Abstract
Non-O157 Shiga toxin-producing Escherichia coli (STEC) strains are clinically significant food-borne pathogens. However, there is a dearth of information on serotype prevalence and virulence gene distribution, data essential for the development of public health protection monitoring and control activities for the meat and dairy industries. Thus, the objective of this study was to examine the prevalence of non-O157 STEC on beef and dairy farms and to characterize the isolates in terms of serotype and virulence markers. Bovine fecal samples (n = 1,200) and farm soil samples (n = 600) were collected from 20 farms throughout Ireland over a 12-month period. Shiga toxin-positive samples were cultured and colonies examined for the presence of stx1 and/or stx2 genes by PCR. Positive isolates were serotyped and examined for a range of virulence factors, including eaeA, hlyA, tir, espA, espB, katP, espP, etpD, saa, sab, toxB, iha, lpfAO157/OI-141, lpfAO113, and lpfAO157/OI-154. Shiga toxin and intimin genes were further examined for known variants. Significant numbers of fecal (40%) and soil (27%) samples were stx positive, with a surge observed in late summer-early autumn. One hundred seven STEC isolates were recovered, representing 17 serotypes. O26:H11 and O145:H28 were the most clinically significant, with O113:H4 being the most frequently isolated. However, O2:H27, O13/O15:H2, and ONT:H27 also carried stx1 and/or stx2 and eaeA and may be emerging pathogens.
INTRODUCTION
Shiga toxin-producing Escherichia coli (STEC), also referred to as verocytotoxogenic Escherichia coli (VTEC), has emerged as a group of highly pathogenic Escherichia coli strains characterized by the production of one or more Shiga toxins (stx1, stx2, or their variants). STEC is common in ruminants and related foods (4, 5, 15, 30, 47). Clinical manifestations in humans range from hemorrhagic colitis (HC) to hemolytic uremic syndrome (HUS) and thrombocytopenic purpura (TTP) (3) and are directly related to the virulence genes present in the causative agent. To date, STEC prevalence studies have focused primarily on E. coli O157:H7, because of its initial predominance in human clinical infection. Culture and molecular methods for the detection of STEC have thus been developed and optimized for that serotype, with little attention to and resultant underestimation of the risks posed by non-O157 serogroups. However, non-O157 infections are of primary concern in several countries (19), with half of all confirmed STEC infections in Europe caused by non-O157 STEC (3), and non-O157 infections outnumber O157 cases in the United States (10). Ireland has the highest per capita incidence (5.7 STEC cases per 100,000) in Europe (28).
E. coli O157:H7, the most extensively investigated serotype, has long been associated with ruminants, but more-recent work has suggested that these animals are also important reservoirs for other STEC serotypes (29). Six non-O157 O groups have been identified by the Centers for Disease Control and Prevention (CDC) as being responsible for over 70% of non-O157 STEC-associated illness (O26, O45, O103, O111, O121, and O145) (10), and both the European Food Safety Authority (EFSA) and the U.S. Department of Agriculture (USDA) have issued recommendations for laboratory testing for these pathogens (17, 19). However, serotype alone does not necessarily provide an accurate assessment of the ability to cause illness or the severity of disease, which requires virulence gene data. In addition to the stx genes, other virulence factors encoded by eaeA (intimin), tir (espE) (translocated intimin receptor), espA (EspA protein), and espB (EspB protein) are required for intimate attachment and the formation of the attaching and effacing (A/E) lesions characteristic of STEC infection (10, 13, 79). Other clinically significant virulence markers include hlyA (pO157 enterohemolysin releasing hemoglobin from red blood cells), katP (pO157 catalase peroxidase that defends the cell against oxidative damage), etpD (pO157, encodes part of a type II secretory pathway transporting proteins across the outer membrane) (73), lpf (chromosomal long polar fimbriae) (72), espP (pO157 extracellular serine protease autotransporter), saa (pO113 STEC agglutinating adhesion) (55), sab (pO113 STEC autotransporter) (33), toxB (pO157 adhesion), and iha (chromosomal iron-regulated gene A homolog adhesin) (68, 70).
Despite the increasing general recognition of the clinical significance of non-O157 STEC, there is a lack of data on (i) prevalence at the farm level, (ii) distribution of virulence factors, and (iii) emerging serotypes for inclusion in future monitoring programs. The objective of this study was to contribute to addressing these data gaps. Furthermore, as the interaction between different virulence gene products in pathogenesis is poorly understood, the presence of virulence genes in farm serotypes in this study that are not associated with human infection would also provide an important insight into the relative importance of virulence factors required for human illness.
MATERIALS AND METHODS
Sample collection.
Twenty bovine (beef and dairy) farms located throughout the Republic of Ireland were selected on the basis of geographical location (covering the whole country) for inclusion in this study. Each farm was sampled bimonthly over a 1-year period (July 2007 to July 2008). Animals were largely on pasture for the duration of the study, although they were housed during the winter months. At each visit, 10 fresh fecal samples (from the ground) and 5 soil samples (taken from areas where the animals congregated, such as beside a water trough) were aseptically transferred into sterile containers (Sterilin Ltd., Medical Supply Co., Mulhuddard, Dublin, Ireland). A total of 1,200 fecal and 600 soil samples were taken throughout the study. Samples were stored at 4°C for no more than 24 h prior to analysis.
Bacterial cultures.
The E. coli control strains used are described in Table 1. Control strains used in the typing of the Shiga toxin genes, STEC strain O111:H− (EC132) and STEC strain O113:H21 (98NK2), were kindly provided by Helge Karch (University of Münster, Germany) and Adrienne Patton (University of Adelaide, Australia), respectively.
Table 1.
Origin and serotype of isolates used as positive controls
| Strain | Serotype | Origin | Target gene | Reference/source |
|---|---|---|---|---|
| 3653/97 | O78:H− | Patient with diarrhea | stx1c | 77 |
| 7139/96 | O8:H− | Asymptomatic carrier | stx1d | 42 |
| E32511 | O157:H− | Patient with HC | stx2c | 34 |
| EH250 | ONT:H12 | Patient with diarrhea | stx2d (nonactivatable) | 61 |
| B2F1 | O91:H21 | Patient with HUS | stx2dact | 49 |
| 2771/97 | ONT: H− | Patient with diarrhea | stx2e | 50 |
| T4/97 | O128:H2 | Pigeon | stx2f | 67 |
| EC132 | O111:H− | Bovine feces | stx1, stx2, eaeA, and hlyA | Provided by University of Münster, Germany |
| 98NK2 | O113:H21 | Patient with HUS | lpfAO113, saa, sab | 56 |
| 38094 | O157:H7 | Patient with HUS | katP, etpD, tir, espP, espA, espB, lpfAO157/OI154, toxB, and iha | Provided by CDC, Atlanta, GA |
| C9490 | O157:H7 | Patient with HUS | lpfA | 74 |
Detection of vt-positive E. coli.
Each sample (10 g) was homogenized with 90 ml of tryptone soya broth (TSB; CM0129; Oxoid, Basingstoke, United Kingdom) containing 4 μg ml−1 vancomycin hydrochloride (V2002; Sigma-Aldrich, St. Louis, MO) and incubated overnight at 37°C. One-milliliter aliquots of the homogenized enrichment were harvested by centrifugation (7,426 × g for 10 min at 4°C). Genomic DNA was extracted from the resultant pellets (resuspended in maximum-recovery diluent [MRD] CM0733; Oxoid, Basingstoke, United Kingdom) by using the DNeasy tissue kit (69506; Qiagen, Crawley, United Kingdom) according to the manufacturer's instructions. The remainder of the enrichment samples were stored at 4°C. The sample DNA (2 μl) was screened for the presence of stx1 and/or stx2 genes by using PCR in a Peltier thermal cycler (PTC-200; MJ Research Inc., Watertown, MA) with primer sets and reaction conditions as described by Paton and Paton (54) (Table 2). The reaction mixture (reaction mix A) was modified by using 1× Green GoTaq reaction buffer (M891; Promega, Madison, WI) and made up to a final volume of 25 μl. PCR products (10 μl) were separated by electrophoresis on a 1.5% (wt/vol) agarose gel and visualized under UV light (GelDoc 2000 system; Bio-Rad Laboratories, Hercules, CA) by ethidium bromide staining (10 mg ml−1). Product size was determined using a BenchTop 100-bp DNA ladder (G8291; Promega).
Table 2.
Target genes and primer sequences used for the detection of STEC virulence gene markers
| Primer | Sequence (5′–3′) | Target (gene) | Reference |
|---|---|---|---|
| stx1-F | ATAAATCGCCATTCGTTGACTAC | stxA1 | 54 |
| stx1-R | AGAACGCCCACTGAGATCATC | ||
| stx2-F | GGCACTGTCTGAAACTGCTCC | stxA2 | 54 |
| stx2-R | TCGCCAGTTATCTGACATTCTG | ||
| eaeA-F | GACCCGGCACAAGCATAAGC | eaeA | 54 |
| eaeA-R | CCACCTGCAGCAACAAGAGG | ||
| hlyA-F | GCATCATCAAGCGTACGTTCC | hlyA | 54 |
| hlyA-R | AATGAGCCAAGCTGGTTAAGCT | ||
| TIR-F | CATTACCTTCACAAACCGAC | tir | 40 |
| TIR-R | CCCCGTTAATCCTCCCAT | ||
| EspAa | CACGTCTTGAGGAAGTTTGG | espA | 48 |
| EspAb | CCGTTGTTAATGTGAGTGCG | ||
| EspBa | CGATGGTTAATTCCGCTTCG | espB | 48 |
| EspBb | CGATGGTTAATTCCGCTTCG | ||
| ESPPa | AAACAGCAGGCACTTGAACG | espP | 48 |
| ESPPb | AGACAGTTCCAGCGACAACC | ||
| D1 | CGTCAGGAGGATGTTCAG | etpD | 65 |
| D13R | CGACTGCACCTGTTCCTGATTA | ||
| wkat-B | CTTCCTGTTCTGATTCTTCTGG | katP | 11 |
| wkat-F | AACTTATTTCTCGCATCATCC | ||
| lpfO141-F | CTGCGCATTGCCGTAAC | lpfAO157/OI-141 | 69 |
| lpfO141-R | ATTTACAGGCGAGATCGTG | ||
| lpfA-F | ATGAAGCGTAATATTATAG | lpfAO113 | 16 |
| lpfA-R | TTATTTCTTATATTCGAC | ||
| O154-FCT | GCAGGTCACCTACAGGCGGC | lpfAO157/OI-154 | 71 |
| O154-RCT | CTGCGAGTCGGCGTTAGCTG | ||
| SAADF | CGTGATGAACAGGCTATTGC | saa | 55 |
| SAADR | ATGGACATGCCTGTGGCAAC | ||
| toxB.911F | ATACCTACCTGCTCTGGATTGA | toxB | 70 |
| toxB.1468R | TTCTTACCTGATCTGATGCAGC | ||
| iha-I | CAGTTCAGTTTCGCATTCACC | iha | 68 |
| iha-II | GTATGGCTCTGATGCGATG | ||
| LH0147-BamHI | CCCGGATCCGGAAACTCCAAGAGTATTGC | sab | 33 |
| LH0147-EcoRI | CCCGAATTCCCTTGCTTTTCCCTGTTACC | ||
| KS7 | CCCGGATCCATGAAAAAAACATTATTAATAGC | stx1b | 66 |
| KS8 | CCCGAATTCAGCTATTCTGAGTCAACG | ||
| Stx1c-1 | TTTTCACATGTTACCTTTCCT | stxA1c | 77 |
| Stx1c-2 | CATAGAAGGAAACTCATTAGG | ||
| VT1varF | CTTTTCAGTTAATGCGATTGCT | stxA1d | 12 |
| VT1varR | AACCCCATGATATCGACTGC | ||
| LP43 | ATCCTATTCCCGGGAGTTTACG | stxA2 variants | 13 |
| LP44 | GCGTCATCGTATACACAGGAGC | ||
| GK3 | ATGAAGAAGATGTTTATG | stxB2 and stxB2c | 32 |
| GK4 | TCAGTCATTATTAAACTG | ||
| SLT-II-vc | ACCACTCTGCAACGTGTCGC | stx2c and stx2dact | 36 |
| CKS2 | ACTGAATTGTGACACAGATTA | ||
| VT2-cm | AAGAAGATATTTGTAGCGG | stxB2d | 61 |
| VT2-f | TAAACTGCACTTCAGCAAAT | ||
| FK1 | CCCGGATCCAAGAAGATGTTTATAG | stxB2e | 21 |
| FK2 | CCCGAATTCTCAGTTAAACTTCACC | ||
| 128-1 | AGATTGGGCGTCATTCACTGGTTG | stxA2f | 67 |
| 128-2 | TACTTTAATGGCCGCCCTGTCTCC | ||
| 209F | GTTATATTTCTGTGGATATC | stx2g | 44 |
| 781R | GAATAACCGCTACAGTA | ||
| VSAAF | ACTCGCATAATTGGTGGTG | saa variants | 45 |
| VSAAR | ATCATTGGTATTGCTGTCAT | ||
| EaeVF | AGYATTACTGAGATTAAG | eae variants | 64 |
| EaeVR | AAATTATTYTACACARAY | ||
| EaeZetaVR | AGTTTATTTTACGCAAGT | ||
| EaeIotaVR | TTAAATTATTTTATGCAAAC |
Isolation of stx-positive E. coli.
Samples positive for the virulence genes stx1 and/or stx2 were serially diluted in MRD and plated onto Chromocult tryptone bile X-glucuronide agar (TBX; Merck, Germany) supplemented with sterile filtered streptomycin sulfate (10 μg ml−1) (S6501; Sigma-Aldrich, Steinheim, Germany) and sulfamethazine (100 μg ml−1) (S6256; Sigma-Aldrich, Steinheim, Germany). Preliminary studies in our laboratory (data not shown), designed to optimize non-O157 recovery, found that TBX agar gave maximum recovery of non-O157 STEC isolates. After overnight incubation at 37°C, 5 colonies of differing colony morphologies were taken from each sample and streaked onto nutrient agar (NA) (CM0003; Oxoid, Basingstoke, United Kingdom) and eosin methylene blue agar (EMBA) (CM0069; Oxoid, Basingstoke, United Kingdom). Agar plates were incubated overnight at 37°C. Genomic DNA was extracted from presumptive E. coli colonies (those showing a green sheen on EMB plates) by resuspending a single colony from the NA plate in PrepMan Ultra sample preparation reagent (Applied Biosciences, Foster City, CA) and extracting DNA as per the manufacturer's instructions. All isolates were assessed for the presence of stx1, stx2, eaeA, and enterohemorrhagic E. coli (EHEC) hlyA by using 2 μl of the DNA sample and the PCR protocol as described above (Table 2). All STEC isolates were preserved in a cryogenic bead storage system (Protect bacterial preservers; TSC Ltd., Heywood, United Kingdom) at −20°C and were routinely recultured.
Serotyping.
Serotyping of the O (lipopolysaccharide) and H (flagellar) antigens was performed by the E. coli Reference Center at Pennsylvania State University, University Park, PA. The O antigen was determined using antisera produced against available serogroups designated O1 to O181, with the exceptions of O31, O47, O72, O93, O94, and O122, as these are not designated (52). The H antigen was established by PCR-restriction fragment length polymorphism (PCR-RFLP) analysis of the fliC gene, which is responsible for flagella (46).
Detection of putative virulence and adhesion genes.
One cryogenic bead of each STEC isolate was individually cultured in 10 ml of TSB overnight at 37°C. Genomic DNA was extracted from a 1-ml aliquot of culture by using PrepMan Ultra reagent. In addition to stx1, stx2, eaeA, and hlyA genes, the template DNA of each STEC isolate was analyzed using PCR for the presence or absence of other putative virulence and adhesion genes. These include (i) genes associated with the locus of enterocyte effacement (LEE) (tir, espA, and espB), (ii) plasmid-encoding virulence genes associated with STEC O157:H7 (katP, espP, and etpD), and (iii) genes encoding other attachment mechanisms (saa, sab, toxB, iha, lpfAO157/OI-141, lpfAO113, and lpfAO157/OI-154). The primer sets and target genes used in these analyses are also listed in Table 2. Reaction mixture A was used to amplify the katP, etpD, tir, saa, toxB, iha, lpfAO157/OI-141, lpfAO113, and lpfAO157/OI-154 genes. Reaction mixture B was modified from the method of McNally et al. (48), in which 1× Green GoTaq reaction buffer was used to amplify the espA, espB, espP, and sab genes. Reaction mixtures, including 2 μl of DNA, were made up to a final volume of 25 μl, and PCR product (10 μl) was separated by electrophoresis on a 1.5% (wt/vol) agarose gel and visualized under UV light by ethidium bromide staining. Product size was determined using a BenchTop 100-bp DNA ladder.
Molecular characterization of stx variants.
The Shiga toxin genes were subtyped using the primer sets, typing protocols, and PCR conditions given in Table 2. Briefly, isolates were initially examined by PCR using the primer pair KS7 and KS8 to detect stx1 and the genetic variants (stx1c and stx1d) of stx1 (66). Positive isolates were further assessed for the presence of vt1c and vt1d by using the primer pair stx1c-1 and stx1c-2 (77) and primer pair VT1AvarF and VT1varR (12). PCR and RFLP-PCR was used to examine each isolate for the presence of vt2 and its genetic variants (stx2a, stx2c, activatable stx2d [stx2dact], stx2e, stx2f, and stx2g) by using previously published methods (Table 2). In brief, all STEC isolates were examined by PCR with the primer pair LP43 and LP44 for the detection of stx2, stx2c, stx2dact, and stx2e (13); primer pair 128-1 and 128-2 for the detection of vt2f (67); and primer pair 209F and 781R for the detection of vt2g (44). Isolates positive for stx2 were further differentiated by PCR screening using the primer pair GK3 and GK4 (32) to detect stx2, stx2c, and stx2dact. The resulting PCR products were incubated with the HaeIII restriction enzyme (R0107; New England BioLabs, Hertfordshire, United Kingdom) according to the manufacturer's instructions. Bands at 137 bp and 151 bp (32) indicated the presence of the variant stx2c or stx2dact. PCR-RFLP methods were also used to differentiate between stx2c and stx2dact. PCR was preformed using the primer pair SLT-II-vc and CKS2. The resulting PCR product (890 bp) was incubated in the PstI restriction enzyme (R0140; New England BioLabs) according to the manufacturer's instructions. The absence of any band in the amplicon was indicative of stx2dact, with 504-bp and 386-bp bands indicative of stx2 and stx2c, respectively (36).
Molecular characterization of intimin variants.
All intimin-positive STEC isolates were further characterized using intimin typing as described by Ramachandran and colleagues (64). Briefly, a single forward primer (EaeVF) and 3 reverse primers (EaeVR, EaeZetaVR, and EaeIotaVR) (Eurofins MWG Operon, Ebersberg, Germany) (Table 2) were used to amplify an 834- to 876-bp fragment (size varied depending on the variant) representing the 3′-variable regions of all reported intimin variants. The resulting PCR products (10 μl) were incubated along with 3 restriction endonucleases, AluI, RsaI, and CfoI (New England Biosciences, Ipswich, MA), following the manufacturer's instructions. The restriction fragments were separated by agarose gel electrophoresis and visualized using ethidium bromide staining. To determine which intimin type was present in each isolate, the resulting RFLP patterns were compared to published RFLP profiles (64).
Sequencing analysis.
All PCR products for the intimin variants and the vt2g gene were purified using the QIAquick PCR purification kit (28106; Qiagen, Crawley, West Sussex, United Kingdom) by following the manufacturer's guidelines and commercially sequenced (Eurofins MWG Operon, Ebersberg, Germany). Sequences were analyzed using the BLASTN program to search nucleotide databases (2), and sequences were aligned with the sequence of the vt2g gene and intimin variants.
RESULTS
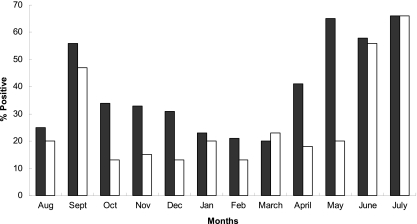
Of the 1,800 samples analyzed, 40% (480/1,200) of fecal and 27% (162/600) of soil samples were stx1 and/or stx2 positive. STEC were cultured from 1.9% (23/1,200) of fecal and 0.7% (4/600) of soil samples, with the majority of the 107 isolates obtained from samples taken from May to September (Fig. 1). The serotypes obtained included O2:H27, O6:H8, O13/O150:H2, O20:H19, O26:H11, O86:H21, O109:H5, O113:H4, O116:H28, O119:H5, O136:H2, O136:H16, O145:H28, O168:H8, O168:H27, O171:H2, and O174:H21 (Table 3). There were 6 isolates which could not be assigned to O-antigen serogroups but had H4, H17, H18, or H27 flagellar antigens. The most frequently occurring serotypes were O113:H4 (29%), O26:H11 (13%), and O2:H27 (12%), and the most widely distributed were O26:H11 (5 farms) and O113:H4 (4 farms).
Fig. 1.
The seasonal distribution of STEC-positive enrichment samples in feces (■) and soil (□).
Table 3.
Serotypes, sources (fecal or soil), number of isolates, virulence factors, and variantsa
| Serotype (source) | No. of isolates | stx1 | stx1c | stx1d | stx2 | stx2c | stx2dact | stx2d | stx2e | stx2f | stx2g | eaeA | hlyA | tir | espA | espB | lpfAO157 | lpfAO113 | espP | saa | toxB | iha |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| O2:H27 (1F) | 1 | + | − | − | − | − | − | − | − | − | − | + (NT) | + | + | − | − | − | + | + | |||
| O2:H27 (2F) | 2 | − | − | − | + | − | − | + | − | − | − | − | + | − | − | − | − | − | − | − | + | + |
| O2:H27 (2F) | 2 | − | − | − | + | − | − | + | − | − | − | − | + | − | − | − | − | − | − | − | − | + |
| O2:H27 (2F, 1S) | 3 | − | − | − | + | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| O2:H27 (4F, 4S) | 5 | − | − | − | + | − | − | + | − | − | − | − | + | − | − | − | − | − | − | − | − | − |
| O6:H8 (1S) | 1 | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | − | − | − | + |
| O13/O150:H2 (2F) | 2 | + | − | − | + | − | − | + | − | − | − | + (ζ) | + | − | − | − | − | + | + | − | − | + |
| O20:H19 (1F) | 1 | + | − | − | + | − | − | + | − | − | − | − | + | − | − | − | − | + | + | + | − | + |
| O26:H11 (6F) | 6 | + | − | − | − | − | − | − | − | − | − | + (β1) | + | + | − | − | − | + | + | − | + | + |
| O26:H11 (7F) | 7 | + | − | − | + | − | − | + | − | − | − | + (β1) | + | + | − | − | − | + | + | − | + | + |
| O26:H11 (1S) | 1 | + | − | − | − | − | − | − | − | − | − | + (β1) | + | − | − | − | − | + | + | − | + | + |
| O86:H21 (1F) | 1 | − | − | − | + | − | − | + | − | − | − | − | − | − | − | − | − | + | + | + | − | + |
| O109:H5 (1F) | 1 | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | − | − | − | − | + |
| O113:H4 (3S, 23F) | 26 | + | − | − | + | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | + |
| O113:H4 (3F) | 3 | − | − | − | + | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | + |
| O113:H4 (1S, 1F) | 2 | + | − | − | + | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| O116:H28 (4F, 2S) | 6 | − | − | − | + | − | − | + | − | − | + | − | − | − | − | − | − | + | − | − | − | − |
| O119:H5 (3F, 3S) | 6 | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | − | − | − | − | − |
| O136:H2 (1F) | 1 | − | − | − | + | − | − | + | − | − | + | − | − | − | − | − | − | + | − | − | − | − |
| O136:H16 (2F) | 2 | − | − | − | + | − | − | + | − | − | + | − | − | − | − | − | − | + | − | − | − | − |
| O145:H28 (1F) | 1 | + | − | − | − | − | − | − | − | − | − | + (γ) | − | + | + | + | + | − | + | − | + | + |
| O168:H8 (7F) | 7 | − | − | − | + | − | + | + | − | − | − | − | − | − | − | − | − | + | − | − | − | + |
| O168:H8 (2F) | 2 | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | − | − | − | + |
| O168:H27 (F) | 1 | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | − | − | − | + |
| O171:H2 (4F) | 4 | − | − | − | + | − | + | + | − | − | − | − | − | − | − | − | − | + | − | − | − | + |
| O174:H21 (7F) | 7 | − | − | − | + | − | − | + | − | − | − | − | − | − | − | − | − | + | − | − | − | + |
| ONT:H4 (3F, 1S) | 3 | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | − | + | + | − | + |
| ONT:H17 (1S) | 1 | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| ONT:H18 (1F) | 1 | − | − | − | + | − | − | + | − | − | − | − | + | − | − | − | − | + | + | + | − | + |
| ONT:H27 (1F) | 1 | + | − | − | − | − | − | − | − | − | − | + (θ) | − | − | − | − | − | − | + | + | − | − |
F, fecal sample; S, soil sample. Numbers of samples from each source are also indicated. NT, nontypeable.
stx1, stx2, and a combination of both were present in 22%, 42%, and 36% of isolates, respectively. The stx1c and stx1d gene variants were not detected, but stx2d, stx2dact, and stx2g were present in 77%, 11.2%, and 8.4% of isolates, respectively. The distribution of target virulence genes in the farm isolates is shown in Table 3. The intimin gene (eaeA) was present in 18% of isolates: serotypes O2:H27 (variant untypeable), O13/O150:H2 (ζ), O26:H11 (β1), O145:H28 (γ), and ONT:H27 (θ). Other virulence genes detected included hlyA (26%; O2:H27, O13/O150:H2, O20:H19, and O26:H11), tir (14%; O2:H27, O26:H11, and O145:H28), espA (0.9%; O145:H28), espB (0.9%; O145:H28), lpfO157/OI-141 (10.3%; O109:H5, O119:H5, O145:H28, and ONT:H4), lpfAO113 (48.5%; O2:H27, O6:H8, O13/O150:H2, O20:H19, O26:H11, O86:H21, O109:H5, O116:H28, O136:H2, O136:H16, O168:H8, O168:H27, O171:H2, O174:H21, ONT:H4, and ONT:H18), espP (23%; O2:H27, O13/O150:H2, O20:H19, O26:H11, O86:H21, O116:H28, O136:H2, O136:H16, O168:H8, O168:H27, O171:H2, O174:H21, ONT:H4, and ONT:H18), saa (5.6%; O20:H19, O86:H21, ONT:H4, and ONT:H18), toxB (15.9%; O2:H27, O26:H11, and O145:H28), and iha (73.8%; O2:H27, O6:H8, O13/O150:H2, O20:H19, O26:H11, O86:H21, O109:H5, O113:H4, O145:H28, O168:H8, O168:H27, O171:H2, O174:H21, ONT:H4, ONT:H17, and ONT:H18). Virulence genes katP, etpD, lpfAO157/OI-154, and sab were not detected in any of the isolates.
Interestingly, different virulence gene profiles were detected within strains from the same serotype; for example, O26:H11 (10 isolates) displayed 3 different virulence profiles: stx1 stx2 stx2d eaeA hlyA tir lpfAO113 espP toxB iha (7 isolates), stx1 eaeA hlyA tir lpfAO113 espP toxB iha (2 isolates), and stx1 eaeA hlyA lpfAO113 espP toxB iha (1 isolate).
DISCUSSION
Forty percent of bovine fecal and 27% of farm soil samples were positive for Shiga toxin genes (stx1 and/or stx2), with culture-based prevalences of 1.9% and 0.7%, respectively. The difference in the molecular and culture-based analyses was attributed to the relative sensitivities of the two methods and the presence of phage DNA. The increased prevalence in late summer-early autumn has been widely reported (38, 60, 62) and corresponds to an increase in human cases for both O157 and non-O157 STEC (31).
Seventeen different serotypes were isolated from bovine feces and beef farm soil samples, including O2:H27, O6:H8, O13/O150:H2, O20:H19, O26:H11, O86:H21, O109:H5, O113:H4, O116:H28, O119:H5, O136:H2, O136:H16, O145:H28, O168:H8, O168:H27, O171:H2, and O174:H21. Of these, O20:H19, O26:H11 O113:H4, and O171:H2 are common in cattle (7). Indeed the most prevalent serogroup in our study, O113, is the predominant serogroup reported in the majority of European bovine surveys (79). However, our study also discovered a range of new serotypes (O6:H8, O13/O150:H2, O86:H21, O109:H5, O116, H28, O119:H5, and ONT:H17) not previously reported in the lists of non-O157 strains previously published (8, 35, 79). Furthermore, 6 of the 107 STEC strains were O nontypeable, highlighting the need for new antisera for the detection of emerging STEC serogroups. In total, 55% of the strains belonged to 5 serotypes (O2:H27, O26:H11, O113:H4, O136:H2, and O174:H21) previously associated with disease in humans. Moreover, the second most prevalent serotype (O26:H11) has been widely associated with HUS.
STEC strains from patients suffering severe disease such as HC or HUS are frequently stx2 and eaeA positive and many also carry the hlyA gene (23). The majority of the strains obtained in this study were stx2 positive, with 77% of isolates carrying the stx2 and stx2d variants, 13% stx2dact, and 8% stx2g. A high prevalence of stx2 genes in bovine STEC has been previously reported (6, 10), in particular stx2, stx2d, and stx2g (78). In more general terms, the high incidence of stx2 genes observed in this and other studies is a matter of some concern, as the carriage of stx2 genes and in particular stx2c and stx2dact has been linked to more-severe E. coli infection (25), and the presence of stx2c and stx2dact in O168:H8, O171:H2, and ONT:H18 is of particular concern, as all three serotypes, while eaeA negative, possessed an alternative attaching mechanism (lpfAO113).
stx1 was present in 11% of isolates, but neither stx1c nor stx1d was detected. STEC strains harboring stx1c or stx1d are more commonly associated with ovine sources (41) and are rarely recovered from patients suffering from HC or HUS (23, 24).
The eaeA gene was detected in less than one-fifth of isolates (18/107), a finding consistent with previous studies which reported a lower frequency in bovine than in human strains (8). Intimin-positive serotypes included O2:H27, O13/O150:H2, O26:H11, and O145:H28 as well as ONT:H27, with the O26:H11 strains carrying type β1. This subtype has been previously associated with O26:H11 and O157:H7 (8, 64), and its high specificity for both bovine and human cells may underlie its association with clinically significant STEC (9). O145:H2 and ONT:H27 strains carried intimin γ and θ, respectively, which along with β1 are commonly recovered from HC and HUS outbreak patients (53, 64). Different intimin types may have different host cell tropisms (73), and differentiation of different intimin alleles is an important diagnostic and epidemiological tool. The O2:H27 intimin variant was untypeable, suggesting there are more subtypes yet to be identified and characterized.
With the exception of those mentioned above, most STEC serotypes were eaeA negative and therefore unlikely to be associated with large outbreaks and HUS (39), a situation previously reported for the majority of bovine STEC (75, 76). However, while there is a strong association between the carriage of eaeA and the capacity to cause severe human disease (14), non-eaeA strains have been responsible for sporadic cases and small outbreaks in both the United States and Australia (20, 59) as well as the recent outbreak associated with intimin-negative O104:H4 in Germany (18). Attachment of eaeA-negative human pathogenic strains is therefore mediated by alternative adherence factors such as the STEC autoagglutinating adhesion (Saa), found in 5.6% of the strains in this study. Furthermore, to the best of our knowledge, this is the first study reporting the presence of saa in serotypes O20:H19, O86:H21, ONT:H4, and ONT:H18. All of the saa-positive strains were eaeA negative, supporting the hypothesis that these virulence factors are mutually exclusive (1, 37, 43, 58, 71, 78, 79). The eaeA gene is located in the LEE region of the chromosome, while the saa gene is found on pO113; thus there is no known reason as to why both could not be present in the same STEC strain. Further research is required.
The hemolysin gene, hlyA, was present in 30% of our strains, while the etpD and katP genes were absent in all isolates, despite all 3 genes being carried on pO157. Several studies have shown that these 3 genes are almost always present in O157:H7 but occur sporadically in non-O157 strains (22). Indeed, the etpD gene is rarely detected in bovine STEC (22). All of the O113:H4 (the most prevalent serotype) isolates were eaeA negative, a characteristic particularly associated with the O113 serogroup (57). In recent years, intimin-negative STEC O113:H4 has been associated with clinical cases in Ireland (26, 27). Intimin-negative O113:H4 has also been implicated in sporadic cases in other countries, where patients displayed an array of symptoms, including colitis and watery diarrhea (63).
Conclusion.
Despite the low prevalence of STEC using culture-based methods, the diversity of serotypes provides further evidence that cattle are an important reservoir of STEC. The isolation of eaeA-positive O2:H27, O13/O150:H2 O26:H11, O145:H28, and ONT:H27 represents a risk to public health, as does the presence of phage-mediated HUS-associated stx2dact genes in 6% of our isolates. Moreover, cattle are also an important source of atypical STEC, with this study reporting several previously unknown saa-positive STEC serotypes. Our work also highlights several knowledge gaps, including the limitation of current intimin typing and O-serotyping methods as well as in our understanding of virulence factors, specifically why eaeA and saa are rarely if ever found in the same strains.
ACKNOWLEDGMENT
This project was funded by the Department of Agriculture, Fisheries and Food (DAFF) under the Food Institutional Research Measure (FIRM), Ireland.
Footnotes
Published ahead of print on 14 October 2011.
REFERENCES
- 1. Aidar-Ugrinovich L., et al. 2007. Serotypes, virulence genes, and intimin types of Shiga toxin-producing Escherichia coli (STEC) and enteropathogenic E. coli (EPEC) isolated from calves in Sao Paulo, Brazil. Int. J. Food Microbiol. 115:297–306 [DOI] [PubMed] [Google Scholar]
- 2. Altschul S. F., et al. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389–3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Anonymous 2011. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2009. EFSA J. 9:2090 [Google Scholar]
- 4. Bettelheim K. A., Kuzevski A., Gilbert R., Krause D., McSweeney C. 2005. The diversity of Escherichia coli serotypes and biotypes in cattle faeces. J. Appl. Microbiol. 98:699–709 [DOI] [PubMed] [Google Scholar]
- 5. Beutin L., et al. 1997. Epidemiological relatedness and clonal types of natural populations of Escherichia coli strains producing Shiga toxins in separate populations of cattle and sheep. Appl. Environ. Microbiol. 63:2175–2180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Beutin L., et al. 2007. Relationship between O-antigen subtypes, bacterial surface structures and O-antigen gene clusters in Escherichia coli O123 strains carrying genes for Shiga toxins and intimin. J. Med. Microbiol. 56:177–184 [DOI] [PubMed] [Google Scholar]
- 7. Blanco J., et al. 2001. 2001 Epidemiology of verocytotoxigenic Escherichia coli (STEC) in ruminants, p. 113–148 In Duffy G., Garvey P., McDowell D. A. (ed.), Inc., Trumbull, CT: Verocytotoxigenic E. coli. Food and Nutrition Press [Google Scholar]
- 8. Blanco M., et al. 2004. Serotypes, virulence genes, and intimin types of Shiga toxin (verotoxin)-producing Escherichia coli isolates from cattle in Spain and identification of a new intimin variant gene (eae-). J. Clin. Microbiol. 42:645–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bolton D. J. 2011. Verocytotoxigenic (Shiga toxin-producing) Escherichia coli: virulence factors and pathogenicity in the farm to fork paradigm. Foodborne Pathog. Dis. 8:357–365 [DOI] [PubMed] [Google Scholar]
- 10. Bosilevac J. M., Koohmaraie M. 2011. Prevalence and characterization of non-O157 Shiga toxin-producing Escherichia coli isolated from commercial ground beef in the United States. Appl. Environ. Microbiol. 77:2103–2112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brunder W., Schmidt H., Karch H. 1996. KatP, a novel catalase-peroxidase encoded by the large plasmid of enterohaemorrhagic Escherichia coli O157:H7. Microbiology 142:3305–3315 [DOI] [PubMed] [Google Scholar]
- 12. Burk C., et al. 2003. Identification and characterization of a new variant of Shiga toxin 1 in Escherichia coli ONT:H19 of bovine origin. J. Clin. Microbiol. 41:2106–2112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cebula T. A., Payne W. L., Feng P. 1995. Simultaneous identification of strains of Escherichia coli serotype O157:H7 and their Shiga-like toxin type by mismatch amplification mutation assay-multiplex PCR. J. Clin. Microbiol. 33:248–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cobbold R., Desmarchelier P. 2001. Characterisation and clonal relationships of Shiga-toxigenic Escherichia coli (STEC) isolated from Australian dairy cattle. Vet. Microbiol. 79:323–335 [DOI] [PubMed] [Google Scholar]
- 15. Djordjevic S. P., et al. 2001. Virulence properties and serotypes of Shiga toxin-producing Escherichia coli from healthy Australian slaughter-age sheep. J. Clin. Microbiol. 39:2017–2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Doughty S., et al. 2002. Identification of a novel fimbrial gene cluster related to long polar fimbriae in locus of enterocyte effacement-negative strains of enterohemorrhagic Escherichia coli. Infect. Immun. 70:6761–6769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Eblen D. R. 2006. Public health importance of non-O157 Shiga toxin-producing Escherichia coli (non-O157 STEC) in the US food supply. http://www.fsis.usda.gov/PDF/STEC_101207.pdf
- 18. ECDC 2011. Shiga toxin/verotoxin-producing Escherichia coli in humans, food and animals in the EU/EAA, with special reference to the German outbreak strain STEC O104. ECDC/EFSA joint technical report. http://www.ecdc.europa.eu/en/publications/Publications/1106_TER_EColi_joint_EFSA.pdf
- 19. EFSA 2007. Scientific opinion of the panel on biological hazards on a request from EFSA on monitoring of verotoxigenic Escherichia coli (STEC) and identification of human pathogenic types. EFSA J. 579:1–61 [Google Scholar]
- 20. Feng P., Weagant S. D., Monday S. R. 2001. Genetic analysis for virulence factors in Escherichia coli O104:H21 that was implicated in an outbreak of hemorrhagic colitis. J. Clin. Microbiol. 39:24–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Franke S., Gunzer F., Wieler L. H., Baljer G., Karch H. 1995. Construction of recombinant Shiga-like toxin-IIv (SLT-IIv) and its use in monitoring the SLT-IIv antibody status of pigs. Vet. Microbiol. 43:41–52 [DOI] [PubMed] [Google Scholar]
- 22. Fratamico P. M., et al. 2011. The complete DNA sequence and analysis of the virulence plasmid and of five additional plasmids carried by Shiga toxin-producing Escherichia coli O26:H11 strain H30. Int. J. Med. Microbiol. 301:192–203 [DOI] [PubMed] [Google Scholar]
- 23. Friedrich A., et al. 2002. Escherichia coli harboring Shiga toxin 2 gene variants: frequency and association with clinical symptoms. J. Infect. Dis. 185:74–84 [DOI] [PubMed] [Google Scholar]
- 24. Friedrich A. W., et al. 2003. Shiga toxin 1c-producing Escherichia coli strains: phenotypic and genetic characterization and association with human disease. J. Clin. Microbiol. 41:2448–2453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fuller C. A., Pellino C. A., Flagler M. J., Strasser J. E., Weiss A. A. 2011. Shiga toxin subtypes display dramatic differences in potency. Infect. Immun. 79:1329–1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Garvey P., McKeown P., Carroll A., McNamara E. 2008. Epidemiology of verotoxigenic E. coli in Ireland, 2006. Epi-Insight 9:2–3 [Google Scholar]
- 27. Garvey P., McKeown P., Carroll A., McNamara E. 2009. Epidemiology of verotoxigenic E. coli in Ireland, 2008. Epi-Insight 10:1–7 [Google Scholar]
- 28. Garvey P., McKeown P., Carroll A., McNamara E. 2010. Epidemiology of verotoxigenic E. coli in Ireland, 2009. Epi-Insight 11:1–6 [Google Scholar]
- 29. Gill A., O G. C. 2010. Non-O157 verotoxigenic Escherichia coli and beef: a Canadian perspective. Can. J. Vet. Res. 74:161–169 [PMC free article] [PubMed] [Google Scholar]
- 30. Gilmour M. W., et al. 2007. Isolation and genetic characterization of a coinfection of non-O157 Shiga toxin-producing Escherichia coli. J. Clin. Microbiol. 45:3771–3773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Griffin P. M., Tauxe R. V. 1991. The epidemiology of infections caused by Escherichia coli O157: H7, other enterohemorrhagic E. coli, and the associated hemolytic uremic syndrome. Epidemiol. Rev. 13:60–98 [DOI] [PubMed] [Google Scholar]
- 32. Gunzer F., et al. 1992. Molecular detection of sorbitol-fermenting Escherichia coli O157 in patients with hemolytic-uremic syndrome. J. Clin. Microbiol. 30:1807–1810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Herold S., Paton J. C., Paton A. W. 2009. Sab, a novel autotransporter of locus of enterocyte effacement-negative Shiga toxigenic Escherichia coli O113:H21, contributes to adherence and biofilm formation. Infect. Immun. 77:3234–3243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hii J. H., et al. 1991. Development of verotoxin 2- and verotoxin 2 variant (VT2v)-specific oligonucleotide probes on the basis of the nucleotide sequence of the B cistron of VT2v from Escherichia coli E32511 and B2F1. J. Clin. Microbiol. 29:2704–2709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hussein H. S. 2007. Prevalence and pathogenicity of Shiga toxin-producing Escherichia coli in beef cattle and their products. J. Anim. Sci. 85:E63–72 [DOI] [PubMed] [Google Scholar]
- 36. Jelacic J. K., et al. 2003. Shiga toxin-producing Escherichia coli in Montana: bacterial genotypes and clinical profiles. J. Infect. Dis. 188:719–729 [DOI] [PubMed] [Google Scholar]
- 37. Jenkins C., et al. 2003. Subtyping intimin genes from enteropathogenic Escherichia coli associated with outbreaks and sporadic cases in the United Kingdom and Eire. Mol. Cell. Probes 17:149–156 [DOI] [PubMed] [Google Scholar]
- 38. Jenkins C., et al. 2002. An eight-month study of a population of verocytotoxigenic Escherichia coli (STEC) in a Scottish cattle herd. J. Appl. Microbiol. 93:944–953 [DOI] [PubMed] [Google Scholar]
- 39. Karmali M. A., et al. 2003. Association of genomic O island 122 of Escherichia coli EDL 933 with verocytotoxin-producing Escherichia coli seropathotypes that are linked to epidemic and/or serious disease. J. Clin. Microbiol. 41:4930–4940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kobayashi H., et al. 2001. Prevalence and characteristics of Shiga toxin-producing Escherichia coli from healthy cattle in Japan. Appl. Environ. Microbiol. 67:484–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Koch C., Hertwig S., Lurz R., Appel B., Beutin L. 2001. Isolation of a lysogenic bacteriophage carrying the stx1OX3 gene, which is closely associated with Shiga toxin-producing Escherichia coli strains from sheep and humans. J. Clin. Microbiol. 39:3992–3998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kuczius T., Bielaszewska M., Friedrich A. W., WenLan Z. 2004. A rapid method for the discrimination of genes encoding classical Shiga toxin (Stx) 1 and its variants, Stx1c and Stx1d, in Escherichia coli. Mol. Nutr. Food Res. 48:515–521 [DOI] [PubMed] [Google Scholar]
- 43. Kumar H. S., et al. 2004. Characterisation of Shiga toxin-producing Escherichia coli (STEC) isolated from seafood and beef. FEMS Microbiol. Lett. 233:173–178 [DOI] [PubMed] [Google Scholar]
- 44. Leung P. H. M., et al. 2003. A newly discovered verotoxin variant, VT2g, produced by bovine verocytotoxigenic Escherichia coli. Appl. Environ. Microbiol. 69:7549–7553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lucchesi P. M. A., Kruger A., Parma A. E. 2006. Distribution of saa gene variants in verocytotoxigenic Escherichia coli isolated from cattle and food. Res. Microbiol. 157:263–266 [DOI] [PubMed] [Google Scholar]
- 46. Machado J., Grimont F., Grimont P. A. D. 2000. Identification of Escherichia coli flagellar types by restriction of the amplified fliC gene. Res. Microbiol. 151:535–546 [DOI] [PubMed] [Google Scholar]
- 47. Mathusa E., Chen C. Y., Enache E., Hontz L. 2010. Non-O157 Shiga toxin producing Escherichia coli in foods. J. Food Prot. 73:1721–1736 [DOI] [PubMed] [Google Scholar]
- 48. McNally A., et al. 2001. Differences in levels of secreted locus of enterocyte effacement proteins between human disease-associated and bovine Escherichia coli O157. Infect. Immun. 69:5107–5114 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 49. Melton-Celsa A. R., Darnell S. C., O'Brien A. D. 1996. Activation of Shiga-like toxins by mouse and human intestinal mucus correlates with virulence of enterohemorrhagic Escherichia coli O91:H21 isolates in orally infected, streptomycin-treated mice. Infect. Immun. 64:1569–1576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Muniesa M., Recktenwald J., Bielaszewska M., Karch H., Schmidt H. 2000. Characterization of a Shiga toxin 2e-converting bacteriophage from an Escherichia coli strain of human origin. Infect. Immun. 68:4850–4855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Reference deleted.
- 52. Orskov I., Orskov F., Jann B., Jann K. 1977. Serology, chemistry, and genetics of O and K antigens of Escherichia coli. Microbiol. Mol. Biol. Rev. 41:667–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Oswald E., et al. 2000. Typing of intimin genes in human and animal enterohemorrhagic and enteropathogenic Escherichia coli: characterization of a new intimin variant. Infect. Immun. 68:64–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Paton A. W., Paton J. C. 1998. Detection and characterization of Shiga toxigenic Escherichia coli by using multiplex PCR assays for stx1, stx2, eaeA, enterohemorrhagic E. coli hlyA, rfbO111, and rfbO157. J. Clin. Microbiol. 36:598–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Paton A. W., Paton J. C. 2002. Direct detection and characterization of Shiga toxigenic Escherichia coli by multiplex PCR for stx1, stx2, eae, ehxA, and saa. J. Clin. Microbiol. 40:271–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Paton A. W., Paton J. C. 1999. Molecular characterization of the locus encoding biosynthesis of the lipopolysaccharide O antigen of Escherichia coli serotype O113. Infect. Immun. 67:5930–5937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Paton A. W., et al. 1996. Molecular microbiological investigation of an outbreak of hemolytic-uremic syndrome caused by dry fermented sausage contaminated with Shiga-like toxin-producing Escherichia coli. J. Clin. Microbiol. 34:1622–1627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Paton A. W., Srimanote P., Woodrow M. C., Paton J. C. 2001. Characterization of Saa, a novel autoagglutinating adhesin produced by locus of enterocyte effacement-negative Shiga-toxigenic Escherichia coli strains that are virulent for humans. Infect. Immun. 69:6999–7009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Paton A. W., Woodrow M. C., Doyle R. M., Lanser J. A., Paton J. C. 1999. Molecular characterization of a Shiga toxigenic Escherichia coli O113:H21 strain lacking eae responsible for a cluster of cases of hemolytic-uremic syndrome. J. Clin. Microbiol. 37:3357–3361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Pearce M. C., et al. 2006. Prevalence and virulence factors of Escherichia coli serogroups O26, O103, O111, and O145 shed by cattle in Scotland. Appl. Environ. Microbiol. 72:653–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Pierard D., Muyldermans G., Moriau L., Stevens D., Lauwers S. 1998. Identification of new verocytotoxin type 2 variant B-subunit genes in human and animal Escherichia coli isolates. J. Clin. Microbiol. 36:3317–3322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Pradel N., et al. 2000. Prevalence and characterization of Shiga toxin-producing Escherichia coli isolated from cattle, food, and children during a one-year prospective study in France. J. Clin. Microbiol. 38:1023–1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Prager R., Annemuller S., Tschape H. 2005. Diversity of virulence patterns among Shiga toxin-producing Escherichia coli from human clinical cases-need for more detailed diagnostics. Int. J. Med. Microbiol. 295:29–38 [DOI] [PubMed] [Google Scholar]
- 64. Ramachandran V., et al. 2003. Distribution of intimin subtypes among Escherichia coli isolates from ruminant and human sources. J. Clin. Microbiol. 41:5022–5032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Schmidt H., Henkel B., Karch H. 1997. A gene cluster closely related to type II secretion pathway operons of Gram-negative bacteria is located on the large plasmid of enterohemorrhagic in Escherichia coli O157 strains. FEMS Microbiol. Lett. 148:265–272 [DOI] [PubMed] [Google Scholar]
- 66. Schmidt H., et al. 1994. Prevalence of attaching and effacing Escherichia coli in stool samples from patients and controls. Zentralbl. Bakteriol. 281:201–213 [DOI] [PubMed] [Google Scholar]
- 67. Schmidt H., et al. 2000. A New Shiga toxin 2 variant (Stx2f) from Escherichia coli isolated from pigeons. Appl. Environ. Microbiol. 66:1205–1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Schmidt H., et al. 2001. Identification and characterization of a novel genomic island integrated at selC in locus of enterocyte effacement-negative, Shiga toxin-producing Escherichia coli. Infect. Immun. 69:6863–6873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Szalo I. M., et al. 2002. Presence in bovine enteropathogenic (EPEC) and enterohaemorrhagic (EHEC) Escherichia coli of genes encoding for putative adhesins of human EHEC strains. Res. Microbiol. 153:653–658 [DOI] [PubMed] [Google Scholar]
- 70. Tarr C. L., et al. 2002. Molecular characterization of a serotype O121:H19 clone, a distinct Shiga toxin-producing clone of pathogenic Escherichia coli. Infect. Immun. 70:6853–6859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Toma C., et al. 2004. Distribution of putative adhesins in different seropathotypes of Shiga toxin-producing Escherichia coli. J. Clin. Microbiol. 42:4937–4946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Torres A. G., et al. 2002. Identification and characterization of lpfABCC'DE, a fimbrial operon of enterohemorrhagic Escherichia coli O157:H7. Infect. Immun. 70:5416–5427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Torres A. G., Zhou X., Kaper J. B. 2005. Adherence of diarrheagenic Escherichia coli strains to epithelial cells. Infect. Immun. 73:18–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Wells J. G., et al. 1983. Laboratory investigation of hemorrhagic colitis outbreaks associated with a rare Escherichia coli serotype. J. Clin. Microbiol. 18:512–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. WHO 1999. Zoonotic non-O157 Shiga toxin-producing Escherichia coli (STEC), p. 1–30 Report of a WHO Scientific Working Group meeting, 23 to 26 June 1998, Berlin, Germany [Google Scholar]
- 76. Wilson J., et al. 1998. Verocytotoxigenic Escherichia coli infection in dairy farm families. Can. Commun. Dis. Rep. 24:17–20 [PubMed] [Google Scholar]
- 77. Zhang W., Bielaszewska M., Kuczius T., Karch H. 2002. Identification, characterization, and distribution of a Shiga toxin 1 gene variant (stx1c) in Escherichia coli strains isolated from humans. J. Clin. Microbiol. 40:1441–1446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Zweifel C., Blanco J. E., Blanco M., Blanco J., Stephan R. 2004. Serotypes and virulence genes of ovine non-O157 Shiga toxin-producing Escherichia coli in Switzerland. Int. J. Food Microbiol. 95:19–27 [DOI] [PubMed] [Google Scholar]
- 79. Zweifel C., et al. 2005. Phenotypic and genotypic characteristics of non-O157 Shiga toxin-producing Escherichia coli (STEC) from Swiss cattle. Vet. Microbiol. 105:37–45 [DOI] [PubMed] [Google Scholar]