Abstract
Geobacter sulfurreducens PCA completely oxidized lactate and reduced iron or an electrode, producing pyruvate and acetate intermediates. Compared to the current produced by Shewanella oneidensis MR-1, G. sulfurreducens PCA produced 10-times-higher current levels in lactate-fed microbial electrolysis cells. The kinetic and comparative analyses reported here suggest a prominent role of G. sulfurreducens strains in metal- and electrode-reducing communities supplied with lactate.
TEXT
There is great interest in the study of bacteria capable of transferring electrons to solid electron acceptors, such as metal oxides or electrodes, in different types of bioelectrochemical systems (BESs). The two most-studied exoelectrogenic bacteria are Geobacter sulfurreducens PCA and Shewanella oneidensis MR-1 (1, 2, 9, 23), but these microorganisms have never been studied under exactly the same conditions in BESs. S. oneidensis MR-1 can grow anaerobically using lactate, but it produces acetate, which it cannot further oxidize under these conditions. 16S rRNAs sequences similar to those found with G. sulfurreducens are commonly found in mixed-culture microbial fuel cells (MFCs) and sediments supplied with lactate, while sequences similar to those found with the Shewanella genus are relatively rare in such studies (13–15, 28). It has been suggested that the presence of sequences matching those found with G. sulfurreducens was primarily due to acetate consumption sustained by other bacteria that incompletely oxidized lactate to acetate (13–15, 28). This assumption was probably based on earlier work indicating that G. sulfurreducens PCA could not oxidize lactate, as the authors reported similar levels of Fe(II) in both lactate-supplied cultures and negative controls after 5 days (3). Although G. sulfurreducens PCA was thought not to respire with lactate (3), its genome includes genes for putative lactate permeases (GSU1622 and GSU0226) and lactate dehydrogenases (GSU1620 and GSU1621), which are highly conserved among lactate-oxidizing bacteria, including those of the Shewanella genus (25).
It is now known that several Geobacter species can oxidize lactate (11, 24, 27), and it has recently been shown that G. sulfurreducens PCA can oxidize lactate with fumarate reduction (21, 30). Gene regulation in lactate- versus acetate-fed cultures of G. sulfurreducens PCA has been examined (30), but no data have yet been presented on lactate oxidation kinetics or intermediate product formation by strain PCA. There are indications of direct lactate oxidation in previous MFC studies with communities containing sequences matching those found with G. sulfurreducens (13, 15). For example, there was immediate current production by established mixed-culture MFC biofilms at the onset of lactate fed-batch cycles (13, 14, 16, 20, 29). Immediate current generation is unlikely to be a result of lactate conversion to acetate, as indirect conversion results in a lag of current generation of several hours. Direct current production from lactate oxidation is more plausible in these MFCs, yet bacteria capable of this type of metabolism, such as Shewanella species, produce lower current densities than mixed cultures under identical conditions (12, 14, 31). Furthermore, many Shewanella species use electron shuttles (22) that would be removed when the medium was replaced prior to fed-batch operation. This suggests that strains of G. sulfurreducens capable of high current production directly from lactate oxidation exist within mixed-community biofilms fed with lactate.
In order to better understand lactate utilization in BESs, we examined current generation by G. sulfurreducens PCA and S. oneidensis MR-1 in microbial electrolysis cells (MECs) under identical conditions (medium, electrode, and temperature). Iron reduction by G. sulfurreducens PCA was also examined to relate lactate oxidation kinetics to previously reported rates of acetate oxidation.
Electrode reduction using lactate.
Biofilms of G. sulfurreducens PCA (strain obtained from laboratory stocks of ATCC 51573 frozen at −80°C) were initially established on graphite plate anodes (anode specific surface area [AA] = 92 m2/m3) in serum bottle (5 ml)-based MECs (4, 18) using stainless steel mesh cathodes (cathode specific surface area [AC] = 86 m2/m3) (5). MECs (triplicate reactors) were supplied with acetate (10 mM) in a freshwater (FW) medium (0.1 g KCl, 0.3 g NH4Cl, 0.6 g NaH2PO4, 2.5 g NaHCO3, 10 ml vitamins, and 10 ml minerals per liter; headspace of 80:20 mixture of N2/CO2) (6) and operated in fed-batch mode at an applied voltage (EAP) of 0.7 V at 30°C (Fig. 1A). This applied voltage was chosen because it allowed for relatively fast cycle times (<2 days) compared to those obtained with lower applied voltages, and this voltage was previously used for culturing Geobacter sp. in MECs (6).
Fig. 1.
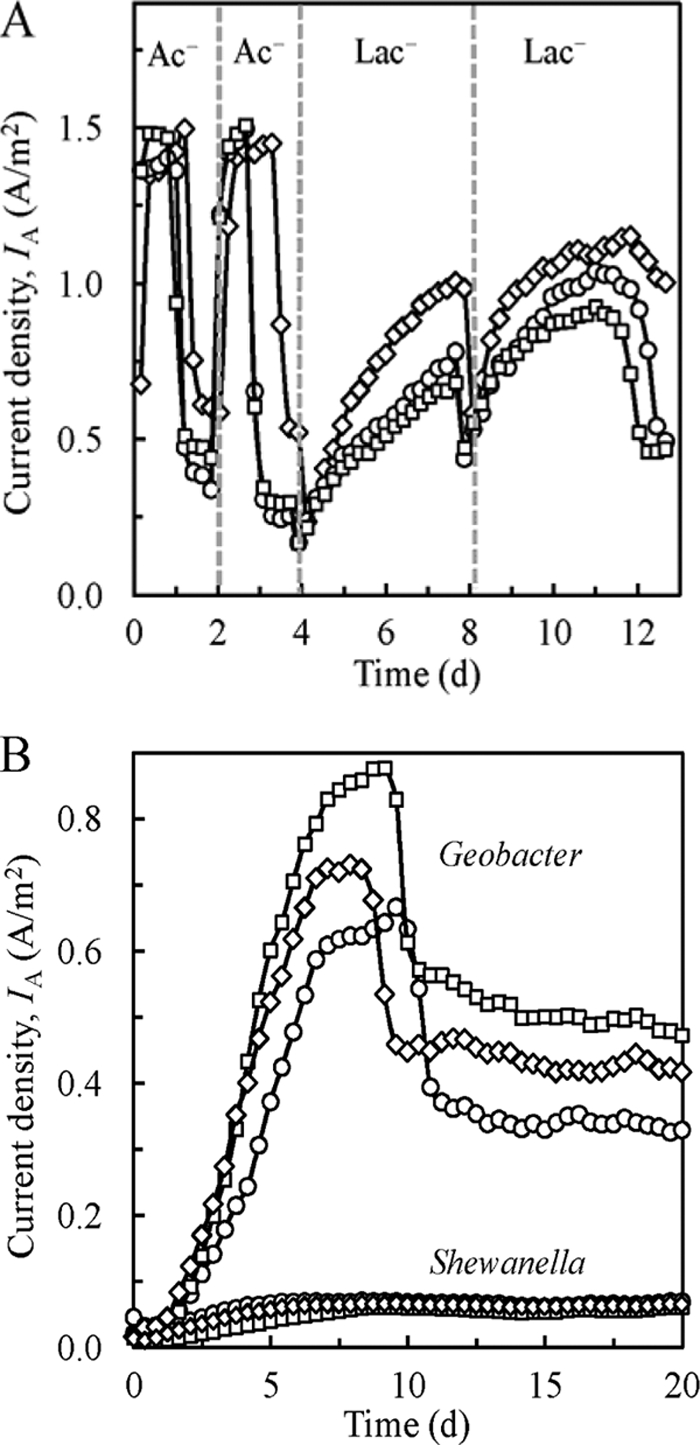
(A) Current densities of G. sulfurreducens in MECs fed acetate (Ac−) or lactate (Lac−) at an EAP of 0.7 V. Batch cycles were operated in triplicate in the order shown, with dotted lines representing times of replacement of medium. (B) Current densities of G. sulfurreducens and S. oneidensis in MECs fed lactate during an initial batch cycle at an EAP of 0.7 V. Both cultures were operated in triplicate.
After current production stabilized, the electron donor was switched to lactate (10 mM). The current density (IA; normalized to total anode surface area) reached a maximum of 0.8 ± 0.2 A/m2 (mean ± standard deviation) over a period of 4 days, which was ca. 50% less than the maximum current produced when the cultures were supplied with acetate. When the MECs were emptied, sparged with sterile, anaerobic gas, and refreshed with new medium, 0.3 ± 0.1 A/m2 was produced immediately after applying the voltage, supporting direct conversion of lactate into current. Improvements in current production for the triplicate samples were noted for the second fed-batch cycle, possibly due to evolution of G. sulfurreducens PCA for more efficient lactate utilization. Electrons were recycled via hydrogen gas, as shown by estimated Coulombic efficiencies (CE) above 100% for both acetate-fed (140% ± 15%) and lactate-fed (140% ± 33%) MECs. To eliminate the possibility that residual hydrogen produced in the MECs was the main contributor to current production, G. sulfurreducens PCA was also tested in a two-chamber reactor incorporating a membrane separator (Nafion) between the working and counter chambers (12-cm electrode spacing). The internal resistance of this two-chamber reactor is large, which results in a low current. Therefore, to increase current in this device and to better define the anode conditions, the potential of the anode (graphite rod, 14.5 cm2) was controlled at +0.4 V (versus a standard hydrogen electrode). This potential was selected to allow comparison with previous reports of acetate-fed reactors at this potential (1, 32). After 20 days, the current reached a maximum of ca. 0.2 A/m2 (data not shown) with an estimated CE of 12% ± 1%, confirming that lactate could serve as the sole electron donor for current generation. The higher current densities in the MEC tests (single chamber) than in the two-chamber tests may have been due to different operating conditions (fixed whole-cell potential versus fixed anode potential), a decrease in internal resistance due to closer electrode spacing and no membrane, or the presence of hydrogen, which could serve as an additional electron donor.
The use of lactate by G. sulfurreducens PCA allowed for the direct comparison of its electrode-reducing rates to those of S. oneidensis MR-1 under identical conditions. S. oneidensis MR-1 was grown overnight in Luria-Bertani medium at 30°C to an optical density at 600 nm (OD600) of 1.1 ± 0.0, centrifuged, washed three times in sterile FW medium, and inoculated into sterile MECs (in triplicate) containing the same medium used for testing G. sulfurreducens PCA. G. sulfurreducens PCA was also inoculated into new, sterile reactors (in triplicate) for comparison. The current production reached a maximum of 0.06 A/m2 for S. oneidensis MR-1, compared to 0.8 A/m2 for G. sulfurreducens PCA (Fig. 1B). The low current densities observed here for S. oneidensis MR-1 are consistent with previous reports of low current generation by this bacterium in MFCs and MECs (12, 14, 31).
Iron reduction using lactate.
Cells of G. sulfurreducens PCA were thawed from frozen stocks, grown at 30°C in anaerobic culture tubes containing acetate (10 mM) and ferric citrate (15 mM) in FW medium (10 ml) until complete Fe(III) reduction occurred, and transferred (10%) three times in FW medium containing lactate (2 mM) and ferric citrate (15 mM) under Fe(III)-limiting conditions. On the fourth transfer, the lactate, acetate, and pyruvate concentrations were recorded using high-performance liquid chromatography (HPLC), the Fe(II) production was measured using a ferrozine assay (19), and cell counts were done using an acridine orange staining procedure (10). After 10 days, G. sulfurreducens PCA produced 12.8 ± 0.6 mM Fe(II) and removed 2.6 ± 0.0 mM lactate, indicating a stoichiometry of Fe(II) production to lactate consumption of 4.9 ± 0.2 (Fig. 2). These results are not consistent with incomplete lactate oxidation to acetate via equation 1 (below), as this would have required a stoichiometric ratio of less than four when including substrate conversion to biomass.
| (1) |
| (2) |
| (3) |
Additional tests under conditions of limited lactate [1 mM lactate, 30 mM Fe(III); conducted in triplicate] confirmed that complete oxidation of lactate occurred. Pyruvate was detected, suggesting lactate oxidation via equation 2 with the transfer of two electrons, followed by pyruvate oxidation to acetate (equation 3). This result is consistent with pyruvate oxidation by G. sulfurreducens PCA, as described previously (26).
Fig. 2.
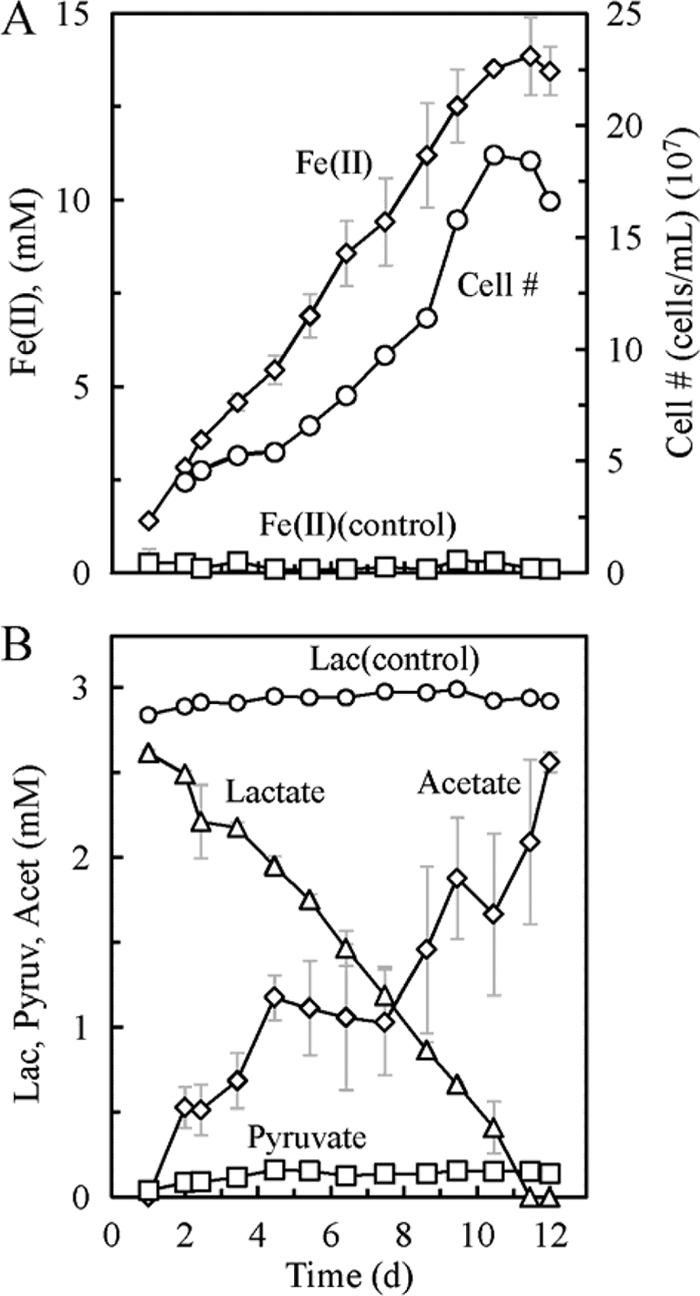
Fe(II) concentrations and cell numbers (A) and lactate (Lac), acetate (Acet), and pyruvate (Pyruv) concentrations (B) of G. sulfurreducens cells oxidizing lactate as the sole electron donor under conditions of limited Fe(III). Experiments were performed in triplicate with two uninoculated controls. Error bars show standard deviations.
All cultures were tested for purity by extracting DNA, conducting PCR of the 16S rRNA genes using forward primer 530F (5′-GTCCCAGCMGCCGCGG-3′) and reverse primer 1490R (5′-GGTTACCTTGTTACGACTT-3′), and sequencing the purified PCR products. All sequences returned clean and were confirmed as pure using the BLASTn algorithm at the National Center for Biotechnology Information (NCBI) website.
Implications.
These results provide the kinetics of lactate oxidation by G. sulfurreducens PCA coupled with either electrode or Fe(III) reduction along with the first direct comparison to a member of the Shewanella genus. Although sequences matching G. sulfurreducens in mixed communities supplied with lactate may be associated with strains capable of lactate oxidation, researchers have previously concluded that these Geobacter species relied on acetate production from incomplete lactate utilization by other members of the community (13–15), probably due to the previous conclusion by Caccavo et al. that G. sulfurreducens PCA did not utilize lactate (3). The results shown here expand upon more recent findings that strain PCA can grow using lactate and show, based on current generation in MECs, that electrode-reducing rates are substantially higher than those obtained with strain MR-1 under the same conditions. Elucidating the contribution of G. sulfurreducens to lactate oxidation within mixed communities warrants further investigation, along with examining the functional role of the genes (lactate permease and dehydrogenase) associated with lactate utilization.
Expanding the substrate diversity of G. sulfurreducens PCA is promising for the further development of MFCs. Bacterial respiration rates can be a limiting factor in MFCs and other BESs, as it is well known that certain substrates (e.g., acetate) result in higher current and power densities than others (e.g., butyrate and complex substrates, such as wastewaters) (8, 17). Therefore, methods to improve microbial kinetics in BESs can help to improve system performance. Adaptive evolution of G. sulfurreducens PCA for high-current-producing strains has been shown with acetate (33), but wastewaters and cellulosic fermentation effluents contain a diverse range of substrates. Although mixed communities can generate current from these waste streams (15), using strains of G. sulfurreducens that are highly adapted to acetate, lactate, pyruvate, and formate (7) may allow for increased current production compared to the levels obtained in mixed cultures.
Acknowledgments
This research was funded by the National Science Foundation Graduate Research Fellowship Program and by award no. KUS-I1-003-13 from the King Abdullah University of Science and Technology.
Footnotes
Published ahead of print on 14 October 2011.
REFERENCES
- 1. Bond D. R., Lovley D. R. 2003. Electricity production by Geobacter sulfurreducens attached to electrodes. Appl. Environ. Microbiol. 69:1548–1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bretschger O., et al. 2007. Current production and metal oxide reduction by Shewanella oneidensis MR-1 wild type and mutants. Appl. Environ. Microbiol. 73:7003–7012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Caccavo F., Jr., et al. 1994. Geobacter sulfurreducens sp. nov., a hydrogen- and acetate-oxidizing dissimilatory metal-reducing microorganism. Appl. Environ. Microbiol. 60:3752–3759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Call D., Logan B. E. 2008. Hydrogen production in a single chamber microbial electrolysis cell lacking a membrane. Environ. Sci. Technol. 42:3401–3406 [DOI] [PubMed] [Google Scholar]
- 5. Call D. F., Logan B. E. 2011. A method for high throughput bioelectrochemical research based on small scale microbial electrolysis cells. Biosens. Bioelectron. 26:4526–4531 [DOI] [PubMed] [Google Scholar]
- 6. Call D. F., Wagner R. C., Logan B. E. 2009. Hydrogen production by Geobacter species and a mixed consortium in a microbial electrolysis cell. Appl. Environ. Microbiol. 75:7579–7587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Coppi M. V., et al. 2007. Involvement of Geobacter sulfurreducens SfrAB in acetate metabolism rather than intracellular, respiration-linked Fe(III) citrate reduction. Microbiology 153:3572–3585 [DOI] [PubMed] [Google Scholar]
- 8. Hays S., Zhang F., Logan B. E. 2011. Performance of two different types of anodes in membrane electrode assembly microbial fuel cells for power generation from domestic wastewater. J. Power Sources 196:8293–8300 [Google Scholar]
- 9. Heidelberg J. F., et al. 2002. Genome sequence of the dissimilatory metal ion-reducing bacterium Shewanella oneidensis. Nat. Biotechnol. 20:1118–1123 [DOI] [PubMed] [Google Scholar]
- 10. Hobbie J. E., Daley R. J., Jasper S. 1977. Use of Nuclepore filters for counting bacteria by fluorescence microscopy. Appl. Environ. Microbiol. 33:1225–1228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Holmes D. E., Nicoll J. S., Bond D. R., Lovley D. R. 2004. Potential role of a novel psychrotolerant member of the family Geobacteraceae, Geopsychrobacter electrodiphilus gen. nov., sp. nov., in electricity production by a marine sediment fuel cell. Appl. Environ. Microbiol. 70:6023–6030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hu H., Fan Y., Liu H. 2008. Hydrogen production using single-chamber membrane-free microbial electrolysis cells. Water Res. 42:4172–4178 [DOI] [PubMed] [Google Scholar]
- 13. Jung S., Regan J. 2007. Comparison of anode bacterial communities and performance in microbial fuel cells with different electron donors. Appl. Microbiol. Biotechnol. 77:393–402 [DOI] [PubMed] [Google Scholar]
- 14. Kan J., Hsu L., Cheung A. C. M., Pirbazari M., Nealson K. H. 2011. Current production by bacterial communities in microbial fuel cells enriched from wastewater sludge with different electron donors. Environ. Sci. Technol. 45:1139–1146 [DOI] [PubMed] [Google Scholar]
- 15. Kiely P. D., Rader G., Regan J. M., Logan B. E. 2011. Long-term cathode performance and the microbial communities that develop in microbial fuel cells fed different fermentation endproducts. Biores. Technol. 102:361–366 [DOI] [PubMed] [Google Scholar]
- 16. Lanthier M., Gregory K. B., Lovley D. R. 2008. Growth with high planktonic biomass in Shewanella oneidensis fuel cells. FEMS Microbiol. Lett. 278:29–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu H., Cheng S., Logan B. E. 2005. Production of electricity from acetate or butyrate using a single-chamber microbial fuel cell. Environ. Sci. Technol. 39:658–662 [DOI] [PubMed] [Google Scholar]
- 18. Logan B. E., et al. 2008. Microbial electrolysis cells for high yield hydrogen gas production from organic matter. Environ. Sci. Technol. 42:8630–8640 [DOI] [PubMed] [Google Scholar]
- 19. Lovley D. R., Phillips E. J. P. 1986. Availability of ferric iron for microbial reduction in bottom sediments of the freshwater tidal Potomac River. Appl. Environ. Microbiol. 52:751–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lovley D. R., Phillips E. J. P., Lonergan D. J. 1989. Hydrogen and formate oxidation coupled to dissimilatory reduction of iron or manganese by Alteromonas putrefaciens. Appl. Environ. Microbiol. 55:700–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lovley D. R., Summers Z. M., Haveman S. A., Izallalen M. 2011. Geobacter strains that use alternate organic compounds, methods of making, and methods of use thereof. U.S. patent 20,110,151,544
- 22. Marsili E., et al. 2008. Shewanella secretes flavins that mediate extracellular electron transfer. Proc. Nat. Acad. Sci. 105:3968–3973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Methe B. A., et al. 2003. Genome of Geobacter sulfurreducens: metal reduction in subsurface environments. Science 302:1967–1969 [DOI] [PubMed] [Google Scholar]
- 24. Nevin K. P., et al. 2005. Geobacter bemidjiensis sp. nov. and Geobacter psychrophilus sp. nov., two novel Fe(III)-reducing subsurface isolates. Int. J. Syst. Evol. Microbiol. 55:1667–1674 [DOI] [PubMed] [Google Scholar]
- 25. Pinchuk G. E., et al. 2009. Genomic reconstruction of Shewanella oneidensis MR-1 metabolism reveals a previously uncharacterized machinery for lactate utilization. Proc. Natl. Acad. Sci. U. S. A. 106:2874–2879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Segura D., Mahadevan R., Juárez K., Lovley D. R. 2008. Computational and experimental analysis of redundancy in the central metabolism of Geobacter sulfurreducens. PLoS Comput. Biol. 4:e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shelobolina E. S., Vrionis H. A., Findlay R. H., Lovley D. R. 2008. Geobacter uraniireducens sp. nov., isolated from subsurface sediment undergoing uranium bioremediation. Int. J. Syst. Evol. Microbiol. 58:1075–1078 [DOI] [PubMed] [Google Scholar]
- 28. Snoeyenbos-West O. L., Nevin K. P., Anderson R. T., Lovley D. R. 2000. Enrichment of Geobacter species in response to stimulation of Fe(III) reduction in sandy aquifer sediments. Microb. Ecol. 39:153–167 [DOI] [PubMed] [Google Scholar]
- 29. Tang Y. J., et al. 2009. Metabolic flux analysis of Shewanella spp. reveals evolutionary robustness in central carbon metabolism. Biotechnol. Bioeng. 102:1161–1169 [DOI] [PubMed] [Google Scholar]
- 30. Ueki T., Lovley D. R. 2010. Genome-wide gene regulation of biosynthesis and energy generation by a novel transcriptional repressor in Geobacter species. Nucleic Acids Res. 38:810–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Watson V. J., Logan B. E. 2010. Power production in MFCs inoculated with Shewanella oneidensis MR-1 or mixed cultures. Biotechnol. Bioeng. 105:489–498 [DOI] [PubMed] [Google Scholar]
- 32. Wei J., Liang P., Cao X., Huang X. 2010. A new insight into potential regulation on growth and power generation of Geobacter sulfurreducens in microbial fuel cells based on energy viewpoint. Environ. Sci. Technol. 44:3187–3191 [DOI] [PubMed] [Google Scholar]
- 33. Yi H., et al. 2009. Selection of a variant of Geobacter sulfurreducens with enhanced capacity for current production in microbial fuel cells. Biosens. Bioelectron. 24:3498–3503 [DOI] [PubMed] [Google Scholar]


