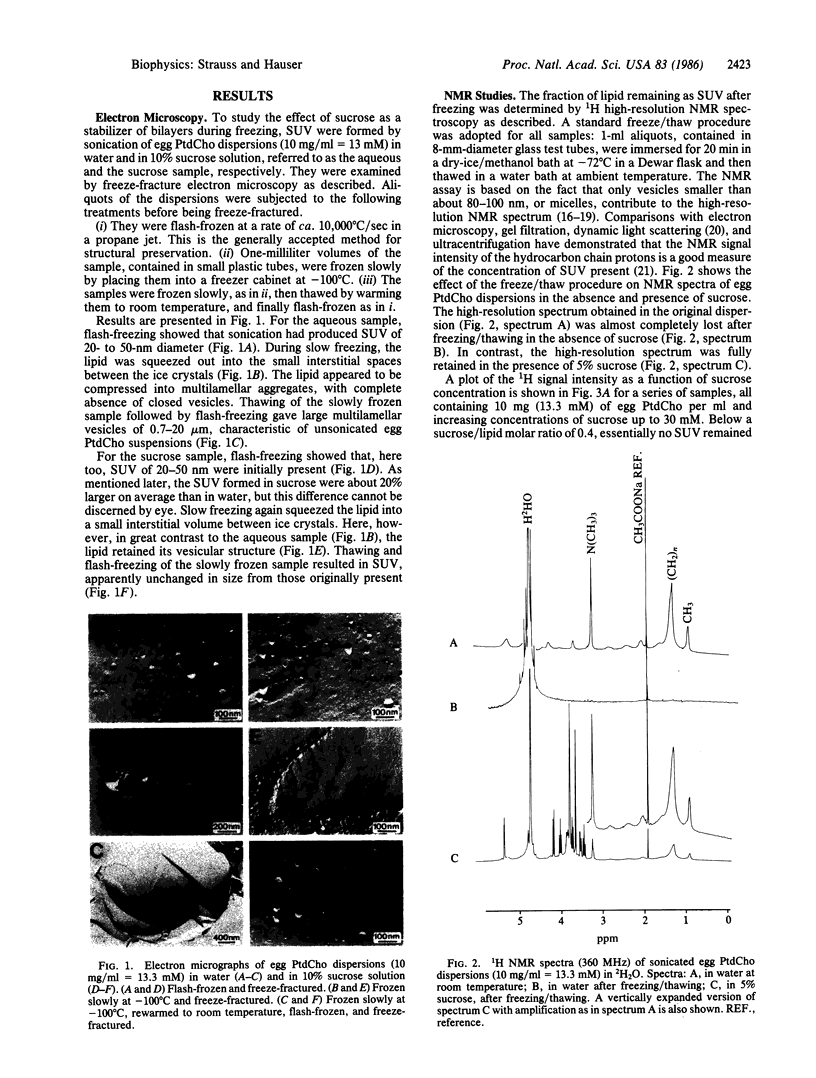
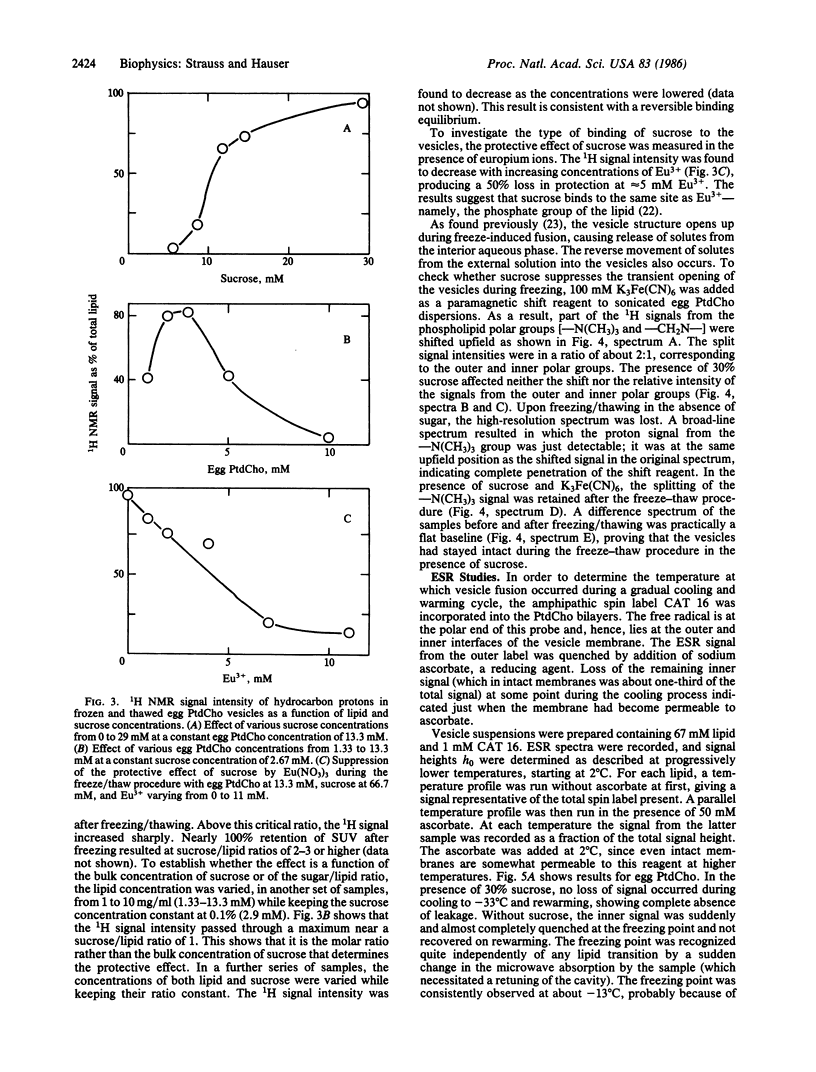
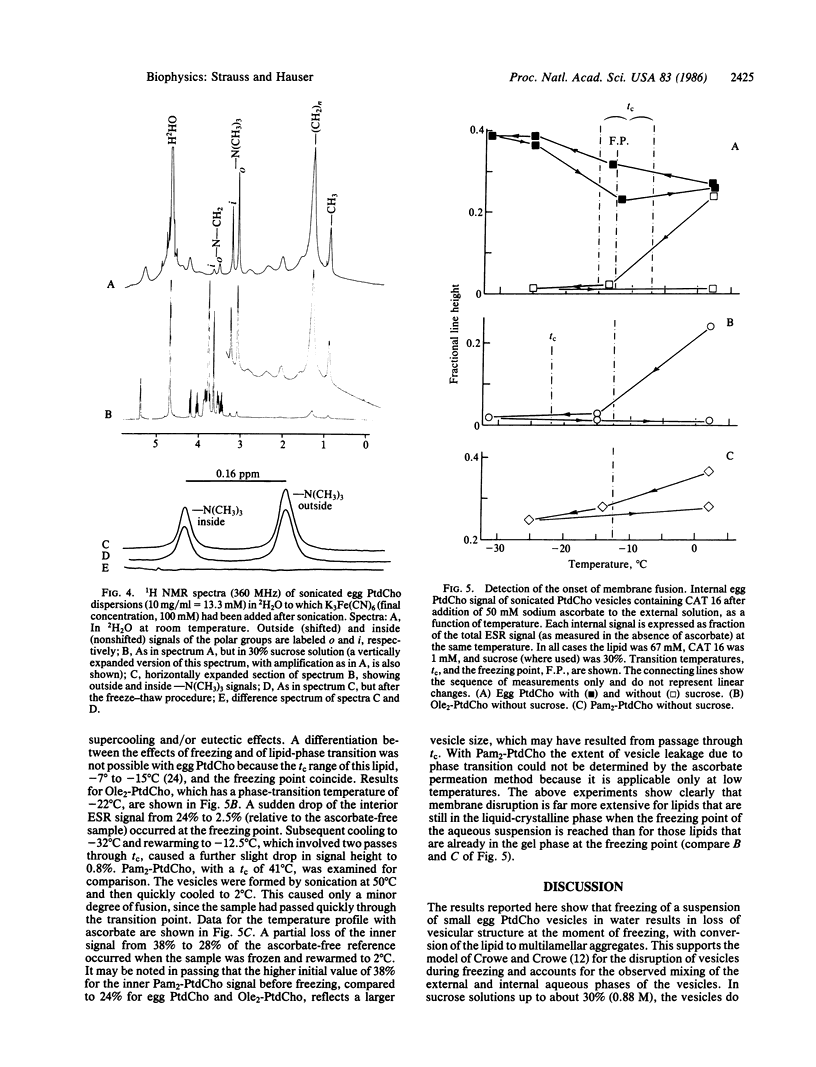
Abstract
The freeze-induced fusion and leakage of small unilamellar vesicles (SUV) of natural and synthetic phosphatidylcholines and the suppression of these processes by sucrose was studied by electron microscopy, by high-resolution NMR, and by ESR techniques. During slow freezing of SUV suspensions in water, the lipid was compressed into a small interstitial volume and transformed into a multilamellar aggregate without vesicular structure. When frozen in sucrose solution, the lipid also was compressed between the ice crystals but remained in the form of vesicles. The fractional amount of lipid remaining as SUV after freezing was found to increase significantly only at sucrose/lipid molar ratios above 0.4. Eu3+ displaced sucrose from the lipid by competitive binding. During freezing in the absence of sucrose, the vesicles became transiently permeable to ions. ESR studies showed that fusion of vesicles in the absence of sucrose is far more extensive when they are frozen while above their phase-transition temperature (tc) than when frozen while below their tc. It is concluded that the extent of membrane disruption depends on the membrane mobility at the moment of freezing and that sucrose exerts its protective effect by binding to the membrane interface and/or by affecting the water structure.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bloom M., Burnell E. E., Valic M. I., Weeks G. Nuclear magnetic resonance line shapes in lipid bilayer model membranes. Chem Phys Lipids. 1975 Apr;14(2):107–112. doi: 10.1016/0009-3084(75)90052-3. [DOI] [PubMed] [Google Scholar]
- Crowe J. H., Crowe L. M. Induction of anhydrobiosis: membrane changes during drying. Cryobiology. 1982 Jun;19(3):317–328. doi: 10.1016/0011-2240(82)90160-2. [DOI] [PubMed] [Google Scholar]
- Crowe J. H., Crowe L. M., Mouradian R. Stabilization of biological membranes at low water activities. Cryobiology. 1983 Jun;20(3):346–356. doi: 10.1016/0011-2240(83)90023-8. [DOI] [PubMed] [Google Scholar]
- Crowe J. H., Whittam M. A., Chapman D., Crowe L. M. Interactions of phospholipid monolayers with carbohydrates. Biochim Biophys Acta. 1984 Jan 11;769(1):151–159. doi: 10.1016/0005-2736(84)90018-x. [DOI] [PubMed] [Google Scholar]
- Crowe L. M., Mouradian R., Crowe J. H., Jackson S. A., Womersley C. Effects of carbohydrates on membrane stability at low water activities. Biochim Biophys Acta. 1984 Jan 11;769(1):141–150. doi: 10.1016/0005-2736(84)90017-8. [DOI] [PubMed] [Google Scholar]
- Düzgünes N., Paiement J., Freeman K. B., Lopez N. G., Wilschut J., Papahadjopoulos D. Modulation of membrane fusion by ionotropic and thermotropic phase transitions. Biochemistry. 1984 Jul 17;23(15):3486–3494. doi: 10.1021/bi00310a016. [DOI] [PubMed] [Google Scholar]
- Finer E. G., Flook A. G., Hauser H. Mechanism of sonication of aqueous egg yolk lecithin dispersions and nature of the resultant particles. Biochim Biophys Acta. 1972 Jan 27;260(1):49–58. doi: 10.1016/0005-2760(72)90073-2. [DOI] [PubMed] [Google Scholar]
- Hauser H., Gains N., Müller M. Vesiculation of unsonicated phospholipid dispersions containing phosphatidic acid by pH adjustment: physicochemical properties of the resulting unilamellar vesicles. Biochemistry. 1983 Sep 27;22(20):4775–4781. doi: 10.1021/bi00289a025. [DOI] [PubMed] [Google Scholar]
- Hauser H., Gains N. Spontaneous vesiculation of phospholipids: a simple and quick method of forming unilamellar vesicles. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1683–1687. doi: 10.1073/pnas.79.6.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser H., Pascher I., Pearson R. H., Sundell S. Preferred conformation and molecular packing of phosphatidylethanolamine and phosphatidylcholine. Biochim Biophys Acta. 1981 Jun 16;650(1):21–51. doi: 10.1016/0304-4157(81)90007-1. [DOI] [PubMed] [Google Scholar]
- Hauser H., Phillips M. C. Conformation of the lecithin polar group in charged vesicles. Nature. 1976 Jun 3;261(5559):390–394. doi: 10.1038/261390a0. [DOI] [PubMed] [Google Scholar]
- Ladbrooke B. D., Chapman D. Thermal analysis of lipids, proteins and biological membranes. A review and summary of some recent studies. Chem Phys Lipids. 1969 Dec;3(4):304–356. doi: 10.1016/0009-3084(69)90040-1. [DOI] [PubMed] [Google Scholar]
- Lis L. J., McAlister M., Fuller N., Rand R. P., Parsegian V. A. Interactions between neutral phospholipid bilayer membranes. Biophys J. 1982 Mar;37(3):657–665. [PMC free article] [PubMed] [Google Scholar]
- MacKenzie A. P. The physico-chemical basis for the freeze-drying process. Dev Biol Stand. 1976 Oct;36:51–67. [PubMed] [Google Scholar]
- Müller M., Meister N., Moor H. Freezing in a propane jet and its application in freeze-fracturing. Mikroskopie. 1980 Sep;36(5-6):129–140. [PubMed] [Google Scholar]
- Papahadjopoulos D., Vail W. J., Pangborn W. A., Poste G. Studies on membrane fusion. II. Induction of fusion in pure phospholipid membranes by calcium ions and other divalent metals. Biochim Biophys Acta. 1976 Oct 5;448(2):265–283. doi: 10.1016/0005-2736(76)90241-8. [DOI] [PubMed] [Google Scholar]
- Parsegian V. A., Fuller N., Rand R. P. Measured work of deformation and repulsion of lecithin bilayers. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2750–2754. doi: 10.1073/pnas.76.6.2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips M. C., Graham D. E., Hauser H. Lateral compressibility and penetration into phospholipid monolayers and bilayer membranes. Nature. 1975 Mar 13;254(5496):154–156. doi: 10.1038/254154a0. [DOI] [PubMed] [Google Scholar]
- Pick U. Liposomes with a large trapping capacity prepared by freezing and thawing of sonicated phospholipid mixtures. Arch Biochem Biophys. 1981 Nov;212(1):186–194. doi: 10.1016/0003-9861(81)90358-1. [DOI] [PubMed] [Google Scholar]
- Sheetz M. P., Chan S. I. Effect of sonication on the structure of lecithin bilayers. Biochemistry. 1972 Nov 21;11(24):4573–4581. doi: 10.1021/bi00774a024. [DOI] [PubMed] [Google Scholar]
- Stockton G. W., Polnaszek C. F., Tulloch A. P., Hasan F., Smith I. C. Molecular motion and order in single-bilayer vesicles and multilamellar dispersions of egg lecithin and lecithin-cholesterol mixtures. A deuterium nuclear magnetic resonance study of specifically labeled lipids. Biochemistry. 1976 Mar 9;15(5):954–966. doi: 10.1021/bi00650a003. [DOI] [PubMed] [Google Scholar]
- Wilschut J., Düzgüneş N., Hoekstra D., Papahadjopoulos D. Modulation of membrane fusion by membrane fluidity: temperature dependence of divalent cation induced fusion of phosphatidylserine vesicles. Biochemistry. 1985 Jan 1;24(1):8–14. doi: 10.1021/bi00322a002. [DOI] [PubMed] [Google Scholar]



