Abstract
Water bodies often receive agrochemicals and animal waste carrying fecal indicator bacteria (FIB) and zoonotic pathogens, but we know little about the effects of agrochemicals on these microbes. We assessed the direct effects of the pesticides atrazine, malathion, and chlorothalonil and inorganic fertilizer on Escherichia coli and enterococcal survival in simplified microcosms held in the dark. E. coli strain composition in sediments and water column were positively correlated, but none of the agrochemicals had significant direct effects on E. coli strain composition or on densities of culturable FIBs. In a companion study, microcosms with nondisinfected pond water and sediments were exposed to or shielded from sunlight to examine the potential indirect effects of atrazine and inorganic fertilizer on E. coli. The herbicide atrazine had no effect on E. coli in dark-exposed microcosms containing natural microbial and algal communities. However, in light-exposed microcosms, atrazine significantly lowered E. coli densities in the water column and significantly increased densities in the sediment compared to controls. This effect appears to be mediated by the effects of atrazine on algae, given that atrazine significantly reduced phytoplankton, which was a positive and negative predictor of E. coli densities in the water column and sediment, respectively. These data suggest that atrazine does not directly affect the survival of FIB, rather that it indirectly alters the distribution and abundance of E. coli by altering phytoplankton and periphyton communities. These results improve our understanding of the influence of agricultural practices on FIB densities in water bodies impacted by agricultural runoff.
INTRODUCTION
Within the last century, agriculture has become a significant, and in some areas the dominant, form of land use (38). This agricultural intensification has had detrimental effects on neighboring freshwater and marine ecosystems, leading to the loss of biodiversity, blooms of harmful algal species, and shifts in the composition of food webs (52). In recent years, agricultural practices have been subjected to increased scrutiny for their potential contribution to human health risks (23, 31, 47, 67). For instance, many endemic and emerging human pathogens are derived from or associated with livestock, including pathogenic E. coli strains, Salmonella enterica, Campylobacter jejuni, Cryptosporidium spp., and zoonotic influenza viruses (53, 58, 59). Many of these agriculturally derived pathogens are waterborne and can enter water bodies via the introduction of runoff containing waste from livestock, such as cattle, swine, and poultry (6, 10, 23).
Due to the multitude of potential fecally derived pathogens in water, which include bacteria, protozoa, and viruses, testing directly for each pathogen is prohibitively costly and time-consuming. Consequently, regulatory agencies have relied on the density of indicator organisms (fecal indicator bacteria [FIB]) for over a century to detect fecal contamination and thus an increased likelihood of the presence of human pathogens (65). Many epidemiological studies in recreational waters have supported the association between elevated FIB densities and the risk of contracting gastroenteritis (14, 28, 61).
Storm water and agricultural runoff regularly introduce both microbial contaminants and agrochemicals, such as fertilizers and pesticides, to water bodies. Through direct or indirect mechanisms, agrochemicals may affect bacterial populations in beneficial or adverse ways (12). Possible direct effects include agrochemicals acting as nutrients (beneficial) or toxic compounds (adverse). Agrochemicals can also have indirect effects that are mediated by another species or by interactions with other factors. For instance, agrochemicals can alter the traits of an organism, such as behavior, immunity, physiology, or morphology, thereby affecting its interactions with other species. This effect has been seen in multicellular organisms (39–41, 43, 45) and microorganisms (15–17). Alternatively, agrochemicals may cause density-mediated, rather than trait-mediated, indirect effects via direct toxicity to a target species' food resources, parasites, competitors, or predators (18, 21, 22, 36, 41, 44–46, 51). FIBs, pathogens, and other organisms ranging from bacterivorous protozoa to algae to aquatic animals are exposed to agrochemicals in environmental waters that receive agricultural runoff, but we know very little about the effect of the chemicals on the fate of microorganisms in such systems.
Here, we assess the direct effects of the pesticides atrazine, malathion, and chlorothalonil and inorganic fertilizer (all at expected environmental concentrations) on Escherichia coli and enterococcal survival in simplified microcosms held in the dark. Based on our previous work (49), only atrazine and fertilizer were predicted to have indirect effects on E. coli. Hence, in a separate study, we assessed whether these agrochemicals had indirect effects on E. coli densities by incubating half of the microcosms in the dark to prevent the growth of phototrophs, while the other half were exposed to natural light. Each pesticide was selected because it was among the top two in usage within the United States for its pesticide type (e.g., herbicide, insecticide, synthetic fungicide [27]).
Fertilizer and atrazine both had positive effects on FIBs in our previous outdoor mesocosm experiment, where direct and indirect effects were combined, and thus we hypothesized that fertilizer and atrazine might impact FIB densities through either direct or indirect mechanisms (49). Previous research showed that soils amended with fertilizer resulted in greater persistence of E. coli O157:H7, Salmonella enterica serovar Typhimurium, nonpathogenic E. coli, and enterococci (31, 32, 47), suggesting a potential direct mechanism. Similarly, positive direct effects were anticipated for atrazine, as past in vitro studies have shown that E. coli is chemotactic toward atrazine (33) and can directly utilize atrazine as a nutrient (30, 37, 66). Atrazine and fertilizer might also cause indirect effects by influencing the algal populations within water bodies. Fertilizer often increases algal growth, potentially increasing the reservoir for bacteria and nutrient availability to heterotrophs (4, 5, 11, 64). Atrazine is typically associated with decreases in phytoplankton that result in an increase in UV light penetration (45), which could stress bacteria in the water column (34, 48). Additionally, atrazine is often associated with increases in periphyton that could provide additional nutrients to bacteria in the sediment. Therefore, we hypothesize that atrazine will alter the algal community under light-exposed conditions, which in turn will result in a reduction in bacterial densities in the water column but an increase in densities in the sediment. Neither malathion nor chlorothalonil had significant effects on FIBs in our previous outdoor mesocosm experiment, where direct and indirect effects were combined, and thus we hypothesized that they would not have direct effects on FIBs in this experiment.
MATERIALS AND METHODS
Direct effect experiment.
We established microcosms at the University of South Florida Botanical Garden (Tampa, FL) in an outdoor greenhouse to examine exclusively direct effects of four agrochemicals (atrazine, chlorothalonil, fertilizer, and malathione), and all their pairwise combinations, on the survival of FIBs. Replication was achieved by repeating each 12-microcosm block (controls, single agrochemical treatments, and pairwise combinations) four times (four temporal blocks). The first temporal block (block A) was run from 3 to 10 June 2009, block B was run from 17 to 24 June, block C was run from 1 to 8 July, and block D was run from 29 July through 5 August. Overall, 48 separate microcosms were established to examine direct effects. Air temperatures during the temporal blocks ranged from 21 to 34°C for block A, from 23 to 40°C for block B, from 22 to 37°C for block C, and from 18 to 39°C for block D. The microcosms consisted of 11.3-liter Rubbermaid plastic trash cans with opaque sides (29.97 by 22.86 by 33.65 cm), and each contained disinfected water (2 liters) and sediment (1 liter). Sediment was collected from the lower Hillsborough River (Tampa, FL). Each microcosm was disinfected prior to bacterial inoculation via the addition of 2 liters of a 30% (vol/vol) bleach solution made with deionized (DI) water. The bleach solution was then neutralized through the addition of 45 ml of a 10% sodium thiosulfate (Na2S2O3) solution (0.225% final concentration), and served as the water column for each microcosm (60). Culturing on selective differential media (as described below) verified that the sediment and water column contained undetectable levels of E. coli and enterococci. Microcosms were covered individually with aluminum foil, and a dark tarp covered all microcosms to prevent exposure to light. The water column of each microcosm was inoculated with three E. coli strains and five Enterococcus sp. strains. Two of the E. coli strains were originally isolated from wastewater in Tampa, FL, and the third was E. coli 9637 (American Type Culture Collection). Inoculated Enterococcus strains included environmental water isolates (two Enterococcus casseliflavus strains and one E. faecalis strain isolated from Siesta Key, FL, and one E. faecium strain isolated from a University of South Florida pond, Tampa, FL) as well as E. faecalis 19433 (American Type Culture Collection). E. coli and enterococcal species/strains were selected based on differential survival observed in a previous study (1). This combination of “survivor” (exhibiting prolonged survival) and “nonsurvivor” (becoming unculturable in a relatively short time frame) strains was selected to determine whether or not strains previously demonstrated to exhibit differing degrees of robustness in secondary environments would all react similarly to the application of agrochemical treatments. One of the wastewater strains and ATCC 9637 were previously shown to be survivors, while the other wastewater strain was a nonsurvivor. The two E. casseliflavus strains and the E. faecium strain were observed to be survivors, while both E. faecalis strains were nonsurvivors.
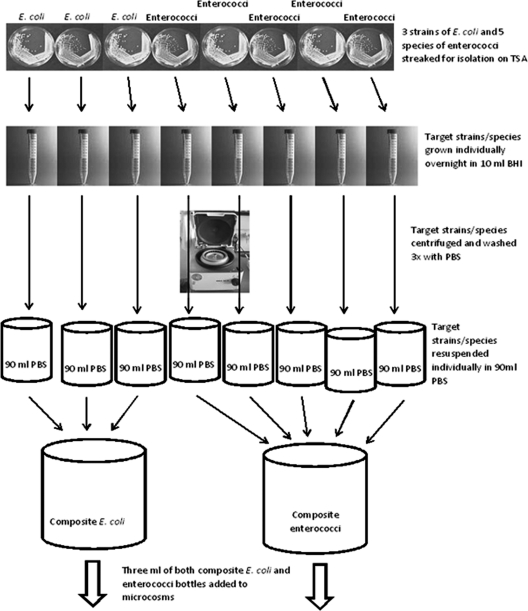
The E. coli and Enterococcus sp. strains were streaked for isolation on Trypticase soy agar (TSA) and incubated for 24 h at 37°C (Fig. 1, flow chart). Isolated colonies were inoculated into 10 ml of brain heart infusion (BHI) broth and incubated for 24 h at 37°C. Each of these cultures was then centrifuged at 5,000 rpm for 5 min (IEC Multi, Thermo Scientific), and the supernatant was discarded. Each culture was then resuspended and washed in 10 ml of sterile buffered water (0.0425 g · liter−1 KH2PO4 and 0.4055 g · liter −1 MgCl2) twice (20). Each of these 10-ml suspensions was then added to a bottle containing 90 ml of sterile buffered water. Bottles containing E. coli strains were then combined, as were bottles containing Enterococcus sp. strains. From both the bottles containing E. coli and the bottle containing enterococci, 3 ml of mixed culture was aseptically added to each microcosm (∼107 CFU/100 ml for both E. coli and enterococci).
Fig. 1.
Schematic of the procedure for used to isolate, grow, centrifuge, and inoculate the target bacteria. PBS, phosphate-buffered saline.
One hour after FIB inoculations, the 12 agrochemical treatments were assigned randomly to the microcosms. There were two control treatments, one consisting of DI water and a second in which DI water was amended with 0.002% acetone (used as a solvent for all agrochemicals). The remaining 10 microcosms received technical-grade agrochemicals: the herbicide atrazine, the insecticide malathion, the fungicide chlorothalonil, or inorganic fertilizer (sodium nitrate and sodium phosphate, purity >97%; Chemservice, West Chester, PA), either alone at their expected environmental concentrations (EEC, 102 μg/liter for atrazine, 101 μg/liter for malathion, 170 μg/liter for chlorothalonil, and 4,400 μg/liter N and 440 μg/liter P for fertilizer, calculated using the U.S. Environmental Protection Agency's GENEEC v2 software) or in pairwise combinations at their EEC. Each microcosm was then covered with a layer of aluminum foil, and all 12 were then covered with a dark tarp (1.676 m × 2.286 m) to prevent light penetration. The experiment was carried out in four sequential temporal blocks (see above for the specific dates for each temporal block). All microcosms were thoroughly cleaned and disinfected with 10% bleach following sampling at 1 week, prior to the establishment of the another replication of the 12 treatments.
Water and sediment samples were collected from each microcosm 1 h after FIB inoculations but immediately before agrochemical applications to obtain baseline (preagrochemical) measurements of FIB densities (time zero [T0]). Water and sediment samples were also collected from each microcosm 24 h and 1 week after inoculation. In total, microcosms were sampled three times (T0, T24, and T168) in each of the four temporal blocks.
Indirect effect experiment.
We established 30 microcosms at the University of South Florida in an outdoor greenhouse, as described above, to test for indirect effects of atrazine and fertilizer on E. coli. These microcosms consisted of 2-liter glass beakers containing autoclaved DI water (1.5 liter), sediment collected from the lower Hillsborough River (0.5 liter disinfected by baking at 350°F for 24 h), and pond water collected from a eutrophic pond at the University of South Florida (0.5 liter). Half of the microcosms were completely covered with aluminum foil to prevent light penetration, while the other half were covered only at the top with plastic wrap. Algal populations from the seeded pond water were given 2 weeks to establish before treatment and E. coli applications.
In addition to bacteria already present in the pond water, the water column of each microcosm was inoculated with four strains of E. coli: E. coli 9637 (American Type Culture Collection) and three strains isolated from wastewater in Tampa, FL. These strains were prepared as described for the direct effect experiment (∼107 CFU/100 ml).
One hour after FIB inoculations, the microcosms were randomly assigned one of three agrochemical treatments: a water control, inorganic fertilizer, or atrazine at 1× EEC (as described above). Microcosms were arranged so that there were five replicates (in spatial blocks conducted concurrently) of each agrochemical treatment for both light-exposed and dark conditions.
Water and sediment samples were collected from each microcosm 1 h after FIB inoculations but immediately before agrochemical applications to obtain baseline (preagrochemical) measurements of FIB densities (time zero). Water and sediment samples were also collected from each microcosm after 2, 7, 9, 14, and 28 days after inoculation. The sampling period used for this experiment was longer than that in the direct effect experiment because any indirect effects on the algal populations would require a longer period to become evident, as observed in our previous study (49). We used an AquaPen AP100 m (Photon Systems Instruments, Brno, Czech Republic) to quantify the effects of the treatments on suspended algae (phytoplankton). We measured chlorophyll a (F0) and photosynthetic efficiency (QY) initially from each microcosm and then at days 7, 14, and 28.
Sample collection and filtration.
For both the direct and indirect effect experiments, water samples were aseptically collected in centrifuge tubes. Sediment samples were collected using a sterile 50-ml centrifuge tube to scoop the top 1 to 2 cm of the sediment across the length of the microcosm. Sterile gloves were worn when collecting samples, and the microcosms were shallow enough that no connection between human skin and the microcosms occurred. Samples were placed on ice for transport to the laboratory. Transit time to the laboratory was approximately 10 min, and samples were processed immediately upon arrival.
Water samples were filtered through a nitrocellulose membrane filter (0.45-μm pore size, 47-mm diameter; Fisher Scientific). Before filtration, sediment samples were weighed and diluted 1:10 (wt/vol) in sterile buffered water (20) and sonicated for 30 s at 14 W (sonic dismembrator model 100; Fisher Scientific) to dislodge bacterial particles attached to the sediment for the direct effect microcosms (1, 29). For the indirect effect experiment, sediment samples were shaken for 2 min instead of being subjected to sonication (8). Sediment suspensions were allowed to settle for several minutes before the supernatant was pipetted off and filtered as detailed above.
Culturable bacteria were enumerated by standard membrane filtration methods. For the direct effect microcosms, E. coli colonies were enumerated on mTEC agar at 35°C for 2 h, followed by 22 h of incubation at 44.5°C (57); enterococcal colonies were enumerated on mEI agar after 24 h of incubation at 41°C (56). For the indirect effect experiment, fecal coliforms were enumerated on mFC agar at 44.5°C for 24 h (13).
E. coli genetic typing.
No genetic typing was done on colonies isolated from the indirect effect experiment; however, E. coli isolates cultured from the direct effect experiment after 1 week of incubation were selected for genetic typing from each time block. Twenty isolates were randomly selected for each agrochemical treatment in both water and sediment samples, where possible. Colonies were picked with sterile toothpicks and transferred into wells of microtiter plates containing EC broth amended with 4-methylumbelliferyl-β-d-glucuronide (MUG; 50 μg/ml). Fluorescence under UV light was used to assess MUG cleavage, which is characteristic of E. coli and differentiates it from the remainder of the coliform group (2, 7). Cultures in microtiter plates were stored at −80°C after the addition of 3 drops of glycerol, used as a cryopreservant, to each well of the microtiter plate.
Strains were revived from freezer storage by streaking for isolation on TSA, followed by incubation for 24 h at 37°C. A single, well-isolated colony from each plate was then inoculated into 750 μl of BHI and incubated for 24 h at 37°C. To extract DNA, suspensions were centrifuged at 14,000 rpm for 1 min, and the supernatant was discarded. Cells were washed with autoclaved buffered water twice and then resuspended in 500 μl of autoclaved DI water and boiled for 5 min. This suspension was used as the template for PCRs. Negative extraction controls consisting of sterile DI water were run with each group of cultures subjected to DNA extraction.
Horizontal fluorophore-enhanced repetitive extragenic palindromic PCR (HFERP) was used to provide unique DNA banding patterns for each isolate (26). A positive control (E. coli 9637) and negative controls, including the extraction control described above and a no-template control, were run with each set of reactions. PCR products were loaded onto 1.5% agarose gels and run at 90 V for 4 h at room temperature. Gels were visualized using a Typhoon 9410 variable-mode imager (Molecular Dynamics/Amersham Biosciences, Sunnyvale, CA). Banding patterns were compared using BioNumerics (Applied Maths) and verified by eye. Typing of the water column isolates was done for the first three temporal blocks, and typing of the sediment samples was done for only the third temporal block because of financial and time constraints.
Statistical analysis.
All response variables were log transformed, and block was included in all analyses. The residuals were always carefully scrutinized to ensure that the assumptions of the analysis were met.
(i) Direct effect experiment. (a) Treatment effects on FIB abundance.
We conducted repeated-measures, regression-based, multivariate analysis of variance (MANOVA), where the repeated-measures factor was FIB density, on the three sampling intervals (times 0, 24, and 168 h [9]). In these analyses, we always included interactions between among- and within-tank (repeated-measures) factors. This allowed us to test for treatment-by-time interactions. In all MANOVAs, the response variables were the density of E. coli and enterococci on each sampling date. We first conducted a MANOVA to ensure that there were no differences in FIB densities among microcosms before agrochemical applications. We then tested for a difference between the water and solvent controls to determine whether we could pool these treatments. Finally, our full model included the main effects of sample location (sediment or water column) and the four agrochemicals, two-way and three-way interactions (excluding three-way interactions among agrochemicals given that we had only pairwise combinations), and repeated-measures main effects and interactions. ANOVAs were also conducted on each FIB to ensure that we did not miss any significant univariate effects (3).
(b) Treatment effects on FIB composition.
To test for treatment effects on FIB composition, we arcsine square root transformed the proportions of E. coli colonies that were of the three inoculated strains and conducted a MANOVA test for the main effects of agrochemicals and their two-way interactions (50). Isolates from only three out of four of the blocks were typed (isolates from the fourth temporal block, block D, were not genetically typed).
(c) Relationship between FIB composition in the sediment and water column.
To test for a relationship between FIB composition in the sediment and water column, for each microcosm in the third temporal block, we calculated the proportion of total colonies from the sediment and water column that were each of the three E. coli strains. Treating these three strains as independent of one another would have inappropriately tripled our sample size. Hence, we bootstrapped the relationship between FIB composition in the sediment and water column by randomly selecting paired sediment and water column samples, with replacement, until we reached the sample size for the block. We then calculated the Pearson's correlation coefficient for these randomly selected, paired sediment and water column samples. We did this 1,000 times and attained our probability value for the null model by calculating the proportion of these 1,000 correlation coefficients that were ≤0.
(ii) Indirect effect experiment.
We conducted repeated-measures, regression-based, analysis of variance (ANOVA), where the repeated-measures factor was E. coli density on the five sampling intervals (times 2, 7, 9, 14, and 28 days). In these analyses, we always included interactions between among- and within-tank (repeated-measures) factors, as well as utilizing the day 0 densities as a continuous covariate to control for stochastic differences at the outset of the experiment. This allowed us to test for treatment-by-time interactions. In all ANOVAs, the response variables were the density of E. coli on each sampling date. Our full model included the main effects of light (present or absent) and the three agrochemical treatments (water, fertilizer, or atrazine), two-way interactions, and repeated-measures main effects and interactions. Separate ANOVAs were conducted for densities in the water column and the sediment. We used a repeated-measures multivariate analysis of variance (MANOVA) to evaluate the factorial effects of the treatments and light exposure on phytoplankton F0 and QY measurements on days 7, 14, and 28, controlling for initial phytoplankton abundance. We used a regression analysis to test whether phytoplankton abundance on day 28 (end of the experiment) was predictive of E. coli densities in the water column and sediment on day 28. We always conducted separate ANOVAs and MANOVAs excluding the fertilizer treatment to verify that significant differences were driven by a difference between the water control and atrazine and not by differences between fertilizer and atrazine treatments.
RESULTS
Direct effects of agrochemicals (dark conditions).
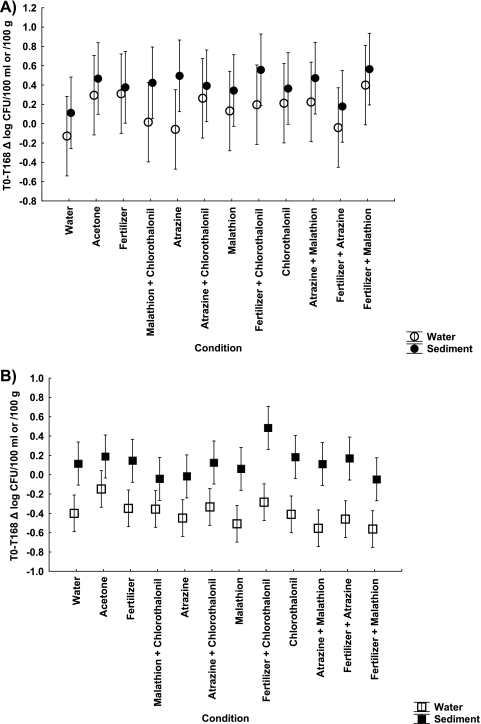
Initial densities of E. coli in dark conditions before the addition of agrochemicals were ∼107 CFU/100 ml in the water column and ∼105 CFU/100 g (wet weight) in the sediment. Enterococcus densities were initially ∼107 CFU/100 ml in the water column and ∼106 CFU/100 g (wet weight) in the sediment. Average decay rates per day were calculated for E. coli and enterococci in both water and sediment by subtracting the density at T168 from the initial density and dividing by 7. Decay rates for E. coli (Fig. 2A) and enterococci (Fig. 2B) were not significantly different among treatments in either the water column or sediment.
Fig. 2.
Average decay rates per day (mean Δ log CFU per 100 ml or 100 g ± standard error [SE], n = 4) in water and sediment averaged over all four temporal blocks for E. coli (A) and enterococci (B).
Mean values from all temporal blocks showed E. coli densities in the water column and sediment ranging from ∼106 to ∼109 CFU per 100 ml or 100 g across all treatments. Enterococcus densities after 1 week in the water column ranged from ∼104 to ∼106 CFU/100 ml, and sediment densities ranged from ∼104 to ∼108 CFU/100 g. When averaged over all treatments and all blocks after a week, E. coli densities were significantly higher than enterococcus densities in both water and sediment matrices.
We found no significant differences in FIB density among microcosms before agrochemical applications and no significant difference between the water and solvent controls after agrochemical applications (P > 0.05). Hence, we pooled the water and solvent controls for subsequent analyses. There were significant multivariate effects of block (F6,152 = 28.96, P < 0.001), sampling intervals (F2,76 = 38.43, P < 0.001), and sampling location (F2,76 = 7.69, P < 0.001), i.e., the sediment had higher densities than the water column for enterococci (F1,76 = 28.23, P < 0.001) but not for E. coli (F1,76 = 0.82, P = 0.367). There were no significant main effects or interaction associated with any of the agrochemicals (all P values were >0.115).
MANOVA revealed that agrochemical treatments did not influence the genotype composition of E. coli strains (number of isolates of any given genotype recovered) or the proportion of each genotype recovered (all main effects and interactions had P values of >0.213). However, bootstrapping analysis revealed that there was a significant positive multivariate correlation between the genotypes recovered from the sediment and water column (mean r = 0.532, P = 0.018) (Fig. 3).
Fig. 3.
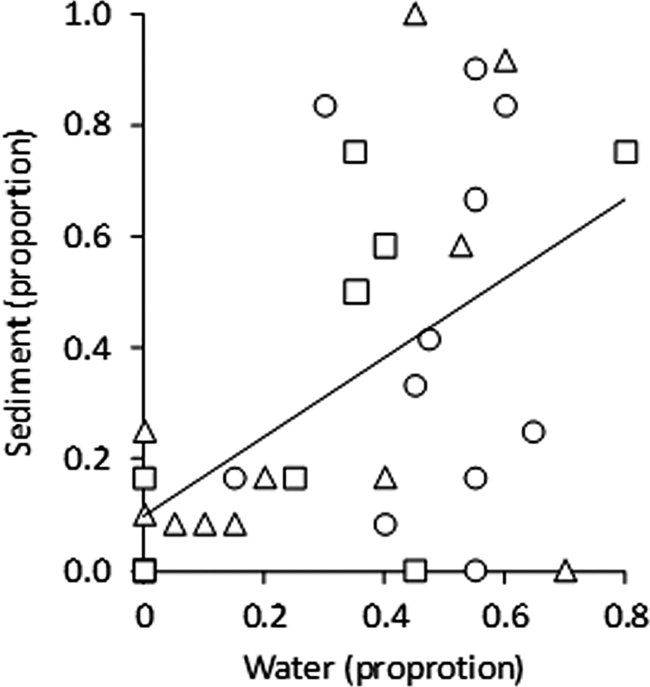
The relationship between strain composition in the sediment and water column for three strains of E. coli (strain 14, squares; strain 19, triangles; strain 9637, circles). The three strains are not independent of one another because they were sampled from the same mesocosms. To calculate the multivariate correlation between composition in the water column and sediment, we bootstrapped the relationship, ensuring that our sample size for each of the 1,000 iterations matched the number of replicates. These results revealed an average Pearson's r of 0.532 and a probability value for the null model of 0.018.
Indirect effects of agrochemicals (light conditions).
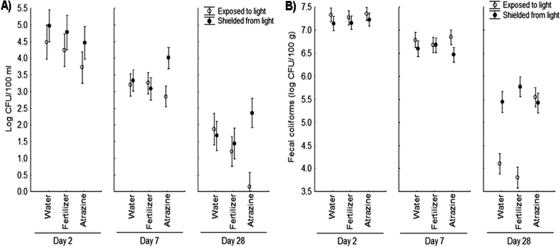
Initially, and prior to agrochemical additions, E. coli densities in the water column were ∼107 CFU/100 ml. After 2 days, E. coli densities in the water column had dropped ∼3 logs in the light-exposed tanks and ∼ 2 logs in the darkened tanks (Fig. 4A), demonstrating the adverse effects of light exposure on water column E. coli. The decline in the E. coli densities slowed over the remainder of the experiment with declines of ∼0.5 to 1 log/week for all tanks in either light or dark conditions.
Fig. 4.
E. coli densities in the water (A) and sediment (B) for each treatment (mean ± SE, n = 5) at days 2, 7, and 28.
Within the water column (Fig. 4A), ANOVA revealed significant main effects of light (F1,19 = 5.36, P = 0.031) and block (F4,19 = 4.29, P = 0.012) and a significant interaction between light exposure and treatment (F2,19 = 4.05, P = 0.034). An additional ANOVA was run excluding the fertilizer treatment, and a significant effect of light by treatment was still observed (F1,11 = 8.24, P = 0.015), meaning that the effect was the result of a difference between the water control and atrazine treatments, not the result of differences between the atrazine and fertilizer treatments. Under lit conditions, atrazine treatments resulted in significantly lower E. coli densities in the water column compared to control treatments, but atrazine had no significant effect on E. coli densities in the dark (for dark treatments at day 7: P = 0.28; at day 24: P = 0.10) (Fig. 4A). No significant effect was observed for fertilizer treatments relative to controls. No significant effects were seen for the repeated-measures main effect or interactions.
E. coli densities within the sediments of all treatments were initially ∼107 CFU/100 g prior to agrochemical additions. No decline was seen between day 0 and day 2, although between days 2 and 7, a decline of approximately half a log was observed in the sediments of all treatments, and E. coli densities continued to decline more slowly than in the water column (Fig. 4B). E. coli densities in the sediments of the dark fertilizer treatment experienced a slightly slower, albeit not statistically significant, decay rate relative to other treatments. ANOVA revealed no significant main effect of light or block for the sediment densities; however, there was a significant main effect of treatment (F2,19 = 3.57, P = 0.048) and a significant light-by-treatment interaction (F2,19 = 7.77, P = 0.003). An additional ANOVA excluding fertilizer revealed a significant main effect of treatment (F1,11 = 5.71, P = 0.036) and light-by-treatment interaction (F1,11 = 6.10, P = 0.031). In other words, the significant effects of treatment and light by treatment were driven by differences between the water control and atrazine treatments, not by differences between the atrazine and fertilizer treatments. Under lit conditions, atrazine treatments resulted in significantly higher E. coli densities in the sediment after 28 days compared to control treatments, but atrazine had no significant effect on E. coli densities in the dark. No significant effect was observed for fertilizer treatments relative to controls. A significant main effect was observed for the repeated-measures factor (F2,38 = 4.94, P = 0.012) as well as a significant interaction between time by light (F2,38 = 14.72, P < 0.001), time by treatment (F4,38 = 4.95, P = 0.003), and time by light by treatment (F4,38 = 7.64, P < 0.001).
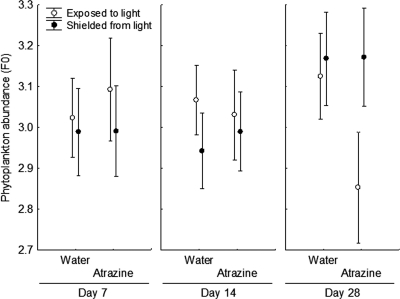
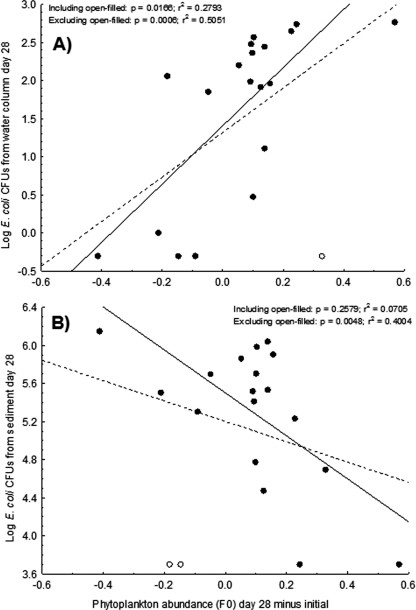
We quantified phytoplankton levels (chlorophyll a) in each microcosm to evaluate whether treatment effects on E. coli densities could be explained by indirect effects mediated by algae. There was a significant time-by-treatment-by-light interaction for the algal responses (F8,40 = 2.20, P = 0.048), and this interaction remained significant when the fertilizer treatment was excluded (F4,12 = 4.17, P = 0.024), indicating that phytoplankton were responding differently in atrazine-treated and control microcosms. This interaction was driven by atrazine having no effect on phytoplankton abundance in dark or lit conditions until day 28, when chlorophyll a measures were lower in the atrazine-treated microcosms than the control microcosms under lit conditions only (Fig. 5). Furthermore, algal abundance at the end of the experiment was a significant positive predictor of E. coli densities in the water column regardless of whether an outlier was included or excluded (Fig. 6A). In contrast, algal abundance at the end of the experiment was negatively associated with E. coli densities in the sediment (Fig. 6B).
Fig. 5.
Phytoplankton abundance (measured as F0) with and without atrazine (least squares mean ± SE, n = 5) on days 7, 14, and 28 when controlling for initial phytoplankton levels.
Fig. 6.
Relationship between phytoplankton abundance (controlling for initial abundance estimates) and E. coli densities (measured as CFU/100 ml or CFU/100 g) in the water column (A) and sediment (B).
DISCUSSION
The overall net effect (direct plus indirect mechanisms) of agrochemicals on the fate of allochthonous bacteria in water bodies and the underlying sediment has been greatly understudied. Our previous research suggested that the presence of fertilizer or atrazine has positive effects on FIB densities in the sediment (49). However, our previous study did not investigate potential impacts of agrochemicals in the water column and, due to the complexity of the mesocosms used in that study, it was not possible to say definitively whether the effects observed were due to direct mechanisms, indirect mechanisms, or a combination of the two. The present study utilized simple microcosms, inoculated with several known strains of E. coli and Enterococcus spp., to further examine the impact that agrochemicals have on the fate of bacteria in both water and sediment as well as to distinguish between direct and indirect mechanisms. The initial bacterial densities (∼107 CFU/100 ml), though certainly elevated, have been observed in environmental water samples in a previous study (63). While the addition of bacteria to the microcosms would add some nutrients to the microcosms, cells were washed prior to inoculation into the microcosms to limit the transfer of excess nutrients and were added to all of the microcosms at similar densities.
To examine direct effects of agrochemicals, our microcosms were designed specifically to exclude potential indirect confounders, specifically algal growth and protozoan predation. Under these conditions, none of the agrochemicals, alone or in combination, had significant effects on the densities of E. coli or enterococci in either the water column or the underlying sediment. These results suggest that the agrochemicals used in this experiment do not have any direct impact, positive or negative, on FIB densities in either the water or sediment matrix. However, when seeded with pond water (containing algae and protozoan predators), significant effects were observed on E. coli densities in both the water column and the sediment when atrazine was present and microcosms were exposed to light, indicating that the effects of atrazine on E. coli were indirect because they depended on the presence of other species.
Given that the effect of atrazine on E. coli depended on light availability, the indirect effect is almost certainly at least partially mediated by phototrophs. Atrazine is directly toxic to phytoplankton (45). The present experiment supports this finding as phytoplankton density decreased in lit microcosms containing atrazine relative to controls (Fig. 5). Further, light attenuates exponentially as a function of chlorophyll a in phytoplankton, potentially resulting in an exponential decrease in light penetration in response to a small increase in chlorophyll a (24). Therefore, as the phytoplankton dies off, the water column receives increased light, which would result in greater UV stress to bacteria in the water column, explaining the significantly lower density of E. coli in the water column of the light-exposed atrazine tanks (34, 48). The algal data support this conclusion, as greater amounts of phytoplankton were predictive of higher E. coli densities in the water column (Fig. 6A). Increased light penetration has also been shown to stimulate the growth of periphyton in sediment biofilms, thus increasing available carbon (25, 35, 44). Therefore, as phytoplankton decreased in the lit atrazine microcosms, periphyton likely increased (although this was not measured). In support of this assertion, we observed significantly higher E. coli densities in the sediments of lit atrazine microcosms and a strong negative trend for the correlation between phytoplankton in the water column and E. coli densities in the sediments (Fig. 6B). All of these findings support the conclusion that algal dynamics mediated the indirect effects of atrazine on E. coli. However, it should be noted that not all algal species respond identically to atrazine. As algal species can differ in sensitivity to atrazine, the initial algal community present in a water body may well modify the direct and indirect algal-mediated effects on the entire community (39). Further, it should be noted that atrazine, beyond phytotoxic effects, can exert effects on higher trophic levels in impacted communities. While the ability of atrazine to directly cause mortality is controversial, a meta-analysis has shown that atrazine consistently influences metamorphosis, antipredator behavior, and immunity of freshwater fish and amphibians (42). These wide-ranging effects of atrazine exposure could have further implications for microbial fate in water bodies exposed to the herbicide.
In contrast to findings from our previous study conducted with nutrient poor sediments and water (49), no significant increase in FIB densities was observed for the fertilizer treatment. The sediments used in this experiment were taken from the Hillsborough River and would therefore be expected to contain large amounts of phosphorus as well as multiple nitrogen species (62). Additionally, the pond water seeded into the microcosms was taken from a eutrophic pond at the University of South Florida. Therefore, we hypothesize that the seeded pond water and sediments resulted in microcosms that were neither phosphorus nor nitrogen limited, preventing us from detecting an increase in FIBs with fertilizer addition.
Although we detected no direct effect of the agrochemicals on overall E. coli and enterococcus densities, we also hypothesized that changes in strain composition may be induced by agrochemical treatments. Particular strains of E. coli and Enterococcus spp. have been shown in previous studies to exhibit extended survival in microcosms (1), so it was thought that individual strains may respond differently to agrochemical treatments. However, these experiments did not support this hypothesis, as the proportion of each E. coli strain recovered from water and sediment in light-free microcosms was independent of treatments, and no one strain persisted better overall than any other. We do not suggest that these results should be generalized to all E. coli strains, as only three were included in this study.
Despite the lack of direct effects of the agrochemicals on the abundance or population structure of FIBs, we did detect a positive multivariate correlation between E. coli genotype abundances (observations of a particular genotype) in the sediment and water column. These results suggest that the distribution of E. coli genotypes in the sediment influence the population structure within the water column and/or vice versa. Regulatory standards consider FIB densities only in the water column (54, 55), but E. coli and enterococci are clearly also present in the sediments, which can act as important reservoirs of FIBs and possibly of pathogens (1, 4, 5, 19).
The effects of agrochemicals on the survival of FIBs in environmental waters are greatly understudied. Based on the results of this study, agrochemicals had no direct effect on FIB growth, although the presence of atrazine did mediate significant indirect effects. The significant effects observed in the presence relative to the absence of light and the associations between phytoplankton abundance and E. coli densities suggest that the presence of atrazine alters algal dynamics that affect light and nutrient levels leading to reductions in E. coli in the water column but increases in the sediment. While these results are novel and suggest a mechanism of action for the effect of atrazine on FIB densities, it is unknown whether agrochemical treatments will have any direct or indirect effects on other bacterial pathogens or viruses. Further examination of both the direct and indirect effects of agrochemicals on pathogen survival in the environment is needed to better understand and manage potential risks to human health.
ACKNOWLEDGMENTS
We thank Laurie Walker, Gordon Fox, and the USF Botanical Gardens for greenhouse space and logistical support.
Funding was provided by USDA-NIFA Water and Watershed program grant 2009-35102-05043.
Footnotes
Published ahead of print on 14 October 2011.
REFERENCES
- 1. Anderson K. L., Whitlock J. E., Harwood V. J. 2005. Persistence and differential survival of fecal indicator bacteria in subtropical waters and sediments. Appl. Environ. Microbiol. 71:3041–3048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Anderson M. A., Whitlock J. E., Harwood V. J. 2006. Diversity and distribution of Escherichia coli genotypes and antibiotic resistance phenotypes in feces of humans, cattle, and horses. Appl. Environ. Microbiol. 72:6914–6922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Armstrong R., Hilton A. 2004. The use of analysis of variance (ANOVA) in applied microbiology. Microbiologist December:18–21 [Google Scholar]
- 4. Badgley B. D., Nayak B. S., Harwood V. J. 2010. The importance of sediment and submerged aquatic vegetation as potential habitats for persistent strains of enterococci in a subtropical watershed. Water Res. 44:5857–5866 [DOI] [PubMed] [Google Scholar]
- 5. Badgley B. D., Thomas F. I., Harwood V. J. 2010. The effects of submerged aquatic vegetation on the persistence of environmental populations of Enterococcus spp. Environ. Microbiol. 12:1271–1281 [DOI] [PubMed] [Google Scholar]
- 6. Berry E. D., et al. 2007. Incidence and persistence of zoonotic bacterial and protozoan pathogens in a beef cattle feedlot runoff control vegetative treatment system. J. Environ. Qual. 36:1873–1882 [DOI] [PubMed] [Google Scholar]
- 7. Bitton G., Koopman B., Jung K. 1995. An assay for the enumeration of total coliforms and Escherichia coli in water and wastewater. Water Environ. Res. 67:906–909 [Google Scholar]
- 8. Boehm A. B., et al. 2009. Faecal indicator bacteria enumeration in beach sand: a comparison study of extraction methods in medium to coarse sands. J. Appl. Microbiol. 107:1740–1750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bray J. H., Maxwell S. E. 1985. Multivariate analysis of variance. Sage, Newbury Park, CA [Google Scholar]
- 10. Brooks J. P., Adeli A., Read J. J., McLaughlin M. R. 2009. Rainfall simulation in greenhouse microcosms to assess bacterial-associated runoff from land-applied poultry litter. J. Environ. Qual. 38:218–229 [DOI] [PubMed] [Google Scholar]
- 11. Byappanahalli M. N., Shively D. A., Nevers M. B., Sadowsky M. J., Whitman R. L. 2003. Growth and survival of Escherichia coli and enterococci populations in the macro-alga Cladophora (Chlorophyta). FEMS Microbiol. Ecol. 46:203–211 [DOI] [PubMed] [Google Scholar]
- 12. Clements W. H., Rohr J. R. 2009. Community responses to contaminants: using basic ecological principles to predict ecotoxicological effects. Environ. Toxicol. Chem. 28:1789–1800 [DOI] [PubMed] [Google Scholar]
- 13. Clesceri L. S., Greenberg A. E., Eaton A. D. (ed.). 1998. Standard methods for the examination of water and wastewater, 20th ed American Public Health Association, Washington, DC [Google Scholar]
- 14. Craun G. F., Calderon R. L. 2006. Observational epidemiological studies of endemic waterborne risks: cohort, case-control, time-series, and ecological studies. J. Water Health 4:101–119 [DOI] [PubMed] [Google Scholar]
- 15. Debenest T., Silvestre J., Coste M., Pinelli E. 2010. Effects of pesticides on freshwater diatoms. Rev. Environ. Contam. Toxicol. 203:87–103 [DOI] [PubMed] [Google Scholar]
- 16. De Laender F., Soetaert K., Middelburg J. J. 2010. Inferring chemical effects on carbon flows in aquatic food webs: methodology and case study. Environ. Pollut. 158:1775–1782 [DOI] [PubMed] [Google Scholar]
- 17. DeLorenzo M. E., Scott G. I., Ross P. E. 2001. Toxicity of pesticides to aquatic microorganisms: a review. Environ. Toxicol. Chem. 20:84–98 [DOI] [PubMed] [Google Scholar]
- 18. Dive D., Leclerc H., Persoone G. 1980. Pesticide toxicity on the ciliate protozoan Colpidium campylum: possible consequences of the effect of pesticides in the aquatic environment. Ecotoxicol. Environ. Saf. 4:129–133 [DOI] [PubMed] [Google Scholar]
- 19. Droppo I. G., et al. 2009. Dynamic existence of waterborne pathogens within river sediment compartments. Implications for water quality regulatory affairs. Environ. Sci. Technol. 43:1737–1743 [DOI] [PubMed] [Google Scholar]
- 20. Eaton A. D., Clesceri L. S., Greenberg A. E. (ed.). 1995. Standard methods for the examination of water and wastewater, 19th ed American Public Health Association, Washington, DC [Google Scholar]
- 21. Ekelund F. 1999. The impact of the fungicide fenpropimorph (Corbel (R)) on bacterivorous and fungivorous protozoa in soil. J. Appl. Ecol. 36:233–243 [Google Scholar]
- 22. Foit K., Chatzinotas A., Liess M. 2010. Short-term disturbance of a grazer has long-term effects on bacterial communities-relevance of trophic interactions for recovery from pesticide effects. Aquat. Toxicol. 99:205–211 [DOI] [PubMed] [Google Scholar]
- 23. Fratamico P. M., Bagi L. K., Bush E. J., Solow B. T. 2004. Prevalence and characterization of shiga toxin-producing Escherichia coli in swine feces recovered in the National Animal Health Monitoring System's Swine 2000 study. Appl. Environ. Microbiol. 70:7173–7178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gallegos C. L., Moore K. A. 2000. Factors contributing to water-column light attenuation, p. 16–27 In Batiuk R. A., et al. (ed.), Chesapeake Bay submerged aquatic vegetation water quality and habitat-based requirements and restoration targets: a second technical synthesis. U.S. Environmental Protection Agency, Chesapeake Bay Program, Annapolis, MD [Google Scholar]
- 25. Herman D., Kaushik N. K., Solomon K. R. 1986. Impact of atrazine on periphyton in fresh-water enclosures and some ecological consequences. Can. J. Fish Aquat. Sci. 43:1917–1925 [Google Scholar]
- 26. Johnson L. K., et al. 2004. Sample size, library composition, and genotype diversity among natural populations of Escherichia coli from different animals influence accuracy of determining sources of fecal pollution. Appl. Environ. Microbiol. 70:4478–4485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kiely T., Donaldson D., Grube A. 2004. Pesticide industry sales and usage: 2000 and 2001 market estimates. U.S. Environmental Protection Agency, Washington, DC [Google Scholar]
- 28. Kite-Powell H., et al. 2008. Linking the oceans to public health: current efforts and future directions. Environ. Health 7:S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Korajkic A., Badgley B. D., Brownell M. J., Harwood V. J. 2009. Application of microbial source tracking methods in a Gulf of Mexico field setting. J. Appl. Microbiol. 107:1518–1527 [DOI] [PubMed] [Google Scholar]
- 30. Koutsotoli A. D., Dimou D. S., Alamanos Y. P., Maipa V. E. 2005. Inductive effects of environmental concentration of atrazine on Escherichia coli and Enterococcus faecalis. Folia Microbiol. (Praha) 50:283–287 [DOI] [PubMed] [Google Scholar]
- 31. Kudva I. T., Blanch K., Hovde C. J. 1998. Analysis of Escherichia coli O157:H7 survival in ovine or bovine manure and manure slurry. Appl. Environ. Microbiol. 64:3166–3174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lau M. M., Ingham S. C. 2001. Survival of faecal indicator bacteria in bovine manure incorporated into soil. Lett. Appl. Microbiol. 33:131–136 [DOI] [PubMed] [Google Scholar]
- 33. Liu X., Parales R. E. 2009. Bacterial chemotaxis to atrazine and related s-triazines. Appl. Environ. Microbiol. 75:5481–5488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Noble R. T., Lee I. M., Schiff K. C. 2004. Inactivation of indicator micro-organisms from various sources of faecal contamination in seawater and freshwater. J. Appl. Microbiol. 96:464–472 [DOI] [PubMed] [Google Scholar]
- 35. Pratt J. R., Melendez A. E., Barreiro R., Bowers N. J. 1997. Predicting the ecological effects of herbicides. Ecol. Appl. 7:1117–1124 [Google Scholar]
- 36. Raffel T. R., Martin L. B., Rohr J. R. 2008. Parasites as predators: unifying natural enemy ecology. Trends Ecol. Evol. 23:610–618 [DOI] [PubMed] [Google Scholar]
- 37. Rhine E. D., Fuhrmann J. J., Radosevich M. 2003. Microbial community responses to atrazine exposure and nutrient availability: linking degradation capacity to community structure. Microb. Ecol. 46:145–160 [DOI] [PubMed] [Google Scholar]
- 38. Robinson R. A., Sutherland W. J. 2002. Post-war changes in arable farming and biodiversity in Great Britain. J. Appl. Ecol. 39:157–176 [Google Scholar]
- 39. Rohr J. R., Crumrine P. W. 2005. Effects of an herbicide and an insecticide on pond community structure and processes. Ecol. Appl. 15:1135–1147 [Google Scholar]
- 40. Rohr J. R., et al. 2003. Lethal and sublethal effects of atrazine, carbaryl, endosulfan, and octylphenol on the streamside salamander (Ambystoma barbouri). Environ. Toxicol. Chem. 22:2385–2392 [DOI] [PubMed] [Google Scholar]
- 41. Rohr J. R., Kerby J. L., Sih A. 2006. Community ecology as a framework for predicting contaminant effects. Trends Ecol. Evol. 21:606–613 [DOI] [PubMed] [Google Scholar]
- 42. Rohr J. R., McCoy K. A. 2010. A qualitative meta-analysis reveals consistent effects of atrazine on freshwater fish and amphibians. Environ. Health Perspect. 118:20–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rohr J. R., Palmer B. D. 2005. Aquatic herbicide exposure increases salamander desiccation risk eight months later in a terrestrial environment. Environ. Toxicol. Chem. 24:1253–1258 [DOI] [PubMed] [Google Scholar]
- 44. Rohr J. R., Raffel T. R., Sessions S. K., Hudson P. J. 2008. Understanding the net effects of pesticides on amphibian trematode infections. Ecol. Appl. 18:1743–1753 [DOI] [PubMed] [Google Scholar]
- 45. Rohr J. R., et al. 2008. Agrochemicals increase trematode infections in a declining amphibian species. Nature 455:1235–1239 [DOI] [PubMed] [Google Scholar]
- 46. Rohr J. R., Swan A., Raffel T. R., Hudson P. J. 2009. Parasites, info-disruption, and the ecology of fear. Oecologia 159:447–454 [DOI] [PubMed] [Google Scholar]
- 47. Semenov A. V., van Overbeek L., van Bruggen A. H. C. 2009. Percolation and survival of Escherichia coli O157:H7 and Salmonella enterica serovar Typhimurium in soil amended with contaminated dairy manure or slurry. Appl. Environ. Microbiol. 75:3206–3215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sinton L. W., Hall C. H., Lynch P. A., Davies-Colley R. J. 2002. Sunlight inactivation of fecal indicator bacteria and bacteriophages from waste stabilization pond effluent in fresh and saline waters. Appl. Environ. Microbiol. 68:1122–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Staley Z. R., Rohr J. R., Harwood V. J. 2010. The effect of agrochemicals on indicator bacteria densities in outdoor mesocosms. Environ. Microbiol. 12:3150–3158 [DOI] [PubMed] [Google Scholar]
- 50. Steel R. G. D., Torrie J. H. 1960. Principles and procedures of statistics. McGraw-Hill, New York, NY [Google Scholar]
- 51. Sumpono P. P., Belean A., Forestier C., Lavedrine B., Bohatier J. 2003. Effect of Diuron on aquatic bacteria in laboratory-scale wastewater treatment ponds with special reference to Aeromonoas species studied by colony hybridization. Chemosphere 50:445–455 [DOI] [PubMed] [Google Scholar]
- 52. Tilman D. 1999. Global environmental impacts of agricultural expansion: the need for sustainable and efficient practices. Proc. Natl. Acad. Sci. U. S. A. 96:5995–6000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. U.S. Environmental Protection Agency 2005. Detecting and mitigating the environmental impact of fecal pathogens originating from confined animal feeding operations: Rev. EPA/600/R-06/021. U.S. Environmental Protection Agency, Washington, DC [Google Scholar]
- 54. U.S. Environmental Protection Agency 1983. Health effects criteria for marine recreational waters. EPA-600/1-80-031. U.S. Environmental Protection Agency, Washington, DC [Google Scholar]
- 55. U.S. Environmental Protection Agency 2002. Implementation guidance for ambient water quality criteria for bacteria. EPA-823-B-02-003. U.S. Environmental Protection Agency, Washington, DC [Google Scholar]
- 56. U.S. Environmental Protection Agency 2002. Method 1600: enterococci in water by membrane filtration using membrane-enterococcus indoxy-B-d-glucoside agar (mEI). EPA 821-R-02-022. U.S. Environmental Protection Agency, Washington, DC [Google Scholar]
- 57. U.S. Environmental Protection Agency 2002. Method 1603: Escherichia coli (E.coli) in water by membrane filtration using modified membrane-thermotolerant Escherichia coli agar (modified mTEC). EPA 821-R-02-023. U.S. Environmental Protection Agency, Washington, DC [Google Scholar]
- 58. U.S. Environmental Protection Agency 2009. Review of published studies to characterize relative risks from different sources of fecal contamination in recreational water. EPA 822-R-09-001. U.S. Environmental Protection Agency, Washington, DC [Google Scholar]
- 59. U.S. Environmental Protection Agency 2009. Review of zoonotic pathogens in ambient waters. EPA 822-R-09-002. U.S. Environmental Protection Agency, Washington, DC [Google Scholar]
- 60. U.S. Environmental Protection Agency 2001. USEPA manual of methods for virology. U.S. Environmental Protection Agency, Washington, DC [Google Scholar]
- 61. Wade T. J., et al. 2008. High sensitivity of children to swimming-associated gastrointestinal illness: results using a rapid assay of recreational water quality. Epidemiology 19:375–383 [DOI] [PubMed] [Google Scholar]
- 62. Wang P. F., Martin J., Morrison G. 1999. Water quality and eutrophication in Tampa Bay, Florida. Estuar. Coast. Shelf Sci. 49:1–20 [Google Scholar]
- 63. Weidhass J. L., Tamzen W. M., Olsen R. L., Harwood V. J. 2011. Correlation of quantitative PCR for a poultry-specific Brevibacterium marker gene with bacterial and chemical indicators of water pollution in a watershed impacted by land application of poultry litter. Appl. Environ. Microbiol. 77:2094–2102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Whitman R. L., Shively D. A., Pawlik H., Nevers M. B., Byappanahalli M. N. 2003. Occurrence of Escherichia coli and enterococci in cladophora (chlorophyta) in nearshore water and beach sand of Lake Michigan. Appl. Environ. Microbiol. 69:4714–4719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wolf H. W. 1972. The coliform count as a measure of water quality, p. 333–345 In Mitchell R. (ed.), Water pollution microbiology. Wiley Interscience, New York, NY [Google Scholar]
- 66. Yanze-Kontchou C., Gschwind N. 1994. Mineralization of the herbicide atrazine as a carbon source by a Pseudomonas strain. Appl. Environ. Microbiol. 60:4297–4302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ziemer C. J., et al. 2010. Fate and transport of zoonotic, bacterial, viral, and parasitic pathogens during swine manure treatment, storage, and land application. J. Anim. Sci. 88:E84–E94 [DOI] [PubMed] [Google Scholar]