Abstract
Bacterial biofilms are crucial to the pathogenesis of many important infections and are difficult to eradicate. Streptococcus suis is an important pathogen of pigs, and here the biofilm-forming ability of 32 strains of this species was determined. Significant biofilms were completely formed by 10 of the strains after 60 h of incubation, with exopolysaccharide production in the biofilm significantly higher than that in the corresponding planktonic cultures. S. suis strain SS2-4 formed a dense biofilm, as revealed by scanning electron microscopy, and in this state exhibited increased resistance to a number of antibiotics (ampicillin, amoxicillin, ciprofloxacin, kanamycin, and rifampin) compared to that of planktonic cultures. A bacteriophage lysin, designated LySMP, was used to attack biofilms alone and in combination with antibiotics and bacteriophage. The results demonstrated that the biofilms formed by S. suis, especially strains SS2-4 and SS2-H, could be dispersed by LySMP and with >80% removal compared to a biofilm reduction by treatment with either antibiotics or bacteriophage alone of less than 20%; in addition to disruption of the biofilm structure, the S. suis cells themselves were inactivated by LySMP. The efficacy of LySMP was not dose dependent, and in combination with antibiotics, it acted synergistically to maximize dispersal of the S. suis biofilm and inactivate the released cells. These data suggest that bacteriophage lysin could form part of an effective strategy to treat S. suis infections and represents a new class of antibiofilm agents.
INTRODUCTION
Streptococcus suis is an important pathogen of pigs, causing arthritis, endocarditis, meningitis, pneumonia, and septicemia (11). Thirty-five serotypes (1 to 34 and 1/2) have been identified on the basis of capsular antigens, and of these, serotype 2 is considered the most virulent and is a prevalent isolate recovered from diseased pigs in China (35). S. suis serotype 2 is also an important zoonotic agent infecting humans who work in close contact with pigs or their products and causes endocarditis and meningitis (1, 34). There have been three outbreaks of human S. suis infections that have led to fatalities, occurring in 1998, 1999, and 2005 (35).
Biofilms are surface-associated communities of microorganisms encased in a protective extracellular matrix. Biofilm formation by pathogenic microorganisms is a mechanism that allows them to become persistent colonizers, resist clearance by the innate and adaptive host immune system, enhance resistance to antibiotics, and promote the exchange of genetic material (5, 32). Although the extent to which S. suis forms biofilms in animalia is unknown, it is likely to be significant and contribute to disease persistence in the face of antibiotic treatment regimens.
Lytic bacteriophages can be an alternative or adjunct to antibiotics for bacterial infections, particularly for biofilm reduction or disruption; the use of bacteriophages against Staphylococcus epidermidis biofilms on surgical implants is a good example of this application (4). Phage SMP is an S. suis serotype 2 lytic bacteriophage isolated from nasal swabs of healthy Bama minipigs (23). Although SMP can lyse planktonic S. suis cultures efficiently (23), its efficacy against S. suis biofilms is unknown. Bacteriophage-encoded lysins (cell wall hydrolases) are important for the release of lytic phages from the infected bacterial host cell (35). They have been investigated as therapeutic agents due to their ability to lyse susceptible Gram-positive bacteria and have been applied to a range of pathogens, such as Bacillus anthracis, Streptococcus pneumoniae, Staphylococcus aureus, and S. suis (17, 21, 27, 30, 35). The putative lysin produced by phage SMP and named LySMP has been purified and tested for activity against S. suis (35). As the next step toward its development as a therapeutic agent, we determined its ability to disrupt S. suis in an established biofilm form and to act synergistically with antibiotics.
MATERIALS AND METHODS
Bacterial strains, bacteriophages, and growth media.
S. suis strains SS2, SS7, and SS9 were isolated from diseased pigs between 1998 and 2005 in China. Streptococcus equi subsp. zooepidemicus reference strain ATCC 35246 was originally isolated in Sichuan in 1976. All of the strains were grown in Todd-Hewitt broth (THB) or agar medium supplemented with 2% (vol/vol) newborn bovine serum at 37°C. Plasmid pET-28a(+) containing the LySMP gene was propagated in Escherichia coli BL21 in Luria-Bertani broth (LB) containing 50 μg ml−1 kanamycin (35). Bacteriophage SMP (23) was propagated on S. suis SS2-H using double-layer agar plates as previously described (35).
Twenty-four-well plate biofilm assay.
The capacity for biofilm formation was tested in a 24-well plate assay modified for use with a 96-well microplate (18). Briefly, 200 μl of a 37°C overnight culture in THB was added to the wells of a 24-well polystyrene tissue culture plate each containing 1.8 ml of THB and incubated at 37°C for 3 days. After incubation, media and planktonic bacteria were removed by aspiration and the wells were washed twice with sterile phosphate-buffered saline (PBS; pH 7.2). The remaining attached bacteria were fixed with 500 μl of methanol for 30 min. After drying in air, the biofilms were stained with 0.1% crystal violet (500 μl) for 30 min at room temperature. The wells were washed twice with tap water to remove unbound crystal violet stain and dried for 2 h at 70°C. After the addition of 500 μl of 33% (vol/vol) glacial acetic acid to each well, the plate was shaken for 30 min to release the stain from the biofilms, and 200-μl aliquots were added to wells of a 96-well microplate before the determination of optical density at 600 nm (OD600) using an absorbance reader.
EPS assay.
Exopolysaccharide (EPS) produced by 10 S. suis strains which could form biofilms was extracted as previously described (26, 28), with some modifications. Briefly, planktonic bacteria were grown in THB with shaking, while biofilm-grown cells were incubated statically in a 24-well plate at 37°C for 72 h and then harvested by centrifugation (10,000 × g, 20 min, 4°C). The cells were washed once with buffer (1 mol/liter NaCl, 10 mmol/liter EDTA, pH 8.0) to release cell-bound EPS, and this was added to the culture supernatant, which contained any unbound EPS. The supernatant mixtures were extracted with 2 volumes of cold (−20°C) isopropanol for 24 h at 4°C to precipitate EPS, which was collected by centrifugation (10,000 × g, 20 min, 4°C). The bacterial protein contaminants in the EPS were precipitated with 25% (wt/vol) trichloroacetic acid on ice for 2 h and removed by centrifugation. EPS in the resulting supernatant was reprecipitated with two volumes of cold isopropanol as described above and air dried. The carbohydrate content of EPS was estimated by the phenol-sulfuric acid method of Dubois et al. (8) using d-glucose as the standard. The experiment was performed three times.
Observation of biofilm structure by SEM.
The structural architecture of the SS2-4 biofilm was visualized by scanning electron microscopy (SEM). Bacterial cells were inoculated in a 6-well culture plate containing glass coverslips, and the microscopic sample was prepared as described previously (24). The biofilm that formed on the glass coverslips was examined using a JEOL-JSM-6380LV scanning electron microscope.
Effects of antibiotics on S. suis biofilm.
Determination of the MICs and minimal bactericidal concentrations (MBCs) of ampicillin, amoxicillin, ciprofloxacin, kanamycin, and rifampin for 10 planktonic cultures was conducted according to the method of Grenier et al. (12), with some modifications. Briefly, 2-fold serial dilutions of antibiotics were prepared in THB culture and 20-μl volumes of the dilutions were inoculated into wells of sterile 96-well polystyrene plates. A 180-μl volume of an overnight culture of SS2-4 diluted in fresh THB to an OD600 of 0.2 was inoculated into each well containing a dilution of the antibiotics. After 24 h of incubation at 37°C, the MIC was determined by monitoring the OD600. To determine MBCs, 100-μl volumes of culture were recovered from wells with no visible growth and spread on Todd-Hewitt agar plates. The MBC was the lowest concentration of antibiotic at which no colonies were obtained on the agar medium.
For the measurement of the susceptibility of biofilms to antimicrobial agents, the biofilm was prepared in a 96-well microplate as previously described (18). After the medium was removed, the 72-h preformed biofilm was exposed to ampicillin or amoxicillin over a range of 0.125 to 1,280 μg ml−1. After 24 h of incubation at 37°C, the biofilm was suspended by scraping and the MIC was estimated by recording the OD600. The MBCs for the biofilm-grown cells were determined by incubating the biofilm for 24 h with antibiotics, followed by suspension in THB and spreading of 100 μl on Todd-Hewitt agar plates (12).
Effect of phage SMP on S. suis biofilms.
Bacteriophage treatment has been proposed as one method for controlling bacterial biofilms (4, 25). SMP is an S. suis serotype 2 (SS2) lytic bacteriophage which can lyse S. suis strains SS2-H and SS2-4. To determine the effect of phage SMP on preformed biofilm, biofilms were formed by 10 S. suis strains in a 24-well plate for 72 h as described above. Planktonic bacteria were removed, phage was added to the wells at 109 PFU/well, and the plate was incubated for 24 h at 37°C. The phage effect was quantified by adding appropriate phage dilutions in SM buffer (5.8 g of NaCl, 2 g of MgSO4·7H2O, 50 ml of 1 M Tris-HCl [pH 7.5], and 0.1 g of gelatin in a final volume of 1 liter of double-distilled H2O) to wells to provide a range of 1 × 106 to 1 × 109 PFU/well to treat biofilms of S. suis strain SS2-4. The 24-well plates were incubated at 37°C for 4, 8, 12, 24, 36, or 48 h and then washed and stained, and OD600s were measured as described above. The viable count was also determined by reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (9). Briefly, a stock solution (100 mg MTT, 20 ml 10 mM PBS) was filter sterilized and kept for no more than 2 weeks at 4°C. The S. suis culture in exponential phase was centrifuged (12,000 × g, 10 min) and washed once with PBS (pH 7.2). Then the S. suis culture was diluted and 100 μl was added to 20 μl of MTT in a 96-well plate and incubated for 2 h at 37°C. After incubation, 100 μl of dimethyl sulfoxide was added, the OD570 was measured, and the viable count was determined from a standard curve. The experiment was performed in triplicate.
Effect of LySMP on S. suis biofilm.
Lysin, encoded by most double-stranded DNA (dsDNA) bacteriophages, is essential for bacterial host cell lysis. Phage SMP has been identified as a dsDNA phage (23), and its putative lysin, designated LySMP, was expressed and purified as previously described, with some modification (35). Briefly, E. coli BL21 harboring the plasmid pET-lys, coding for LySMP, was inoculated into LB containing 50 μg/ml kanamycin and grown at 27°C with vigorous shaking, followed by 0.5 mM isopropyl-β-d-thiogalactopyranoside (IPTG) induction for 4 h when the OD600 reached 0.5. The induced culture was centrifuged (4,700 × g, 30 min, 4°C), suspended in 25 ml ice-cold lysis buffer (pH 6.8), and sonicated on ice (40 cycles of 3 s on and 13 s off at 400 W) to produce crude extracts of LySMP in the supernatant after centrifugation (10,000 × g, 20 min, 4°C). Purified LySMP was obtained by nickel affinity chromatography and characterized by SDS-PAGE, zymogram analysis (35), and Western blotting (31). To evaluate the percentage of the purified LySMP protein that retained muralytic activity, the relative LySMP protein concentrations before and after purification were determined by SDS-PAGE. The lytic efficacies (IU) of purified LySMP and the crude extracts were also tested using a turbidity reduction assay (35), enabling the percentage of purified LySMP retaining activity to be calculated.
The ability of purified LySMP to disrupt the biofilm was tested. E. coli BL21 harboring plasmid pET but without insertion of the LySMP gene was treated using the same protocol as for purification of LySMP to serve as a LySMP negative control. Wells containing biofilms grown for 72 h were filled with purified LySMP (100 IU/well), ampicillin (20 μg/well), amoxicillin (20 μg/well), ciprofloxacin (20 μg/well), or a mixture of LySMP and the antibiotics at these concentrations, together with appropriate control preparations. The 24-well plates were incubated at 37°C for intervals of up to 24 h. As previously stated, the relationship with the concentration of these biofilm-disrupting agents was determined using S. suis SS2-4 as the representative strain. After incubation, biofilm integrity was determined as described above. The viability of the cells in the disrupted biofilm was determined by dividing the plates into two groups; one group was washed with sterile distilled water to remove the free cells, the other group was left untreated, and the viable counts were compared. To visualize the degradation process, bacterial cells were inoculated into a 6-well culture plate containing glass coverslips, and photomicrographs of S. suis SS2-4 biofilms stained with crystal violet were taken at 1-h intervals after treatment with purified LySMP or elution buffer as a control.
RESULTS
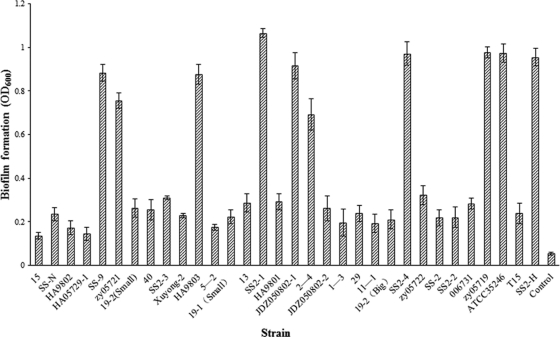
Thirty-two strains of S. suis, comprising 1 serotype 7 strain, 1 serotype 9 strain, and 30 serotype 2 strains were tested for the ability to form biofilms. Ten strains (SS-9, zy05721, HA9803, SS2-1, JDZ050802-1, 2–4, SS2-4, zy05719, ATCC 35246, and SS2-H) were identified as forming significant biofilm on the basis of final OD600 values in a range 0.7 to 1.1 to indicate the extent of crystal violet retention by surface-adherent cells (Fig. 1). This compares with an OD600 of 0.05 for the uninoculated controls and values of <0.4 for the remaining 22 strains (Fig. 1). The time course of biofilm formation revealed that significant/complete S. suis biofilms were formed after 60 h of incubation (see Fig. S1 in the supplemental material). These 10 S. suis strains were selected for further study.
Fig. 1.
Capacity of 32 strains of S. suis to form biofilms as determined by 24-well microplate crystal violet assay. Ten strains were identified as forming significant biofilms on this basis. All assays were performed in triplicate, and the data are expressed as means ± standard deviations.
The EPS content of the biofilm culture was significantly higher than that in the corresponding planktonic culture for each of the 10 strains, although the magnitude of the difference varied considerably between strains (data not shown).
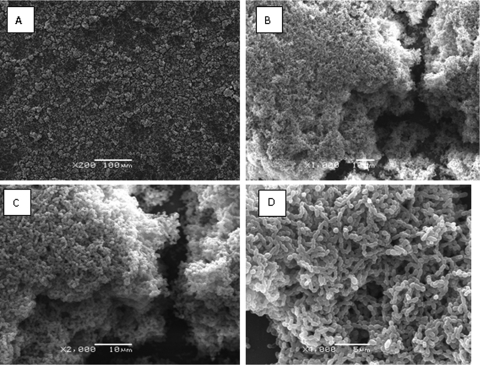
The structural architecture of a typical S. suis biofilm is presented in Fig. 2 and shows aggregates and microcolonies covering the surface of the coverslip to produce a compact and dense biofilm structure.
Fig. 2.
Scanning electron micrograph of S. suis SS2-4 biofilm formed after 72 h of growth. Aggregates and microcolonies of S. suis SS2-4 covered the surface of the coverslip to produce a compact and dense biofilm structure. Panels A to D show different magnifications.
The MICs and MBCs of ampicillin, amoxicillin, ciprofloxacin, kanamycin, and rifampin for the 10 S. suis strains are listed in Table 1. Five of the strains tested were fully resistant to these antibiotics when grown either as biofilm or planktonic cultures, and this emphasizes the need to develop alternative control agents for this pathogen. For the remainder, relatively low concentrations of these five antibiotics inhibited the growth of planktonic cultures, but the cells grown in the biofilm configuration were more resistant to each antibiotic by several orders of magnitude.
Table 1.
MICs and MBCs of 5 antibiotics for planktonic and biofilm-grown S. suis strains
| Strain and growth condition | MIC, MBC (μg/ml) |
||||
|---|---|---|---|---|---|
| Ampicillin | Amoxicillin | Ciprofloxacin | Kanamycin | Rifampin | |
| SS2-4 | |||||
| Planktonic | 0.0625, 0.125 | 0.0625, 0.125 | 0.125, 0.25 | 0.125, 0.25 | 0.0625, 0.125 |
| Biofilm | 160, >640 | 160, >640 | 160, >640 | 160, >640 | 160, >640 |
| SS2-H | |||||
| Planktonic | 0.0625, 0.125 | 0.0625, 0.125 | 0.125, 0.25 | 0.125, 0.25 | 0.0625, 0.125 |
| Biofilm | 160, >640 | 160, >640 | 160, >640 | 160, >640 | 160, >640 |
| 2–4 | |||||
| Planktonic | 0.0625, 0.125 | 0.125, 0.25 | 0.125, 0.25 | 0.125, 0.25 | 0.0625, 0.125 |
| Biofilm | 160, >640 | 160, >640 | 160, >640 | 160, >640 | 160, >640 |
| zy05719 | |||||
| Planktonic | 0.25, 0.5 | 0.25, 0.5 | 0.125, 0.25 | 160, >640 | 0.125, 0.25 |
| Biofilm | 320, >640 | 320, >640 | 160, >640 | 160, >640 | 160, >640 |
| ATCC 35246 | |||||
| Planktonic | 0.125, 0.25 | 0.125, 0.25 | 0.125, 0.25 | 160, >640 | 0.125, 0.25 |
| Biofilm | 320, >640 | 320, >640 | 160, >640 | 160, >640 | 160, >640 |
| JDZ050802-1 | |||||
| Planktonic | >640, >640 | >640, >640 | >640, >640 | >640, >640 | >640, >640 |
| Biofilm | >640, >640 | >640, >640 | >640, >640 | >640, >640 | >640, >640 |
| zy05721 | |||||
| Planktonic | >640, >640 | >640, >640 | >640, >640 | >640, >640 | >640, >640 |
| Biofilm | >640, >640 | >640, >640 | >640, >640 | >640, >640 | >640, >640 |
| HA9803 | |||||
| Planktonic | >640, >640 | >640, >640 | >640, >640 | >640, >640 | >640, >640 |
| Biofilm | >640, >640 | >640, >640 | >640, >640 | >640, >640 | >640, >640 |
| SS2-1 | |||||
| Planktonic | >640, >640 | >640, >640 | >640, >640 | >640, >640 | >640, >640 |
| Biofilm | >640, >640 | >640, >640 | >640, >640 | >640, >640 | >640, >640 |
| SS-9 | |||||
| Planktonic | >640, >640 | >640, >640 | >640, >640 | >640, >640 | >640, >640 |
| Biofilm | >640, >640 | >640, >640 | >640, >640 | >640, >640 | >640, >640 |
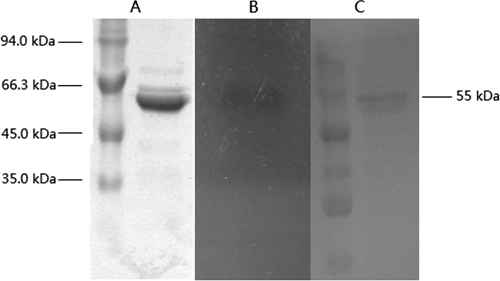
Phage therapy is often cited as a potential strategy to treat antibiotic-resistant bacterial infections, but here we could find no evidence of efficacy. There was no significant reduction of the S. suis biofilm (see Fig. S2A in the supplemental material) or viable counts of biofilm-grown bacteria (see Fig. S2A) after treatment with phage SMP, which is capable of infecting S. suis SS2-H and SS2-4 in conventional plaque assay (see Fig. S4 in the supplemental material). Furthermore, increasing the phage titer (see Fig. S2A) was ineffective and this phage, at least, appears to have no future as a therapeutic agent for S. suis biofilm disruption. Phage lysins may be more effective, and here the purified lysin from phage SMP (LySMP) was prepared and confirmed by SDS-PAGE to comprise a band of approximately 55 kDa (Fig. 3A) with activity, as determined by zymogram analysis (Fig. 3B). Its identity as LySMP was further confirmed by Western blotting using specific mouse LySMP antibody (Fig. 3C). It was calculated that ca. 79% of the LySMP protein retained muralytic activity after purification on the basis of a turbidimetric assay.
Fig. 3.
SDS-PAGE, zymogram analysis, and Western blotting of purified LySMP. The size of LySMP is ca. 55 kDa as shown on the right. (A) SDS-PAGE stained with Coomassie brilliant blue stain. (B) Zymogram analysis. A boiled cell suspension of S. suis SS2-4 was overlaid on the gel for the band to act against; lytic activity appears as a translucent band on the opaque background. (C) Western blotting with mouse LySMP antibody.
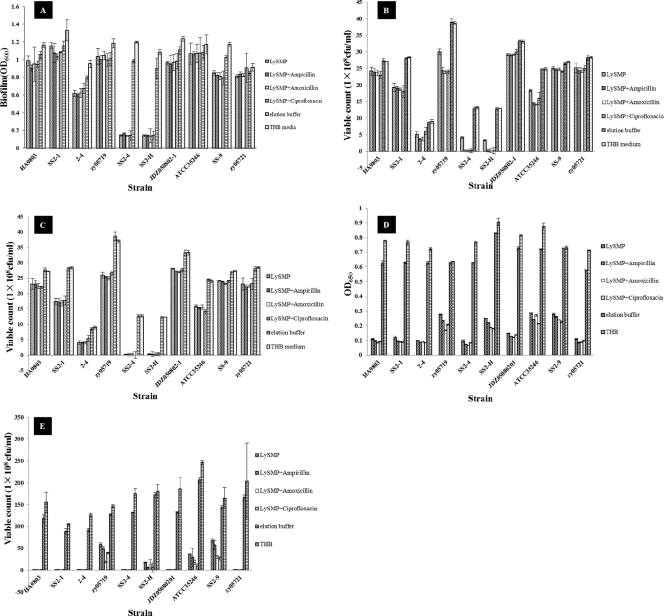
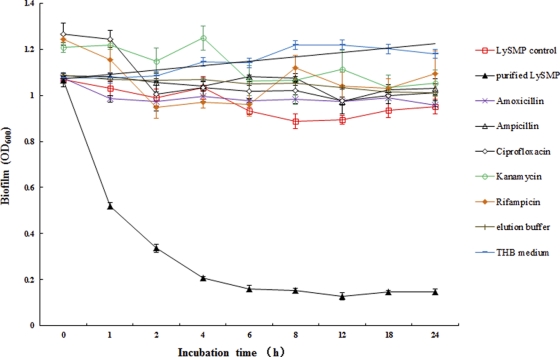
One hundred international units of LySMP was added to planktonic bacteria or preformed biofilms in 24-well plates with and without antibiotics, respectively, and the effect on viability (planktonic cultures) or biofilm disruption was determined. The results showed that LySMP has very strong lytic activity against 10 planktonic S. suis cultures (Fig. 4D and E) and against the biofilms formed by strains SS2-4 and SS2-H and a disrupting effect on the biofilms of some other strains, although the latter was limited (Fig. 4A, B, and C). Almost complete biofilm degradation could be achieved within 4 h when S. suis strain SS2-4 was used, and the reduction was up to 80% compared to the control (Fig. 5). After treatment with LySMP resulting in biofilm degradation, viable cells of S. suis remained (Fig. 4B and C). In the presence of antibiotics, however, viability was virtually eliminated, demonstrating that LySMP and antibiotics have an overall synergetic antimicrobial effect and the combination can kill both cells within the biofilm and those desquamated from the biofilm posttreatment (Fig. 4B). In contrast, the therapeutic dose of amoxicillin, ampicillin, or ciprofloxacin effective for planktonic cultures had no significant effect on biofilm structure or cell viability in the biofilm itself (see Fig. S3 in the supplemental material).
Fig. 4.
(A) Effect of purified LySMP on S. suis biofilms 12 h after treatment. (B) Viable counts of biofilm-grown bacteria 12 h after LySMP treatment and without removal of suspension. (C) Viable counts of biofilm-grown bacteria 12 h after LySMP treatment with the free cells removed from suspension. The viable counts of washed residual bacteria are lower than those in panel B, showing that free viable cells remain in suspension after treatment with LySMP. (D) Turbidity reduction assay for planktonic bacteria after treatment with LySMP. (E) Viable counts of planktonic bacteria after treatment with LySMP. Comparison of these data with those in panels B and C shows that LySMP alone or mixed with antibiotics had a more significant bactericidal effect on planktonic bacteria than on biofilm-grown bacteria. All assays were performed in triplicate, and the data are expressed as means ± standard deviations.
Fig. 5.
Analysis of the effect of LySMP on S. suis SS2-4 biofilm disruption. Up to 80% biofilm degradation could be achieved by LySMP within 4 h. LySMP control (□), which is the product expressed by the plasmid lacking the lysin gene insert, had little influence on the biofilm. Purified LySMP (▴) eliminated almost all of the biofilms, and elution buffer (+) had little or no effect on the biofilms. Amoxicillin (×), ampicillin (▵), ciprofloxacin (⋄), kanamycin (○), and rifampin (♦) all had minimal effects. THB (−) had no effect. All assays were performed in triplicate, and the data are expressed as means ± standard deviations.
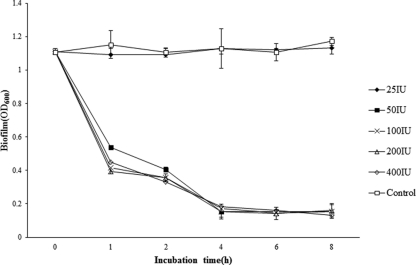
The disrupting effect of LySMP on S. suis SS2-4 was confirmed by observing stained biofilms under a light microscope over a period of 4 h, and the results show that the preformed biofilm was progressively dispersed by LySMP, while the control showed little change (Fig. 6). Furthermore, LySMP was effective at relatively low doses; there was no reduction in efficacy between 400 and 50 IU under the conditions of these experiments (Fig. 7).
Fig. 6.
Photomicrographs of S. suis SS2-4 biofilms stained with crystal violet at different times after treatment with purified LySMP or elution buffer as a control. The preformed biofilm was progressively dispersed by LySMP, while the control showed little change.
Fig. 7.
Dose-effect relationship between LySMP and S. suis SS2-4 biofilm integrity. All assays were performed in triplicate, and the data are expressed as means ± standard deviations. LySMP was effective at relatively low doses; there was no reduction in efficacy between 400 and 50 IU under the conditions in these experiments.
DISCUSSION
S. suis is a major pathogen of pigs worldwide and an early colonizer of the respiratory tract leading to septicemia, meningitis, and endocarditis. Thirty-five serotypes (1 to 34 and 1/2) have been identified, of which serotype 2 is the most common isolate from diseased pigs and has the strongest virulence, followed by serotypes 1, 4, 7, 9, and 1/2 (35). S. suis serotype 2 is an important zoonotic pathogen for humans and pigs, and biofilm formation may contribute to resistance to antibiotics and clearance by the immune system. A significant number of the strains tested (10 of 32) exhibited biofilm-forming ability in vitro, and this could be a contributory factor in the observation that S. suis can persist in pigs for a long time, although the exact nature of biofilm formation in vivo still needs to be determined. The biofilm-forming ability of pathogenic S. suis strain SS2-4 isolated from a diseased pig and its susceptibility to lysis by SMP bacteriophage were the factors that led to its selection as the model here. Gilbert et al. (10) proposed that biofilm-grown cells were up to 1,000 times more resistant to antimicrobial agents, and Grenier et al. (12) have made similar observations with S. suis. Here we observed much-increased resistance to amoxicillin and ampicillin in biofilm versus planktonic cultures of S. suis and a very limited, if any, ability of these antibiotics to disrupt the S. suis biofilm in vitro. Although high concentrations of antibiotics are able to kill biofilm bacteria, it is both impractical and inadvisable to administer antibiotics at the very high doses that would be required. Extracellular polysaccharide production and accumulation are established features of stable bacterial biofilms (5) and can protect cells against heat shock, desiccation, predation by protozoa (in the environment) or macrophages (in the animal host), and bacteriophage attack, and biofilms can adsorb/exclude chemical antimicrobial agents (20). In this study, we found that S. suis biofilms were indeed associated with a high concentration of EPS, while planktonic cultures produced significantly or dramatically less EPS.
In the last decade, the continued emergence of antibiotic-resistant bacteria has led to increasing interest in phage therapy. Phages have been examined as potential agents for biofilm control (5, 13, 14, 15, 16, 30, 33); for example, phage T4 can infect and replicate within E. coli biofilms and disrupt biofilm topography by killing bacterial cells (3, 6, 7). Bacteriophage SMP is the only lytic phage of S. suis described thus far (35), and our failure here to disrupt S. suis biofilms with this lytic phage was disappointing, although not totally unexpected. Knezevic and Petrovic (19) suggested that although lytic phages showed considerable inhibitory effects on growth and biofilm formation in Pseudomonas aeruginosa, their effect on mature biofilms was very limited. While that may be due to extracellular polymeric substances (EPS) preventing phage access to the bacteria and/or the receptors for phage infection, there are other reports indicating that EPS has no obvious inhibitory effect on phage invasion (2). Engineering bacteriophage to express a biofilm-degrading enzyme during infection is one possible solution, and there are precedents. Lu and Collins cloned the gene for dispersin B (dspB) into T7, an E. coli-specific phage, to express dspB and produced an engineered enzymatic phage which was more efficacious than wild-type phage at attacking biofilms (22). Alternatively, phage lysins, or endolysins, are possible antimicrobial agents against Gram-positive bacteria and have been applied to a variety of pathogens. Lytic activity of recombinant bacteriophage φ11 and φ12 endolysins on whole cells and biofilms of Staphylococcus aureus has been reported (29), but to our knowledge, no phage lysin has been reported to disrupt S. suis biofilms. Our data for phage LySMP (Fig. 5 and 7) are very encouraging because the purified lysin almost completely eliminated the S. suis SS2-4 and SS2-H biofilms, with amoxicillin, ampicillin, and ciprofloxacin in combination completing the inactivation of any released cells (Fig. 4). Photomicroscopy confirmed the efficacy of LySMP in disrupting the biofilm, and exploitation of this novel antimicrobial agent is promoted by the absence of any reduction of activity at low doses. With the efficacy of LySMP against preformed S. suis biofilms in vitro having been established, further investigation of the therapeutical potential of this bacteriophage-derived product is now warranted.
Supplementary Material
ACKNOWLEDGMENTS
We thank Alan McCarthy of the University of Liverpool for assistance in manuscript preparation.
This research was supported by the Special Fund for Public Welfare Industry of Chinese Ministry of Agriculture (200803016), the National Natural Science Foundation of China (31172381), and funds from the State Key Laboratory of Veterinary Etiological Biology (SKLVEB2010KFKT004).
Footnotes
Supplemental material for this article may be found at http://aem.asm.org/.
Published ahead of print on 7 October 2011.
REFERENCES
- 1. Arends J. P., Zanen H. C. 1988. Meningitis caused by Streptococcus suis in humans. Rev. Infect. Dis. 10: 131–137 [DOI] [PubMed] [Google Scholar]
- 2. Briandet R., Lacroix P., Renault M. 2008. Fluorescence correlation spectroscopy to study diffusion and reaction of bacteriophages inside biofilms. Appl. Environ. Microbiol. 74: 2135–2143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Corbin B. D., McLean R. J., Aron G. M. 2001. Bacteriophage T4 multiplication in a glucose-limited Escherichia coli biofilm. Can. J. Microbiol. 47: 680–684 [PubMed] [Google Scholar]
- 4. Curtin J. J., Donlan R. M. 2006. Using bacteriophages to reduce formation of catheter-associated biofilms by Staphylococcus epidermidis. Antimicrob. Agents Chemother. 50: 1268–1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Donlan R. M., Costerton J. W. 2002. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 15: 167–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Doolittle M. M., Cooney J. J., Caldwell D. E. 1995. Lytic infections of Escherichia coli biofilms by bacteriophage T4. Can. J. Microbiol. 41: 12–18 [DOI] [PubMed] [Google Scholar]
- 7. Doolittle M. M., Cooney J. J., Caldwell D. E. 1996. Tracing the interaction of bacteriophage with bacterial biofilms using fluorescent and chromogenic probes. J. Ind. Microbiol. 16: 331–341 [DOI] [PubMed] [Google Scholar]
- 8. Dubois M., Gilles K., Hamilton J. K., Rebers P. A., Smith F. A. 1956. Colorimetric method for the determination of sugars and related substances. Anal. Chem. 28: 350–356 [DOI] [PubMed] [Google Scholar]
- 9. Foongladda S., Roengsanthia D., Arjrattanakool W. 2002. Rapid and simple MTT method for rifampicin and isoniazid susceptibility testing of Mycobacterium tuberculosis. Int. J. Tuberc. Lung Dis. 6: 1118. [PubMed] [Google Scholar]
- 10. Gilbert P., Das J., Folez I. 1997. Biofilm susceptibility to antimicrobials. Adv. Dent. Res. 11: 160–167 [DOI] [PubMed] [Google Scholar]
- 11. Gottschalk M., Segura M. 2000. The pathogenesis of the meningitis caused by Streptococcus suis: the unresolved questions. Vet. Microbiol. 76: 259–272 [DOI] [PubMed] [Google Scholar]
- 12. Grenier D., Grignon L., Gottschalk M. 2009. Characterisation of biofilm formation by a Streptococcus suis meningitis isolate. Vet. J. 179: 292–295 [DOI] [PubMed] [Google Scholar]
- 13. Hanlon G. W., Denyer S. P., Olliff C. J., Ibrahim L. J. 2001. Reduction in exopolysaccharide viscosity as an aid to bacteriophage penetration through Pseudomonas aeruginosa biofilms. Appl. Environ. Microbiol. 67: 2746–2753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hanlon G. W. 2007. Bacteriophages: an appraisal of their role in the treatment of bacterial infections. Int. J. Antimicrob. Agents. 30: 118–128 [DOI] [PubMed] [Google Scholar]
- 15. Hughes K. A., Sutherland I. W., Jones M. V. 1998. Biofilm susceptibility to bacteriophage attack: the role of phage-borne polysaccharide depolymerase. Microbiology 144: 3039–3047 [DOI] [PubMed] [Google Scholar]
- 16. Hughes K. A., Sutherland I. W., Jones M. V. 1998. Bacteriophage and associated polysaccharide depolymerases-novel tools for study of bacterial biofilms. J. Appl. Microbiol. 85: 583–598 [DOI] [PubMed] [Google Scholar]
- 17. Jado I., et al. 2003. Phage lytic enzymes as therapy for antibiotic-resistant Streptococcus pneumoniae infection in a murine sepsis model. J. Antimicrob. Chemother. 52: 967–973 [DOI] [PubMed] [Google Scholar]
- 18. Jin H., et al. 2006. Biofilm formation by field isolates and reference strains of Haemophilus parasuis. Vet. Microbiol. 118: 117–123 [DOI] [PubMed] [Google Scholar]
- 19. Knezevic P., Petrovic O. 2008. A colorimetric microtiter plate method for assessment of phage effect on Pseudomonas aeruginosa biofilm. J. Microbiol. Methods 74: 114–118 [DOI] [PubMed] [Google Scholar]
- 20. Landini P. 2009. Cross-talk mechanisms in biofilm formation and responses to environmental and physiological stress in Escherichia coli. Res. Microbiol. 160: 259–266 [DOI] [PubMed] [Google Scholar]
- 21. Loeffler J. M., Nelson D., Fischetti V. A. 2001. Rapid killing of Streptococcus pneumoniae with a bacteriophage cell wall hydrolase. Science 294: 2170–2172 [DOI] [PubMed] [Google Scholar]
- 22. Lu T. K., Collins J. J. 2007. Dispersing biofilms with engineered enzymatic bacteriophage. Proc. Natl. Acad. Sci. U. S. A. 104: 11197–11202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ma Y., Lu C. 2008. Isolation and identification of a bacteriophage capable of infecting Streptococcus suis type 2 strains. Vet. Microbiol. 132: 340–347 [DOI] [PubMed] [Google Scholar]
- 24. Marrie T. J., Costerton J. W. 1984. Scanning and transmission electron microscopy of in situ bacterial colonization of intravenous and intraarterial catheters. J. Clin. Microbiol. 19: 687–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Merril C. R., Scholl D., Adhya S. L. 2003. The prospect for bacteriophage therapy in Western medicine. Nat. Rev. Drug Discov. 2: 489–497 [DOI] [PubMed] [Google Scholar]
- 26. Ngwai Y. B., Adachi Y., Ogawa Y., Hara H. 2006. Characterization of biofilm-forming abilities of antibiotic-resistant Salmonella typhimurium DT104 on hydrophobic abiotic surfaces. J. Microbiol. Immunol. Infect. 39: 278–291 [PubMed] [Google Scholar]
- 27. Rashel M., et al. 2007. Efficient elimination of multidrug-resistant Staphylococcus aureus by cloned lysin derived from bacteriophage ΦMR11. J. Infect. Dis. 196: 1237–1247 [DOI] [PubMed] [Google Scholar]
- 28. Read R. R., Costerton J. W. 1987. Purification and characterization of adhesive exopolysaccharides from Pseudomonas putida and Pseudomonas fluorescens. Can. J. Microbiol. 33: 1080–1090 [DOI] [PubMed] [Google Scholar]
- 29. Sass P., Bierbaum G. 2007. Lytic activity of recombinant bacteriophage φ11 and φ12 endolysins on whole cells and biofilms of Staphylococcus aureus. Appl. Environ. Microbiol. 73: 347–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schuch R., Nelson D., Fischetti V. A. 2002. A bacteriolytic agent that detects and kills Bacillus anthracis. Nature 418: 884–889 [DOI] [PubMed] [Google Scholar]
- 31. Sharma S. D., Mullenax J., Araujo F. G., Erlich H. A., Remington J. S. 1983. Western blot analysis of the antigens of Toxoplasma gondii recognized by human IgM and IgG antibodies. J. Immunol. 131: 977–983 [PubMed] [Google Scholar]
- 32. Stoodley P., Sauer K., Davies D. G., Costerton J. W. 2002. Biofilms as complex differentiated communities. Annu. Rev. Microbiol. 56: 187–209 [DOI] [PubMed] [Google Scholar]
- 33. Sutherland I. W., Hughes K. A., Skillman L. C., Tait K. 2004. The interaction of phage and biofilm. FEMS Microbiol. Lett. 232: 1–6 [DOI] [PubMed] [Google Scholar]
- 34. Trottier S., Higgins R., Brochu G., Gottschalk M. 1991. A case of human endocarditis due to Streptococcus suis in North America. Rev. Infect. Dis. 13: 1251–1252 [DOI] [PubMed] [Google Scholar]
- 35. Wang Y., Sun J. H., Lu C. P. 2009. Purified recombinant phage lysin LySMP: an extensive spectrum of lytic activity for swine streptococci. Curr. Microbiol. 58: 609–615 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.