Abstract
Methanotrophs play a key role in the global carbon cycle, in which they affect methane emissions and help to sustain diverse microbial communities through the conversion of methane to organic compounds. To investigate the microbial interactions that cause positive effects on methanotrophs, cocultures were constructed using Methylovulum miyakonense HT12 and each of nine nonmethanotrophic bacteria, which were isolated from a methane-utilizing microbial consortium culture established from forest soil. Three rhizobial strains were found to strongly stimulate the growth and methane oxidation of M. miyakonense HT12 in cocultures. We purified the stimulating factor produced by Rhizobium sp. Rb122 and identified it as cobalamin. Growth stimulation by cobalamin was also observed for three other gammaproteobacterial methanotrophs. These results suggest that microbial interactions through cobalamin play an important role in methane oxidation in various ecosystems.
INTRODUCTION
Methane is the second most important greenhouse gas, and mitigating emissions of methane has become a major global concern (17). Aerobic methanotrophs are the major terrestrial methane sink and are widespread in a large variety of ecosystems (26). They belong to the Gammaproteobacteria (type I), Alphaproteobacteria (type II), and Verrucomicrobia (26). Methanotrophs utilize methane as a single source of carbon and energy, but only some methanotrophic strains in the class Alphaproteobacteria can assimilate substrates with C-C bonds (8).
Mutual interactions that occur between methanotrophs and other organisms, ranging from microbes to plants and animals, may affect the global methane cycle in various ways. Stable isotope probing (SIP) experiments revealed that methane-derived carbon was incorporated into methylotrophic or heterotrophic bacteria when they were incubated with methanotrophs (5, 14, 20, 22, 23), indicating an important role of methanotrophs in supplying nutrients in the form of carbon sources to other nonmethanotrophic organisms. At deep-sea hydrothermal vents and cold seeps, invertebrates form symbiotic associations with gammaproteobacterial methanotrophs living in their tissues (21, 26). Some invertebrates can derive most of their carbon nutrition from methane, indeed acquiring it from methane-derived metabolites of methanotrophs or by digestion of methanotrophs. Inversely, the hosts provide methanotrophs with simultaneous access to methane and oxygen by positioning themselves appropriately and also by providing a stable environment. In peat bogs, Sphagnum mosses associate with alphaproteobacterial methanotrophs and utilize carbon dioxide that is generated from methane by methanotrophic symbionts (18, 24). Although previous studies have demonstrated that methanotrophs serve as food suppliers for other organisms, less attention has been paid to the specific benefits that methanotrophs acquire from such interactions.
Previously, we established a forest soil-based microbial consortium utilizing methane as the single carbon and energy source, from which we isolated a new obligate methanotroph, Methylovulum miyakonense HT12 (15). The aim of the present study was to clarify the beneficial factors for methanotrophic growth and methane oxidation provided by other bacteria in the methane-grown microbial consortium to further understand interactions between methanotrophs and microorganisms.
MATERIALS AND METHODS
Methanotrophic strains and growth conditions.
M. miyakonense HT12 was a laboratory stock (15). Methylococcus capsulatus Bath, Methylobacter luteus, Methylomonas methanica S1, and Methylosinus trichosporium OB3b were obtained from the culture collection of NCIMB (strain no. 11132, 11914, 11130, and 11131, respectively). Details of the isolation of methanotrophic strains OS501, R4F, B3R, SH31p, and SS2C will be described elsewhere (H. Iguchi, I. Sato, M. Sakakibara, H. Yurimoto, and Y. Sakai, unpublished data). The methanotrophic strains were grown in nitrate mineral salts (NMS) medium (28) under a 20:80 methane-to-air atmosphere at 28°C with shaking in 25-ml vials capped with butyl rubber stoppers. In order to compensate for slow growth, M. miyakonense HT12, strain OS501, and strain R4F were subcultured in medium supplemented with Bacto tryptone (Becton Dickinson and Company) at 0.01% (wt/vol).
Isolation of bacterial strains grown in the consortium culture.
The methane-enriched culture of forest soil (15) was subcultured every month over 2 years. The culture was serially diluted and spread onto agar plates: the media used were modified Luria-Bertani (LB) broth (0.2% tryptone, 0.1% yeast extract, 0.1% sodium chloride, 0.1% glucose), 10-fold-diluted tryptone soy broth (Oxoid, Cambridge, United Kingdom), 5-fold-diluted nutrient broth (Becton Dickinson and Company), and NMS medium with 0.1% methanol. A single colony grown on a plate at 28°C was isolated. The 16S rRNA gene was amplified by PCR, using the 27f-1492r primer set (27), and then sequenced. A similarity search for the nucleotide sequences of the 16S rRNA genes of the isolates was carried out using the BLAST program.
Mixed culture experiments.
The cells of the heterotrophic isolates were prepared from liquid cultures grown in modified LB broth. Because of its inability to grow in this medium, strain Rb122 was grown in TY medium (0.5% tryptone, 0.3% yeast extract, 0.083% calcium chloride). Methanotroph cells were prepared from liquid cultures in NMS medium with methane. Cells of the methanotroph and the heterotroph were washed with water, mixed to adjust the ratio of optical densities at 600 nm (OD600 values) to 10:1 (OD600 values of 0.0025 and 0.00025, respectively), and cultivated in NMS medium with methane. For the second-generation culture, the cells were harvested by centrifugation from the 8-day-old mixed culture, washed with water, and inoculated into fresh NMS medium at an OD600 value of 0.0025. Triplicate samples were prepared for each culture and each time point, because after the methane concentration was measured, the vial was opened to measure the OD600.
Methane concentrations were determined using a Shimadzu GC-14B gas chromatograph (Shimadzu, Kyoto, Japan) equipped with a flame ionization detector and a Porapak Q column (Shinwa Chemical Industries, Kyoto, Japan). Nitrogen gas was used as the carrier. Analytical temperatures of the oven, injector, and detector were 100, 120, and 225°C, respectively.
qPCR.
Quantitative PCR (qPCR) was carried out using a LightCycler system (Roche Diagnostics, Tokyo, Japan) and SYBR Premix ExTaq (Takara Bio, Shiga, Japan) according to the manufacturers' instructions. Genomic DNA, which was extracted from the culture by a method using SDS and proteinase K as described previously (16), was used as the template. Primers specific for the 16S rRNA gene of each strain were designed. The primer sets were ht-fw (TGGCCCCAATTATGGGGTAA) and ht-re (AGGGATCTCTGCCGAATCCA) for M. miyakonense HT12 and rb-fw (GTCGGGCAGTTGACTGTTCG) and rb-re (TACCGTCTCCGGTAACCGCGA) for strain Rb122. A standard curve for copy number calculation was generated with plasmid DNA (pGEM-T Easy [Promega, Madison, WI] harboring the 16S rRNA gene).
Preparation of spent medium and cell extracts.
The pure culture of M. miyakonense HT12 and the coculture of M. miyakonense HT12 and strain Rb122 were grown in NMS medium with methane. Strain Rb122 was grown in Rhizobium minimal medium (RMM) with glucose (13). Bacterial cells were removed from the culture by centrifugation, and the spent medium was filter sterilized using a 0.45-μm filter. Until use, the samples were stored at −80°C.
For quantification, cobalamins were twice extracted from the lyophilized culture or the harvested cells by using methanol with incubation at 80°C. The extracts were lyophilized, dissolved in water, and filter sterilized using a 0.45-μm filter.
Stimulatory activity assay of methanotrophic growth (HT12 assay).
M. miyakonense HT12 was grown on methane in NMS medium containing a test sample. The stimulatory activity of the sample was evaluated based on the OD600 value after cultivation for 48 to 72 h. We confirmed that the addition of NMS medium, RMM, or methanol extracts of the media did not stimulate growth. For quantification of the stimulatory activity, a calibration curve was established using a cyanocobalamin (CN-Cbl) standard (Wako Pure Chemical Industries, Osaka, Japan).
Bioassay of cobalamin (Lactobacillus assay).
Cobalamin was quantified by the growth of Lactobacillus delbrueckii NBRC 3073 on B12 assay medium (Becton Dickinson and Company) according to the procedures in the Difco and BBL manual.
Purification of cobalamins from Rb122 culture.
Four 500-ml batches of stationary-phase cultures of strain Rb122 were prepared by growth in RMM with glucose at 28°C with shaking. The cells were removed by centrifugation, and the resulting 1.9-liter supernatant was incubated with potassium cyanide to convert cobalamins to cyanocobalamin. This was performed to facilitate purification and detection because cobalamins may occur in different forms in cells and are sensitive to light. The solution was evaporated with ethanol and extracted with methanol.
The extracts were evaporated and dissolved in water. The sample was applied to an Amberlite XAD-2 column (Sigma-Aldrich, St. Louis, MO). The column was eluted with 50% methanol after washing with water. The eluate was evaporated, dissolved in water, and applied to a Sep-Pak C18 cartridge column (Waters, Milford, MA). The column was eluted with 30% acetonitrile after washing with 10% acetonitrile.
Nucleotide sequence accession numbers.
The partial 16S rRNA gene sequences determined in this study have been deposited in GenBank under accession numbers AB636288 to AB636303.
RESULTS
Isolation of bacterial strains from methane-grown consortium culture.
Previously, we established a stable microbial consortium utilizing methane as the single carbon and energy source from a forest soil sample which has been maintained for over 2 years (15). From this consortium culture, the methanotroph M. miyakonense HT12 was isolated (15). Sequence analysis based on the 16S rRNA gene identified another methanotroph (a Methylocystis sp.) and 10 heterotrophic bacteria in the consortium (Table 1). Nine of these 10 heterotrophic strains were isolated in pure culture (Table 1). They were all Gram-negative bacteria belonging to either the Proteobacteria or Bacteroidetes. Strain NS1203 was a facultative methylotroph that grew on methanol, while the other eight strains were nonmethylotrophic heterotrophs. Among the strains identified in the consortium, Methylocystis sp. and Sphingobacterium sp. could not be isolated (Table 1).
Table 1.
Bacterial strains identified in the consortium culture
| Strain or clone (GenBank accession no.) | Phylum | Closest relativeb (GenBank accession no.) | % Similarityb |
|---|---|---|---|
| HT12 (AB501287) | Gammaproteobacteria | Methylovulum miyakonense HT12 (AB501287) | 100 |
| NM120a (AB636288) | Alphaproteobacteria | Methylocystis sp. 18-2 (AB007841) | 99 |
| NL123 (AB636289) | Alphaproteobacteria | Mesorhizobium sp. WSM3872 (FJ827044) | 99 |
| Rb122 (AB636290) | Alphaproteobacteria | Rhizobium sp. BZ3 (HQ588847) | 97 |
| NS1202 (AB636291) | Alphaproteobacteria | Sinorhizobium sp. J1 (DQ294628) | 100 |
| NL127 (AB636292) | Alphaproteobacteria | Xanthobacter flavus (EF592179) | 100 |
| NL121 (AB636293) | Betaproteobacteria | Hydrogenophaga sp. AH-24 (AB300163) | 99 |
| NS1203 (AB636294) | Betaproteobacteria | Ideonella sp. B513 (AB049107) | 99 |
| NS1204 (AB636295) | Betaproteobacteria | Lutiella nitroferrum 2002 (AY609199) | 93 |
| NL124 (AB636296) | Bacteroidetes | Flavobacterium sp. WG2 (FN547416) | 95 |
| NL128 (AB636297) | Bacteroidetes | Emticicia ginsengisoli Gsoil 085 (AB245370) | 98 |
| NC22a (AB636298) | Bacteroidetes | Sphingobacterium sp. P-7 (AM411964) | 94 |
Not isolated.
Closest relatives and similarities are based on 16S rRNA gene sequences.
Methane oxidation and growth of M. miyakonense HT12 were stimulated in mixed cultures.
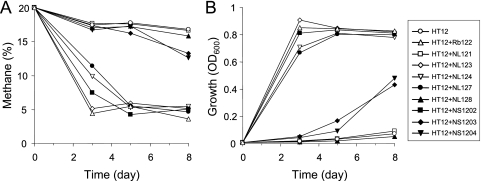
Each of the isolated strains was added to the culture of M. miyakonense HT12 to test for positive effects on growth and methane oxidation. Five strains, Rb122, NL123, NS1202, NL124, and NL127, caused significant effects on methane oxidation (Fig. 1). The concomitant increase in methane consumption with the cell density indicated that M. miyakonense HT12 showed improved growth on methane in the mixed culture. Interestingly, three of the five strains were members of the order Rhizobiales. We used the representative rhizobial strain Rb122, which is closely related to the genus Rhizobium (Table 1), for an in-depth analysis of the stimulatory effect on methane oxidation by M. miyakonense HT12.
Fig. 1.
Stimulatory effects of bacterial inocula on growth (A) and methane oxidation (B) of M. miyakonense HT12. Mixed cultures were constructed by inoculating M. miyakonense HT12 together with each of the bacterial strains into the medium. To generate stable bacterial cocultures, mixed cultures grown on methane for 8 days were transferred to fresh medium. Values shown are the data for these second cultures. The data are the means for triplicate samples.
Analysis of coculture consisting of M. miyakonense HT12 and Rhizobium sp. Rb122.
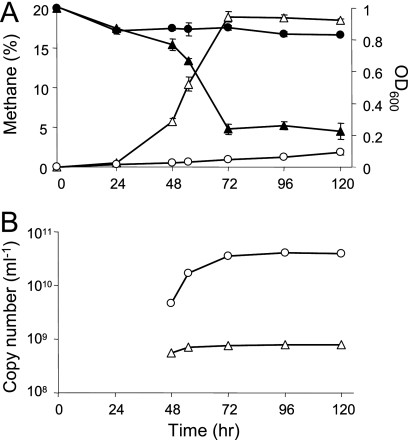
Growth of the coculture of M. miyakonense HT12 and Rhizobium sp. Rb122 was analyzed by qPCR. Although we could not quantify cell mass during the early growth phase, the cell masses of both strains certainly increased in the mixed culture, and the number of cells was estimated to be 1 to 2 orders of magnitude higher for M. miyakonense HT12 than for Rhizobium sp. Rb122 (Fig. 2B). M. miyakonense HT12 exhibited slow growth in pure culture (Fig. 3A), while the growth of the coculture with Rhizobium sp. Rb122 was stable even after repetitive subculturing with methane. These results indicate that Rhizobium sp. Rb122 plays a critical role in stimulating the growth of M. miyakonense HT12.
Fig. 2.
Growth profiles of M. miyakonense HT12 in pure culture and coculture with Rhizobium sp. Rb122. (A) Methane oxidation (closed symbols) and growth (open symbols) were compared between the pure culture (circles) and the coculture (triangles). The data are the means for triplicate samples. (B) Cell masses of M. miyakonense HT12 (circles) and Rhizobium sp. Rb122 (triangles) in the coculture were determined by quantitative PCR targeting the 16S rRNA gene. Representative data from triplicate samples are shown.
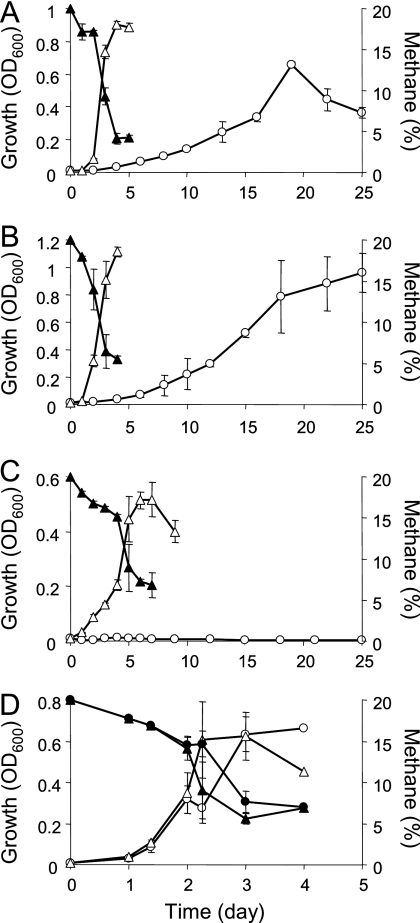
Fig. 3.
Comparison of growth on methane between pure methanotroph culture (circles) and coculture of a methanotroph with Rhizobium sp. Rb122 (triangles). (A) M. miyakonense HT12; (B) strain OS501; (C) strain R4F; (D) M. methanica. Open symbols indicate growth. Methane consumption (closed symbols) in the coculture demonstrates that growth was dependent on the methanotrophic activity. The data are the means for triplicate samples.
Rhizobium sp. Rb122 produces a methanotrophic growth-stimulating factor.
We hypothesized that the observed positive effect on M. miyakonense HT12 growth by Rhizobium sp. Rb122 was due to the production of some growth-stimulating factor(s). To verify this, we examined filtered spent medium from Rhizobium sp. Rb122 for a stimulatory effect. The quantitative assay method to evaluate the growth-stimulatory effect on M. miyakonense HT12 was established as described in Materials and Methods (designated the HT12 assay).
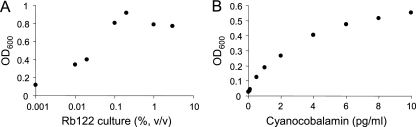
Both the spent medium from the coculture of M. miyakonense HT12 and Rhizobium sp. Rb122 and the spent medium from the pure culture of Rhizobium sp. Rb122 (Fig. 4A) showed stimulatory activity, whereas the spent medium from the M. miyakonense HT12 culture did not. These results indicate that Rhizobium sp. Rb122 produces growth-stimulating compounds for M. miyakonense HT12 in the medium.
Fig. 4.
Stimulatory activity of the Rb122 culture supernatant (A) and cyanocobalamin (B) on the growth of M. miyakonense HT12. The cells were grown on methane in NMS medium containing the indicated concentrations of additives. Plotting the OD600 values of the culture measured at a time point (48 to 72 h) with respect to the concentrations of the additives showed dose-dependent growth-stimulatory activity.
Next, we characterized the chemical properties of the growth-stimulating factor present in the spent medium of Rhizobium Rb122. Growth stimulation of M. miyakonense HT12 was observed by addition of the spent medium at a 0.01% (vol/vol) concentration. Neither autoclaving (121°C, 20 min) nor enzyme treatments with proteinase K or lipase significantly changed the stimulatory activity. The activity could be extracted from the spent medium with methanol or butanol but not with ethyl acetate. These observations suggested that the factor is a heat-stable, relatively polar microelement for cells.
Based on these data, we tested the following bioactive compounds for stimulation of the growth of M. miyakonense HT12: thiamine, riboflavin, pyridoxine, cyanocobalamin, niacin, calcium pantothenate, biotin, folic acid, inositol, ascorbic acid, pyrroloquinoline quinone, glutathione, and amino acids. Among them, cyanocobalamin was found to have a clear stimulatory activity, in a dose-dependent manner (Fig. 4B), whereas the other compounds had no activity. Vitamin B12 is used to describe compounds of the cobalamin group, which include cyanocobalamin (CN-Cbl), methylcobalamin (Me-Cbl), hydroxocobalamin (OH-Cbl), and adenosylcobalamin (Ado-Cbl). Me-Cbl and OH-Cbl also exhibited a stimulatory effect equivalent to that of CN-Cbl (data not shown).
Bacto tryptone, which is used to stimulate the growth of M. miyakonense HT12 in pure culture, was also found to contain cobalamins (93 ng/g) by the Lactobacillus assay (12).
Cobalamin is the growth-stimulating factor produced by Rhizobium sp. Rb122.
The cobalamin content in the spent culture was quantified by the Lactobacillus assay. Cobalamin was detected in both the coculture and the Rb122 culture, and the amount detected in the coculture was 2 orders of magnitude lower than that in the Rb122 culture (Table 2). On the other hand, cobalamin was not detected in the M. miyakonense HT12 culture (Table 2). These results show that Rhizobium sp. Rb122 releases cobalamin into the medium, but M. miyakonense HT12 does not produce it.
Table 2.
Cobalamin production determined by the Lactobacillus assay
| Culture | Cultivation time (days) | OD600 | Cobalamin concn (pg/ml culture)a |
|
|---|---|---|---|---|
| Extracellular | Intracellular | |||
| Coculture of HT12 | 1 | 0.041 | 3.79 | 2.77 × 10 |
| and Rb122 | 2 | 0.520 | 1.34 × 10 | 6.70 × 10 |
| 3 | 0.918 | 9.56 | 1.15 × 102 | |
| 4 | 0.873 | 1.29 × 10 | 1.28 × 102 | |
| Rb122 culture | 1 | 0.142 | 7.68 × 10 | 1.53 × 102 |
| 2 | 0.738 | 1.24 × 103 | 4.91 × 103 | |
| 3 | 0.714 | 2.52 × 103 | 4.82 × 103 | |
| 4 | 0.689 | 2.39 × 103 | 4.24 × 103 | |
| HT12 culture | 7 | 0.108 | ND | ND |
| 12 | 0.301 | ND | ND | |
ND, not detected.
In order to identify the cobalamin compound produced by Rhizobium sp. Rb122 and to confirm its growth-stimulatory activity, we purified cobalamin compounds according to the standard method for vitamin B12 (29). Rhizobium sp. Rb122 was grown in RMM with glucose, and the culture supernatant was incubated with potassium cyanide to convert cobalamins to CN-Cbl. The resultant solution was evaporated, extracted with methanol, and purified using an Amberlite XAD-2 column and a Sep-Pak C18 column. The purified compounds exhibited stimulatory activity based on the HT12 assay (Table 3). The activity detected in the HT12 assay and the cobalamin content assessed by the Lactobacillus assay were nearly identical throughout the purification step (Table 3), indicating that cobalamin is the principal methanotrophic growth-stimulating compound produced by Rhizobium sp. Rb122.
Table 3.
Cobalamin activity of the purified compound from the culture of Rhizobium sp. Rb122
| Sample | Amt of cobalamin (μg) |
|
|---|---|---|
| HT12 assay | Lactobacillus assay | |
| Culture supernatant | 7.52 | 4.38 |
| Amberlite XAD-2 eluate | 2.75 | 2.03 |
| Sep-Pak C18 eluate | 1.16 | 1.03 |
The final preparation was subjected to liquid chromatography-mass spectrometry (LC-MS) analysis with CN-Cbl as the standard. The final preparation generated a peak at 10.5 min that was identical to the CN-Cbl standard based on the retention time (see Fig. S1A in the supplemental material). The MS profile showed that this peak yielded an m/z 678.90 fragment ion, which corresponded to [CN-Cbl + 2H]2+ (see Fig. S1B). The LC eluate of the purified compounds was examined for growth-stimulating activity for M. miyakonense HT12, and only the fraction including the peak at 10.5 min was found to possess the activity. Based on these results, we concluded that cobalamin is the growth-stimulating factor for M. miyakonense HT12 produced by Rhizobium sp. Rb122.
Stimulatory activity for methanotrophs.
M. miyakonense HT12 is in a new phylogenetic lineage (15), so we raised the question of whether Rhizobium sp. Rb122 as well as cobalamin exerts a stimulating effect on other methanotrophs. Growth and methane consumption rates were compared between three cultures of methanotrophs: a culture with addition of Rhizobium sp. Rb122 (coculture), a culture with addition of cyanocobalamin, and a culture with no additive. Four methanotrophic strains from the culture collection and five methanotrophic isolates obtained by our laboratory were used in this experiment.
Both Rhizobium sp. Rb122 and cobalamin stimulated three methanotrophic strains, OS501, R4F, and M. methanica S1 (Table 4 and Fig. 3). The growth profiles revealed that the slow growth and deficient growth of strain OS501 and strain R4F, respectively, were significantly stimulated by Rhizobium sp. Rb122 (Fig. 3B and C). No growth-stimulating effects were observed for the other methanotrophs by either Rhizobium sp. Rb122 or cobalamin, although M. luteus showed a slight stimulation of growth and methane oxidation by Rhizobium sp. Rb122.
Table 4.
Stimulatory activity and cobalamin production for methanotrophs
| Strain | Proteobacterial subgroup | Stimulatory activitya |
Cobalamin productionc (ng/ml) | |
|---|---|---|---|---|
| Coculture with Rb122 | Cobalaminb | |||
| Methylovulum miyakonense HT12T | Gamma | ++ | ++ | ND |
| Methylococcaceae bacterium strain OS501 | Gamma | ++ | ++ | ND |
| Methylomonas sp. R4F | Gamma | ++ | ++ | NT |
| Methylomonas methanica S1T | Gamma | + | + | 2.76 |
| Methylobacter luteusT | Gamma | + | − | 2.58 |
| Methylococcus capsulatus Bath | Gamma | − | − | 2.16 |
| Methylosinus trichosporium OB3bT | Alpha | − | − | 4.32 |
| Methylosinus sp. B3R | Alpha | − | − | 6.26 |
| Methylocystis sp. SS2C | Alpha | − | − | 5.05 |
| Methylocystis sp. SH31p | Alpha | − | − | 3.65 |
Stimulatory activity was evaluated by growth and methane oxidation. ++, strong stimulation; +, weak stimulation; −, no stimulation.
Cyanocobalamin was added to the medium at a final concentration of 100 pg/ml.
Cobalamin in the whole culture at stationary phase was quantified by the Lactobacillus assay. ND, not detected; NT, not tested due to the inability to grow without cobalamin.
Cobalamin production was quantified for the methanotrophs grown on methane by the Lactobacillus assay. All of the tested methanotrophs except for strain OS501 produced cobalamins (Table 4). Accordingly, cobalamin was determined to be the stimulating factor in the coculture with Rhizobium sp. Rb122 for strain OS501, strain R4F, and M. methanica S1, but some other factors were likely provided for M. luteus in the coculture. We screened stimulating factors from the bioactive compounds as performed for M. miyakonense HT12 and found that thiamine, pyridoxine, and calcium pantothenate slightly stimulated the growth of M. luteus.
The phylogenetic tree based on the 16S rRNA gene sequences showed that the methanotrophs tested (Table 4) were located divergently in the tree, and no obvious phylogenetic correlation could be found with respect to the stimulatory effect or cobalamin production of methanotrophs, except that the methanotrophs for which growth stimulation was observed belonged to the Gammaproteobacteria (see Fig. S2 in the supplemental material).
DISCUSSION
We investigated the microbial interaction that caused a positive effect on methanotrophic growth and methane oxidation of M. miyakonense HT12 and showed that five bacterial strains related to the genera Rhizobium, Sinorhizobium, Mesorhizobium, Xanthobacter, and Flavobacterium stimulated the growth of M. miyakonense HT12 (Fig. 1 and Table 1). Further analysis of the stimulatory mechanism exerted by Rhizobium sp. Rb122 identified cobalamin as the growth-stimulating factor. We also detected cobalamin production by four other strains in pure cultures, namely, NS1202, NL123, NL127, and NL124 (see Table S1 in the supplemental material), indicating that the pivotal stimulatory mechanism in these cocultures is mediated by cobalamin. The degree of variation in the stimulatory effect observed in the coculture experiments (Fig. 1) is thought to be dependent on the growth of the cocultured bacterium and its cobalamin productivity (Table 2; see Table S1). Strains Rb122, NS1202, and NL123 excreted a considerable amount of cobalamin into the medium, while strains NL127 and NL124 did not. Such extracellular production of cobalamin may be the cause of the strong stimulatory effect on methanotrophic growth exerted by rhizobia.
With respect to methylotrophs (methanol utilizers incapable of growth on methane) employing the ribulose monophosphate (RuMP) pathway, Methylophaga species were reported to show cobalamin auxotrophy (9), while Methylobacillus flagellatus KT is deficient in cobalamin synthesis but can grow on methanol without cobalamin (11). However, cobalamin auxotrophy related to methanotrophic growth has not been investigated. It is worthwhile to note that among the tested methanotrophs, methanotrophic growth of four gammaproteobacterial methanotrophs that employ the RuMP pathway was stimulated by cobalamin (Table 4), and their slow or deficient growth in pure culture was correlated with the ability to synthesize cobalamin de novo (Fig. 3 and Table 4). On the other hand, alphaproteobacterial methanotrophs and methylotrophs, which employ the serine cycle, require ethylmalonyl-coenzyme A (ethylmalonyl-CoA) mutase, an Ado-Cbl-dependent enzyme, for methylotrophic growth (1, 6). Indeed, all of the tested alphaproteobacterial methanotrophs were shown to produce cobalamin de novo (Table 4). Therefore, although the positive effect of cobalamin on methanotrophic growth seems to be limited to gammaproteobacterial methanotrophs, cobalamin should have significant functions in methanotrophic metabolism.
Bowman and Sayler (3) reported that cobalamin increases the soluble methane monooxygenase (sMMO) activity of M. trichosporium OB3b. However, activation of sMMO cannot explain the growth stimulation of M. miyakonense HT12, since cobalamin also stimulates the growth of this strain on methanol. For some organisms (4, 7), cobalamin auxotrophy was reported to be stimulated by addition of methionine, because methionine synthase (MetH) requires cobalamin as a cofactor (25). However, addition of methionine had no effect on the growth of M. miyakonense HT12 on methane. Further work is needed to elucidate the specific function of cobalamin in methanotrophic metabolism.
Interestingly, M. miyakonense HT12 seemed to obtain some growth factors from Rhizobium sp. 122 other than cobalamin, since the culture supernatant of Rhizobium sp. 122 exhibited higher cobalamin activity in the HT12 assay than in the Lactobacillus assay (Table 3). The presence of another growth-stimulatory factor was also suggested for M. luteus (Table 4). Vitamins and organic acids were reported to enhance the growth rate of the methanotroph (30), and amino acids could be utilized as a nitrogen source for methanotrophs (10). Production of such compounds by Rhizobium sp. 122 may support methanotrophic growth in cocultures.
In natural environments, organisms lacking the ability to synthesize cobalamin must rely on cobalamin producers such as bacteria and archaea (25). Cobalamin availability in the environment may thus restrict the growth of such organisms (2). The four tested methanotrophic strains were isolated from different habitats (HT12 from forest soil, OS501 from lake water, R4F from rice root, and S1 from an aquatic plant), and cobalamin-producing microorganisms, including rhizobia, are found ubiquitously in these environments (19, 25). Therefore, we suggest that microbial interactions with methanotrophs through cobalamin are distributed in various natural environments and play an important role in the methane cycle.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported in part by grants-in-aid for scientific research to Y.S. (B; 22380052) and H.Y. (B; 22310046) from the Japan Society for the Promotion of Science. This work was also supported in part by research grant programs for natural science from the Asahi Glass Foundation to Y.S.
Footnotes
Supplemental material for this article may be found at http://aem.asm.org/.
Published ahead of print on 7 October 2011.
REFERENCES
- 1. Alber B. E. 2010. Biotechnological potential of the ethylmalonyl-CoA pathway. Appl. Microbiol. Biotechnol. 89:17–25 [DOI] [PubMed] [Google Scholar]
- 2. Bertrand E. M., et al. 2007. Vitamin B12 and iron colimitation of phytoplankton growth in the Ross Sea. Limnol. Oceanogr. 52:1079–1093 [Google Scholar]
- 3. Bowman J. P., Sayler G. S. 1994. Optimization and maintenance of soluble methane monooxygenase activity in Methylosinus trichosporium OB3b. Biodegradation 5:1–11 [DOI] [PubMed] [Google Scholar]
- 4. Bretscher A. P., Kaiser D. 1978. Nutrition of Myxococcus xanthus, a fruiting myxobacterium. J. Bacteriol. 133:763–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cebron A., et al. 2007. Nutrient amendments in soil DNA stable isotope probing experiments reduce the observed methanotroph diversity. Appl. Environ. Microbiol. 73:798–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chistoserdova L. 2011. Modularity of methylotrophy, revisited. Environ. Microbiol. doi:10.1111/j.1462-2920.2011.02464.x [DOI] [PubMed] [Google Scholar]
- 7. Croft M. T., Lawrence A. D., Raux-Deery E., Warren M. J., Smith A. G. 2005. Algae acquire vitamin B12 through a symbiotic relationship with bacteria. Nature 438:90–93 [DOI] [PubMed] [Google Scholar]
- 8. Dedysh S. N., Dunfield P. F. 2011. Facultative and obligate methanotrophs how to identify and differentiate them. Methods Enzymol. 495:31–44 [DOI] [PubMed] [Google Scholar]
- 9. Doronina N., Darmaeva T., Trotsenko Y. 2003. Methylophaga natronica sp. nov., a new alkaliphilic and moderately halophilic, restricted-facultatively methylotrophic bacterium from Soda Lake of the Southern Transbaikal Region. Syst. Appl. Microbiol. 26:382–389 [DOI] [PubMed] [Google Scholar]
- 10. Green P. N. 1992. Taxonomy of methylotrophic bacteria, p. 23–84 In Murrell J. C., Dalton H. (ed.), Methane and methanol utilizers. Plenum Press, New York, NY [Google Scholar]
- 11. Hendrickson E. L., et al. 2010. Expressed genome of Methylobacillus flagellatus as defined through comprehensive proteomics and new insights into methylotrophy. J. Bacteriol. 192:4859–4867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hoffmann C. E., Stokstad E. L. R., Hutchings B. L., Dornbush A. C., Jukes T. H. 1949. The microbiological assay of vitamin B12 with Lactobacillus leichmannii. J. Biol. Chem. 181:635–644 [PubMed] [Google Scholar]
- 13. Hooykaas P. J. J., Klapwijk P. M., Nuti M. P., Schilperoort R. A., Rorsch A. 1977. Transfer of the Agrobacterium tumefaciens TI plasmid to avirulent agrobacteria and to Rhizobium ex planta. J. Gen. Microbiol. 98:477–484 [Google Scholar]
- 14. Hutchens E., Radajewski S., Dumont M. G., McDonald I. R., Murrell J. C. 2004. Analysis of methanotrophic bacteria in Movile Cave by stable isotope probing. Environ. Microbiol. 6:111–120 [DOI] [PubMed] [Google Scholar]
- 15. Iguchi H., Yurimoto H., Sakai Y. 2011. Methylovulum miyakonense gen. nov., sp. nov., a novel type I methanotroph from a forest soil in Japan. Int. J. Syst. Evol. Microbiol. 61:810–815 [DOI] [PubMed] [Google Scholar]
- 16. Iguchi H., Yurimoto H., Sakai Y. 2010. Soluble and particulate methane monooxygenase gene clusters of the type I methanotroph Methylovulum miyakonense HT12. FEMS Microbiol. Lett. 312:71–76 [DOI] [PubMed] [Google Scholar]
- 17. Intergovernmental Panel on Climate Change 2007. Climate change 2007: the physical science basis. Summary for policymakers. Contribution of Working Group I to the fourth assessment report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, United Kingdom [Google Scholar]
- 18. Kip N., et al. 2010. Global prevalence of methane oxidation by symbiotic bacteria in peat-moss ecosystems. Nat. Geosci. 3:617–621 [Google Scholar]
- 19. Martens J. H., Barg H., Warren M., Jahn D. 2002. Microbial production of vitamin B12. Appl. Microbiol. Biotechnol. 58:275–285 [DOI] [PubMed] [Google Scholar]
- 20. Murase J., Frenzel P. 2007. A methane-driven microbial food web in a wetland rice soil. Environ. Microbiol. 9:3025–3034 [DOI] [PubMed] [Google Scholar]
- 21. Petersen J. M., Dubilier N. 2009. Methanotrophic symbioses in marine invertebrates. Environ. Microbiol. Rep. 1:319–335 [DOI] [PubMed] [Google Scholar]
- 22. Qiu Q., Conrad R., Lu Y. 2009. Cross-feeding of methane carbon among bacteria on rice roots revealed by DNA-stable isotope probing. Environ. Microbiol. Rep. 1:355–361 [DOI] [PubMed] [Google Scholar]
- 23. Radajewski S., et al. 2002. Identification of active methylotroph populations in an acidic forest soil by stable-isotope probing. Microbiology 148:2331–2342 [DOI] [PubMed] [Google Scholar]
- 24. Raghoebarsing A. A., et al. 2005. Methanotrophic symbionts provide carbon for photosynthesis in peat bogs. Nature 436:1153–1156 [DOI] [PubMed] [Google Scholar]
- 25. Roth J. R., Lawrence J. G., Bobik T. A. 1996. Cobalamin (coenzyme B12) synthesis and biological significance. Annu. Rev. Microbiol. 50:137–181 [DOI] [PubMed] [Google Scholar]
- 26. Semrau J. D., DiSpirito A. A., Yoon S. 2010. Methanotrophs and copper. FEMS Microbiol. Rev. 34:496–531 [DOI] [PubMed] [Google Scholar]
- 27. Weisburg W. G., Barns S. M., Pelletier D. A., Lane D. J. 1991. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 173:697–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Whittenbury R., Phillips K. C., Wilkinson J. F. 1970. Enrichment, isolation and some properties of methane-utilizing bacteria. J. Gen. Microbiol. 61:205–218 [DOI] [PubMed] [Google Scholar]
- 29. Wilhelm F. 1988. Vitamin B12, p. 837–928 In Vitamins. Walter de Gruyter, Berlin, Germany [Google Scholar]
- 30. Xing X.-H., Wu H., Luo M.-F., Wang B.-P. 2006. Effects of organic chemicals on growth of Methylosinus trichosporium OB3b. Biochem. Eng. J. 31:113–117 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.