Abstract
Intensive aquaculture releases large quantities of nutrients into aquatic bodies, which can lead to eutrophication. The objective of this study was the development of a biological recirculatory wastewater treatment system with a diazotrophic cyanobacterium, Aulosira fertilissima, and simultaneous production of valuable product in the form of poly-β-hydroxybutyrate (PHB). To investigate this possible synergy, batch scale tests were conducted under a recirculatory aquaculture system in fiber-reinforced plastic tanks enhanced by several manageable parameters (e.g., sedimentation, inoculum size, depth, turbulence, and light intensity), an adequate combination of which showed better productivity. The dissolved-oxygen level increased in the range of 3.2 to 6.9 mg liter−1 during the culture period. Nutrients such as ammonia, nitrite, and phosphate decreased to as low as zero within 15 days of incubation, indicating the system's bioremediation capability while yielding valuable cyanobacterial biomass for PHB production. Maximum PHB accumulation in A. fertilissima was found in sedimented fish pond discharge at 20-cm culture depth with stirring and an initial inoculum size of 80 mg dry cell weight (dcw) liter−1. Under optimized conditions, the PHB yield was boosted to 92, 89, and 80 g m−2, respectively for the summer, rainy, and winter seasons. Extrapolation of the result showed that a hectare of A. fertilissima cultivation in fish pond discharge would give an annual harvest of ∼17 tons dry biomass, consisting of 14 tons of PHB with material properties comparable to those of the bacterial polymer, with simultaneous treatment of 32,640 m3 water discharge.
INTRODUCTION
Over the last 2 decades, aquaculture has gone through major changes, growing from small-scale homestead level activities to large-scale commercial farming, which is driving the industry toward more intensive practices. One of the major drawbacks of this trend lies in the waste derived from fish feed and its metabolic end products. These include uneaten food, feces and excreta, and dissolved inorganic nutrients, which are transported in water in various concentrations and can cause eutrophication of the water resources that receive them (23). Therefore, to combat this problem, a novel technique, i.e., a recirculatory aquaculture system (RAS), has been proposed, which is based on conservation of the limited water and abatement of water pollution caused by the expanding aquaculture industries. The most prominent characteristic of this system is the presence of a biofilter, which treats water contaminated by dissolved organics and ammonia rather than discharging these contaminants directly into bodies of water.
Cyanobacteria (blue-green algae) are O2-evolving photosynthesizing prokaryotes that can be successfully cultivated in wastewater due to their ability to use inorganic nitrogen and phosphorus for their multiplication. For example, Spirulina platensis (5, 10), Spirulina maxima (25), Romeria sp. (3), Synechococcus sp. strain PCC 7942 (16), Schizothrix calcicola, Phormidium tenue, Phormidium subfuscum, and Oscillatoria sp. (6) have been found to be efficient in bioremediation of wastewater rich in phosphate, nitrate, ammonia, and urea. Phormidium bohneri Schmidle, a tropical cyanobacterium, shows self-aggregation and an ability to sediment that lends itself to serving as a biotreatment system for wastewater in temperate and warm climates (18).
At present, problems concerning nondegradable plastic materials have generated much interest in the development of biodegradable polymers. In addition to various bacterial species, cyanobacteria are emerging as potential prokaryotic candidates karyotes for production of poly-β-hydroxybutyrate (PHB), which occurs as insoluble cytoplasmic inclusions for carbon/energy-reducing power (39). PHB has various properties, such as being elastomeric, insoluble in water, nontoxic, biocompatible, and piezoelectric, with a high degree of polymerization (14), which make it suitable for a variety of applications in the fields of agricultural, environmental, and biomedical sciences (27). A few cyanobacterial species, viz., Synechococcus sp. strain MA19, Nostoc muscorum, and Synechocystis sp. strain PCC 6803, have been found to accumulate a considerable amount of PHB (≥40% of dry cell weight [dcw]) under specific culture conditions (24, 26, 36). Most recently, with an N2-fixing cyanobacterium, Aulosira fertilissima, an accumulation of up to 85% (dcw) was observed, the highest value recorded so far for PHB-accumulating cyanobacterial species (34). In the present study, our effort has been focused on exploring the efficiency of A. fertilissima as a biofiltrant and PHB producer in a recirculatory aquaculture system. Such integrated efforts to clean up wastewater with cyanobacteria will significantly enhance the environmental and economic benefits of the technology of PHB production by minimizing the additional cost of nutrients and by saving precious freshwater resources.
MATERIALS AND METHODS
Organism and growth conditions.
Axenic cultures of the filamentous nitrogen-fixing cyanobacterium A. fertilissima were grown in nitrate-free BG-11 medium (33) as detailed by Samantaray and Mallick (34). The logarithmic-phase cultures were transferred to fiber-reinforced plastic (FRP) tanks containing the above-mentioned medium for acclimatization of the test organism to the outdoors for further study.
RAS and operation mode.
Experiments were conducted in a RAS at the Agricultural and Food Engineering Department, Indian Institute of Technology Kharagpur, Kharagpur, West Bengal, India. The waste discharge from a large open fish pond was first pumped into a settling tank (volume, 1,000 liters) to remove large solids in the discharge. After 24 h, the supernatant was siphoned into FRP tanks (length, 125 cm; width, 60 cm; depth, 45 cm) for culturing of the test cyanobacterium through an inclined-plate settler to remove other fine solids. The plates used in the inclined-plate settler were made of Plexiglas with a thickness of 2 mm. The plates were inserted in the tank at 45° angles by means of prefabricated fiber channels pasted onto the glass surface (35). The experiments were carried out under the shade of translucent acrylic Plexiglas sheets using natural sunlight.
Various physical parameters, such as the effects of sedimentation, inoculum size, and mixing on bioremediation and PHB production by A. fertilissima, were examined. To study the effect of sedimentation, one set of 3 FRP tanks were filled with fish pond discharge (FPD) to about a 10-cm depth after passing through the settling tank and inclined-plate settler. Another set of FRP tanks were filled with FPD directly from the fish pond. To study the effect of inoculum size, various concentrations (40, 80, 100, and 120 mg dcw liter−1) of 8- to 9-day-old logarithmic-phase A. fertilissima cultures grown in FRP tanks (outdoors) filled with nitrate-free BG-11 medium were taken. To study the effects of mixing on the biomass and PHB yield, mixing of the culture was done by mechanical stirring and aeration. In this study, stirring was performed by stirrers with pitched blades (model RQ-124A; Remi Instruments Ltd., Vasai, India) intermittently at a rotation speed of about 20 × g. Aeration was provided to another set of tanks by sparging with compressed air (12 liter min−1) intermittently through porous polyvinyl chloride (PVC) tubes spread on the bottoms of the tanks. The combined effects of aeration and stirring were also studied in FRP tanks filled with FPD about 10 cm deep.
To study the seasonal variation in the growth, PHB content, and bioremediation potential of A. fertilissima, experiments were conducted in three seasons: summer (April to June 2009), the rainy season (July to August 2009), and winter (December 2009 to January 2010). In each season, the effects of culture depth on biomass, PHB yield, and bioremediation were also studied. For this, four sets of FRP tanks were filled with FPD up to 5, 10, 20, and 30 cm deep after passing through the inclined-plate settler (38, 75, 150, and 225 liters, respectively).
Analytical methods.
The FPD was characterized for pH (Microcontroller pH analyzer model pHA-5; Loba Chemie, Colaba, Mumbai, India), dissolved oxygen (DO) (DO meter model 55-12 FT; YSI Inc., OH), biological oxygen demand (BOD), chemical oxygen demand (COD), total suspended solids (TSS), and nutrients, such as total organic carbon (TOC), PO43−, NH4+, NO3−, and NO2−, following the standard protocols of the American Public Health Association (APHA) (1). A. fertilissima growth was monitored by estimating the chlorophyll a content following the method of Mackinney (21). The dry cell weight of A. fertilissima was determined gravimetrically following the method of Rai et al. (28). The corresponding dcw for the chlorophyll a content was then plotted in the form of a standard graph. The biomass concentration was estimated from the standard graph by monitoring the chlorophyll a content at regular time intervals. PHB extraction was carried out following the protocol of Yellore and Desia (44) as detailed by Bhati et al. (2), and quantification was done by gas chromatography following the method of Riis and Mai (31).
Chemical analysis of the polymer produced.
Chemical analysis of the polymer was done using 1H nuclear magnetic resonance (NMR) and Fourier transform infrared (FTIR) spectroscopy. The polymer (1 mg) was dissolved in 1 ml of CDCl3, and the spectra of the sample, as well as the standard, were obtained with a Bruker-Advanced 400 Spectrometer at 400 MHz. The FTIR spectrum of the polymer was obtained by using an FTIR spectrophotometer (Nexus-870; Thermo Nicolet Co.). The polymer and KBr were mixed at a ratio of 1:100 and pressed into tablet form (12-mm diameter; 1-mm thickness). The FTIR spectrum was recorded in the spectral range of 500 to 4,000 cm−1.
The thermal properties of the polymer were studied with a Pyris Diamond Differential Scanning Calorimeter (Perkin-Elmer) equipped with a liquid nitrogen cooling accessory. Samples (5 mg) were exposed to −50 to 200°C at a heating rate of 10°C min−1 for the first run. The samples were maintained at 200°C for 1 min and then rapidly quenched in liquid nitrogen to −150°C. The samples were again analyzed during a second heating scan from −50 to 200°C at a heating rate of 10°C min−1. The melting temperature (Tm) was determined from the differential scanning calorimetry (DSC) endotherms. The glass transition temperature (Tg)was taken as the midpoint of the heat capacity change. The degree of crystallinity (Xc) was calculated following the method of Lupke et al. (20).
All the experiments were performed in triplicate and repeated at least twice to check the reproducibility. The results were analyzed statistically by Student's t test and Duncan's new multiple-range test.
RESULTS AND DISCUSSION
Effect of sedimentation.
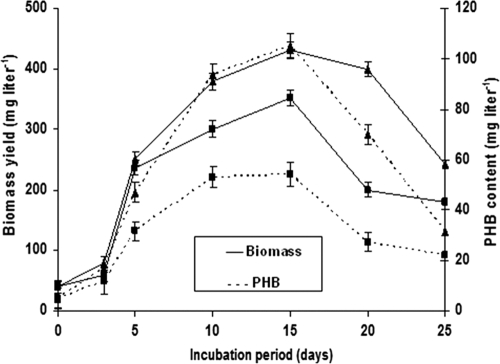
FPD has a very high load of solid particles in suspension, which contributes to an increase in turbidity. Thus, experiments were carried out to examine the nutrient removal efficiency and PHB accumulation by A. fertilissima in sedimented and nonsedimented FPD. The concentration of nutrients in FPD decreased significantly during the experimental period, indicating that A. fertilissima has high nutrient removal efficiency in FRP tanks with sedimented FPD (Table 1). Sedimentation of FPD reduced the TSS content by 59%. NH4+ removal occurred rapidly from 2.1 to 0.2 mg liter−1 after 15 days of incubation in FRP tanks with sedimentation, leading to 90% removal. This likely occurs because NH4+ is the most reduced form of inorganic nitrogen and thus the most preferred form of nitrogen for algal growth (19). The removal rate (75%) of orthophosphate in sedimented FPD was observed to be of the same order of magnitude as reported by De la Noüe and Proulx (8) while treating urban wastewater with Phormidium sp. In contrast, there was a considerable rise in the NO3− level in the tanks during the first week of the experiment, with a declining trend thereafter. According to Jones et al. (17), this increase may be related to the decomposition and mineralization of the organic materials by bacteria present in the waste discharge. Further, nitrification of ammonia could also contribute to increasing nitrate concentrations (32). TOC, COD, and BOD levels showed a declining trend with the growth of A. fertilissima, which was more pronounced for the sedimented than the nonsedimented FPD. There was a significant difference in DO content between the sedimented and nonsedimented FPD during the experimental period. This pronounced difference could be ascribed to the oxygen consumed by bacteria and zooplankton in nonsedimented tanks (22). After 15 days, reduction in the DO rise rate could be attributable to the declining phase of A. fertilissima. A maximum biomass yield of 432 mg liter−1 was recorded after 15 days of incubation in FRP tanks with sedimented FPD compared to 352 mg liter−1 in nonsedimented tanks (Fig. 1). This could be due to the presence of high concentrations of suspended solid particles in the nonsedimented tanks, which limits light penetration and affects the photosynthetic efficiency of microalgae, and consequently their growth (45). Adsorption of A. fertilissima filaments to these suspended particles was followed by sedimentation, and thus, hampering of growth also could not be ruled out. Moreover, the presence of more zooplankton and bacteria in the nonsedimented FPD could also play a role in the reduced biomass yield, which warrants further experimentation. Maximum PHB accumulation (105 mg liter−1) was observed in the sedimented FPD after 15 days of incubation (Fig. 1), after which a declining trend was observed. Therefore, further experiments were designed with sedimented FPD.
Table 1.
Concentration changes in sedimented and nonsedimented FPD after inoculation of A. fertilissima
| Parameter | Treatment | Value after incubation for (days)a: |
|||||
|---|---|---|---|---|---|---|---|
| 0 | 5 | 10 | 15e | 20 | 25 | ||
| Orthophosphate (mg liter−1) | Sb | 2.8 ± 0.16h | 1.1 ± 0.07g | 0.9 ± 0.02g | 0.7 ± 0.03g | NDd | ND |
| NSc | 2.8 ± 0.21 | 2.4 ± 0.12 | 1.9 ± 0.10 | 1.4 ± 0.08 | 1.1 ± 0.05 | ND | |
| Ammonium (mg liter −1) | S | 2.1 ± 0.18h | 1.6 ± 0.07e | 0.9 ± 0.02g | 0.2 ± 0.01g | ND | ND |
| NS | 2.1 ± 0.11 | 1.9 ± 0.09 | 1.7 ± 0.08 | 1.5 ± 0.06 | 1.4 ± 0.05 | 0.8 ± 0.01 | |
| Nitrate (mg liter−1) | S | 12.2 ± 0.42h | 17.2 ± 0.69h | 14.2 ± 0.52f | 12.9 ± 0.47f | 8.9 ± 0.36g | 4.8 ± 0.21g |
| NS | 12.2 ± 0.49 | 16.5 ± 0.62 | 15.3 ± 0.58 | 14.2 ± 0.52 | 13.9 ± 0.48 | 13.1 ± 0.45 | |
| Nitrite (mg liter−1) | S | 3.2 ± 0.15h | 1.4 ± 0.12g | 0.3 ± 0.01g | ND | ND | ND |
| NS | 3.2 ± 0.17 | 2.8 ± 0.19 | 1.9 ± 0.15 | 0.6 ± 0.02 | ND | ND | |
| TOC (mg liter−1) | S | 15.6 ± 0.57e | 13.6 ± 0.48g | 11.9 ± 0.33g | 9.6 ± 0.29g | 6.8 ± 0.24g | 2.8 ± 0.15g |
| NS | 18.2 ± 0.64 | 17.4 ± 0.59 | 16.5 ± 0.47 | 14.2 ± 0.42 | 11.2 ± 0.38 | 7.5 ± 0.25 | |
| COD (mg liter−1) | S | 218.7 ± 2.33e | 159.2 ± 1.83e | 99.1 ± 1.32g | 82.4 ± 1.18g | 71.1 ± 1.09g | 61.9 ± 1.02g |
| NS | 238.6 ± 3.16 | 201.2 ± 2.09 | 181.6 ± 1.93 | 142.5 ± 1.71 | 127.1 ± 1.59 | 99.3 ± 1.39 | |
| BOD (mg liter−1) | S | 102.7 ± 1.61e | 97.6 ± 1.41f | 88.7 ± 1.20g | 73.6 ± 1.08g | 64.2 ± 1.01g | 39.8 ± 0.86g |
| NS | 113.9 ± 1.52 | 104.2 ± 1.43 | 98.3 ± 1.27 | 78.9 ± 1.10 | 66.5 ± 1.07 | 59.3 ± 0.99 | |
| DO (mg liter−1) | S | 3.2 ± 0.23h | 4.7 ± 0.29e | 5.2 ± 0.33e | 6.3 ± 0.41g | 6.4 ± 0.42g | 6.6 ± 0.41g |
| NS | 3.2 ± 0.18 | 4.1 ± 0.25 | 4.2 ± 0.20 | 3.9 ± 0.21 | 3.7 ± 0.22 | 3.6 ± 0.21 | |
| pH | S | 7.8 ± 0.36h | 8.1 ± 0.34h | 8.4 ± 0.37h | 8.8 ± 0.41h | 8.9 ± 0.40f | 8.7 ± 0.39e |
| NS | 7.8 ± 0.38 | 8.0 ± 0.35 | 8.1 ± 0.34 | 8.2 ± 0.36 | 7.9 ± 0.34 | 7.8 ± 0.35 | |
| TSS (mg liter−1) | S | 99.5 ± 20.3f | 87.5 ± 25.7f | 76.5 ± 18.9g | 52.3 ± 17.2g | 68.2 ± 21.4g | 73.4 ± 25.6g |
| NS | 245.3 ± 29.3 | 223.7 ± 23.6 | 210.9 ± 25.8 | 196.1 ± 19.1 | 198.1 ± 27.2 | 198.5 ± 29.4 | |
The values represent the averages ± standard errors of data based on three independent determinations.
S, sedimented.
NS, nonsedimented.
ND, not detected.
t significant at P < 0.05 (Student's t test).
t significant at P < 0.01 (Student's t test).
t significant at P < 0.001 (Student's t test).
Not significant.
Fig. 1.
Effects of sedimentation on the biomass and PHB yield of A. fertilissima. ▴, sedimented FPD; ■, nonsedimented FPD. The error bars indicate standard error.
Effect of inoculum size.
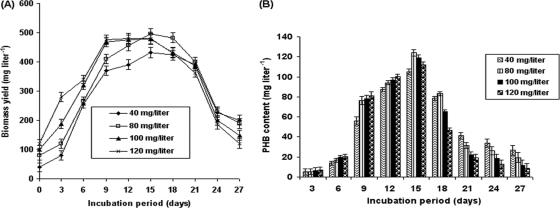
Cell density represents a key factor governing culture productivity, since it critically affects the availability of light to the cells. The effects of the different inoculum sizes (40, 80, 100, and 120 mg dcw liter−1) evaluated in this study on biomass and PHB accumulation are shown in Fig. 2. Cultures with initial inoculum sizes of 40 and 80 mg dcw liter−1 took 15 days to reach the stationary growth phase, while those with inoculum sizes of 100 and 120 mg dcw liter−1 took only 9 days. The culture with an initial inoculum size of 80 mg dcw liter−1 reached a maximum biomass yield of 496 mg liter−1 after 15 days of incubation (Fig. 2A). Maximum PHB accumulation up to 124 mg liter−1 was also recorded at the initial inoculum size of 80 mg dcw liter−1 (Fig. 2B). No further rise in biomass and PHB content was observed at increasing inoculum sizes. The possible explanation could be that, when the cell concentration is below the optimum value, all the light energy is not captured by the cells. Above the optimal concentration, a proportion of the cells are in the dark due to self-shading, and light intensity can influence the growth of microalgae (46). Another explanation for this observation that PHB content tended to be lower in the FPD inoculated with higher densities of A. fertilissima is that growth under the conditions imposed by the FPD (excess carbon) favors PHB accumulation, and thus, the cells that were most actively growing (those in the FPD inoculated with lower densities of A. fertilissima) accumulated the most PHB (40).
Fig. 2.
Relationship between A. fertilissima inoculum size (mg dry cell weight liter−1) and biomass (A) and PHB yield (B) over a 27-day period. The error bars indicate standard error.
Effect of mixing.
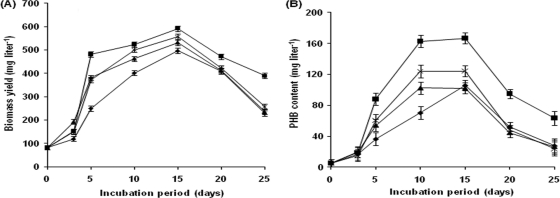
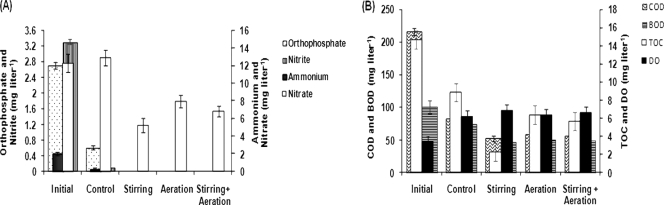
In general, a rise in biomass yield was evident under mixing conditions, reaching a maximum of 592 mg liter−1 in FRP tanks with stirrers, followed by 556 mg liter−1 in tanks with stirrers and aeration and 531 mg liter−1 in air-purged tanks after 15 days of incubation compared to the yield of 496 mg liter−1 under control conditions (Fig. 3A). Mixing is necessary to prevent sedimentation of the algae, to ensure that all cells of the population are equally exposed to light and nutrients, to avoid stratification, and to improve gas exchange between the culture medium and the atmosphere (29). If mixing is not adequate, the cells on the surface also suffer photoinhibition, resulting in low biomass yield (42). A maximum PHB yield of 166 mg liter−1 was also recorded in the tanks that were provided with stirring versus 124 mg liter−1 for the control (Fig. 3B). Similar to biomass and PHB yield, the bioremediation efficiencies of A. fertilissima in FRP tanks were found to be in the following order: stirring > stirring plus aeration > aeration > control (Fig. 4). Thus, further experiments were designed with stirrers.
Fig. 3.
Effects of mixing on the biomass (A) and PHB yield (B) of A. fertilissima. ♦, control; ■, stirring; ▴, aeration; ×, aeration plus stirring. The error bars indicate standard error.
Fig. 4.
Changes in PO43−, NH4+, NO3−, and NO2− (A) and in BOD, COD, TOC, and DO (B) values over a 15-day period under various mixing conditions. The error bars indicate standard error.
Effects of seasonal variation and culture depth on the bioremediation efficiency, biomass, and PHB yield of A. fertilissima.
Cultures were managed in similar manners during different seasons (summer, rainy, and winter). The parameters that changed in each season were temperature, hours of sunshine, and light intensity. The biomass yield depends not only on the total irradiance impinging on the culture surface, but also, and more importantly, on the amount of energy available at the cell level (30). Thus, the extent of mutual shading, which is a function of the population density and the culture depth, is the major factor determining the amount of solar radiation available to the cells in the culture. Experiments were conducted in each season with an optimum cell concentration of 80 mg dcw liter−1 in sedimented FPD at different culture depths (5, 10, 20, and 30 cm) with stirring.
The average productivity during summer was higher than that during the rainy and winter seasons (Table 2). During summer, the average light intensity over the course of the entire day over the season was 1,762 μE m−2 s−1, and the average temperature was 37.8°C. No major changes were observed in the light intensity throughout the experiment. In summer, when the culture depth was reduced from 10 to 5 cm, the biomass yield increased from 593 to 610 mg liter−1. However, no significant difference in biomass yields was observed at culture depths of 10 and 20 cm, i.e., they were 593 and 590 mg liter−1, respectively, after 15 days of incubation. Increasing the culture depth to 30 cm decreased the biomass yield significantly, which may be due to low light penetration at increasing depths. However, the overall productivity in terms of total yield or areal density was maximal at a 20-cm depth; an areal density of 118 g m−2 (total yield, 88 g) was recorded. In contrast to the biomass yield, a rise in PHB content was observed by increasing the culture depth from 5 cm to 10 to 20 cm. This could be partially attributable to the presence of cells under shaded conditions. In a dark/shaded state, an increase in PHB accumulation could possibly be due to conversion of glycogen to acetyl-coenzyme A (CoA) for PHB biosynthesis, as reported for Gloeothece sp. strain PCC 6909 (39). Moreover, the shaded condition reduced the light availability, which directs a greater flux of carbon through the heterotrophic pathway, leading to greater PHB accumulation. The presences of various organic compounds, as reflected in the high TOC, BOD, and COD values, might be utilized by A. fertilissima in mixotrophic and heterotrophic modes (40). A maximum PHB yield of 41 g m−2 was observed at a 20-cm culture depth, but increasing the culture depth to 30 cm decreased the PHB yield significantly, which could be directly related to the reduced biomass yield of the test cyanobacterium at increasing culture depths (Table 2).
Table 2.
Biomass and PHB yield of A. fertilissima grown in different seasons in sedimented FPD with a stirrer at different culture depths after 15 days of incubationa
| Season | Culture depth (cm) | Biomassb |
PHBb |
Avg sunshinec (h) | Avg tempc (°C) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Volumetric yield (mg liter−1) | Areal density (g m−2) | Total yield (g) | Volumetric yield (mg liter−1) | Areal density (g m−2) | Total yield (g) | ||||
| Summer (May–June) | 5 | 610.2 ± 1.09J | 30.5 ± 0.25B | 22.8 ± 0.19B | 157.1 ± 1.09G | 7.9 ± 0.09B | 5.9 ± 0.07B | 13.3 ± 0.2 | 37.8 ± 2 |
| 10 | 592.7 ± 2.11I | 59.3 ± 0.47E | 44.4 ± 0.35D | 166.7 ± 1.02H | 16.6 ± 0.12D | 12.5 ± 0.11D | |||
| 20 | 589.8 ± 2.23I | 117.9 ± 0.91J | 88.4 ± 0.74H | 205.4 ± 1.17K | 41.0 ± 0.29I | 30.8 ± 0.32H | |||
| 30 | 311.8 ± 1.66C | 93.5 ± 0.82GH | 70.1 ± 0.62F | 92.1 ± 0.98C | 27.6 ± 0.21F | 20.7 ± 0.29EF | |||
| Rainy (July–August) | 5 | 572.6 ± 1.72H | 28.6 ± 0.35AB | 21.4 ± 0.17AB | 132.5 ± 1.21E | 6.7 ± 0.08AB | 5.0 ± 0.06AB | 12.5 ± 0.4 | 31.2 ± 2 |
| 10 | 554.3 ± 2.03G | 55.4 ± 0.38D | 41.6 ± 0.39C | 161.7 ± 1.06GH | 16.1 ± 0.10D | 12.1 ± 0.07D | |||
| 20 | 551.4 ± 2.03G | 110.3 ± 0.85I | 82.7 ± 0.76G | 198.4 ± 1.03J | 39.6 ± 0.18H | 29.7 ± 0.12G | |||
| 30 | 297.8 ± 1.88B | 89.1 ± 0.77G | 67.0 ± 0.59F | 81.7 ± 0.93B | 24.5 ± 0.16E | 18.3 ± 0.11E | |||
| Winter (December–January) | 5 | 480.9 ± 1.94D | 24.0 ± 0.23A | 18.0 ± 0.18A | 107.4 ± 1.02D | 5.4 ± 0.06A | 4.1 ± 0.02A | 10.5 ± 0.1 | 19.5 ± 3 |
| 10 | 486.4 ± 1.98E | 48.6 ± 0.37C | 36.4 ± 0.41C | 149.5 ± 1.10F | 14.9 ± 0.09C | 11.2 ± 0.05C | |||
| 20 | 501.3 ± 1.03F | 100.2 ± 0.79H | 75.1 ± 0.52G | 169.7 ± 0.9I | 33.9 ± 0.17G | 25.4 ± 0.09F | |||
| 30 | 246.5 ± 1.97A | 73.9 ± 0.62F | 55.4 ± 0.59E | 55.7 ± 0.62A | 16.7 ± 0.14D | 12.5 ± 0.06D | |||
The values represent the averages ± standard errors of data based on three independent determinations.
The values in columns followed by different letters are significantly different from each other (P < 0.05; Duncan's new multiple-range tests). Separate analysis was done for each column.
The meteorological data were obtained from the Physics and Meteorology Department, Indian Institute of Technology Kharagpur, Kharagpur, India.
A highly positive correlation (r = 0.99; P < 0.01) between the hours of sunshine and the biomass yield demonstrated that the rise in biomass yield during the summer season is due to increased hours of sunshine. The daytime temperature approached an average of 37.8°C in summer, although the water temperature in the tanks was a few degrees lower due to evaporation. Thus, in summer, the medium temperature was also close to the optimum for the growth of the cyanobacterium (25 to 35°C). Maximal algal biomass productivity in summer was also reported by many researchers (7, 11).
Table 3 shows the seasonal variation in bioremediation efficiency of A. fertilissima after 15 days of incubation compared with the permissible limits of fish culture ponds, i.e., the water quality needed to support fish (13, 38). The bioremediation efficiencies of A. fertilissima in different seasons were found to be in the following order: summer > rainy > winter. Aquaculture operations demand precise control of DO, ammonium, and nitrite for susceptibility of fish. After 15 days of experimentation, approximately 100% PO43−, NH4+, and NO2− removal was observed in all seasons. A profound rise in the DO level was also observed. The water quality parameters in all seasons were found to be within the permissible limits of fish culture ponds.
Table 3.
Seasonal variations in bioremediation efficiency of A. fertilissima at 20-cm culture depth after 15 days of incubationa
| Parameter | Initial concn | Concn inb: |
Aquaculture limits (13, 38) | ||
|---|---|---|---|---|---|
| Summer | Rainy | Winter | |||
| Orthophosphate (mg liter−1) | 3.0 ± 0.14A | ND | ND | ND | 0.025–0.2 |
| Ammonium (mg liter−1) | 2.3 ± 0.16B | ND | ND | ND | 0.05–1.0 |
| Nitrate (mg liter−1) | 11.8 ± 0.41D | 1.4 ± 0.04A | 1.9 ± 0.04B | 2.3 ± 0.08C | 0.1–3.0 |
| Nitrite (mg liter−1) | 3.5 ± 0.13A | ND | ND | ND | 0–0.25 |
| TOC (mg liter−1) | 14.9 ± 0.54C | 1.8 ± 0.03A | 2.2 ± 0.04B | 2.3 ± 0.03B | |
| COD (mg liter−1) | 228.6 ± 2.29D | 41.3 ± 0.55A | 48.1 ± 0.35B | 51.9 ± 0.66C | <200 |
| BOD (mg liter−1) | 93.7 ± 1.62C | 18.7 ± 0.58A | 20.4 ± 0.24A | 46.2 ± 0.68B | |
| DO (mg liter−1) | 3.1 ± 0.15A | 6.9 ± 0.05C | 6.7 ± 0.07C | 6.3 ± 0.06B | ≥6 |
| pH | 8.0 ± 0.29A | 8.9 ± 0.13B | 8.7 ± 0.11B | 8.8 ± 0.12B | 6.5–9.0 |
The values represent the averages ± standard errors of data based on three independent determinations. The values in rows followed by different letters are significantly different from each other (P < 0.05; Duncan's new multiple-range tests). Separate analysis was done for each row.
ND, not detected.
Biomass and PHB yield of A. fertilissima under optimized conditions.
In a laboratory batch culture study with A. fertilissima grown in nitrate-free BG-11 medium (34), optimization of the process variables by response surface methodology (RSM) resulted in PHB accumulation of 85% (dcw) at 0.26% citrate, 0.28% acetate, and 5.58 mg liter−1 K2HPO4 for an incubation period of 5 days. Thus, A. fertilissima cultures grown in FPD for 15 days were harvested and subsequently transferred to the same volume of nitrate-free BG-11 medium containing 0.26% citrate, 0.28% acetate, and 5.58 mg liter−1 K2HPO4 in FRP tanks at a culture depth of 20 cm for 5 days. The PHB yield was recorded at up to 92 g m−2 in summer. During the rainy and winter seasons, the yields were 89 and 80 g m−2, respectively (Table 4). Supplementation of acetate stimulated PHB accumulation, due to direct utilization of acetate to increase the intracellular acetyl-CoA pool at the expense of free CoA by means of the usual pathway operating in cyanobacteria (12). Addition of citrate enhanced the availability of NADPH, a prerequisite for the activity of the enzyme acetoacetyl-CoA reductase for conversion of acetoacetyl-CoA to β-hydroxybutyryl-CoA. As observed by Chen et al. (4), citrate supplementation decreased the activity of the enzyme phosphofructokinase by chelating Mg2+, which inhibited the reaction from fructose-6-phosphate to fructose-1,6-bisphosphate in the glycolytic pathway, resulting in an elevated pool of fructose-6-phosphate. A rise in the glucose-6-phosphate pool was observed by conversion of fructose-6-phosphate to glucose-6-phosphate by the enzyme isomerase, which led to increased activity of 6-phosphoglucose dehydrogenase, the first enzyme of the pentose phosphate pathway, thus facilitating the production of more reduced cofactor, NADPH. Moreover, a role for phosphate limitation also could not be ruled out (26).
Table 4.
Seasonal variation in biomass and PHB yields of A. fertilissima under optimized conditionsa
| Season | Biomass |
PHB |
||||
|---|---|---|---|---|---|---|
| Volumetric yield (mg liter−1) | Areal density (g m−2) | Total yield (g) | Volumetric yield (mg liter−1) | Areal density (g m−2) | Total yield (g) | |
| Summer | 572.7 ± 2.19C | 114.5 ± 0.93C | 85.9 ± 0.89C | 460.3 ± 1.89C | 92.0 ± 0.91C | 69.0 ± 0.59C |
| Rainy | 550.3 ± 2.06B | 110.1 ± 0.89B | 82.5 ± 0.81B | 445.2 ± 1.82B | 89.0 ± 0.83B | 66.8 ± 0.57B |
| Winter | 498.2 ± 1.93A | 99.6 ± 0.85A | 74.7 ± 0.76A | 398.6 ± 1.74A | 79.7 ± 0.75A | 59.7 ± 0.51A |
Optimized conditions were as follows. A. fertilissima cultures grown in FPD for 15 days were harvested and subsequently transferred to the same volume of nitrate-free BG-11 medium containing 0.26% citrate, 0.28% acetate, and 5.58 mg liter−1 K2HPO4 in FRP tanks at a culture depth of 20 cm for 5 days (34). The values represent the averages ± standard errors of data based on three independent determinations. The values in columns followed by different letters are significantly different from each other (P < 0.05; Duncan's new multiple-range tests). Separate analysis was done for each column.
Chemical analysis of the polymer produced.
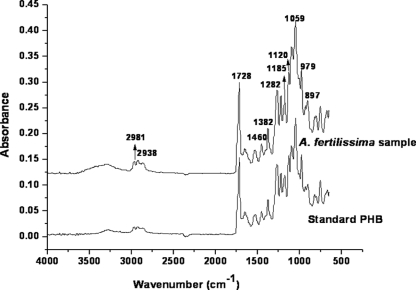
The FTIR spectrum of the extracted polymer is presented in Fig. 5. The band found at 1,460 cm−1 corresponds to the asymmetrical C—H bending vibration in the CH3 group, while the one found at 1,120 cm−1 is equivalent to CH2 asymmetrical bending vibration. The strong absorption bands at 1,728 cm−1 indicated stretching of the C O bond, and the band at 1,282 cm−1 corresponded to the —CH group. The series of bands located around 1,000 to 1,200 cm−1 correspond to the stretching of the C—O bond of the ester group. The absorption bands around 3,000 cm−1 correspond to the terminal OH group. The IR absorption peaks obtained correlated with the values in the literature (37) and with the spectrum of pure PHB standard, thus identifying the polymer as PHB. FTIR analysis was then used to measure the degree of crystallinity of the polymer (9). The crystallinity index (CI) is defined as the ratio of the intensities of the bands at 1,382 cm−1 (CH3) to that at 1,185 cm−1 (C—O—C). The CI of PHB was found to be 0.78, which was much lower than the previously reported value of 0.949 for PHB (9). Lower crystallinity of the extracted polymer is a favorable property for use in tissue-engineering applications (43).
O bond, and the band at 1,282 cm−1 corresponded to the —CH group. The series of bands located around 1,000 to 1,200 cm−1 correspond to the stretching of the C—O bond of the ester group. The absorption bands around 3,000 cm−1 correspond to the terminal OH group. The IR absorption peaks obtained correlated with the values in the literature (37) and with the spectrum of pure PHB standard, thus identifying the polymer as PHB. FTIR analysis was then used to measure the degree of crystallinity of the polymer (9). The crystallinity index (CI) is defined as the ratio of the intensities of the bands at 1,382 cm−1 (CH3) to that at 1,185 cm−1 (C—O—C). The CI of PHB was found to be 0.78, which was much lower than the previously reported value of 0.949 for PHB (9). Lower crystallinity of the extracted polymer is a favorable property for use in tissue-engineering applications (43).
Fig. 5.
FTIR spectra of standard poly(3-hydroxybutyric acid) and the polymer isolated from A. fertilissima.
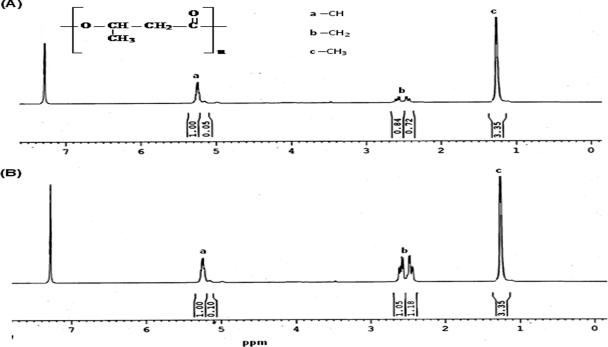
The NMR spectrum identified the polymer as an isocratic homopolymer (Fig. 6). The spectrum revealed the presence of three groups of signal characteristic of PHB. The doublet at 1.25 ppm was attributed to the methyl group coupled to one proton, the doublet of the quadruplet around 2.54 ppm to the methylene group adjacent to an asymmetric carbon atom bearing a single proton, and the singlet at 5.24 ppm to the methylene group. Deuterated chloroform (CDCl3) gave a chemical shift signal at 7.26 ppm.
Fig. 6.
1H NMR spectra of standard poly(3-hydroxybutyric acid) (A) and an A. fertilissima sample (B).
To corroborate the results described above and to analyze possible differences in the PHB obtained from A. fertilissima grown in FPD, the polymer was examined by differential scanning calorimetry. The thermal properties of PHB are quite in tune with those of the bacterial polymer, exhibiting Tm and Tg values of 171 and 0.6°C, respectively (Table 5). The Xc value was 59.3%. There was no significant difference in the properties of PHB from A. fertilissima grown in FPD and BG-11 medium. Lower Tm and crystallinity values compared to the bacterial polymer indicate better processibility.
Table 5.
Thermal characterization of PHB extracted from A. fertilissima
| Sample | Tm (°C) | Tg (°C) | Xc (%) |
|---|---|---|---|
| Bacterial PHBa | 171–182 | 0–5 | 60–80 |
| PHB (BG-11 medium) | 174 | 0.6 | 60.7 |
| PHB (fish pond discharge) | 171 | 0.6 | 59.3 |
Conclusions.
In aquaculture ponds, ammonium and nitrite are highly toxic to fish. A reduction in the level of DO in the pond water can increase the susceptibility of fish to ammonia toxicity. Therefore, aquaculture operation demands precise control of DO, ammonium, and nitrite. This study demonstrated that A. fertilissima has a high nutrient removal capacity in all seasons, reaching a maximum in summer. The DO level rose significantly during the culture period. Nutrients such as ammonium, nitrite, and phosphate reached as low as zero within 15 days of incubation, indicating the system's bioremediation capability while yielding valuable cyanobacterial biomass for PHB extraction. The optimum conditions for maximum productivity of Aulosira cultures was observed in an initial inoculum size of 80 mg dcw liter−1 at a culture depth of 20 cm, augmented by sedimentation and mixing with a stirrer, and harvesting schedules of 15 days. Extrapolation of the results showed that a hectare of A. fertilissima cultivation in FPD would give an annual harvest of ∼17 tons dry biomass consisting of 14 tons of PHB with simultaneous treatment of 32,640 m3 water discharge.
ACKNOWLEDGMENT
Financial support from the Department of Biotechnology, New Delhi, India, is gratefully acknowledged.
Footnotes
Published ahead of print on 7 October 2011.
REFERENCES
- 1. American Public Health Association 1998. Standard methods for the examination of water and wastewater, 20th ed American Public Health Association, Washington, DC [Google Scholar]
- 2. Bhati R., Samantaray S., Sharma L., Mallick N. 2010. Poly-β-hydroxybutyrate accumulation in cyanobacteria under photoautotrophy. Biotechnol. J. 5:1181–1185 [DOI] [PubMed] [Google Scholar]
- 3. Burford M. A. 2005. Relative uptake of urea and ammonium by dinoflagellates or cyanobacteria in shrimp mesocosms. Hydrobiologia 549:297–303 [Google Scholar]
- 4. Chen Y., et al. 2010. The mechanisms of citrate on regulating the distribution of carbon flux in the biosynthesis of uridine 5′-monophosphate by Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 86:75–81 [DOI] [PubMed] [Google Scholar]
- 5. Cheunbarn S., Peerapornpisal Y. 2010. Cultivation of Spirulina platensis using anaerobically swine wastewater treatment effluent. Int. J. Agric. Biol. 12:586–590 [Google Scholar]
- 6. Chevalier P., Proulx D., Lessard P., Vincent W. F., de la Noüe J. 2000. Nitrogen and phosphorus removal by high latitude mat-forming cyanobacteria for potential use in tertiary wastewater treatment. J. Appl. Phycol. 12:105–112 [Google Scholar]
- 7. Converti A., Casazza A. A., Ortiz E. Y., Perego P., Del Borghi M. 2009. Effect of temperature and nitrogen concentration on the growth and lipid content of Nannochloropsis oculata and Chlorella vulgaris for biodiesel production. Chem. Eng. Process 48:1146–1151 [Google Scholar]
- 8. De la Noüe J., Proulx D. 1988. Biological tertiary treatment of urban wastewater with chitosan-immobilized Phormidium. Appl. Microbiol. Biotechnol. 29:292–297 [Google Scholar]
- 9. Galego N., et al. 2000. Characterization and application of poly(β-hydroxyalkanoates) family as composite biomaterials. Polymer Testing 19:485–492 [Google Scholar]
- 10. Gantar M., Obreht Z., Dalmacija B. 1991. Nutrient removal and algal succession during the growth of Spirulina platensis and Scenedesmus quadricauda on swine wastewater. Bioresour. Technol. 36:167–171 [Google Scholar]
- 11. García-González M., Moreno J., Manzano J. C., Florencio F. J., Guerrero M. G. 2005. Production of Dunaliella salina biomass rich in 9-cis-β-carotene and lutein in a closed tubular photobioreactor. J. Biotechnol. 115:81–90 [DOI] [PubMed] [Google Scholar]
- 12. Gibson J. 1981. Movement of acetate across the cytoplasmic membrane of the unicellular cyanobacteria Synechococcus and Aphanocapsa. Arch. Microbiol. 130:175–179 [DOI] [PubMed] [Google Scholar]
- 13. Hajek B. F., Boyd C. E. 1994. Rating soil and water information for aquaculture. Aquacult. Eng. 13:115–128 [Google Scholar]
- 14. Hocking J., Marchessault R. H. 1994. Biopolyesters, p. 48–96 In Griffin G. F. L. (ed.), Chemistry and technology of biodegradable polymers. Chapman and Hall, London, United Kingdom [Google Scholar]
- 15. Holmes P. A. 1988. Biologically produced (R)-3-hydroxyalkanoate polymers and co-polymers, p. 1–65 In Basset D. C. (ed.), Developments in crystalline polymers-2. Elsevier, London, United Kingdom [Google Scholar]
- 16. Hu Q., Westerhoff P., Vermaas W. 2000. Removal of nitrate from groundwater by cyanobacteria: quantitative assessment of factors influencing nitrate uptake. Appl. Environ. Microbiol. 66:133–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jones A. B., Dennison W. C., Preston N. P. 2001. Integrated mariculture of shrimp effluent by sedimentation, oyster filtration and macroalgal absorption: a laboratory scale study. Aquaculture 193:155–178 [Google Scholar]
- 18. Laliberte G., Lessard P., de la Noue J., Sylvestre S. 1997. Effect of phosphorus addition on nutrient removal from wastewater with the cyanobacterium Phormidium bohneri. Bioresour. Technol. 59:227–233 [Google Scholar]
- 19. Lobban C. S., Harrison P. J. 1997. Seaweeds ecology and physiology, p. 366 Cambridge University Press, Cambridge, United Kingdom [Google Scholar]
- 20. Lupke T., Radusch H. J., Metzner K. 1998. Solid-state processing of PHB-powders. Macromol. Symp. 127:227–240 [Google Scholar]
- 21. Mackinney G. 1941. Absorption of light by chlorophyll solution. J. Biol. Chem. 140:315–322 [Google Scholar]
- 22. Marinho-Soriano E., Nunes S. O., Carneiro M. A. A., Pereira D. C. 2008. Nutrients removal from aquaculture wastewater using the macroalgae Gracilaria birdiae. Biomass. Bioenerg. 33:327–331 [Google Scholar]
- 23. Marinho-Soriano E., Panucci R. A., Carneiro M. A. A., Pereira D. C. 2009. Evaluation of Gracilaria caudata J. Agardh for bioremediation of nutrients from shrimp farming wastewater. Bioresour. Technol. 100:6192–6198 [DOI] [PubMed] [Google Scholar]
- 24. Nishioka M., Nakai K., Miyake M., Asada Y., Taya M. 2001. Production of poly-β-hydroyxybutyrate by thermophilic cyanobacterium, Synechococcus sp. MA19, under phosphate limitation. Biotechnol. Lett. 23:1095–1099 [Google Scholar]
- 25. Olguin E. J., et al. 1994. Simultaneous high-biomass protein production and nutrient removal using Spirulina maxima in sea water supplemented with anaerobic effluents. World J. Microbiol. Biotechnol. 10:576–578 [DOI] [PubMed] [Google Scholar]
- 26. Panda B., Mallick N. 2007. Enhanced poly-β-hydroxybutyrate accumulation in a unicellular cyanobacterium, Synechocystis sp. PCC 6803. Lett. Appl. Microbiol. 44:194–198 [DOI] [PubMed] [Google Scholar]
- 27. Philip S., Keshavarz T., Roy I. 2007. Polyhydroxyalkanoates: biodegradable polymers with a range of applications. J. Chem. Technol. Biotechnol. 82:233–247 [Google Scholar]
- 28. Rai L. C., Mallick N., Singh J. B., Kumar H. D. 1991. Physiological and biochemical characteristics of a copper tolerant and a wild type strain of Anabaena doliolum under copper stress. J. Plant Physiol. 138:68–74 [Google Scholar]
- 29. Richmond A., Grobbelaar J. U. 1986. Factors affecting the output rate of Spirulina platensis with reference to mass cultivation. Biomass 10:253–264 [Google Scholar]
- 30. Richmond A. 1996. Efficient utilization of high irradiance for production of photoautotropic cell mass: a survey. J. Appl. Phycol. 8:381–387 [Google Scholar]
- 31. Riis V., Mai W. 1988. Gas chromatographic determination of poly-β-hydroxybutyric acid in microbial biomass after hydrochloric acid propanolysis. J. Chromatogr. 445:285–289 [Google Scholar]
- 32. Rijn J. V. 1996. The potential for integrated biological treatment systems in recirculating fish culture: a review. Aquaculture 139:181–201 [Google Scholar]
- 33. Rippka R., Deruelles J., Waterbury J. B., Herdman M. 1979. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J. Gen. Microbiol. 111:1–61 [Google Scholar]
- 34. Samantaray S., Mallick N. 2011. Production and characterization of poly-β-hydroxybutyrate (PHB) polymer from Aulosira fertilissima. J. Appl. Phycol. doi:10.1007/s10811-011-9699-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sarkar S., Kamilya D., Mal B. C. 2007. Effect of geometric and process variables on the performance of inclined plate settlers in treating aquacultural waste. Water Res. 41:993–1000 [DOI] [PubMed] [Google Scholar]
- 36. Sharma L., Singh A. K., Panda B., Mallick N. 2007. Process optimization for poly-β-hydroxybutyrate production in a nitrogen fixing cyanobacterium, Nostoc muscorum using response surface methodology. Bioresour. Technol. 98:987–993 [DOI] [PubMed] [Google Scholar]
- 37. Silverstein R. M., Bassler G. C., Morril T. C. 1991. Spectrometric identification of organic compounds, 5th ed John Wiley and Sons Inc., Singapore [Google Scholar]
- 38. Srebotnjak T., Carr G., de Sherbinin A., Rickwood C. 2011. A global Water Quality Index and hot-deck imputation of missing data. Ecol. Indic. doi:10.1016/j.ecolind.2011.04.023 [Google Scholar]
- 39. Stal L. J. 1992. Poly(hydroxyalkanoates) in cyanobacteria: a review. FEMS Microbiol. Rev. 103:169–180 [Google Scholar]
- 40. Straub C. P. 1989. Practical handbook of environmental control. CRC Press Inc., Boca Raton, FL [Google Scholar]
- 41. Vincenzini M., De Philippis R. 1999. Polyhydroxyalkanoates, p. 292–312 In Cohen Z. (ed.), Chemicals from microalgae. Taylor and Francis Inc., New York, NY [Google Scholar]
- 42. Vonshak A., Guy R. 1987. Photo-inhibition as a limiting factor in outdoor cultivation of Spirulina platensis, p. 365–370 In Stadler T. (ed.), Algal biotechnology. Elsevier-Applied Science Publishers, London, United Kingdom [Google Scholar]
- 43. Yang S., Leong K. F., Du Z., Chua C. K. 2001. The design of scaffolds for use in tissue engineering. Part I. Traditional factors. Tissue Eng. 7:679–689 [DOI] [PubMed] [Google Scholar]
- 44. Yellore V., Desia A. 1998. Production of poly-β-hydroxybutyrate from lactose and whey by Methylobacterium sp. ZP24. Lett. Appl. Microbiol. 26:391–394 [DOI] [PubMed] [Google Scholar]
- 45. Zar J. H. 1999. Biostatistical analysis, 4th ed Prentice-Hall Inc., Upper Saddle River, NJ [Google Scholar]
- 46. Zhang K., Miyachi S., Kurano N. 2001. Evaluation of a vertical flat-plate photobioreactor for outdoor biomass production and carbon dioxide bio-fixation: effects of reactor dimensions, irradiation and cell concentration on the biomass productivity and irradiation utilization efficiency. Appl. Microbiol. Biotechnol. 55:428–433 [DOI] [PubMed] [Google Scholar]