Abstract
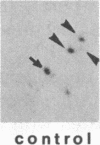
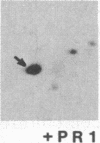
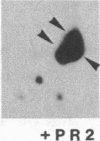
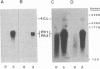
Administration of a cell-wall preparation from the fungus Phytophthora megasperma f. sp. glycinea, which acts as an elicitor of phytoalexin production in cell suspension cultures of parsley (Petroselinum crispum), also results in a rapid and dramatic increase in the relative amounts of mRNAs coding for a number of small proteins having low isoelectric points. According to various operational criteria, the translation products are classified as “pathogenesis-related” (PR) proteins. Here we report that the cDNA inserts of two pBR322-derived plasmids, pcPR1 and pcPR2, are homologous to mRNAs coding for one (PR1) and three (PR2) of these proteins in hybrid-selected in vitro translation experiments. Nuclear run-off transcription studies show that activation of the corresponding genes is extremely rapid; we observed a 4-fold increase in the transcription rate of the PR1 gene within 5 min and a 3-fold increase for the PR2 gene within 20 min following elicitation. Subsequent increases in the amounts of PR1 and PR2 mRNAs indicate that regulation of PR protein synthesis occurs at the transcriptional level.
Keywords: two-dimensional gels, cloned cDNAs, RNA blot hybridization, nuclear run-off transcription
Full text
PDF


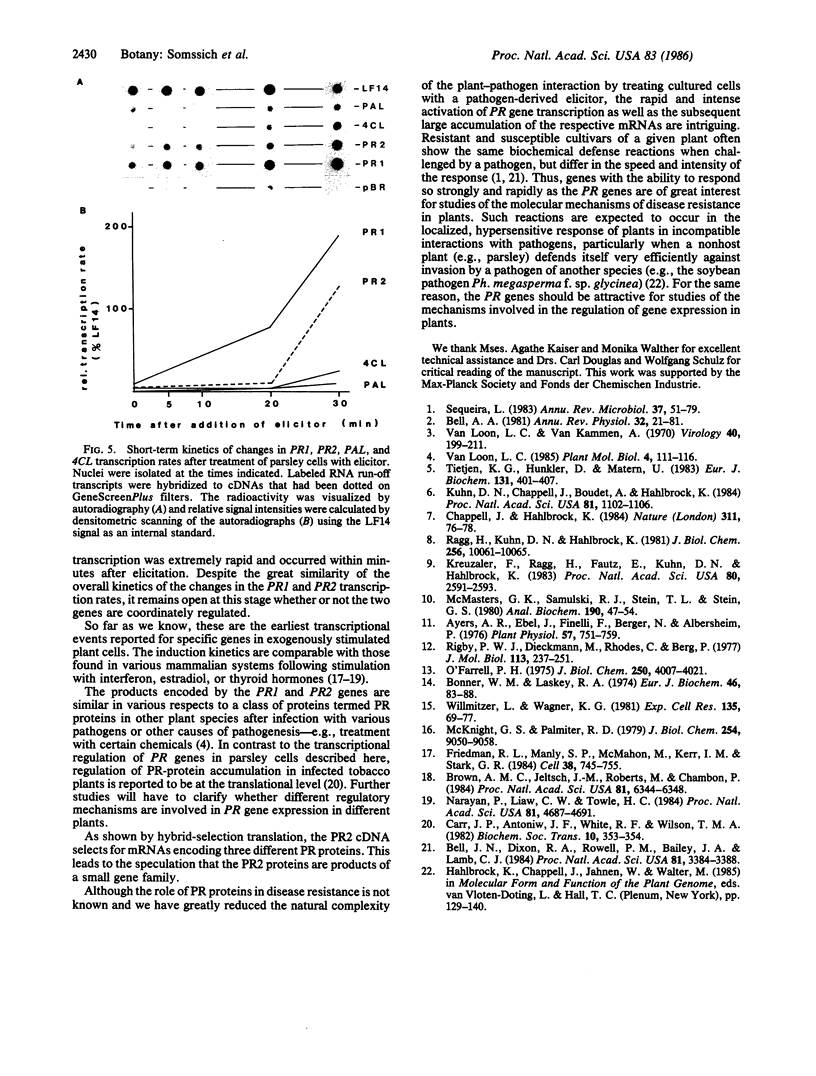
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayers A. R., Ebel J., Finelli F., Berger N., Albersheim P. Host-Pathogen Interactions: IX. Quantitative Assays of Elicitor Activity and Characterization of the Elicitor Present in the Extracellular Medium of Cultures of Phytophthora megasperma var. sojae. Plant Physiol. 1976 May;57(5):751–759. doi: 10.1104/pp.57.5.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell J. N., Dixon R. A., Bailey J. A., Rowell P. M., Lamb C. J. Differential induction of chalcone synthase mRNA activity at the onset of phytoalexin accumulation in compatible and incompatible plant-pathogen interactions. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3384–3388. doi: 10.1073/pnas.81.11.3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Brown A. M., Jeltsch J. M., Roberts M., Chambon P. Activation of pS2 gene transcription is a primary response to estrogen in the human breast cancer cell line MCF-7. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6344–6348. doi: 10.1073/pnas.81.20.6344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman R. L., Manly S. P., McMahon M., Kerr I. M., Stark G. R. Transcriptional and posttranscriptional regulation of interferon-induced gene expression in human cells. Cell. 1984 Oct;38(3):745–755. doi: 10.1016/0092-8674(84)90270-8. [DOI] [PubMed] [Google Scholar]
- Kreuzaler F., Ragg H., Fautz E., Kuhn D. N., Hahlbrock K. UV-induction of chalcone synthase mRNA in cell suspension cultures of Petroselinum hortense. Proc Natl Acad Sci U S A. 1983 May;80(9):2591–2593. doi: 10.1073/pnas.80.9.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn D. N., Chappell J., Boudet A., Hahlbrock K. Induction of phenylalanine ammonia-lyase and 4-coumarate:CoA ligase mRNAs in cultured plant cells by UV light or fungal elicitor. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1102–1106. doi: 10.1073/pnas.81.4.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight G. S., Palmiter R. D. Transcriptional regulation of the ovalbumin and conalbumin genes by steroid hormones in chick oviduct. J Biol Chem. 1979 Sep 25;254(18):9050–9058. [PubMed] [Google Scholar]
- McMaster G. K., Samulski R. J., Stein J. L., Stein G. S. Rapid purification of covalently closed circular DNAs of bacterial plasmids and animal tumor viruses. Anal Biochem. 1980 Nov 15;109(1):47–54. doi: 10.1016/0003-2697(80)90008-1. [DOI] [PubMed] [Google Scholar]
- Narayan P., Liaw C. W., Towle H. C. Rapid induction of a specific nuclear mRNA precursor by thyroid hormone. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4687–4691. doi: 10.1073/pnas.81.15.4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Ragg H., Kuhn D. N., Hahlbrock K. Coordinated regulation of 4-coumarate:CoA ligase and phenylalanine ammonia-lyase mRNAs in cultured plant cells. J Biol Chem. 1981 Oct 10;256(19):10061–10065. [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Sequeira L. Mechanisms of induced resistance in plants. Annu Rev Microbiol. 1983;37:51–79. doi: 10.1146/annurev.mi.37.100183.000411. [DOI] [PubMed] [Google Scholar]
- Tietjen K. G., Hunkler D., Matern U. Differential response of cultured parsley cells to elicitors from two non-pathogenic strains of fungi. 1. Identification of induced products as coumarin derivatives. Eur J Biochem. 1983 Mar 15;131(2):401–407. doi: 10.1111/j.1432-1033.1983.tb07277.x. [DOI] [PubMed] [Google Scholar]
- Willmitzer L., Wagner K. G. The isolation of nuclei from tissue-cultured plant cells. Exp Cell Res. 1981 Sep;135(1):69–77. doi: 10.1016/0014-4827(81)90300-1. [DOI] [PubMed] [Google Scholar]
- van Loon L. C., van Kammen A. Polyacrylamide disc electrophoresis of the soluble leaf proteins from Nicotiana tabacum var. "Samsun" and "Samsun NN". II. Changes in protein constitution after infection with tobacco mosaic virus. Virology. 1970 Feb;40(2):190–211. doi: 10.1016/0042-6822(70)90395-8. [DOI] [PubMed] [Google Scholar]