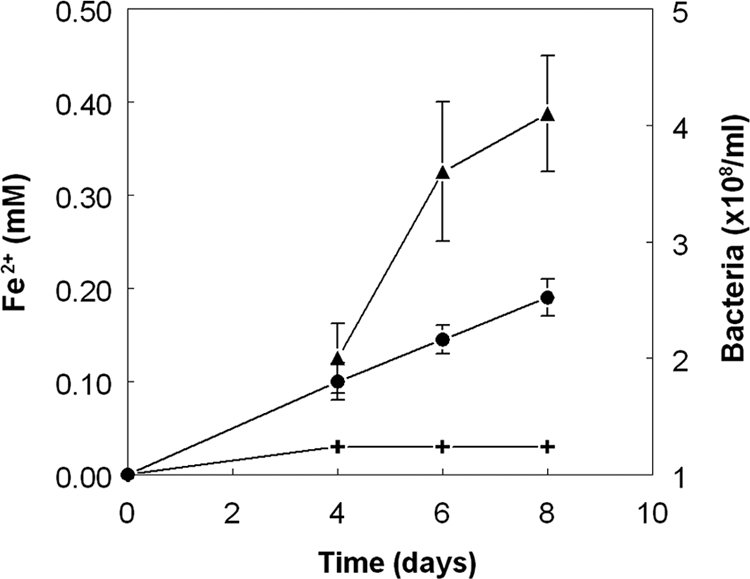
Reductive dissolution of amorphous ferric hydroxide by the Curtobacterium sp. isolated from mine tailings and changes in cell numbers during anaerobic growth by ferric iron respiration. •, ferrous iron (inoculated cultures); +, ferrous iron (control cultures); ▴, bacterial numbers. Data points show the mean values for duplicate cultures, and error bars indicate the range of values.
DISCUSSION
In the current work, we have sought to determine whether bacteria that catalyze the dissimilatory reduction of iron or sulfate and are sustained by organic carbon derived from acidophilic algae can minimize or reverse the impact of acid-generating, metal-mobilizing acidophiles in reactive mine tailings. Prior removal of residual alkali materials and inoculation of all mesocosms (except controls) with active populations of mineral-oxidizing bacteria ensured that the tailings were chemically and biologically primed for oxidizing pyrite and other sulfides, and presented a “worst-case” scenario in regard to their potential for mineral dissolution and metal release. The use of small-scale mesocosms allowed several synchronized microbial permutations to be set up and evaluated. Evidence for both oxidative (e.g., dissolution of pyrite) and reductive (formation of sulfides) processes, as well as the isolation of aerobes and anaerobes from the same mesocosms, pointed to the existence of microsites with contrasting oxygen contents, redox potentials and possibly pHs within the tailings.
Washing the tailings with strong mineral acid did not appear to eliminate indigenous iron-oxidizing or heterotrophic bacteria. While the origin of these bacteria is uncertain, the identification of bacteria related to C. ammoniigenes as the dominant aerobic heterotrophic isolate and to Alicyclobacillus sp. strain AGC-2 as the dominant anaerobic heterotrophic isolate (neither of which was present in the inocula used) strongly suggests that these bacteria (and, by inference, the At. ferrooxidans isolated from noninoculated mesocosms) were indigenous to the tailings. This is the first report of a Curtobacterium sp. proliferating in an acidic, metal-rich mineral environment. C. ammoniigenes is an ammonium-oxidizing species of the class Actinobacteria that was isolated from acidic (pH 2 to 4) swamps in Vietnam (1). The current isolate was partially characterized as a moderate acidophile that, like Acidiphilium, Acidocella, and Acidobacterium spp., catalyzed the dissimilatory reduction of ferric iron, which is another trait previously not identified for this genus. There are no published data on Alicyclobacillus sp. strain AGC-2, though its GenBank entry (accession number AF450135) states that it was isolated from a hot spring in Alaska. Some Alicyclobacillus spp. are facultative anaerobes that can grow by ferric iron respiration, though none are known to catalyze the dissimilatory reduction of sulfate to sulfide (16). While none of the SRB strains included in the inocula were subsequently isolated from the tailings, there was geochemical evidence for sulfidogenesis within alga-inoculated mesocosms (discussed below).
The mesocosm experiments illustrated the how ecological engineering could be used to improve long-term management of reactive mine tailings by minimizing their generation of acidic, metal-rich effluents. Iron-reducing bacteria and SRB were inoculated in order to promote processes that are, essentially, the reverse of those carried out by pyrite-oxidizing autotrophic bacteria. Although none of the inoculation regimes entirely prevented the oxidative dissolution of the reactive sulfidic minerals present in the tailings (as illustrated by the larger concentrations of soluble iron present on any sampling occasions than in the acid-washed material), there was clear evidence of mitigation of this process in those mesocosms that had been inoculated with heterotrophic iron-reducing bacteria and SRB, more particularly when acidophilic algae were also inoculated. Most acidophilic iron-reducing bacteria that have been characterized, and the few acidophilic SRB that have been described, are heterotrophic (4, 17, 19). Therefore, in order to stimulate the growth and activities of both iron- and sulfate-reducing acidophilic bacteria, a supply of organic carbon is often essential. While adding suitable organic carbon from an extraneous source is one possible scenario, this is not desirable (or economical) in a mine waste context, and continuous provision of small concentrations of organic carbon from a sustainable source is preferable for mine waste management. Monosaccharides identified in cell-free cultures of the acidophilic algae used in the current work (Euglena and Chlorella) are metabolized by many species of heterotrophic acidophiles (N̆ancucheo and Johnson, unpublished data). Acidophilic algae have been relatively little studied, partly because they are not perceived to have any role in the major biotechnology (“biomining”), which involves the use of prokaryotic consortia. Das et al. (6) highlighted the significance of eukaryotic microorganisms in mine waters and alluded to the possible role of algae in sustaining populations of SRB. More recently, Senko et al. (25) have provided evidence for the role of algae in enhancing iron reduction in AMD environments. Oxygen evolution by acidophilic algae can also influence iron and sulfur cycling and was probably the reason why ferric iron precipitates accumulated immediately below the algal surface layer in TV mesocosms (see Fig. S1d in the supplemental material).
The current data suggest that these bacteria influence tailings geochemistry directly by redox transformations of iron and sulfur rather than indirectly by inhibiting the growth of pyrite-oxidizing bacteria, the numbers of which were similar in mesocosms that were inoculated with acidophilic heterotrophs or not. The net positive effects highlighted with the heterotrophic bacterium/acidophilic alga consortium were as follows: (i) production of alkalinity, as evidenced by higher pH values; (ii) lower redox potentials, which correlated with higher concentrations of ferrous iron; and (iii) immobilization of copper and zinc released via dissolution of residual sulfide minerals in the tailings. Lower redox potentials and greater concentrations of soluble ferrous iron provided evidence of the activities of iron-reducing heterotrophs within the alga-inoculated tailings, while the presence of nascent sulfide minerals (H2S evolution when hydrochloric acid was added) indicated that sulfidogens were also metabolically active in the same mesocosms. Additional evidence for the latter came from the decreasing concentration of sulfate with incubation time and also from the relative degrees of immobilization of copper, zinc, and nickel within TV tailings. These three metal sulfides have very different solubility products (the log Ksp values for CuS, ZnS, and NiS are −35.9, −24.5, and −21.0, respectively [8]), and the fact that soluble copper declined by 97% and zinc by 83% (values calculated by comparing concentrations of soluble metals at month 12 to the maximum determined during the experiment) while nickel concentrations remained relatively unchanged provided convincing evidence that these metals were precipitated as sulfides rather than, e.g., being immobilized by adsorption. In an earlier report, Fortin and coworkers (11) also found that cycling of iron and sulfur occurred within mine tailings in field samples, and they proposed that removal of sulfate in the tailings was mediated by SRB rather than by chemical precipitation.
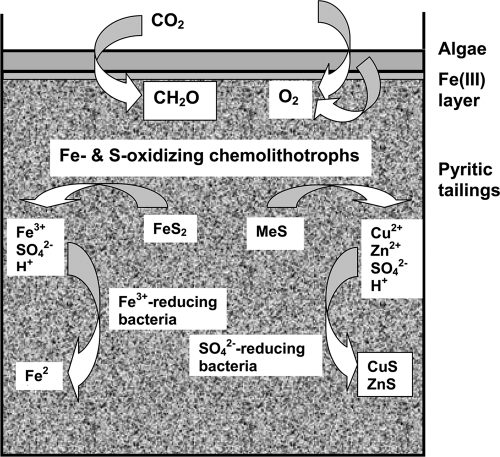
A model of the biogeochemical transformations implied to have occurred within the mesocosms that were inoculated with chemolithotrophic and heterotrophic bacteria and phototrophic algae is shown in Fig. 8. In the context of mine tailings management, the results of the current work suggest that by ensuring that acidophilic/acid-tolerant species of iron-reducing bacteria, SRB, and algae are present in tailings lagoons (e.g., by inoculating them with suitable strains of these microorganisms, as the tailings are being deposited) the environmental impact of these potentially hazardous mine wastes could be much reduced. Surface growths of algae are aided by the widespread current practice of storing tailings under a water cover. However, growth of the algae would also be enhanced by adding small amounts of inorganic nitrogen and phosphorus to nutrient-poor mine tailings.
Fig. 8.
Proposed biogeochemical transformations of carbon, iron, and sulfur in incubated tailings mesocosms inoculated with iron-oxidizing bacteria, aerobic and anaerobic heterotrophic bacteria, and acidophilic algae.
Supplementary Material
ACKNOWLEDGMENT
Ivan N̆ancucheo is grateful to the Mecesup Programme of the Chilean Government for supporting his doctoral study program.
Footnotes
Supplemental material for this article may be found at http://aem.asm.org/.
Published ahead of print on 30 September 2011.
REFERENCES
- 1. Aizawa T., et al. 2007. Curtobacterium ammoniigenes sp. nov., an ammonia-producing bacterium isolated from plants inhabiting acidic swamps in actual acid sulfate soil areas of Vietnam. Int. J. Syst. Evol. Microbiol. 57: 1447–1452 [DOI] [PubMed] [Google Scholar]
- 2. Bridge T. A. M., Johnson D. B. 2000. Reductive dissolution of ferric iron minerals by Acidiphilium SJH. Geomicrobiol. J. 17: 193–206 [Google Scholar]
- 3. Bruneel O., et al. 2011. Characterization of the active bacterial community involved in natural attenuation processes in arsenic-rich creek sediments. Microb. Ecol. 61: 793–810 [DOI] [PubMed] [Google Scholar]
- 4. Coupland K., Johnson D. B. 2008. Evidence that the potential for dissimilatory ferric iron reduction is widespread among acidophilic heterotrophic bacteria. FEMS Microbiol. Lett. 279: 30–35 [DOI] [PubMed] [Google Scholar]
- 5. Dacey P. W., Colbourn P. 1979. An assessment of methods for the determination of iron pyrites in coal mine spoil. Recl. Rev. 2: 113–121 [Google Scholar]
- 6. Das B. K., et al. 2009. Occurrence and role of algae and fungi in acid mine drainage environment with special reference to metals and sulfate immobilization. Water Res. 43: 883–894 [DOI] [PubMed] [Google Scholar]
- 7. Diaby N., et al. 2007. Microbial communities in a porphyry copper tailings impoundment and their impact on the geochemical dynamics of the mine waste. Environ. Microbiol. 9: 298–307 [DOI] [PubMed] [Google Scholar]
- 8. Diaz M. A., Monhemius A. J., Narayanan A. 1997. Consecutive hydroxide-sulphide precipitation treatment of acid mine drainage, p. 1179–1194 In Proceedings of the Fourth International Conference on Acid Rock Drainage, vol III ICARD, Vancouver, BC, Canada [Google Scholar]
- 9. Dold B. 2010. Basic concepts in environmental geochemistry of sulfidic mine-waste management, p. 173–198 In Sunil Kumar E. (ed.), Waste management. InTech, Rijeka, Croatia [Google Scholar]
- 10. Dopson M., Lindstrom E. B. 1999. Potential role of Thiobacillus caldus in arsenopyrite leaching. Appl. Environ. Microbiol. 65: 36–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fortin D., Rioux J.-P., Roy M. 2002. Geochemistry of iron and sulfur in the zone of microbial sulfate reduction in mine tailings. Water Air Soil Pollut. 2: 37–56 [Google Scholar]
- 12. Hallberg K. B., González-Toril E., Johnson D. B. 2010. Acidithiobacillus ferrivorans sp. nov.; facultatively anaerobic, psychrotolerant, iron- and sulfur-oxidizing acidophiles isolated from metal mine-impacted environments. Extremophiles 14: 9–19 [DOI] [PubMed] [Google Scholar]
- 13. Johnson D. B. 1995. Selective solid media for isolating and enumerating acidophilic bacteria. J. Microbiol. Methods 23: 205–218 [Google Scholar]
- 14. Johnson D. B. 2003. Chemical and microbiological characteristics of mineral spoils and drainage waters at abandoned coal and metal mines. Water Air Soil Pollut. Focus 3: 47–66 [Google Scholar]
- 15. Johnson D. B., Hallberg K. B. 2007. Techniques for detecting and identifying acidophilic mineral-oxidising microorganisms, p. 237–262 In Rawlings D. E., Johnson D. B. (ed.), Biomining. Springer-Verlag, Heidelberg, Germany [Google Scholar]
- 16. Johnson D. B., Hallberg K. B. 2009. Carbon, iron and sulfur metabolism in acidophilic micro-organisms. Adv. Microb. Physiol. 54: 202–256 [DOI] [PubMed] [Google Scholar]
- 17. Kimura S., Hallberg K. B., Johnson D. B. 2006. Sulfidogenesis in low pH (3.8-4.2) media by a mixed population of acidophilic bacteria. Biodegradation 17: 159–167 [DOI] [PubMed] [Google Scholar]
- 18. Lovley D. R., Phillips E. J. P. 1987. Rapid assay for microbially reducible ferric iron in aquatic sediments. Appl. Environ. Microbiol. 53: 1536–1540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. N̆ancucheo I., Johnson D. B. 2011. Selective removal of transition metals from acidic mine waters by novel consortia of acidophilic sulfidogenic bacteria. Microb. Biotechnol. doi:10.1111/j.1751-7915.2011.00285.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nordstrom D. K. 2000. Advances in the hydrogeochemistry and microbiology of acid mine waters. Int. Geol. Rev. 42: 499–515 [Google Scholar]
- 21. Rowe O. F., Sánchez-España J., Hallberg K. B., Johnson D. B. 2007. Microbial communities and geochemical dynamics in an extremely acidic, metal-rich stream at an abandoned sulfide mine (Huelva, Spain) underpinned by two functional primary production systems. Environ. Microbiol. 9: 1761–1771 [DOI] [PubMed] [Google Scholar]
- 22. Sanchez Espaňa J., Pamo E. L., Pastor E. S., Andres J. R., Rubi J. A. M. 2005. The natural attenuation of two acidic effluents in Tharsis and La Zarza-Perrunal mines (Iberian Pyrite Belt, Huelva, Spain). Environ. Geol. 49: 253–266 [Google Scholar]
- 23. Sand W., Gehrke T., Jozsa P.-G., Schippers A. 2001. (Bio)chemistry of bacterial leaching—direct vs. indirect bioleaching. Hydrometallurgy 59: 159–175 [Google Scholar]
- 24. Schippers A., et al. 2010. The biogeochemistry and microbiology of sulfidic mine waste and bioleaching dumps and heaps, and novel Fe(II)-oxidizing bacteria. Hydrometallurgy 104: 342–350 [Google Scholar]
- 25. Senko J. M., Bertel D., Quick T. J., Burgos W. D. 2011. The influence of phototrophic biomass on Fe and S redox cycling in an acid mine drainage-impacted system. Mine Water Environ. 30: 38–46 [Google Scholar]
- 26. Wakeman K., Auvinen H., Johnson D. B. 2008. Microbiological and geochemical dynamics in simulated heap leaching of a polymetallic sulfide ore. Biotechnol. Bioeng. 101: 739–750 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.