Abstract
Rotavirus is the leading cause of severe acute gastroenteritis among children worldwide. It is well known that breast-feeding and vaccination afford infants protection. Since breast-feeding has drastically decreased in developed countries, efforts have been focused on the potential use of probiotics as preventive agents. In this study, a novel Bifidobacterium longum subsp. infantis strain was isolated from infant feces and selected, based on its capacity to inhibit in vitro rotavirus Wa replication (up to 36.05% infectious foci reduction) and also to protect cells from virus infection (up to 48.50% infectious foci reduction) in both MA-104 and HT-29 cell lines. Furthermore, studies using a BALB/c mouse model have proved that this strain provides preliminary in vivo protection against rotavirus infection. The strain has been deposited in the Spanish Type Culture Collection under the accession number CECT 7210. This novel strain has the main properties required of a probiotic, such as resistance to gastrointestinal juices, biliary salts, NaCl, and low pH, as well as adhesion to intestinal mucus and sensitivity to antibiotics. The food safety status has been confirmed by the absence of undesirable metabolite production and in acute ingestion studies of mice. Overall, these results demonstrate that Bifidobacterium longum subsp. infantis CECT 7210 can be considered a probiotic able to inhibit rotavirus infection.
INTRODUCTION
Rotavirus infections cause a considerable disease burden throughout the world, in both developed and developing countries, with seasonal peaks according to latitude and climate. Worldwide, rotavirus accounts for an estimated 2 million hospitalizations per year (39). Specifically, in the European region rotavirus infection causes an estimated 6,550 deaths and 146,287 hospital admissions each year in children under 5 years of age. The average percentages of diarrheal disease admissions attributable to rotavirus have been estimated at 26.4% (low-income countries), 21.3% (lower-middle-income countries), 31.7% (upper-middle-income countries), and 39.5% (high-income countries) (51).
The virus is transmitted by the fecal-oral route, with a low infectious dose (<100 virus particles) (27). Rotavirus infects mature enterocytes of the intestinal villus, and consequently crypt cells are spared (22). Once the virus enters epithelial cells, it produces the NSP4 enterotoxin, which mediates phospholipase C-dependent cell signaling and increases intracellular calcium levels, leading to chloride secretion (13). Rotavirus diarrhea has been attributed to different mechanisms, including secondary malabsorption, destruction of enterocytes, villus ischemia, and the enterotoxic role of NSP4, as well as the activation of the enteric nervous system (32). The result is a profuse watery diarrhea lasting 2 to 7 days with loss of fluid and electrolytes, which can cause fatal dehydration. Intensive rehydration with oral or intravenous fluids can correct these imbalances and sustain a child until the diarrhea stops (20). Moreover, viremia has been reported in rotavirus infections, although the clinical consequences of this remain unclear (22).
It is well known that breast-feeding prevents disease in infants, since a large proportion of immunoglobulins excreted in maternal milk are IgA, which mainly protects against enteric infections, such as rotavirus (2). In fact, immunoglobulins can be detected in the stools of breast-fed but not bottle-fed neonates (47). However, the lifestyle in developed countries has led to a drastic decrease in breast-feeding; thus, vaccines and other prevention strategies are becoming increasingly necessary. Although several rotavirus vaccines have been developed and have shown protection against rotavirus (10, 40, 48), a new generation of vaccines and complementary preventive approaches are needed in order to decrease the morbidity and mortality associated with rotavirus diarrhea (9, 20).
Since there are no completely safe methods for preventing diarrhea caused by rotavirus, over the last few years researchers have focused their efforts on the potential use of probiotic agents. According to the expert consultation conducted by the Food and Agriculture Organization (FAO) and the World Health Organization (WHO), probiotics are “live microorganisms which when administered in adequate amounts confer a health benefit on the host” (16). The known benefits of administering probiotic microorganisms include the prevention of several infections, allergic disorders, diarrhea, and inflammatory diseases, such as inflammatory bowel disease (35). Probiotics have been reported as very useful in the prevention and treatment of urogenital and vaginal infections (41). Furthermore, it has been suggested that probiotics or their metabolites play an important role in the formation or establishment of a well-balanced, indigenous, intestinal microbiota in newborn children and adults (25, 44).
Regarding the efficacy of probiotics in the treatment of diarrhea caused by rotavirus, a number of pediatric clinical trials have been reported, with different degrees of success. These studies included different probiotic strains, like Bifidobacterium bifidum and Streptococcus thermophilus (43), Lactobacillus paracasei (45), Lactobacillus rhamnosus GG (23, 36), and the probiotic mixture VSL3 (11). Although direct effects have been reported for the addition of different products, such as isoflavones from soy (1), lactose-based sialyl mimetics (31), and medicinal plants (21), on the inhibition of rotavirus replication, to our knowledge there are very few in vivo data that clearly demonstrate the capacity of a probiotic or a probiotic metabolite to inhibit rotavirus infection. In fact, for all the previously reported cases, the protection mechanisms remain unclear.
The aim of this study was to select a potential probiotic strain that is able to inhibit human rotavirus replication in vitro. Subsequently, an in vivo demonstration of its capacity was performed in a murine model. Further toxicological assays were carried out in order to ensure the harmlessness of the selected strain.
MATERIALS AND METHODS
Cells and viruses.
The rhesus monkey kidney cell line MA-104 was grown in Eagle's minimal essential medium (MEM) supplemented with 10% fetal bovine serum (FBS; Gibco Invitrogen, Paisley, United Kingdom). The human colon carcinoma cell line HT-29 was grown in Dulbecco's modified minimal essential medium (DMEM) supplemented with 10% FBS. Human rotavirus Wa strain (TC adapted; ATCC VR-2018) was obtained from the American Type Culture Collection, and the murine rotavirus McN strain was kindly provided by Richard Ward (Children's Hospital Medical Center, Cincinnati, OH) (33). Viral stocks of this murine rotavirus strain were prepared by orally infecting 8-week-old BALB/c mice. Stools were collected, pooled, homogenized in Earle's balanced saline solution (EBSS), and then aliquoted and stored at −80°C until use. The 50% diarrhea-producing dose (DD50) was determined by oral inoculation of neonatal mice with serial 10-fold dilutions of the viral stock according to the method of Reed and Muench (40a).
Isolation of Bifidobacterium strains.
Thirty Gram-positive, catalase-negative colonies with typical bifidobacterium or lactobacillus cell morphology were obtained from infant feces following a previously described protocol (5) and stored at −80°C in glycerol for further analysis. Of these, six Bifidobacterium spp. strains (numbered OR1 to OR6) were randomly selected for studies of further activity against human rotavirus.
Bacterial strains and growth conditions.
Bifidobacteria isolates OR1 to OR6 were grown using MRS-C medium (de Man Rogosa Sharpe broth [MRS; Oxoid, Basingstoke, United Kingdom] supplemented with 0.05% [wt/vol] cysteine [Sigma-Aldrich, St. Louis, MO]) and incubated anaerobically by means of an AnaeroGen system (Oxoid) at 37°C for 36 to 72 h. The strain L. rhamnosus GG (ATCC 53103), used as a reference strain, was grown on MRS medium and incubated anaerobically at 37°C for 17 to 24 h.
Identification and taxonomic characterization of isolates by sequencing.
DNA from pure culture was extracted using the High Pure PCR kit (Roche Diagnostics GmbH, Mannheim, Germany), spectrophotometrically quantified, and adjusted to a final concentration of 40 ng/μl in ultrapure water (Sigma-Aldrich). An almost-full sequence of the 16S rRNA gene was amplified and sequenced using an ABI Prism BigDye Terminator cycle sequencing ready reaction kit (Applied Biosystems Inc., Foster City, CA). The DNA was checked for purity, using standard methods (50). DNA templates were amplified by PCR on a thermocycler TC-5000 (Bibby Scientific, Stone, United Kingdom) using universal primers that amplified a 1,000-bp region of the 16S rRNA gene: 616V, 5′-AGAGTTTGATYMTGGCTCAG-3′, and 699R 5′-RGGGTTGCGCTCGTT-3′. The amplification mixture (100 μl) comprised 2 μl (50 pmol/μl) of 616V and 699R primers (Thermo Fisher Scientific, Waltham, MA), 0.5 μl (2 U/μl) of Taq DNA polymerase (Finnzymes, Espoo, Finland), 10 μl of 10× reaction buffer (Finnzymes), 10 μl of deoxynucleoside triphosphate mixture containing 1 mM (each) dATP, dGTP, dCTP, and dTTP (Roche Diagnostics GmbH), 70 μl of sterile filtered water (Milli-Q purification system; Millipore, Billerica, MA), and 5.5 μl of DNA template. The DNA templates were amplified by initial denaturation at 94°C for 10 min, followed by 40 cycles of denaturation at 94°C for 1 min, annealing at 55°C for 1 min, extension at 72°C for 1 min, and a final extension at 72°C for 10 min. Negative controls without DNA were simultaneously included in the amplification process. The integrity of the PCR products was confirmed by the presence of single bands following electrophoresis for 1 h at 100 V in 2% (wt/vol) agarose gels in Tris-borate-EDTA buffer. Amplicons were purified using the commercial QIAquick PCR purification kit (Qiagen Inc., Valencia, CA), and subsequent sequencing reactions were performed using the BigDye Terminator v3.1 cycle sequencing kit (premixed format; Applied Biosystems). The resulting sequences were automatically aligned and inspected visually and then compared with the online tool BLAST (http://blast.ncbi.nlm.nih.gov/Blast.cgi). The strain was identified on the basis of the highest scores.
Rotavirus propagation and in vitro inhibition assays. (i) Immunoperoxidase assays.
In order to detect viral antigens in rotavirus-infected HT-29 cell monolayers grown in microtiter plate wells, the cells were washed twice with phosphate-buffered saline (PBS), fixed with methanol-acetone (1:1 [vol/vol]) for 15 min, and washed again with PBS. The anti-VP6 monoclonal antibody 2F3E7 diluted 1/200 in PBS containing 1% (vol/vol) bovine serum albumin (PBS-BSA; 100 μl/well) was added, and the mixture was incubated at 37°C for 1 h. After two washings with PBS, 100 μl/well of a goat anti-mouse IgG antibody conjugated with peroxidase (Sigma) diluted 1/2,000 in PBS-BSA was added and incubated at 37°C for 1 h. After three washings with PBS, 100 μl/well of diamine-benzidine (DAB) substrate was added (6 mg DAB, 9 ml of 50 mM Tris-HCl [pH 7.6], and 0.1 ml H2O2). Infectious, peroxidase-stained foci were counted with an inverted microscope using five defined fields per well. The arithmetic mean was calculated to determine the number of foci per microscopic field, and these values were compared with the number of infectious foci of an untreated virus control to obtain the percentage reduction in virus focus-forming units (FFU).
Rotavirus propagation and in vitro competition assays of bacteria against cellular binding sites.
The rhesus monkey kidney cell line MA-104 was used to propagate the human rotavirus strain Wa in the presence of 1 μg/ml of trypsin (type IX; Sigma). Competition assays were performed with rotavirus Wa in both cell lines HT-29 and MA-104. The infectivities of the rotavirus stocks were activated with 10 μg/ml trypsin for 30 min at 37°C. Cultures were obtained by growing the bifidobacteria in MRS-C medium for 17 h at 37°C under anaerobiosis. Cells were washed with 0.09% (wt/vol) saline solution and standardized in order to obtain a concentration of 108 CFU per milliliter. Monolayers of HT-29 and MA-104 cells were grown in 96-well plates. The assays were performed following two different strategies: (i) incubation of Bifidobacterium cells with trypsin-treated rotavirus followed by infection of cell cultures with this virus-bacteria mixture (strategy A) and (ii) incubation of the bacteria with the cell cultures followed by cellular infection with rotavirus (strategy B).
For the first strategy, 30 μl of the bacterial suspension containing 3 × 106 CFU was mixed with 70 μl of DMEM without serum, and 100 μl of trypsin-treated human rotavirus Wa containing 1 × 107 FFU/well was added to the dilution in DMEM. The virus-bacteria mixtures were incubated at 37°C for 1 h. The cell monolayers were washed three times with PBS, inoculated with the virus-bacteria mixture, and incubated for 18 h at 37°C. To evaluate the inhibitory activity on viral replication, the rotavirus-infected cells were detected in an immunoperoxidase assay as described above. In the second approach, 30 μl of the bacterial suspension containing 3 × 106 CFU was mixed with 70 μl of DMEM without serum. After removal of the maintenance medium, the cellular monolayers were washed three times with PBS. An aliquot of 100 μl per well of the bacterial suspension was added, and after 1 h of incubation at 37°C, 100 μl per well of a viral inoculum in DMEM was added and incubated for 18 h under the same conditions. Rotavirus-infected cells were also identified in the immunoperoxidase assay.
Resistance to gastrointestinal juices.
The assay emulated conditions and exposure times to which bacteria are subjected during their passage through the stomach and intestinal tract in two stages and was performed as described previously by Chenoll et al. (5). Briefly, bacteria were subjected to simulated gastric and pancreatic juices by using pepsin of pig stomach mucous membrane (3 g/liter; Sigma-Aldrich) and pancreatin of pig pancreas (1 g/liter; Sigma-Aldrich), respectively. Samples were counted at 0, 90, and 120 min in the first stage and at 0, 60, and 240 min in the second. As a viability control throughout the process, the strain was inoculated in saline solution and incubated under the same conditions.
Sensitivity to antibiotics.
Strain sensitivities to antibiotics in terms of MICs was determined as recommended by the European Food Safety Authority (EFSA) (14), following the broth dilution test method for antimicrobial susceptibility of the Clinical and Laboratory Standards Institute. The MIC values of 20 antimicrobial agents were determined using the standardized LAB susceptibility test medium broth formulation (LSM), previously described, which is a mixture (9:1 [vol/vol]) of IsoSensitest broth (IST) and MRS broth adjusted to pH 6.7 (28). The antibiotics were tested in a concentration range of 0.125 to 256 mg/liter.
Resistance to biliary salts, NaCl, and low pH.
The resistance levels of the strains to different concentrations of biliary salts (0.5%, 1%, 2%, and 3% [wt/vol] Oxgall; Sigma), NaCl (2%, 3%, 6%, 8%, and 10% [wt/vol] NaCl), and pH (3.0, 2.0, and 1.5) were evaluated by monitoring bacterial growth on 96-well plates according to the methods of Chenoll and coworkers (5). Growth was analyzed at 655 nm in a Multiskan microplate reader (Thermo Fisher Scientific). The percentage resistance was calculated in each case by comparing the final optical density at 655 nm (OD655) results under different growth conditions with the corresponding controls grown in MRS-C.
Adhesion to intestinal mucus.
Bacterial adhesion to mucus was determined in three independent experiments, and each assay was performed in quadruplicate. Pig type III mucin (Sigma-Aldrich) was used at a concentration of 0.5 mg/ml. Bifidobacteria were radioactively labeled by adding 10 μl/ml of tritiated thymidine (5-[3H]thymidine; 120 Ci/mmol; Amersham Biosciences, GE Healthcare, Bio-Sciences Corp.) to the culture medium and incubated at 37°C for 17 h under anaerobiosis. The mucus was dissolved in HEPES buffer (N-2-hydroxyethyl piperazine-N-2-ethanesulfonic acid; 10 mM)–Hanks buffered saline solution (HH; pH 7.4) at the desired concentration and immobilized on polystyrene multiwell plates (Maxisorp; Nunc, Roskilde, Denmark) by adding 100 μl of this solution to each well and incubating at 4°C overnight. The nonimmobilized mucus was removed by two successive washings with 200 μl of HH buffer. After the last washing, 100 μl of HH buffer was added to each well. The radioactively labeled bacteria were centrifuged at 12,000 × g for 7 min and washed twice with HH buffer to eliminate the nonmetabolized thymidine. After this, the concentration of bacteria was determined by measuring the absorbance at 600 nm and adjusting to an OD of 0.25 ± 0.05 (mean ± standard deviation), in order to standardize the number of bacteria to 107 to 108 CFU/ml. An aliquot of 100 μl of labeled cells was added to each of the wells containing the immobilized mucus, and the mixture was incubated for 1 h at 37°C. Nonadhered bacteria were eliminated by two washings of 200 μl with HH buffer. Adhered bacteria were released by scraping the immobilized mucus in each well and were later lysed with 1% (wt/vol) SDS in 0.1 M NaOH (200 μl per well), followed by incubation of the plates at 60°C for 1 h. Cell contents were transferred to Eppendorf tubes containing scintillation liquid in order to measure the radioactivity with a scintillation counter. Adhesion was expressed as the percentage of radioactivity recovered after adhesion compared to the radioactivity of the bacterial suspension added to the immobilized mucus. As controls, three commercial strains (of L. casei, L. rhamnosus GG, and B. longum subsp. infantis) were used.
Lactic acid production.
Lactic acid production was determined using a 24-h supernatant of each strain grown under optimal conditions. Supernatant was obtained by centrifugation at 12,000 × g for 10 min. Lactic acid isomers were quantified by using a commercial kit (d-lactic acid/l-lactic acid; Roche Diagnostics) following the manufacturer's instructions.
Deconjugation of biliary salts.
A bile salt hydrolase activity assay was performed using glycocholate and taurocholate substrates, according to the technique of Kumar and coworkers (29).
Formation of biogenic amines.
The formation of biogenic amines (cadaverine, histamine, putrescine, and tyramine) was determined in MRS-C (CECT 7210) or MRS (LGG) cell-free supernatants from 24-h anaerobic cultures held at 37°C. Formation of amines was determined using the chromatographic method described by Eerola and coworkers (12).
In vivo studies to measure the capacity of bifidobacteria to colonize the mouse intestinal tract and their influence on the immune system.
All the experimental procedures involving animals were conducted in accordance with the regulations established by the European Community Council for the protection of animals with experimental and scientific applications (86/609/EEC).
Anaerobic cultures of the probiotic grown in the MRS-C medium for 17 h at 37°C were washed twice in saline solution. Cells were resuspended in sodium bicarbonate buffer (0.2 M) to an inoculum of 1 × 109 CFU/ml and frozen at −70°C in 20% (vol/vol) glycerol until use.
Two groups of nine 8-week-old BALB/c mice were assayed. In the control group each mouse was treated with a dose of 100 μl of sodium bicarbonate buffer (0.2 M). The experimental group received a dose of 109 CFU of the bifidobacteria resuspended in 100 μl of sodium bicarbonate buffer. Before administration, glycerol was removed from the bacterial suspensions by centrifugation at 12,000 × g for 5 min, and the bacteria were resuspended in sodium bicarbonate buffer. Then, 100-μl aliquots of the suspension were administered to each mouse orally. Fecal samples were collected at different times and homogenized immediately in PBS. Then, they were shaken vigorously, centrifuged at 12,000 × g for 5 min, stored at 4°C, and processed in under 24 h. The supernatants were frozen at −30°C. Inoculations were made on days 1, 2, and 3. Stools were collected 4 and 10 h after the first dose and at days 2, 3, 5, and 8.
Colonization of the intestinal tract by bifidobacteria was evaluated by plate counts of feces in the selective medium for Bifidobacterium, BFM medium (38). Ten-fold serial dilutions of the mixture were prepared in sterile saline solution up to 10−10. Aliquots of 100 μl were spread in triplicate on BFM agar plates and incubated under anaerobic conditions at 37°C for 7 days. IgA antibodies in the fecal samples were detected and measured by enzyme-linked immunosorbent assay (ELISA), as previously described, but with some modifications (2). Polystyrene plate wells (Costar; Corning Inc., Lowell, MA) were coated with 100 μl of a 1/250 dilution of sheep anti-mouse IgA serum (Sigma) diluted in carbonate-bicarbonate buffer (pH 9.6) and incubated for 2.5 h at 37°C and overnight at 4°C until use. After washing the wells three times with 200 μl of PBS containing 0.1% (vol/vol) Tween 20 (PBS-T), 100 μl of the sample diluted in PBS-T buffer containing 1% (wt/vol) bovine serum albumin BSA was added. In order to detect the antibodies, anti-mouse IgA antibodies labeled with peroxidase were used at a dilution of 1/2,000 in PBS-T–BSA. Finally, 100 μl/well of OPD (ortho-phenylenediamine) substrate was added, and after 5 or 10 min of incubation at room temperature the reaction was stopped with 50 μl of H2SO4 (3 M). The absorbance at 492 nm was determined spectrophotometrically. To measure IgA concentrations, a standard curve was prepared with serial dilutions of known concentrations.
Antirotavirus capacities of probiotic cells in vivo.
For evaluation of antirotavirus capacities of probiotic cells, similar groups of mice were used as described for the colonization study. After the first week, a booster dose of probiotic was administered to each mouse orally and repeated over the next 4 days. One oral inoculum of murine rotavirus strain McN containing 100 DD50 in 50 μl of Earle's balanced saline solution (EBSS) was given 10 h after the first booster. Over the following 9 days feces were collected daily, and the presence of bifidobacteria was evaluated by plate counting and viral shedding by ELISA. For postinfection bacterial counts, samples were taken at 10, 33, 58, 106, and 168 h after the first dose. Stools were resuspended in EBSS, homogenized, vortexed, and centrifuged at 12,000 × g for 5 min. Pellets containing bacteria were collected and stored at 4°C to be processed in under 24 h. Supernatants were frozen at −30°C for the viral antigen assay. Bifidobacteria counts were obtained as reported previously.
Quantification of McN rotavirus shedding was determined individually in the group of BALB/c mice previously given the viral inoculum and the bifidobacteria, and results were compared with amount of virus shed by rotavirus-infected control mice. Shedding of the McN rotavirus in feces was assayed in an ELISA. First, a polystyrene 96-well plate was coated with 100 μl of a 1/500 dilution of an antirotavirus sheep serum (Chemicon International, Temecula, CA) in carbonate-bicarbonate buffer (pH 9.6) and incubated for 2.5 h at 37°C and overnight at 4°C. The plates were washed three times with PBS-T, and 100 μl of supernatant of fecal sample was added and treated as described previously to obtain a 1/2 dilution in PBS-T buffer with 1% (wt/vol) BSA. After 1.5 h of incubation at 37°C, the supernatant was eliminated and washed four times with PBS-T buffer, and 100 μl/well of the monoclonal antibody anti-VP6 2F3E7 was added. The mixture was incubated again for 1.5 h at 37°C, and after four washings with PBS-T, 100 μl/well of a 1/2,000 dilution of peroxidase-conjugated anti-mouse IgG antibody in PBS-T–BSA was added and the mixture incubated at 37°C for 1 h. Plates were washed four times with PBS-T, and a solution of OPD was added. The reaction was stopped after 5 to 10 min with 50 μl of 3 M H2SO4 solution. Absorbance was determined at 492 nm. A standard curve was constructed with serial dilutions of a titrated suspension in order to quantify the virus concentration in the samples.
Acute ingestion study of mice.
The acute ingestion study was carried out as previously described (5). Briefly, assays were performed with cells obtained from an anaerobic culture of the bifidobacteria in 2 liters of MRS-C medium for 17 h at 37°C and later freeze-dried. For inoculum preparations, lyophilized bacteria were evaluated by MRS-C plate counts, and aliquots of 109 CFU per 100 μl of Ringers solution were prepared. Assays were carried out with 7-week-old pathogen-free BALB/c males that were randomly assigned to one of the four established groups 5 days before the study (Table 1). Immunosuppression was achieved by intraperitoneal administration of cyclophosphamide (150 mg/kg of body weight) 3 days before the first administration of bifidobacteria and 100 mg/kg 1 day before. Inoculum and placebo (lyophilized skim milk with 5% [wt/vol] sucrose) were administered orally for 6 days. During the study, mortality and morbidity were recorded twice a day. Body weight was registered daily. Feces were collected 1 day before the first administration of bifidobacteria and 10 h after each administration. Fresh feces were homogenized in Ringers solution. Bifidobacteria counts were obtained using BFM as selective medium as described before.
Table 1.
Distribution of experimental groups in the acute ingestion experimenta
| Group | Immunosuppressed? | Dose |
|
|---|---|---|---|
| CFU/mouse | Vol (μl) | ||
| A | No | Placebo | 100 |
| B | No | 1 × 109 | 100 |
| C | Yes | Placebo | 100 |
| D | Yes | 1 × 109 | 100 |
See Materials and Methods for details.
On day 7 of the study, right after the sacrifice, the carcasses were disinfected and dried with gauze. Cardiac blood was obtained with a 25-gauge needle coupled to a 1-ml syringe, and liver, spleen, and mesenteric lymph nodes and the ileum, jejunum, cecum, and colon were extracted and weighed. The organs were then homogenized in BFM medium, and bacteria counts were obtained by plate counts on BFM medium as reported before. In order to perform a histopathological evaluation, on the day of sacrifice the jejunum, cecum, and colon were collected and preserved in 4% (wt/wt) neutral buffered formaldehyde solution (pH 7) for later analysis.
Statistical analysis.
Results of rotavirus propagation and in vitro competition assays of bacteria against cellular binding sites were statistically analyzed by using the Mann-Whitney U test and the SPSS v12.0 software (SPSS Inc., Chicago, IL). Results obtained in mouse assays were analyzed using Statgraphics Plus 5.1 software (Manugistiscs, Rockville, MD). In the latter case, data were subjected to a one-way analysis of variance (ANOVA) using the strain as the variable. Means were compared with a least significant difference test.
Nucleotide sequence accession number.
The 16S rRNA gene sequence corresponding to B. longum subsp. infantis CECT 7210 has been deposited in the EMBL nucleotide database under the accession number HM118564.
RESULTS
In vitro evaluation of the antirotaviral activities of the bifidobacteria collection.
Six randomly selected bifidobacteria strains were evaluated for their antirotaviral activities in vitro. Table 2 shows the results obtained using both HT-29 and MA-104 cell cultures infected with the Wa strain of rotavirus. The results obtained with both infection strategies were very similar. On average, HT-29 cells rendered a higher percent reduction than MA-104 cells in both assays. However, inhibition was observed to increase slightly when the assayed bacteria were incubated with the cell cultures prior to infection with rotavirus. Considering the values obtained, the OR4 strain gave on average higher reduction levels in both cell lines (P < 0.05) (data not shown) and was therefore selected for further studies.
Table 2.
Assay for in vitro activity against human rotavirus Wa in HT-29 and MA-104 cell lines following in vitro competition assays (strategies A and B)a
| Strain | % focus reduction (mean ± SD) in cell line |
|||
|---|---|---|---|---|
| HT-29 |
MA-104 |
|||
| Strategy A | Strategy B | Strategy A | Strategy B | |
| OR1 | 29.45 ± 1.20 | 45.20 ± 2.20 | 13.50 ± 2.16 | 24.80 ± 3.15 |
| OR2 | 26.15 ± 14.44 | 38.80 ± 1.75 | 15.00 ± 1.73 | 23.80 ± 2.11 |
| OR3 | 40.27 ± 2.45 | 40.00 ± 2.34 | 14.70 ± 3.54 | 24.40 ± 1.46 |
| OR4 | 36.05 ± 4.23 | 48.50 ± 1.64 | 18.20 ± 4.41 | 31.80 ± 3.30 |
| OR5 | 49.83 ± 10.43 | 45.00 ± 3.89 | 9.00 ± 1.35 | 24.60 ± 4.25 |
| OR6 | 22.56 ± 15.20 | 39.40 ± 4.74 | 16.40 ± 1.66 | 30.20 ± 2.84 |
Results are from three different experiments and are expressed as the percent reduction in infectious foci.
Taxonomical identification of the CECT 7210 strain.
On the basis of BLAST scores, strain OR4 was identified by the Spanish Type Culture Collection (Burjassot, Spain) as Bifidobacterium longum subsp. infantis. The strain was deposited in the Spanish Type Culture Collection under the accession number CECT 7210 and its nucleotide sequence was deposited in the EMBL nucleotide database (see above).
In vivo analysis of the potential influence of CECT 7210 on the immune response and ability to colonize the murine intestinal tract.
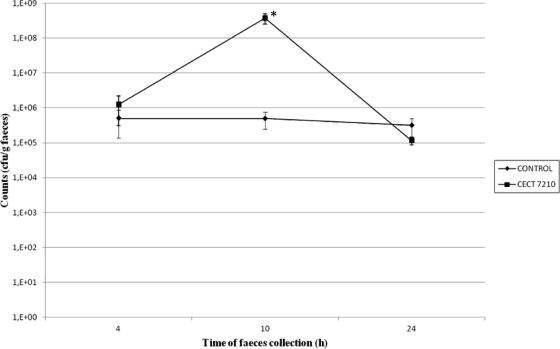
The quantification of IgA antibodies in feces collected at different times indicated that the oral administration of the CECT 7210 strain induced a greater amount of IgA in feces (data not shown). At 96 h after the first dose, stools from those mice fed this strain reached an IgA concentration of 30 mg/g of body weight, compared with a value of 18 mg/g in the nontreated control group. However, these differences between the CECT 7210 strain and the control group at 96 h and 168 h after bifidobacteria administration were not statistically significant. Bifidobacteria counts obtained in feces are shown in Fig. 1. The results showed that CECT 7210 reached maximum viability 10 h after the inoculations, and differences in counts between control and CECT 7210 groups were statistically significant (P < 0.01) at close to 100% viability. Therefore, this time period was chosen for rotavirus inoculation in later tests, as it was considered sufficient to evaluate the possible effects of probiotic activity.
Fig. 1.
Data obtained by BFM medium plate counts from mouse feces at different times in the study. *, significantly different from control group at 10 h (P < 0.01).
In vivo protection afforded by CECT 7210 against experimental infection of mice with the murine rotavirus McN strain.
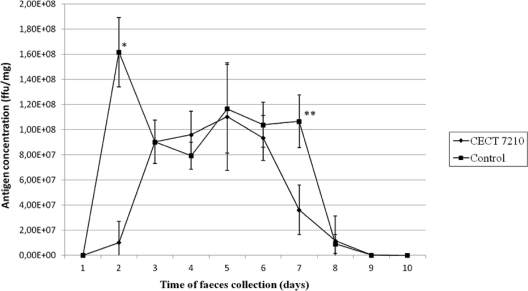
The course of viral infection in mice was evaluated by ELISA and based on the amount of rotavirus antigen shed in feces (Fig. 2). A significant initial delay in rotavirus shedding was detected during the first 48 h postinfection (106 FFU/ml, in comparison with 4 × 107 FFU/ml in the nontreated control group [P < 0.01]). This delay may have been due to an initial reduction in the levels of viral replication in the mice fed CECT 7210, although the virus finally infected all the groups of mice. It is noteworthy that on day 7, antigen concentration was statistically lower in those mice treated with the probiotic (P < 0.05).
Fig. 2.
Rotavirus antigen shedding in feces of mice fed with the selected probiotic compared with a placebo (control group). Results are given as the mean value ± standard deviation of triplicate absorbance values at 492 nm, converted to FFU/mg with the aid of a standard curve, which reflects the viral shedding for each mouse group. *, significantly different from control group (P < 0.01); **, significantly different from control group (P < 0.05).
Functional properties of probiotic relevance in the CECT 7210 strain.
All experiments regarding probiotic relevance were performed using the well-characterized commercial probiotic strain L. rhamnosus GG as a control. Concerning resistance to gastrointestinal juices, the percentage of viability was calculated as the relationship between the number of viable cells obtained at each stage of the assay and the number of viable cells at time zero. Table 3 summarizes the results obtained for the CECT 7210 strain, for which viability dropped after exposure to juices, as also occurred in the control L. rhamnosus GG strain.
Table 3.
In vitro surveillance of gastrointestinal juices in CECT 7210 and commercial probiotic LGG
| Strain | Inoculum (CFU/ml) | Gastric system |
Intestinal system |
||
|---|---|---|---|---|---|
| Count (CFU/ml) | % viable | Count (CFU/ml) | % viable | ||
| CECT 7210 | 1.6 × 108 | 5.5 × 106 | 3.4 | 3.1 × 105 | 0.2 |
| LGG | 3.1 × 108 | 3.0 × 107 | 8.8 | 1.0 × 107 | 2.9% |
Results obtained for antibiotic sensitivities are shown in Table 4. The data obtained were very similar for both strains, with the exception of clarithromycin, erythromycin, and kandamycin, for which the CECT 7210 strain showed higher MIC values. In the case of metronidazole and vancomycin, higher MIC values were obtained for the LGG strain.
Table 4.
CECT 7210 and commercial probiotic LGG resistance to antibiotics
| Antibiotic | MIC (μg/ml) |
|
|---|---|---|
| CECT 7210 | LGG | |
| Amoxicillin | 8 | 4 |
| Ampicillin | 2 | 4 |
| Carbenicillin | 64 | 16 |
| Clarithromycin | >256 | 16 |
| Clindamycin | 1 | 1 |
| Chloramphenicol | 4 | 16 |
| Erythromycin | >256 | 2 |
| Gentamicin | 64 | 16 |
| Kanamycin | >256 | 64 |
| Metronidazole | 4 | >256 |
| Nalidixic acid | >256 | >256 |
| Oxytetracycline | 64 | 4 |
| Penicillin | 0.5 | 1 |
| Polymyxin B | 256 | >256 |
| Rifampin | 0.5 | 1 |
| Streptomycin | 128 | 32 |
| Sulfonamide | >256 | >256 |
| Tetracycline | 128 | 4 |
| Trimethoprim | >256 | >256 |
| Vancomycin | 0.5 | >256 |
Table 5 shows results from cultures of CECT 7210 and L. rhamnosus GG strains grown on MRS-C or MRS broth, respectively, with different concentrations of added Oxgall. The data show that the CECT 7210 strain was able to grow in the presence of different concentrations of biliary salts, similar to the commercial probiotic strain LGG.
Table 5.
Resistance obtained in MRS-C and MRS media with different levels of biliary salts, NaCl, and pH in strains CECT 7210 and LGGa
| Strain | % viable bacteria at: |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Biliary salts (Oxgall) concn (%) |
NaCl concn (%) |
pH |
||||||||||
| 0.5 | 1 | 2 | 3 | 2 | 3 | 6 | 8 | 10 | 1.5 | 2.0 | 3.0 | |
| CECT 7210 | 49.3 ± 4.5 | 51.9 ± 1.0 | 56.7 ± 7.3 | 57.3 ± 15.5 | 100.2 ± 0.5 | 81.1 ± 5.6 | 77.1 ± 0.1 | 18.2 ± 1.2 | 9.3 ± 0.8 | 11.8 ± 0.3 | 12.5 ± 0.1 | 12.8 ± 0.1 |
| LGG | 33.2 ± 3.1 | 34.5 ± 0.6 | 42.7 ± 2.4 | 48.9 ± 3.0 | 99.2 ± 1.7 | 99.5 ± 1.2 | 84.3 ± 1.5 | 31.1 ± 4.7 | 9.5 ± 0.1 | 2.0 ± 0.2 | 11.2 ± 0.4 | 11.1 ± 0.2 |
Strain CECT 7210 was cultured in MRS-C medium, and strain LGG was cultured in MRS medium.
Table 5 shows the resistance obtained with CECT 7210, in the media MRS-C and LGG in MRS, in the presence of different NaCl concentrations. The results showed that the CECT 7210 strain was able to grow on 2% NaCl medium. However, once the concentration increased to 3 to 6%, final growth inhibition was observed. At higher concentrations (8 to 10% NaCl), growth seemed to be inhibited. The results were very similar for the commercial strain LGG.
Results obtained by growing both strains in the respective media at different pH values are represented in Table 5. The results show that growth of CECT 7210 was inhibited at pHs below 3.0. Results were almost identical in the L. rhamnosus GG strain.
Finally, with respect to adhesion to intestinal mucus, the CECT 7210 strain had an adhesion capacity of 9.9%, which was very similar to that obtained with the L. rhamnosus LGG strain (9.8% of adhesion).
Ex vivo toxicological studies: nondesirable metabolite production.
Lactic acid production was determined by using 24-h supernatants of the CECT 7210 and LGG strains grown in MRS-C and MRS, respectively, at 37°C. Results are summarized in Table 6 and show that in both strains d-lactic acid production was much lower than l-lactic acid production.
Table 6.
Undesired metabolite detection in supernatants
| Strain | Undesired metabolite level in supernatant |
||||||
|---|---|---|---|---|---|---|---|
| Lactic acid (g/liter) |
BSH activity (IU/ml of supernatant) |
Biogenic amine (mg/ml of supernatant) |
|||||
| d-Lactic acid | l-Lactic acid | Taurine | Glycine | Cadaverine | Histamine | Tyramine | |
| CECT 7210 | 0.06 ± 0.00 | 2.64 ± 0.01 | 2.10 ± 0.15 | 3.33 ± 0.11 | 0.01 ± 0.00 | 0.00 ± 0.00 | 0.11 ± 0.01 |
| LGG | 0.68 ± 0.06 | 12.10 ± 2.05 | 0.00 ± 0.00 | 0.15 ± 0.02 | 0.93 ± 0.12 | 0.56 ± 0.05 | 2.90 ± 0.24 |
Table 6 shows bile salt hydrolase (BSH) activity in supernatants obtained from 24 h-cultures of both strains. There was no activity in L. rhamnosus GG supernatants when taurocholate was used as substrate. When the reaction substrate was glycocholate, the highest level of activity was obtained for the CECT 7210 strain.
Cadaverine, histamine, putrescine, and tyramine were quantified in cell-free supernatants after 24 h of growth of strains CECT 7210 and LGG. Table 6 summarizes the results obtained. Putrescine was not detected in any case. Regarding cadaverine, histamine, and tyramine, the L. rhamnosus GG strain produced higher levels of these amines, while higher levels of tyramine were obtained in CECT 7210.
In vivo toxicological studies.
The acute ingestion study was performed as described in Materials and Methods, after which animals were sacrificed and examined. White spots were observed in the liver, and an increase in the weight of the spleen was detected in both groups of immunosuppressed mice, although no mortality was recorded during the study period. Regarding body weight gain (Table 7), no significant differences were detected between placebo and CECT 7210 in either nonimmunosuppressed or immunosuppressed mice. These groups showed statistically significant differences with both treatments, the placebo (P < 0.0001) and CECT 7210-treated mice (P = 0.0022).
Table 7.
Body weight gains of mice during the studya
| Group | Immunosuppressed | Dose (CFU/mouse) | Weight gain (g)b |
|---|---|---|---|
| A | No | Placebo | −0.4 (A) |
| B | No | 1 × 109 | −0.8 (B) |
| C | Yes | Placebo | 1.3 (A) |
| D | Yes | 1 × 109 | 1.2 (B) |
Body weight gains are the differences between the final and initial weights. Values sharing the letter A inside parentheses are significantly different at a P value of <0.0001; those sharing the letter B are significantly different at a P value of 0.0022.
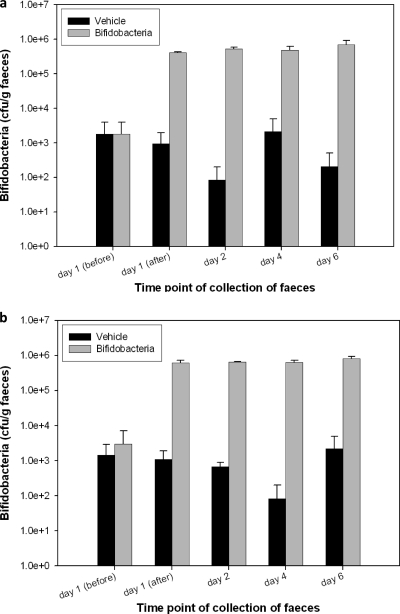
Regarding organ weight, differences were not statistically significant between groups, except in the case of the spleen in both groups of immunosuppressed mice, with a significant increase due to immunosuppression. Figure 3 represents bifidobacteria counts in feces of immunocompetent (Fig. 3a) and immunosuppressed (Fig. 3b) mice. Results indicate that counts were not dependent on immunosuppression. On day 1, an increase in fecal bifidobacteria content could already be observed in mice treated with the CECT 7210 strain. Levels of bifidobacteria remained stable during the study. Regarding the detection of bifidobacteria in organs by plating, no bacterial load of bifidobacteria was found in blood, liver, spleen, or mesenteric lymph nodes in the present study. No intergroup difference considered to be biologically significant was noted in histomorphology between immunocompetent or immune-deficient mice, with or without bifidobacteria administration.
Fig. 3.
Bifidobacteria counts in feces of immunocompetent mice (a) and in immunosupressed mice (b).
DISCUSSION
It is widely accepted that probiotics stimulate the immune system and exert an in vivo antimicrobial effect in humans and other animals. In contrast, most studies of probiotics targeting viruses have involved in vitro assays related to probiotic activities against viral replication. Labadie et al. (30) reported that Bifidobacterium is able to reduce the in vitro growth rate of human HT-29 cells, thus favoring the expression of markers involved in their differentiation into enterocyte-like cells. This effect can contribute to reinforcing epithelial cell resistance to virus-induced lysis in vivo. Another study revealed that probiotics can also block viral attachment by competitive inhibition if they are able to bind viral receptors at the surface of intestinal cells (6). Furthermore, Freitas et al. (18) reported that L. casei DN114 001 and also a strain of Bacteroides thetaiotaomicron can produce a compound that modulates the apical glycosylation pattern of cells and thus partially protect epithelial cells in vitro from rotavirus infection. In our study, the abilities of six Bifidobacterium strains to reduce rotaviral infectivity in a model system were evaluated in vitro. The strain with the best in vitro results was selected for evaluation in an in vivo murine model. Prior to the in vivo studies using a murine model, in vitro assays were carried out to evaluate to what extent Bifidobacterium isolates interfered with the Wa strain of human rotavirus in both MA-104 and HT-29 cell lines. Results showed different inhibition effects in both cell lines. Moreover, when cell cultures were incubated with bifidobacteria prior to infection, the inhibition percentages obtained were higher than when the probiotic was previously incubated with the human rotavirus Wa. These results indicate that the interaction of the probiotic with the mammalian cell surface plays an important role in the inhibition mechanism. This could be related to previously discussed mechanisms (4, 6, 18, 30) and is probably dependent on the infected cells. However, these reasons cannot explain all the mechanisms involved in diminished infection, since infection levels decreased even when the probiotics were added directly to the viral suspension. This fact suggests the existence of several mechanisms that may be involved in the antiviral effect of the probiotic strains, as suggested by Botić and coworkers (4).
Once the in vitro assays demonstrated that several strains were capable of reducing rotavirus infection, the B. longum subsp. infantis CECT 7210 strain was selected based on its ability to inhibit virus infection in both cell lines. Furthermore, this species has been described as one of the most abundant species isolated from breast-fed-infant feces (24). In vivo studies were carried out in order to evaluate its capacity to protect mice against experimental rotavirus infection with the murine rotavirus strain McN, using the adult mouse model, in which the end point is infection rather than illness (49). The results obtained indicated that the administration of the CECT 7210 strain provided preliminary protection against the rotavirus McN infection in the mouse model. Viral shedding in stools was detected later and decreased faster in probiotic-fed mice challenged with viral inocula than in control mice. Under these experimental conditions, the protection afforded by the probiotic was insufficient to completely protect the mice against a highly infectious rotavirus strain, such as the McN strain (33); however, the differences observed in viral shedding between mice given the probiotic and the control group were statistically significant. A similar reduction of McN rotavirus infectivity in the mouse model has been described in animals given bovine macromolecular whey proteins (3). However, further in vitro and in vivo studies are needed in order to clarify the exact mechanisms involved in the antiviral effect of the B. longum subsp. infantis CECT 7210 strain.
The effects of the CECT 7210 strain on the immune response were also analyzed in the murine model. Results showed a preliminary increase in IgA antibody levels after oral administration of the strain. However, IgA antibody levels decreased afterwards and, after 168 h, were similar to control group levels. Increased IgA in the feces of infants receiving bifidobacteria has been widely described (19, 37). This increase in local IgA levels after receiving the probiotic formula may contribute to enhancement of mucosal resistance to gastrointestinal infections.
With a view to its potential use as a probiotic, the B. longum subsp. infantis CECT 7210 strain was screened for several properties directly related to probiotic functions. First, the capacity of the strain to withstand and colonize the digestive system was evaluated. For this purpose, in vitro assays of resistance to gastrointestinal juices, biliary salts, NaCl, and low pH were performed, as well as in vivo studies of the capacity of CECT 7210 to colonize the mouse intestinal tract. Resistance levels to gastrointestinal juices, biliary salts, and NaCl were similar to those for the commercial probiotic L. rhamnosus GG. Regarding pH, the CECT 7210 strain displayed in vitro sensitivity to acidic pH values during long time periods (24 h), similar to L. rhamnosus GG. Nevertheless, different in vivo conditions should be considered. First, the transit time of the probiotic through the stomach, where such a low pH is reached, does not usually take longer than 2 h, after which it passes quickly into the small intestine, where the pH is neutral, thus allowing bacterial growth. Second, the probiotic is ingested together with other compounds that can buffer the effects of the gastric pH (42).
The adherence capacity to the intestinal mucus was also analyzed, as some authors consider this a critical property for probiotics (26). In this study, the value obtained for the B. longum subsp. infantis CECT 7210 strain was the same as for L. rhamnosus GG. Moreover, values obtained in the adhesion assays with the CECT 7210 strain were comparable with those obtained for other Bifidobacterium strains, including the commercial strain B. longum (26).
Although in vitro assays are fast and convenient methods to test gastric resistance and the adherence capacity of potential probiotic strains, in vivo trials are essential to obtain a realistic view of their capacity to withstand and colonize the intestinal tract. Thus, in vivo studies were carried out using a murine model. Results revealed increased bifidobacterial counts in feces 10 h after the oral administration of B. longum subsp. infantis CECT 7210. This demonstrated both the resistance of the CECT 7210 strain to the gastric environment in an in vivo model and the absence of probiotic colonization of the gut at the levels of intake assayed. Consequently, there is need for continued intake of the strain in order to colonize the intestine to the extent necessary to maintain characteristic functional benefits.
B. longum is included in the list of taxonomic units proposed for QPS status (Qualified Presumption of Safety [15]), and several strains of this species have been approved for use in infant formulae by the Food and Drug Administration. However, in order to ensure the safety of the B. longum subsp. infantis CECT 7210 strain, a detailed toxicological study was carried out following the FAO/WHO recommendations (17). Sensitivity to antibiotics was evaluated, and results were similar to those previously reported for other bifidobacteria (7, 8). Regarding the production of undesired metabolites, such as lactic acid isomers, products of BSH activity, and biogenic amines, results showed that production of the d-lactic acid isomer was very low in comparison with l-lactic acid. Regarding BSH activity, values obtained were on the same order as those reported for other probiotics (46). Putrescine and histamine were not detected in any case, and cadaverine and tyramine levels were negligible compared with the maximum levels recommended by FAO OMS. Finally, the acute ingestion study of mice showed neither mortality nor morbidity, even in immunosuppressed mice. Furthermore, differences in bifidobacterial loads in organs and differences in histomorphology between groups with or without bifidobacteria administration were not statistically different. These results, together with the safe background of species of the genus Bifidobacterium (34), indicate that the B. longum subsp. infantis CECT 7210 strain can be considered safe for human consumption, indicating the convenience of proceeding to clinical trials in humans.
In conclusion, strain B. longum subsp. infantis CECT 7210 has been demonstrated to exert a direct in vitro effect on rotavirus infection of the MA-104 and HT-29 cell lines. Furthermore, in vivo trials in an animal assay have shown both antiviral effects and an immunological enhancement tendency. Moreover, characterization as a potential probiotic according to strain-specific traits, as well as results regarding safety considerations, have demonstrated that B. longum subsp. infantis CECT 7210 fulfills the main criteria required for consideration as a probiotic. Further studies will be carried out in order to investigate the molecular basis of its capacity to inhibit rotavirus. Also, human clinical trials are under way.
ACKNOWLEDGMENTS
This study was supported by Ministerio de Industria, Turismo y Comercio grant PROFIT (FIT-0600000-2006-22), and the European Social Fund (grants PTQ06-2-0642 and PTQ05-0101208). José Antonio Moreno Muñoz was cofunded by an ICREA Junior Empresa Grant (Catalonia, Spain). This work was partially funded by Laboratorios Ordesa. José Antonio Moreno Muñoz, Joan Fàbrega, and Montserrat Rivero are employees of Laboratorios Ordesa. Empar Chenoll, Beatriz Casinos, Esther Bataller, Daniel Ramón, and Salvador Genovès are employees of Biópolis.
Footnotes
Published ahead of print on 14 October 2011.
REFERENCES
- 1. Andres A., Donovan S. M., Kuhlenschmidt T. B., Kuhlenschmidt M. S. 2007. Isoflavones at concentrations present in soy infant formula inhibit rotavirus infection in vitro. J. Nutr. 137:2068–2073 [DOI] [PubMed] [Google Scholar]
- 2. Asensi M. T., Martínez-Costa C., Buesa J. 2006. Anti-rotavirus antibodies in human milk: quantification and neutralizing activity. J. Pediatr. Gastroenterol. Nutr. 42:560–567 [DOI] [PubMed] [Google Scholar]
- 3. Bojsen A., et al. 2007. Inhibitory activities of bovine macromolecular whey proteins on rotavirus infections in vitro and in vivo. J. Dairy Sci. 90:66–74 [DOI] [PubMed] [Google Scholar]
- 4. Botíc T., Klingberg T. D., Weingartl H. H., Cencič A. 2007. A novel eukaryotic cell culture model to study antiviral activity of potential probiotic bacteria. Int. J. Food Microbiol. 115:227–234 [DOI] [PubMed] [Google Scholar]
- 5. Chenoll E., et al. 2011. Novel probiotic Bifidobacterium bifidum CECT 7366 strain active against the pathogenic bacterium Helicobacter pylori. Appl. Environ. Microbiol. 77:1335–1343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Colbère-Garapin F., et al. 2007. Prevention and treatment of enteric viral infections: possible benefits of probiotic bacteria. Microbes Infect. 9:1623–1631 [DOI] [PubMed] [Google Scholar]
- 7. D'Aimmo M. R., Modesto M., Biavati B. 2007. Antibiotic resistance of lactic acid bacteria and Bifidobacterium spp. isolated from dairy and pharmaceutical products. Int. J. Food Microbiol. 115:35–42 [DOI] [PubMed] [Google Scholar]
- 8. Delgado S., O'Sullivan E., Fitzgerald G., Mayo B. 2008. In vitro evaluation of the probiotic properties of human intestinal Bifidobacterium species and selection of new probiotic candidates. J. Appl. Microbiol. 104:1119–1127 [DOI] [PubMed] [Google Scholar]
- 9. Demirjian A., Levy O. 2009. Safety and efficacy of neonatal vaccination. Eur. J. Immunol. 39:36–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dennehy P. H. 2008. Rotavirus vaccines: an overview. Clin. Microbiol. Rev. 21:198–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dubey A. P., Rajeshwari K., Chakravarty A., Famularo G. 2008. Use of VSL3 in the treatment of rotavirus diarrhea in children: preliminary results. J. Clin. Gastroenterol. 42(Suppl.):S126–S129 [DOI] [PubMed] [Google Scholar]
- 12. Eerola S., Hinkkanen R., Lindfors E., Hirvi T. 1993. Liquid chromatographic determination of biogenic amines in dry sausages. J. AOAC Int. 76:575–577 [PubMed] [Google Scholar]
- 13. Estes M. K., Kapikian A. 2007. Rotaviruses, p. 1946–1974 In Knipe D. M., Howley P. M. (ed.), Fields virology, 5th ed Lippincott Williams & Wilkins, Philadelphia, PA [Google Scholar]
- 14. European Food Safety Authority 2008. Update of the criteria used in the assessment of bacterial resistance to antibiotics of human or veterinary importance. EFSA J. 732:1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. European Food Safety Authority 2008. Scientific opinion of the Panel on Biological Hazards on the maintenance of the list of QPS microorganisms intentionally added to food or feed. EFSA J. 923:30–48 [Google Scholar]
- 16. FAO/WHO 2001. Health and nutritional properties of probiotics in food, including powder milk with live lactic acid bacteria. Report of a Joint FAO/WHO expert consultation on evaluation of health and nutritional properties of probiotics in food, including powder milk with live lactic acid bacteria. FAO, Córdoba, Argentina [Google Scholar]
- 17. FAO/WHO 2002. Draft guidelines for the evaluation of probiotics in food: report of a joint FAO/WHO working group meeting, London, Ontario, Canada 30 April-1 May 2002. World Health Organization, Geneva, Switzerland [Google Scholar]
- 18. Freitas M., et al. 2003. Host pathogens cross-talk: indigenous bacteria and probiotics also play the game. Biol. Cell 95:503–506 [DOI] [PubMed] [Google Scholar]
- 19. Fukushima Y., Kawata Y., Hara H., Terada A., Mitsuoka T. 1998. Effect of a probiotic formula on intestinal immunoglobulin A production in healthy children. Int. J. Food Microbiol. 42:39–44 [DOI] [PubMed] [Google Scholar]
- 20. Glass R. I., et al. 2006. Rotavirus vaccines: current prospects and future challenges. Lancet 368:323–332 [DOI] [PubMed] [Google Scholar]
- 21. Gonçalves J. L., et al. 2005. In vitro anti-rotavirus activity of some medicinal plants used in Brazil against diarrhea. J. Ethnopharmacol. 99:403–407 [DOI] [PubMed] [Google Scholar]
- 22. Greenberg H. B., Estes M. K. 2009. Rotaviruses: from pathogenesis to vaccination. Gastroenterology 136:1939–1951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Guandalini S., et al. 2000. Lactobacillus GG administered in oral rehydration solution to children with acute diarrhea: a multicenter European study. J. Pediatr. Gastroenterol. Nutr. 30:54–60 [DOI] [PubMed] [Google Scholar]
- 24. Haarman M., Knol J. 2005. Quantitative real-time PCR assays to identify and quantify fecal bifidobacterium species in infants receiving a prebiotic infant formula. Appl. Environ. Microbiol. 71:2318–2324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. He T., et al. 2008. Effects of yogurt and bifidobacteria supplementation on the colonic microbiota in lactose-intolerant subjects. J. Appl. Microbiol. 104:595–604 [DOI] [PubMed] [Google Scholar]
- 26. Izquierdo I., Medina M., Ennahar S., Marchioni E., Sanz Y. 2008. Resistance to simulated gastrointestinal conditions and adhesion to mucus as probiotic criteria for Bifidobacterium longum strains. Curr. Microbiol. 56:613–618 [DOI] [PubMed] [Google Scholar]
- 27. Kapikian A. Z., Hoshino Y., Chanock R. M. 2001. Rotaviruses, p. 1787–1833 In Knipe D. M., Howley P. M. (ed.), Fields virology, 4th ed Lippincott-Raven, Philadelphia, PA [Google Scholar]
- 28. Klare I., et al. 2005. Evaluation of new broth media for microdilution antibiotic susceptibility testing of lactobacilli, pediococci, lactococci, and bifidobacteria. Appl. Environ. Microbiol. 71:8982–8986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kumar R. S., et al. 2006. Structural and functional analysis of a conjugated bile salt hydrolase from Bifidobacterium longum reveals an evolutionary relationship with penicillin V acylase. J. Biol. Chem. 281:32516–32525 [DOI] [PubMed] [Google Scholar]
- 30. Labadie K., Pelletier I., Saulnier A., Martin J., Colbère-Garapin F. 2004. Poliovirus mutants excreted by a chronically infected hypogammaglobulinemic patient establish persistent infections in human intestinal cells. Virology 318:66–78 [DOI] [PubMed] [Google Scholar]
- 31. Liakatos A., Kiefel M. J., Fleming F., Coulson B., von Itzsteina M. 2006. The synthesis and biological evaluation of lactose-based sialylmimetics as inhibitors of rotaviral infection. Bioorg. Med. Chem. 14:739–757 [DOI] [PubMed] [Google Scholar]
- 32. Lundgren O., et al. 2000. Role of the enteric nervous system in the fluid and electrolyte secretion of rotavirus diarrhea. Science 287:491–495 [DOI] [PubMed] [Google Scholar]
- 33. McNeal M. M., Belli J., Basu M., Choi A. H., Ward R. 2004. Discovery of a new strain of murine rotavirus that is consistently shed in large quantities after oral inoculation of adult mice. Virology 320:1–11 [DOI] [PubMed] [Google Scholar]
- 34. Meile L., Le Blay G., Thierry A. 2008. Safety assessment of dairy microorganisms: Propionibacterium and Bifidobacterium. Int. J. Food Microbiol. 126:316–320 [DOI] [PubMed] [Google Scholar]
- 35. Minocha A. 2009. Probiotics for preventive health. Nutr. Clin. Pract. 24:227–241 [DOI] [PubMed] [Google Scholar]
- 36. Misra S., Sabui T. K., Pal N. K. 2009. A randomized controlled trial to evaluate the efficacy of Lactobacillus GG in infantile diarrhea. J. Pediatr. 155:129–132 [DOI] [PubMed] [Google Scholar]
- 37. Mohan R., et al. 2008. Effects of Bifidobacterium lactis Bb12 supplementation on body weight, fecal pH, acetate, lactate, calprotectin, and IgA in preterm infants. Pediatr. Res. 64:418–422 [DOI] [PubMed] [Google Scholar]
- 38. Nebra Y., Blanch A. R. 1999. A new selective medium for Bifidobacterium spp. Appl. Environ. Microbiol. 65:5173–5176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Parashar U. D., Gibson C. J., Bresee M. A., Glass R. I. 2006. Rotavirus and severe childhood diarrhea. Emerg. Infect. Dis. 12:304–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Phua K. B., et al. 2009. Safety and efficacy of human rotavirus vaccine during the first 2 years of life in Asian infants: randomised, double-blind, controlled study. Vaccine 27:5936–5941 [DOI] [PubMed] [Google Scholar]
- 40a. Reed L. J., Muench H. 1938. A simple method of estimating fifty per cent endpoints. Am. J. Hyg. 27:493–497 [Google Scholar]
- 41. Reid G. 2008. Probiotic lactobacilli for urogenital health in women. J. Clin. Gastroenterol. 42(Suppl):S234–S236 [DOI] [PubMed] [Google Scholar]
- 42. Ritter P., Kohler C., von Ah U. 2009. Evaluation of the passage of Lactobacillus gasseri K7 and bifidobacteria from the stomach to intestines using a single reactor model. BMC Microbiol. 9:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Saavedra J. M., Bauman N. A., Oung I., Perman J. A., Yolken R. H. 1994. Feeding of Bifidobacterium bifidum and Streptococcus thermophilus to infants in hospital for prevention of diarrhoea and shedding of rotavirus. Lancet 344:1046–1049 [DOI] [PubMed] [Google Scholar]
- 44. Salazar N., et al. 2009. Exopolysaccharides produced by Bifidobacterium longum IPLA E44 and Bifidobacterium animalis subsp. lactis IPLA R1 modify the composition and metabolic activity of human faecal microbiota in pH-controlled batch cultures. Int. J. Food Microbiol. 135:260–267 [DOI] [PubMed] [Google Scholar]
- 45. Sarker S. A., et al. 2005. Lactobacillus paracasei strain ST11 has no effect on rotavirus but ameliorates the outcome of non-rotavirus diarrhea in children from Bangladesh. Pediatrics 116:e221–e228 [DOI] [PubMed] [Google Scholar]
- 46. Tanaka H., Hashiba H., Kok J., Mierau I. I. 2000. Bile salt hydrolase activity of Bifidobacterium longum: biochemical and genetic characterization. Appl. Environ. Microbiol. 66:2502–2512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Van de Perre P. 2003. Transfer of antibody via mother's milk. Vaccine 21:3374–3376 [DOI] [PubMed] [Google Scholar]
- 48. Vesikari T., et al. 2007. Efficacy of human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in European infants: randomised, double-blind controlled study. Lancet 370:1757–1763 [DOI] [PubMed] [Google Scholar]
- 49. Ward R. L., McNeal M. M., Sheridan J. F. 1990. Development of an adult mouse model for studies on protection against rotavirus. J. Virol. 64:5070–5075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wilfinger W., Mackey M., Chanczynski P. 1997. Effect of pH and ionic strength on the spectrophotometric assessment of nucleic acid purity. Biotechniques 22:474–480 [DOI] [PubMed] [Google Scholar]
- 51. Williams C. J., Lobanov A., Pebody R. G. 2009. Estimated mortality and hospital admission due to rotavirus infection in the WHO European region. Epidemiol. Infect. 137:607–616 [DOI] [PubMed] [Google Scholar]