Abstract
Microbial nitrate-dependent, Fe(II) oxidation (NDFO) is a ubiquitous biogeochemical process in anoxic sediments. Since most microorganisms that can oxidize Fe(II) with nitrate require an additional organic substrate for growth or sustained Fe(II) oxidation, the energetic benefits of NDFO are unclear. The process may also be self-limiting in batch cultures due to formation of Fe-oxide cell encrustations. We hypothesized that NDFO provides energetic benefits via a mixotrophic physiology in environments where cells encounter very low substrate concentrations, thereby minimizing cell encrustations. Acidovorax sp. strain 2AN was incubated in anoxic batch reactors in a defined medium containing 5 to 6 mM NO3−, 8 to 9 mM Fe2+, and 1.5 mM acetate. Almost 90% of the Fe(II) was oxidized within 7 days with concomitant reduction of nitrate and complete consumption of acetate. Batch-grown cells became heavily encrusted with Fe(III) oxyhydroxides, lost motility, and formed aggregates. Encrusted cells could neither oxidize more Fe(II) nor utilize further acetate additions. In similar experiments with chelated iron (Fe(II)-EDTA), encrusted cells were not produced, and further additions of acetate and Fe(II)-EDTA could be oxidized. Experiments using a novel, continuous-flow culture system with low concentrations of substrate, e.g., 100 μM NO3−, 20 μM acetate, and 50 to 250 μM Fe2+, showed that the growth yield of Acidovorax sp. strain 2AN was always greater in the presence of Fe(II) than in its absence, and electron microscopy showed that encrustation was minimized. Our results provide evidence that, under environmentally relevant concentrations of substrates, NDFO can enhance growth without the formation of growth-limiting cell encrustations.
INTRODUCTION
Microbially driven, Fe(III)/Fe(II) redox transitions in the environment have a dramatic impact on iron's solubility, mineralogy, sorption characteristics, and overall geochemical properties (21, 29, 45). Microbially mediated redox transformations of Fe can also affect the biogeochemical cycling of other key nutrients (e.g., C, S, P, and N) (15, 29), trace metals (9, 49), metalloids (12), and the fate of organic pollutants (25) and contaminant metals (8), including those released from industrial and mining areas (30). Anaerobic, microbial Fe(III) reduction has been studied extensively over the last 30 years (31, 45), and much is known about the microbiology and geochemistry of that process.
Chemotrophic, anoxic Fe(II) oxidation under circumneutral conditions, on the other hand, was first described only 15 years ago (42), and little is known about the microbiology of the process or its actual significance in the environment. Microbial nitrate-dependent, Fe(II) oxidation (NDFO) has been demonstrated in a variety of sediments, microbial consortia, and pure cultures, including both autotrophic and heterotrophic cultures. With respect to autotrophic growth via NDFO, for example, the mesophilic enrichment culture originally described by Straub et al. (42) has been maintained for over a decade under autotrophic conditions and is capable of near complete oxidation of Fe(II) and reduction of NO3− to N2 (4). Notwithstanding this example and other reports of autotrophy (18, 46, 48), the majority of known cultures capable of NDFO are phylogenetically diverse, organotrophic, NO3−-reducing bacteria that can oxidize Fe(II) in the presence of a reduced carbon compound, e.g., acetate, as an additional electron donor (5, 16, 22, 23, 26, 27, 34, 43–45). In general, it appears that a subset of organotrophic, NO3−-reducing bacteria can oxidize Fe(II), either cometabolically or through a mixotrophic physiology, and that long-term, continued Fe(II) oxidation by such NO3− reducers requires an organic cosubstrate, e.g., acetate, at low concentration, typically 0.5 to 1.0 mM in batch cultures.
It is not clear whether Fe(II) oxidation confers an energetic benefit or whether Fe(II) oxidation is a fortuitous, side reaction, possible involving both abiotic and enzymatic reactions, that occurs during heterotrophic growth. In recent batch experiments with Acidovorax sp. strain BoFeN1, Muehe et al. demonstrated a slight increase in cell numbers in the presence of Fe(II) and acetate versus cells with acetate alone (34), suggesting that Fe(II) oxidation may provide an energetic benefit. Further experiments are needed to determine whether long-term growth yields can be enhanced by Fe(II) oxidation and whether this enhancement can be realized at environmentally relevant Fe2+, NO3−, and organic C concentrations.
In NDFO batch cultures, encrustation of cells with Fe(III) oxyhydroxides is frequently observed, and Fe(II) oxidation ceases after the cells become heavily encrusted (22). This suggests that encrustation may be growth-limiting and that NDFO may not be a significant, long-term physiology in the environment. Since these batch culture experiments were all conducted with millimolar concentrations of Fe2+ and NO3− (22, 42, 43), it is not known whether encrustations form at lower concentrations.
In the present study, we have characterized a facultatively anaerobic, betaproteobacterium, Acidovorax sp. 2AN, in terms of its ability to oxidize aqueous (uncomplexed) Fe2+, chelated Fe(II) (Fe(II)-EDTA), and solid-phase Fe(II) coupled to nitrate reduction at circumneutral pH. We have also demonstrated NDFO-enhanced growth, both in batch cultures and in a novel, continuous-flow system using micromolar Fe2+ and NO3− concentrations. This is the first report of anoxic, Fe(II)-oxidation-enhanced, chemotrophic growth using environmentally relevant concentrations of Fe2+ and NO3−.
MATERIALS AND METHODS
Bacterial strain and growth conditions.
The bacterium used in these experiments was enriched from a mixture of iron-oxide-bearing sediment from Dorn Creek, WI, that had been used in long-term biogeochemical studies of Fe redox cycling. The studies were conducted in an anoxic sediment column identical to that described elsewhere (36) but operated for several months under fluctuating loading of NO3− and acetate (F. Picardal, unpublished data). Sediment from the column was collected in an anaerobic chamber and serially diluted in medium designed to enrich microorganisms capable of mixotrophic NDFO. The medium (PAFe2N2) contained 5 mM NaNO3, 5 mM FeCl2, 0.5 mM sodium acetate, 0.6 mM CaCl2, 0.2 mM KCl, 0.5 mM MgCl2, 1.0 mM NH4Cl, 0.1 mM KH2PO4, 25 mg of yeast extract (Difco, Detroit, MI)/liter, 2.5 ml of SL10 trace minerals solution (2)/liter, and 5 ml of vitamin solution (41)/liter and was buffered at pH 7 with 5 mM PIPES. All enrichment and culturing was done in anaerobic culture tubes or bottles under an N2 headspace unless otherwise noted. The highest dilution showing visible Fe(II) oxidation was further enriched by multiple passages over several months through similar medium. A pure culture (strain 2AN) was eventually obtained by picking single colonies that grew on anoxic, organotrophic nitrate-reducing medium lacking FeCl2 but containing 5 mM acetate and solidified with 1.5% agar. The plates were incubated inside an anaerobic chamber with an N2 (97%) and H2 (3%) atmosphere. The culture so obtained retained its NDFO physiology when transferred back to the mixotrophic medium described above.
The pure culture was stored at −80°C using 10% dimethyl sulfoxide as the cryoprotectant or maintained anaerobically in medium (AGW) containing 5 mM NO3− as the electron acceptor, 10 mM acetate as the electron donor, 0.6 mM CaCl2, 0.2 mM KCl, 0.5 mM MgCl2, 0.04 mM KH2PO4, 0.19 mM NH4Cl, 2.5 ml of SL10 trace minerals/liter, 5 ml of vitamin solution/liter, and 50 mg of yeast extract/liter. The degassed, O2-free medium was buffered at pH 7 with 10 mM PIPES, and cultures were maintained under an N2 headspace.
Phylogenetic analysis.
Genomic DNA of the isolate was obtained using an UltraClean Microbial DNA isolation kit (MoBio Laboratories, Carlsbad, CA), and the 16S rRNA gene was amplified using the general bacterial primers 8F (5′-AGAGTTTGATCCTGGCTCAG-3′) and 1392R (5′-ACGGGCGGTGTGTRC-3′) from Invitrogen (Carlsbad, CA). The following PCR program was used: 95°C for 5 min, followed by 33 cycles at 95°C for 30 s, 50°C for 40 s, and 72°C for 2 min. After clean-up using a QIAquick nucleotide removal kit (Qiagen, Inc., Valencia, CA), the PCR product was sequenced following an ABI BigDye terminator cycle sequencing reaction using an Applied Biosystems 3730 automated sequencing system (Applied Biosystems, Inc., Foster City, CA). The resultant sequences were edited and aligned using CodonCode Aligner v.2.0.6 (CodonCode Corp., Dedham, MA).
Batch experiments.
Unless otherwise noted, batch experiments were performed using modified, anoxic medium as described above but lacking yeast extract, buffered at pH 6.8 to 6.9 with 30 mM bicarbonate instead of PIPES, and containing experimentally dependent concentrations of 0.5 to 2 mM acetate and 2 to 5 mM NO3−. Fe2+ was added from an anoxic, 1 M stock solution of sterile-filtered FeCl2. As described by Kappler et al. (22), vivianite and siderite produced, respectively, by reaction of Fe2+ with phosphate and bicarbonate were allowed to precipitate overnight in the anoxic medium, followed by filtration (0.2-μm pore size) to remove the solids, leaving 6 to10 mM dissolved Fe2+ in the medium. Portions (50 ml) of anoxic, sterile medium was added to 72-ml serum bottles, sealed with butyl rubber stoppers (Bellco Glass, Inc., Vineland, NJ), and the headspace was replaced with N2:CO2 (80:20 by volume). In experiments with chelated Fe, 5.0 mM Fe(II)-EDTA was used instead of soluble Fe2+ (26). In experiments with solid-phase Fe(II), 10 mM PIPES was used instead of bicarbonate in the modified AGW medium. Three different forms of solid-phase Fe(II), microbially reduced goethite (α-FeOOH), chemically precipitated siderite (FeCO3) (47), and biogenic magnetite (Fe3O4) were added to produce three different sets of media. Biologically reduced minerals were produced, respectively, by reduction of goethite and hydrous ferric oxide by Shewanella putrefaciens strain 200, followed by pasteurization and anoxic washing as previously described (47). Each medium in solid-phase experiments contained 4.5 to 6 mM weak-acid-extractable Fe(II).
For all batch experiments, the inoculum was prepared from an organotrophically grown (10 mM acetate, 5 mM NO3−) culture that was harvested, washed twice under anoxic conditions, and resuspended in bicarbonate-buffered minimal medium. Batch reactors were incubated at 30°C in the dark without shaking. Heat-killed controls were prepared by pasteurizing (80°C, 10 min) the inoculum in a hot water bath.
Continuous-flow experiments.
A continuous-flow system was developed to examine the ability of the isolate to grow with micromolar concentrations of substrates not possible in batch reactors. An organotrophically grown inoculum was prepared as described above with a culture density of ca. 1 × 105 to 2 × 105 cells/ml. A series of sterile-filter cassettes, each containing one 25-mm, 0.22-μm-pore-size, polycarbonate black-stained filter (GE Osmonics, Milwaukee, WI), were anoxically inoculated by injection of 2 ml of the above inoculum through the filter cassette. Seven to eight cassettes containing the immobilized cells were then connected in parallel to a 60-ml sterile syringe containing the medium via a sterile manifold (depicted in Fig. S1 in the supplemental material). The manifold was prepared using sterile Luer-Lok T-connectors. The entire assembly was operated inside an anaerobic chamber (Coy Laboratory Products, Grass Lake, MI). The medium was passed continuously through the filters from the syringe at a flow rate of 2 ml/h using a KDS 220 multisyringe infusion pump (KD Scientific, Inc., Holliston, MA). This resulted in an approximate flow rate of 250 μl/h through each filter. One filter cassette was removed from the manifold every 2 days for cell counting and replaced with a sterile filter cassette to maintain constant flow rates. The syringe was refilled with fresh, anoxic media at the time of each sampling. The sampled filter with attached cells was rinsed by filtration of a 2% glutaraldehyde solution, and fixed cells were counted with an acridine orange direct counting method (24) to monitor growth. In some cases, the filter was also examined by using electron microscopy.
Continuous-flow experiments consisted of side-by-side comparisons of growth in systems containing from 0.0 to 250 μM Fe2+. The medium used was the bicarbonate-buffered medium described above for the batch studies but modified to contain 2 mM bicarbonate, 100 μM nitrate, and 20 μM acetate. Each set of experiments used the identical inoculum in medium with or without Fe2+ to make certain that small differences in growth were due only to the presence of Fe2+ rather than to inoculum differences.
Analytical procedures.
For all of the batch culture experiments, N2-purged syringes were used when collecting samples for acetate or nitrate analysis outside the anaerobic chamber. During sampling for measurement of Fe(II), sampling and processes was done inside the anaerobic chamber. An ∼1.5-ml sample was collected using a syringe while the bottle was shaken vigorously to keep the solids uniformly suspended. Subsequently, samples were centrifuged to separate aqueous Fe2+ and sorbed, weak-acid-extractable Fe2+ (10). Centrifugation and removal of the supernatant was necessary to avoid rapid Fe(II) oxidation by biogenic nitrite under acidic conditions during HCl extraction (47). Aqueous Fe2+ and sorbed Fe2+ extracted from the pellet using 0.5 N HCl were measured using ferrozine as previously described (9, 40). Total Fe(III) in the pellet was measured by the ferrozine method after reduction by hydroxylamine hydrochloride and solid dissolution (14). In experiments using Fe(II)-EDTA, Fe(II) oxidation was quantified using o-phenanthroline as the colorimetric reagent (26). NO3− concentrations were determined either by a nitrate electrode (28) or, more commonly, via a modified version of the colorimetric technique described by Cataldo et al. (6). Colorimetric methods were also used to quantify NO2− (17) and ammonium (20) concentrations. Acetate concentrations were determined by high-performance liquid chromatography (HPLC) using a Waters 2690 Separations Module (Waters Corp., Milford, MA) equipped with a Waters Fast Fruit Juice Column at 55°C and 0.05% phosphoric acid as the mobile phase in combination with a UV detector (λ = 210 nm). Fe2+ in samples for HPLC analysis was removed via air oxidation and filtration prior to sample injection. Cell numbers in batch reactors with precipitated Fe(III) oxides were calculated using an acridine orange direct counting method (37) after dissolving particulate Fe(III) using ammonium oxalate and, if necessary, disrupting cell aggregates by the addition of the surfactant, Tween 80 (Sigma-Aldrich Corp., St. Louis, MO) at a final 0.001% concentration.
SEM.
Samples from batch reactors for scanning electron microscopy (SEM) were withdrawn from reactors and transferred to 0.2-μm-pore-size filters in the anaerobic chamber. Cells on these filters and similar filters from the continuous-flow experiments were fixed anoxically with 2% glutaraldehyde and air dried overnight inside the anaerobic chamber (39). Although sorption of Fe2+ or formation of Fe(III)-oxide encrustations allowed SEM examination without further treatment for cells incubated in the presence of Fe2+, cells from cultures grown organotrophically in the absence of Fe2+ were gold-palladium coated to improve visualization. During imaging, the dried filters were placed directly on the SEM stage and examined using a FEI Quanta 400 FEG SEM (FEI Company, Hillsboro, OR) at 10 and 15 kV under low vacuum conditions using a gaseous secondary electron detector. The stage was at room temperature with a chamber pressure of 80 Pa. A subset of samples was gold coated for better contrast and examined under high vacuum conditions (<6 × 10−4 Pa).
RESULTS
Acidovorax sp. 2AN.
Strain 2AN is a motile rod, ca. 1 to 1.5 μm in length, and is capable of organotrophic growth using acetate as the sole carbon and energy source under aerobic and nitrate-reducing conditions. Using NO3− as an electron acceptor, it is also able to utilize lactate, malate, citrate, and fumarate, but not formate, as growth substrates. A BLAST search of the NCBI database using the 2AN 16S rRNA gene sequence (GenBank accession no. HM625980) revealed the greatest sequence similarity with members of the genus Acidovorax. The closest relatives were A. temperans C23 (GenBank accession no. HQ259690.1, 99% similarity), A. temperans C24 (GenBank accession no. HQ259691.1, 99% similarity), Acidovorax sp. strain R-25074 (GenBank accession no. AM084034.1, 99% similarity), and Acidovorax sp. strain LR05 (GenBank accession no. EU263279.1, 98% similarity). We have therefore classified the isolate as Acidovorax sp. 2AN. A 16S phylogenetic tree is shown in Fig. S2 in the supplemental material.
Anaerobic Fe(II) oxidation and growth in batch systems.
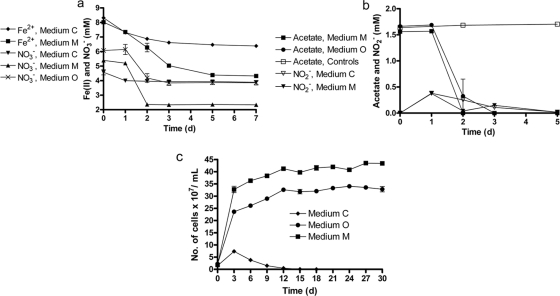
To evaluate patterns of Fe2+ oxidation and growth in batch cultures, Acidovorax sp. 2AN was cultured in 3 different anoxic media, i.e., “organotrophic” medium O (∼ 6 mM NO3− and 1.6 mM acetate), “chemolithoautotrophic” medium C (∼5 mM NO3− and 8 to 8.5 mM Fe2+) that lacked a C source, and “mixotrophic” medium M (5 mM NO3−, 8 to 8.5 mM Fe2+, and 1.6 mM acetate).
Consumption of NO3− (∼3 mM) and Fe(II) oxidation (∼3.5 mM) were more pronounced in replicates supplemented with acetate in comparison to the consumption of NO3− (∼0.5 mM) and Fe(II) oxidation (∼1.7 mM) in reactors without acetate (Fig. 1a). Neither compound was consumed in controls inoculated with heat-killed cultures (see Fig. S4 in the supplemental material). The accumulation of NO2− was observed at day 1 in both sets of Fe2+-containing replicates (medium M and medium C) with concomitant oxidation of Fe2+. In medium M with Fe2+ and acetate, NO2− was depleted by day 2 but was detected again on day 3 (Fig. 1b). No NO2− was produced in controls or in medium O reactors lacking Fe2+. In all cases, acetate was depleted after day 3. The consumption of NO3− and oxidation of Fe2+ ceased by day 5.
Fig. 1.
(a and b) Transformations of NO3− and Fe2+ by Acidovorax sp. 2AN in batch reactors using different media. NO3− was present in all of the replicates at an initial concentration of 4.6 to 6 mM. When present, Fe2+ and acetate were present at initial concentrations of 8 to 8.4 mM and 1.5 to 1.7 mM, respectively. Medium O (organotrophic) contained only acetate as the electron donor, medium C (chemolithotrophic) contained only Fe2+ as the electron donor, and medium M (mixotrophic) contained both acetate and Fe2+. The data for heat-killed controls are presented in the supplemental material. (c) Comparative growth of strain 2AN in different media when sequentially transferred for 30 days. The data are presented as mean ± the standard deviations (n = 3). When not shown, error bars are smaller than the symbol size.
After 5 days of incubation, cell numbers (1.6 × 108 ± 0.1 × 108 cells/ml) in the chemolithoautotrophic medium C were lower than in the other two media containing acetate. Cell numbers in medium M (4.4 × 108 ± 0.2 × 108 cells/ml) containing both Fe2+ and acetate were ca. 29% greater than those in medium O (3.4 × 108 ± 0.2 × 108 cells/ml) which contained only acetate as the electron donor. These statistically significant results (P = 0.0067) suggested that nitrate-dependent, Fe(II) oxidation might be beneficial for growth. Microscopic examination revealed that cells had become aggregated and encrusted with oxide precipitates after several days. These encrustations occurred only in reactors containing Fe2+ and were not observed in medium O (see Fig. 6a and c).
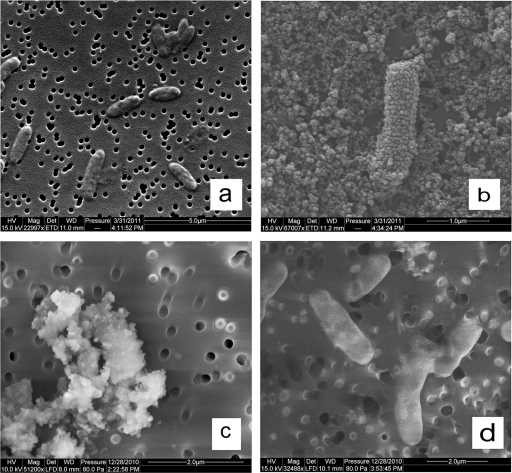
Fig. 6.
SEM images of Acidovorax sp. 2AN grown in different conditions. (a) 4-day-old cells grown on organotrophic medium O in batch culture. (b) A single cell with uniform Fe(III) oxyhydroxide encrustations after 2 h of incubation in mixotrophic medium M in batch culture. (c) Heavily encrusted cells and cell-mineral aggregates after a 4-day incubation in medium M in batch culture. (d) Cells from continuous-flow systems containing 250 μM Fe2+ after 16 days showing no cellular encrustation or cell-mineral aggregates. The images in panels a and b were made on gold-coated samples in high vacuum mode, and the images in panels c and d were made on uncoated samples in low vacuum mode.
In order to confirm our observation that the growth yield of strain 2AN is consistently higher in the mixotrophic medium, we sequentially transferred cultures in the three medium types for 30 days at 3 days intervals. By sequentially transferring 1 ml of 3-day-old culture to 9 ml of fresh medium, we expected to more clearly see small but consistent variations in growth yield and to dilute out those cells growing only with endogenous substrates. In the chemolithoautotrophic medium, cell numbers gradually decreased after the first sampling and were eventually diluted out (Fig. 1c). This suggests that strain 2AN is not able to grow autotrophically using NDFO as the primary energy source. On the other hand, we observed that the growth yield in the mixotrophic medium was consistently ca. 30% higher than in the organotrophic medium for almost all sampling points throughout the 30-day experiment (Fig. 1c).
Acidovorax sp. 2AN was able to oxidize biogenic reduced goethite, chemically precipitated siderite, and biogenic magnetite under mixotrophic conditions with concomitant consumption of NO3− and acetate (see Fig. S3 in the supplemental material). We observed complete oxidation of total Fe(II) [sum of aqueous and weak-acid-extractable Fe(II)] in reduced goethite (∼5 mM) and ca. 85% oxidation of the total Fe(II) in magnetite and siderite (see Fig. S3a in the supplemental material). Acetate was depleted by day 5 and replenished with ca. 1.1 mM acetate (see Fig. S3c in the supplemental material). At day 10, acetate was again depleted.
Effects of Fe(III) oxyhydroxide encrustations on viability and physiology.
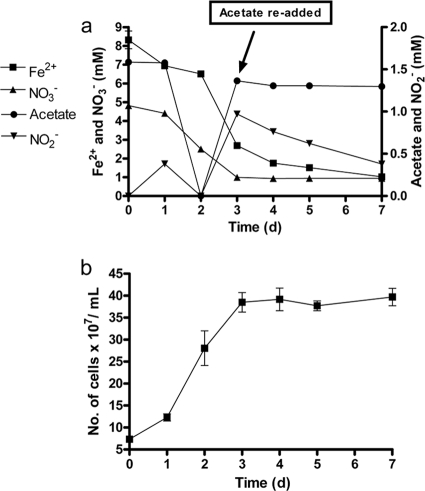
An experiment was conducted to determine whether the encrusted cells observed in batch cultures could resume growth and oxidation of acetate or Fe2+ if acetate was added again to the reactors. Consumption of NO3− (∼4 mM), Fe2+ (6 mM), and acetate (1.6 mM) was observed in cultures during the initial 3 days, along with accumulation of NO2− (Fig. 2a). An additional 1.6 mM acetate was added on day 3 but no further acetate or NO3− consumption occurred. In addition, acetate re-addition did not result in further cell growth (Fig. 2b), suggesting that cells had become metabolically inactive as a result of oxyhydroxide encrustation. After acetate addition, however, Fe2+ and NO2− consumption slowly continued, likely a result of abiotic Fe2+ oxidation by NO2− (7) produced during the initial period of NDFO.
Fig. 2.
(a) Transformations of NO3−, Fe2+, and acetate by Acidovorax sp. 2AN under mixotrophic (containing both Fe2+ and acetate) culture conditions. (b) Cell numbers. Nitrate was present in all of the replicates at an initial concentration of 5 mM. Fe2+ and acetate were present at initial concentrations of 8.3 and 1.6 mM, respectively. An additional 1.6 mM acetate was added on day 3. The data for heat-killed controls were similar to that shown in the supplemental material and is omitted for clarity. The data are presented as mean ± the standard deviations (n = 3). When not shown, error bars are smaller than the symbol size.
Oxidation of Fe(II)-EDTA.
Since Fe2+ oxidation in batch cultures resulted in Fe oxyhydroxide encrustations that apparently limited further growth or Fe(II) oxidation, we evaluated oxidation of chelated Fe(II), i.e., Fe(II)-EDTA, and the effects of such oxidation on the viability and physiology of the cells. In these experiments, the chemolithotrophic medium C and mixotrophic medium M used was modified by replacing FeCl2 with 5.0 mM Fe(II)-EDTA.
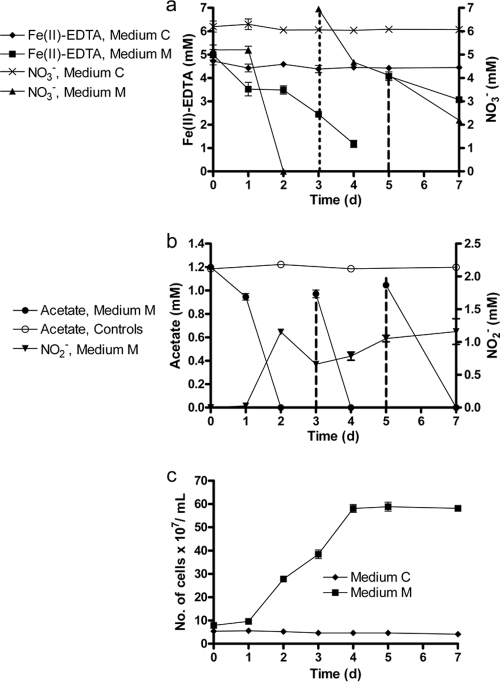
Fe(II)-EDTA and NO3− were not significantly transformed in heat-killed controls (see Fig. S4 in the supplemental material). Although negligible Fe(II)-EDTA oxidation and no NO3− consumption was observed in medium C, NO3− was completely consumed and extensive Fe(II) oxidation occurred over the first 3 days of the experiment in medium M (Fig. 3a). These replicates consumed additional NO3− and Fe(II) when they were replenished with 7 mM NO3− and 4 mM Fe(II)-EDTA on days 3 and 5, respectively. The accumulation of NO2− was observed at day 2 in medium M, concomitant with oxidation of Fe(II)-EDTA, and remained at low millimolar concentrations throughout the experiment (Fig. 3b). Acetate was depleted at day 2 and was replenished on days 3 and 5 (Fig. 3b). Growth was only observed in medium M (Fig. 3c). In contrast to what we had seen in the experiments described above with Fe2+, no cell encrustations or clustering of cells occurred during this experiment. Overall, the results showed that the use of a chelator prevents formation of cell encrustations and allows utilization of repeated additions of electron donor and acceptor.
Fig. 3.
(a) Transformations of NO3− and Fe(II)-EDTA by Acidovorax sp. 2AN in batch cultures. Medium C contained only 5 mM Fe(II)-EDTA as the electron donor. Medium M contained both 5 mM Fe(II)-EDTA and 1.2 mM acetate. All media and controls contained 5 to 6 mM NO3−. The data for heat-killed controls are presented in the supplemental material. The dotted and dashed lines, respectively, represent points when 7 mM nitrate and 4 mM Fe(II)-EDTA were re-added. (b) Consumption of acetate and accumulation of extracellular nitrite in the medium. The dashed lines represent the points when ca. 1 mM acetate was re-added. (c) Growth of strain 2AN in medium C and medium M. The data are presented as mean ± the standard deviations (n = 3). When not shown, error bars are smaller than the symbol size.
Growth of Acidovorax sp. 2AN under continuous-flow conditions.
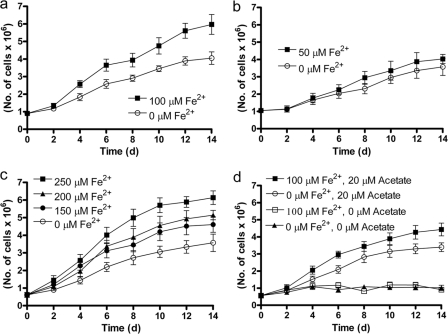
A continuous-flow system using cells immobilized on micropore filters was developed to evaluate Fe(II)-oxidation-dependent growth under μmolar concentration regimes more likely to be encountered in sedimentary environments. A series of side-by-side experiments with 20 μM acetate, 100 μM NO3−, and 0 to 250 μM Fe2+ were performed for 2 weeks using the same inoculum in each experiment. We observed a consistently higher growth rate in all cases when Fe2+ was present than when it was absent (Fig. 4). This was the case even at the lowest Fe2+ concentration, 50 μM, that was utilized (Fig. 4b). In a control experiment lacking Fe2+ and acetate but containing NO3−, no significant growth occurred (Fig. 4d) showing that growth was not due to endogenous electron donors or that they were rapidly exhausted under oligotrophic conditions. In additional controls containing 100 μM Fe2+ but lacking acetate, no growth occurred (Fig. 4d). As shown in Fig. 5, a plot of [Fe2+] versus the initial, mean growth rates from the various experiments depicted in Fig. 4 reveals that growth rate is linearly dependent on [Fe2+].
Fig. 4.
Comparisons of growth curves of Acidovorax sp. 2AN in organotrophic and mixotrophic media with various Fe2+ concentrations. Each panel represents a different set of experiments. The same inoculum was used in each set of experiments as described in the text. All media contained 100 μM NO3− and, except where noted in panel d, 20 μM acetate. The data are presented as mean ± the standard deviations for cell counts in 10 microscopic fields.
Fig. 5.
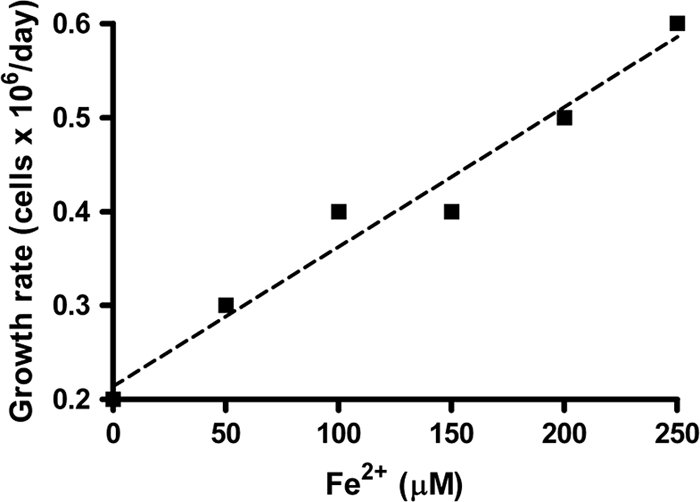
Relationship of initial growth rate of Acidovorax sp. 2AN to [Fe2+] used in the continuous-flow experiments. Mean growth rates were calculated using the cell count data from day 2 to day 4 depicted in Fig. 4. The dashed line represents the regression line with R2 value of 0.966.
It should also be noted that, during epifluorescence microscopy used in this experiment to enumerate cells, we did not notice iron oxyhydroxide cell encrustations at any Fe2+ concentration, a result that could occur if submicron-scale particles formed at these low concentrations and were carried away prior to mineral nucleation and growth of larger particulates. The development of cellular encrustations was also evaluated using SEM (Fig. 6). Compared to 4-day-old, organotrophically grown cells (Fig. 6a), cells from medium M batch reactors (8 mM Fe2+) showed noticeable precipitates after an incubation of only 2 h (Fig. 6b). After a 4-day batch incubation in medium M, encrustations were extensive and individual cells could not be clearly distinguished (Fig. 6c). In continuous-flow systems, however, SEM examination revealed minimal cell coatings, even at 250 μM Fe2+, the highest Fe2+ concentration utilized (Fig. 6d).
DISCUSSION
Batch culture experiments with Acidovorax sp. 2AN clearly showed that the bacterium was able to oxidize aqueous Fe2+, solid-phase Fe(II), and chelated Fe(II). Although EDTA was used as a model chelator in our experiments, we recognize that Fe(II) chelated by natural organic matter may behave differently due to alternate redox potentials and the stability of the metal-ligand complex. To our knowledge, this is the first report of a bacterium able to oxidize all important forms of Fe(II) likely to be present in anoxic sediments. In medium C which lacked organic C, limited Fe(II) oxidation and minimal growth in batch culture suggests that strain 2AN is not capable of lithoautotrophic growth on NO3− and Fe(II). We were also unable to grow this strain lithoautotrophically using H2 as the electron donor (data not shown). Using the draft genome for Acidovorax sp. 2AN (available at http://www.jgi.doe.gov/), we were also unable to find any genes encoding key enzymes involved in CO2 fixation pathways.
Growth in batch cultures containing acetate was consistently greater in the presence of Fe2+ than in its absence, suggesting that Fe(II) oxidation might be energetically beneficial via a mixotrophic physiology in the presence of an organic cosubstrate. Muehe et al. also observed a similarly enhanced growth yield with Acidovorax strain BoFeN1 under mixotrophic conditions in batch culture (34). Fe(II)-oxidation-enhanced growth was also clearly demonstrated when batch cultures of 2AN were sequentially transferred to fresh, mixotrophic medium 10 times over a 30-day period (Fig. 1c). In each transfer, cell numbers were always greater after 3 days when Fe2+ was present.
Although the presence of ca. 8 mM Fe2+ in the mixotrophic medium enhanced growth, cells were always found to be heavily encrusted with Fe(III) oxyhydroxides after approximately 3 to 4 days of incubation in batch cultures. The encrusted cells also lost motility and were always present in aggregates. Encrusted cells were also unable to utilize acetate and NO3− when these compounds were re-added to Fe2+-containing, batch cultures, showing that encrustations prevented use of heterotrophic growth substrates, likely due to blocking of transport into the cell. In the presence of a chelator to prevent precipitation of Fe(III) oxyhydroxides, strain 2AN retained motility and could metabolize further additions of Fe(II)-EDTA and acetate with concomitant growth.
In a series of publications (21, 22, 32, 38), Kappler and coworkers have also described formation of Fe(III) oxyhydroxides precipitates both externally and in the periplasm of Acidovorax strain BoFeN1 and have suggested Fe(II) oxidation occurs periplasmically. We believe that the encrustations formed with Fe2+ in batch reactors are artifacts of the millimolar-level Fe2+ and NO3− concentrations typically used in batch experiments which, until now, have been the only way to showcase the NDFO capability of these strains.
Similar formation of oxyhydroxide precipitates on cells was shown in previous work to result from the abiotic reaction between biogenic NO2− and Fe2+ sorbed onto cell surfaces (7, 10). In the present study, NO2− was measured in cultures containing Fe2+ but not in medium O which contained only acetate as the electron donor. These phenomena could be explained if Fe2+ entered the periplasm via the porous outer membrane and sorbed to periplasmic proteins. The abiotic reaction between sorbed Fe2+ and NO2− produced during NO3− reduction would then lead to the precipitation of Fe(III) oxyhydroxides on periplasmic proteins, including the nitrite reductase, which is typically located in the periplasm (1, 11). Oxyhydroxide accumulations in the periplasm of Acidovorax sp. strain BoFeN1 have indeed been observed by Miot et al. during NDFO (32). Such mineral coatings on the nitrite reductase would decrease the rate of NO2− reduction and therefore lead to the observed accumulation of NO2− in medium containing Fe2+. This proposition is supported by the lack of extracellular NO2− accumulation during organotrophic growth on acetate and nitrate. The relative kinetics of NO3− and NO2− reduction have previously been used by Betlach and Tiedje to explain the variations in NO2− accumulation observed during NO3− reduction (3). NO2− accumulation could also occur if the additional electrons available from Fe(II) oxidation increased rates of NO3− reduction to an extent that rates of NO2− production exceeded NO2− reduction rates. This latter mechanism is supported by the accumulation of NO2− in incubations with Fe(II) EDTA where encrustations were not formed.
Based solely on the use of batch cultures containing millimolar substrate concentrations and the resultant formation of growth-limiting encrustations, it is difficult to argue that microbial NDFO is a significant biogeochemical process in anoxic, subsurface milieus which typically contain much lower concentrations of Fe2+, NO3−, and organic C. The continuous-flow system used in our experiments, however, allowed us to evaluate Fe(II)-oxidation-enhanced growth over longer time periods and at much lower concentrations than are possible in batch cultures. In addition, the continuous-flow experiments clearly demonstrated that growth-limiting Fe(III) oxyhydroxide encrustations are not formed in flowthrough systems at low substrate concentrations. Whereas cell numbers in mixotrophic cultures containing 250 μM Fe2+ were ca. 90% greater than in those lacking Fe2+ in continuous-flow systems (Fig. 4c), the presence of Fe2+ in batch cultures increased cell numbers by only ca. 29%. This further suggests that the cell encrustations in batch systems may have limited the benefits of Fe2+ oxidation. Taken together, these results suggest that mixotrophic NDFO may be a functional metabolism that is energetically beneficial under the low-substrate conditions found in oligotrophic sediments.
It should be noted that we designed the continuous-flow experiments to provide substrates to the filters at rates greatly in excess of the maximum cell specific uptake rates. Using the data for maximal rates of acetate, NO3−, and Fe2+ consumption between days 1 and 2 in the batch reactors (Fig. 2), for example, we calculated the maximal conversion rates (mol/h/cell) for each compound (data not shown) using the available cell number data (Fig. 2b). Although the conversion rates in the continuous-flow system were likely significantly lower than in the batch cultures due to the much lower substrate concentrations, we used maximal conversion rates from the batch cultures, along with the maximum cell numbers on the filters (Fig. 5) to calculate the potential, maximum uptake rate per filter cassette for each substrate. For all three compounds, the mass loading rate (concentration × flow rate) of substrate entering the filter was 4 to 25 times greater than the potential maximum substrate uptake rate, and little or no change of concentration would be detected in the effluent. Therefore, we made no attempt to measure transformation of acetate, NO3−, or Fe2+ in our continuous-flow experiments.
The biochemical mechanisms through which Fe(II) oxidation is coupled to energy generation during NDFO are still obscure. Questions also remain with respect to the fate of the oxidized iron produced in our continuous-flow systems and why oxyhydroxide accumulations were not found on cells or on filters. It is possible that nano-sized particulates were initially formed and that these easily passed through the filters with the effluent. It should be noted that the lithotrophic, Fe(II)-oxidizing enrichment culture of Straub et al. and some phototrophic Fe(II) oxidizers apparently do not form the heavy encrustations observed with Acidovorax sp. strain BoFeN1, although the mechanisms by which they avoid these encrustations is subject to speculation (33, 38). Hegler et al. have suggested formation of a low-pH microenvironment in the immediate vicinity of the cells as a possible means of avoiding the cellular encrustations by some phototrophic Fe(II) oxidizers (19).
Based on our available data, it appears that enhanced growth is a result of the coupling of Fe(II) oxidation to microbial energy generation. The ability to use Fe(II) as an auxiliary energy source would provide a competitive advantage in anoxic, oligotrophic environments where assimilable organic carbon concentrations may be below 10 μM (13, 35). At such very low concentrations, purely organotrophic microorganisms with a limited substrate range typically may use most of the available carbon solely for maintenance energy generation. As described by Egli (13), some organotrophic microorganisms deal with low organic carbon concentrations by being able to simultaneously utilize many different carbon sources, each present at extremely low concentrations, i.e., through a “multivorous” survival strategy. A different strategy could be a mixotrophic metabolism able to utilize Fe(II) as an energy source since more of the limited available C could then be used for biomass generation and growth.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge National Science Foundation grants EAR-0525069 to F.P. and 0525510 to E.E.R.
We thank Melissa Barlett for her assistance with 16S rRNA gene sequencing and phylogenetic analysis. We also acknowledge the help of Barry Stein with sample preparation for SEM imaging assistance and Quanxing Zheng for assistance in early experiments.
Footnotes
Supplemental material for this article may be found at http://aem.asm.org/.
Published ahead of print on 14 October 2011.
REFERENCES
- 1. Alefounder P. R., Ferguson S. J. 1980. The location of dissimilatory nitrite reductase and the control of dissimilatory nitrate reductase by oxygen in Paracoccus denitrificans. Biochem. J. 192:231–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Atlas R. M. 2004. Handbook of media for environmental microbiology, 3rd ed CRC Press, Inc., Boca Raton, FL [Google Scholar]
- 3. Betlach M. R., Tiedje J. M. 1981. Kinetic explanation for accumulation of nitrite, nitric oxide, and nitrous oxide during bacterial denitrification. Appl. Environ. Microbiol. 42:1074–1084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Blothe M., Roden E. E. 2009. Composition and activity of an autotrophic Fe(II)-oxidizing, nitrate-reducing enrichment culture. Appl. Environ. Microbiol. 75:6937–6940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Byrne-Bailey K. G., et al. 2010. Completed genome sequence of the anaerobic iron-oxidizing bacterium Acidovorax ebreus strain TPSY. J. Bacteriol. 192:1475–1476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cataldo D. A., Haroon M., Schrader L. E., Youngs V. L. 1975. Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Commun. Soil Sci. Plant Anal. 6:71–80 [Google Scholar]
- 7. Coby A. J., Picardal F. W. 2005. Inhibition of NO3− and NO2− reduction by microbial Fe(III) reduction: evidence of a reaction between NO2− and cell surface-bound Fe2+. Appl. Environ. Microbiol. 71:5267–5274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cooper D. C., Picardal F., Coby A. J. 2006. Interactions between microbial iron reduction and metal geochemistry: effect of redox cycling on transition metal speciation in iron bearing sediments. Environ. Sci. Technol. 40:1884–1891 [DOI] [PubMed] [Google Scholar]
- 9. Cooper D. C., Picardal F., Rivera J., Talbot C. 2000. Zinc immobilization and magnetite formation via ferric oxide reduction by Shewanella putrefaciens 200. Environ. Sci. Technol. 34:100–106 [Google Scholar]
- 10. Cooper D. C., Picardal F. W., Schimmelmann A., Coby A. J. 2003. Chemical and biological interactions during nitrate and goethite reduction by Shewanella putrefaciens 200. Appl. Environ. Microbiol. 69:3517–3525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Coyne M. S., Arunakumari A., Pankratz H. S., Tiedje J. M. 1990. Localization of the cytochrome cd1 and copper nitrite reductases in denitrifying bacteria. J. Bacteriol. 172:2558–2562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cummings D. E. 1999. Arsenic mobilization by the dissimilatory Fe(III)-reducing bacterium Shewanella alga BrY. Environ. Sci. Technol. 33:723–729 [Google Scholar]
- 13. Egli T. 2010. How to live at very low substrate concentration. Water Res. 44:4826–4837 [DOI] [PubMed] [Google Scholar]
- 14. Ehrenreich A., Widdel F. 1994. Anaerobic oxidation of ferrous iron by purple bacteria, a new type of phototrophic metabolism. Appl. Environ. Microbiol. 60:4517–4526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ehrlich H. L., Newman D. K. (ed.). 2008. Geomicrobiology. CRC Press, Inc., Boca Raton, FL [Google Scholar]
- 16. Finneran K. T., Housewright M. E., Lovley D. R. 2002. Multiple influences of nitrate on uranium solubility during bioremediation of uranium-contaminated subsurface sediments. Environ. Microbiol. 4:510–516 [DOI] [PubMed] [Google Scholar]
- 17. Greenberg A. E., Clesceri L. S., Eaton A. D. (ed.). 1992. Standard methods for the examination of water and wastewater, 18th ed American Public Health Association, Washington, DC [Google Scholar]
- 18. Hafenbradl D., et al. 1996. Ferroglobus placidus gen. nov., sp. nov., a novel hyperthermophilic archaeum that oxidizes Fe2+ at neutral pH under anoxic conditions. Arch. Microbiol. 166:308–314 [DOI] [PubMed] [Google Scholar]
- 19. Hegler F., Schmidt C., Schwarz H., Kappler A. 2010. Does a low-pH microenvironment around phototrophic FeII-oxidizing bacteria prevent cell encrustation by FeIII minerals? FEMS Microbiol. Ecol. 74:592–600 [DOI] [PubMed] [Google Scholar]
- 20. Holmes R. M., Aminot A., Kérouel R., Hooker B. A., Peterson B. J. 1999. A simple and precise method for measuring ammonium in marine and freshwater ecosystems. Can. J. Fish. Aquat. Sci. 56:1801–1808 [Google Scholar]
- 21. Kappler A., Johnson C. M., Crosby H. A., Beard B. L., Newman D. K. 2010. Evidence for equilibrium iron isotope fractionation by nitrate-reducing iron(II)-oxidizing bacteria. Geochim. Cosmochim. Acta 74:2826–2842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kappler A., Schink B., Newman D. K. 2005. Fe(III) mineral formation and cell encrustation by the nitrate-dependent Fe(II)-oxidizer strain BoFeN1. Geobiology 3:235–245 [Google Scholar]
- 23. Kappler A., Straub K. L. 2005. Geomicrobiological cycling of Iron. Rev. Mineral. Geochem. 59:85–108 [Google Scholar]
- 24. Kepner R. L., Jr., Pratt J. R. 1994. Use of fluorochromes for direct enumeration of total bacteria in environmental samples: past and present. Microbiol. Mol. Biol. Rev. 58:603–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kim S., Picardal F. W. 1999. Enhanced anaerobic biodegradation of carbon tetrachloride in the presence of reduced iron oxides. Environ. Toxicol. Chem. 18:2142–2150 [DOI] [PubMed] [Google Scholar]
- 26. Kumaraswamy R., Sjollema K., Kuenen G., von Loosdrecht M. C. M., Muyzer G. 2006. Nitrate-dependent [Fe(II)EDTA]2− oxidation by Paracoccus ferrooxidans sp. nov., isolated from a denitrifying bioreactor. Syst. Appl. Microbiol. 29:276–286 [DOI] [PubMed] [Google Scholar]
- 27. Lack J. G., Chaudhuri S. K., Chakraborty R., Achenbach L. A., Coates J. D. 2002. Anaerobic biooxidation of Fe(II) by Dechlorosoma suillum. Microb. Ecol. 43:424–431 [DOI] [PubMed] [Google Scholar]
- 28. Langmuir D., Jacobson R. I. 1970. Specific ion electrode determination of nitrate in some fresh waters and sewage effluents. Environ. Sci. Technol. 4:835 [Google Scholar]
- 29. Larese-Casanova P., Haderlein S. B., Kappler A. 2010. Biomineralization of lepidocrocite and goethite by nitrate-reducing Fe(II)-oxidizing bacteria: effect of pH, bicarbonate, phosphate, and humic acids. Geochim. Cosmochim. Acta 74:3721–3734 [Google Scholar]
- 30. Lovley D. R. 2000. Fe(III) and Mn(IV) reduction, p. 3–30 In Lovley D. R. (ed.), Environmental microbe-metal interactions. ASM Press, Washington, DC [Google Scholar]
- 31. Lovley D. R., Holmes D. E., Nevin K. P. 2004. Dissimilatory Fe(III) and Mn(IV) reduction. Adv. Microb. Physiol. 49:219–286 [DOI] [PubMed] [Google Scholar]
- 32. Miot J., et al. 2009. Iron biomineralization by anaerobic neutrophilic iron-oxidizing bacteria. Geochim. Cosmochim. Acta 73:696–711 [Google Scholar]
- 33. Miot J., et al. 2009. Extracellular iron biomineralization by photoautotrophic iron-oxidizing bacteria. Appl. Environ. Microbiol. 75:5586–5591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Muehe E. M., Gerhardt S., Schink B., Kappler A. 2009. Ecophysiology and the energetic benefit of mixotrophic Fe(II) oxidation by various strains of nitrate-reducing bacteria. FEMS Microbiol. Ecol. 70:3–11 [DOI] [PubMed] [Google Scholar]
- 35. Münster U. 1993. Concentrations and fluxes of organic carbon substrates in the aquatic environment. Antonie Van Leeuwenhoek 63:243–274 [DOI] [PubMed] [Google Scholar]
- 36. Regberg A., et al. 2011. Electrical conductivity as an indicator of iron reduction rates in abiotic and biotic systems. Water Resource Res. 47:W04509 [Google Scholar]
- 37. Roden E. E., Zachara J. M. 1996. Microbial reduction of crystalline Fe(III) oxides: influence of oxide surface area and potential for cell growth. Environ. Sci. Technol. 30:1618–1628 [Google Scholar]
- 38. Schadler S., et al. 2009. Formation of cell-iron-mineral aggregates by phototrophic and nitrate-reducing anaerobic Fe(II)-oxidizing bacteria. Geomicrobiol. J. 26:93–103 [Google Scholar]
- 39. Schadler S., Burkhardt C., Kappler A. 2008. Evaluation of electron microscopic sample preparation methods and imaging techniques for characterization of cell-mineral aggregates. Geomicrobiol. J. 25:228–239 [Google Scholar]
- 40. Stookey L. L. 1970. Ferrozine: a new spectrophotometric reagent for iron. Anal. Chem. 42:779–781 [Google Scholar]
- 41. Strąpoć D., et al. 2008. Methane-producing microbial community in a coal bed of the Illinois Basin. Appl. Environ. Microbiol. 74:2424–2432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Straub K. L., Benz M., Schink B., Widdel F. 1996. Anaerobic, nitrate-dependent microbial oxidation of ferrous iron. Appl. Environ. Microbiol. 62:1458–1460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Straub K. L., Buchholz-Cleven B. E. E. 1998. Enumeration and detection of anaerobic ferrous iron-oxidizing, nitrate-reducing bacteria from diverse European sediments. Appl. Environ. Microbiol. 64:4846–4856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Straub K. L., Schonhuber W. A., Buchholz-Cleven B. E. E., Schink B. 2004. Diversity of ferrous iron-oxidizing, nitrate-reducing bacteria and their involvement in oxygen-independent iron cycling. Geomicrobiol. J. 21:371–378 [Google Scholar]
- 45. Weber K. A., Achenbach L. A., Coates J. D. 2006. Microorganisms pumping iron: anaerobic microbial iron oxidation and reduction. Nat. Rev. Microbiol. 4:752–764 [DOI] [PubMed] [Google Scholar]
- 46. Weber K. A., et al. 2009. Physiological and taxonomic description of the novel autotrophic, metal oxidizing bacterium, Pseudogulbenkiania sp. strain 2002. Appl. Microbiol. Biotechnol. 83:555–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Weber K. A., Picardal F. W., Roden E. E. 2001. Microbially catalyzed nitrate-dependent oxidation of biogenic solid-phase Fe(II) compounds. Environ. Sci. Technol. 35:1644–1650 [DOI] [PubMed] [Google Scholar]
- 48. Weber K. A., et al. 2006. Anaerobic nitrate-dependent iron(II) bio-oxidation by a novel lithoautotrophic betaproteobacterium, strain 2002. Appl. Environ. Microbiol. 72:686–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zachara J. M., Fredrickson J. K., Smith S. C., Gassman P. L. 2001. Solubilization of Fe(III) oxide-bound trace metals by a dissimilatory Fe(III) reducing bacterium. Geochim. Cosmochim. Acta 65:75–93 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.