Abstract
Simian immunodeficiency virus (SIV) infection in African nonhuman primate (NHP) natural hosts is usually nonpathogenic, despite high levels of virus replication. We have previously shown that chronic SIV infection in sooty mangabeys (SMs) and African green monkeys (AGMs) is associated with low levels of immune activation and bystander T cell apoptosis. To compare these features with those observed in another natural host, the mandrill (MND), we conducted a cross-sectional survey of the 23 SIV-infected and 25 uninfected MNDs from the only semifree colony of mandrills available worldwide. Viral loads (VLs) were determined and phenotypic and functional analysis of peripheral blood- and lymph node-derived lymphocytes was performed. We found that mandrills chronically infected with SIVmnd-1 or SIVmnd-2 have similar levels of viral replication, and we observed a trend toward lower CD4+ T cell counts in chronically SIVmnd-2-infected MNDs than SIVmnd-1-infected MNDs. No correlation between CD4+ T cell counts and VLs in SIV-infected MNDs could be established. Of note, the levels of T cell activation, proliferation, and apoptosis were comparable between SIVmnd-1- and SIVmnd-2-infected MNDs and to those observed in uninfected animals, with the only exception being an increase in tumor necrosis factor alpha-producing CD8+ T cells in SIVmnd-2-infected MNDs. Overall, these findings recapitulate previous observations in SIV-infected SMs and AGMs and lend further evidence to the hypothesis that low levels of immune activation protect natural SIV hosts from disease progression.
INTRODUCTION
Simian immunodeficiency viruses (SIVs) naturally infect over 40 African nonhuman primate (NHP) species (65) and are generally nonpathogenic in their natural hosts (8, 35, 55), with only a few cases of progression to AIDS being reported thus far (45). Numerous studies have investigated the mechanisms by which natural hosts of SIVs limit disease progression and have concluded that lack of AIDS is not due to control of viral replication through exquisite immune responses, as viral loads (VLs) in natural hosts are similar to those observed during pathogenic human immunodeficiency virus (HIV)/SIV infections (46) and SIV-specific adaptive immune responses are not quantitatively or qualitatively different from those mounted during pathogenic infection (10, 13, 14, 25, 26, 68, 70). Instead, the lack of disease progression relies on an adaptation of these natural hosts to control the deleterious indirect consequences of SIV infection, i.e., excessive levels of immune activation, T cell proliferation, and apoptosis (11, 28, 39, 53, 56). More recently, it was proposed that natural SIV infection is characterized by a different pattern of infected cells in vivo that spares central memory CD4+ T cells from virus-mediated killing (1, 4, 33, 37, 67). Regardless of their molecular mechanisms, these adaptations underlining the benign course of SIV infection in natural hosts most likely occurred over millennia (65). In the context of the continuous spread of the HIV pandemic and the absence of an effective cure or vaccine for AIDS, lessons learned from natural hosts of SIVs may be of pivotal importance to develop new strategies to control HIV disease progression (57).
Research in the field of natural SIV infection has primarily been conducted in two models that have provided the vast majority of available data: SIVsmm-infected sooty mangabeys (SMs) and SIVagm-infected African green monkeys (AGMs) (35). Of note, these two models of persistent nonprogressive infection have pathogenic counterparts employing the same viruses, as SIVsmm can be pathogenic in rhesus macaques (RMs) (29, 54) and SIVagm is pathogenic in pigtailed macaques (12, 17, 22; D. Mandell et al., submitted for publication). While these studies of SMs and AGMs have provided a number of important data on the pathophysiology of natural SIV infections (2, 4, 8, 16, 18, 19, 36, 38, 39, 43, 56), it can be argued that an approach focusing only on these two models may skew our view of a phenomenon that involves more than 40 different nonhuman primate species. Indeed, differences between SIV infections in SMs and AGMs have even been observed, and species-specific mechanisms employed for controlling disease progression have been identified (1, 4, 33, 67). Therefore, we believe that studying additional natural SIV host species is useful to advance our understanding of the mechanisms responsible for the control of disease progression. Unfortunately, this type of research has been historically difficult due to a series of logistical challenges. First, most natural SIV host species are not available for comprehensive studies in the United States, their import is problematic, and primate facilities that can carry out in-depth pathogenesis studies in sub-Saharan Africa are virtually nonexistent (35). Second, numerous African monkey species that are natural hosts for SIV are considered endangered, and thus, invasive studies are not permitted in these animals (35). Finally, most of the SIVs naturally infecting African NHP hosts are known only from sequences, and viral isolates for in vitro or in vivo pathogenesis studies are not available (65).
A logical choice to expand our knowledge of the pathophysiology of SIV infection in African natural host species is to use the mandrill (MND; Mandrillus sphinx) model. MNDs are endemic in Gabon and, to date, are the only natural host known to be infected with two types of SIV: SIVmnd type 1 (SIVmnd-1) and SIVmnd type 2 (SIVmnd-2) (23, 59, 62). Similar to HIV type 1 (HIV-1) and HIV-2, which have different structures and different origins resulting from cross-species transmissions of SIVcpz/gor from chimpanzees and gorillas (27, 49, 66) and SIVsmm from SMs (51), respectively, the two SIVmnd types also have different origins and structures (59). SIVmnd-1 (63, 64) belongs to the SIVlhoest lineage (5) and clusters together with viruses naturally infecting guenons belonging to the l'hoesti supergroup: Cercopithecus lhoesti (SIVlhoest) (21), C. solatus (SIVsun) (5), and C. preussi (SIVpre) (69). Similar to the other viruses in this lineage, the genomic structure of SIVmnd-1 comprises only 5 accessory genes, with no vpu or vpx (64). Conversely, SIVmnd-2 is closely related to other Papionini-infecting SIVs (23, 59, 62), such as the viruses naturally infecting Mandrillus leucophaeus (SIVdrl) (9) and Cercocebus torquatus (SIVrcm) (6, 15), and is a recombinant virus (3) harboring gag, pol, vif, vpx, vpr, rev, and tat sequences that are closely related to the sequences of those genes in SIVrcm, while its env and nef sequences are closely related to the env and nef sequences of SIVmnd-1 (59).
In previous studies, we performed experimental infections of MNDs with the SIVmnd-1 (GB1) and SIVmnd-2 (MND14) reference strains and reported that both viruses show a replication pattern characterized by high peaks of viremia followed by robust levels of virus replication that are associated with transient decreases in peripheral CD4+ T cell counts during the chronic phase of infection (30, 31, 43, 60). Of note, this initial assessment involved only the major T cell populations (i.e., CD4+ and CD8+ T cells) and included a relatively short follow-up period (30, 31, 43). To expand on this earlier work, we have now performed a detailed, cross-sectional phenotypic and functional analysis of lymphocyte populations in SIV-infected and uninfected MNDs housed in the International Medical Research Center of Franceville (CIRMF) semifree colony in Gabon, the only colony of known SIVmnd-infected mandrills available worldwide. The aim of this study was to assess the long-term immunological impact of SIV infection and to investigate whether or not the phenotype of low immune activation and limited apoptosis that is associated with control of disease progression in SMs and AGMs (39, 56) is also present in MNDs. We found that, in marked contrast to pathogenic HIV and SIV infections but similar to nonprogressive SIV infections in AGMs and SMs (47, 56), SIVmnd-infected MNDs show limited immune activation and bystander apoptosis and maintain preserved immune regenerative capacity. While SIVmnd-2-infected MNDs showed significantly lower CD4+ T cell counts than SIVmnd-1-infected MNDs, correction for age and gender of the infected animals resulted in loss of statistical significance for this observed difference. Overall, these results demonstrate that the main characteristics of SIV infection in natural hosts are shared between different models and support the paradigm of a major role played by chronic immune activation in the immunopathogenesis of AIDS.
MATERIALS AND METHODS
Animals.
MNDs used in this study were housed at CIRMF, Gabon, and maintained in accordance with NIH guidelines. In uninfected animals, negative SIV PCR of plasma and negative HIV-2 serology confirmed the absence of SIV infection. The study included all 23 SIVmnd-infected MNDs (11 SIVmnd-1 infected, 5 SIVmnd-2 infected, and 7 SIVmnd-1 and SIVmnd-2 coinfected). They were naturally infected (4 SIVmnd-1 and 1 SIVmnd-2 infected) or experimentally infected. The duration of infection ranged from 1 to 21 years for naturally infected MNDs and was at least 4 years for experimentally infected MNDs. Additionally, 25 uninfected MNDs were included as a control group. On the basis of availability, this control group was matched as closely as possible with the group of SIVmnd-infected MNDs.
Plasma SIVmnd-1 and SIVmnd-2 RNA quantification.
RNA was extracted from 150 μl plasma using a QiaAmp viral RNA minikit (Qiagen, Valencia, CA) and eluted in 50 μl TE (Tris-EDTA) buffer, as recommended by the manufacturer. For lymph nodes (LNs), we used a QiaAmp RNA isolation kit (Qiagen) to extract RNA from frozen aliquots of cells. Real-time PCR assays specific for each virus type were developed for SIVmnd quantification, as described previously (31, 60). Briefly, quantification by real-time reverse transcription-PCR (RT-PCR) was performed with 5 μl extracted RNA, using a QuantiTect SYBR green RT-PCR kit (Qiagen) in capillary tubes with the LightCycler system (Roche Diagnostics). For both viral strains, quantification was based on amplification of a 230-bp fragment located in the integrase region. Specific sets of primers were designed for each viral strain. The primers used for SIVmnd-1 were M17IF (5′-AAACAGATTGTGCAAAGTTGCA-3′) and M17IR (5′-CCCATTATCTGTGTGTAGTTTACTAATA-3′). The primers used for SIVmnd-2 quantification were M26IF (5′-GCAAAGGAGATAGTAGCTCAGTGTC-3′) and M26IR (5′-GCCATTATCTGTATGTAAATGTTTCACT-3′). Primers were used at a final concentration of 1 μM, and the final MgCl2 concentration was 2.5 μM. The amplification protocol for SIVmnd-1 quantification consisted of reverse transcription (20 min at 50°C), followed by denaturation and activation of HotStart Taq DNA polymerase (15 min at 95°C) and cDNA amplification (50 cycles of denaturation for 15 s at 95°C, annealing for 15 s at 58°C, and elongation for 22 s at 72°C). For SIVmnd-2 quantification, the conditions were the same, except that the annealing temperature was 60°C. Data were analyzed using LightCycler software, version 3.5. The RNA copy number was determined by comparison with an external standard curve and was expressed as the number of RNA copies/ml. Standards consisting of non-virus-specific tailored competitor RNA complementary to sequences specific for SIVmnd-1 or SIVmnd-2 were added at both the 5′ and 3′ ends. All molecular constructs were produced by Mobidab Molekularbiologie GmbH & Co. The MO4-01 RNA standard for SIVmnd-1 was 230 bp, and the MO4-02 RNA standard for SIVmnd-2 was 233 bp. Each standard was subjected to 10-fold dilutions to generate solutions with 107 to 102 RNA copies. The detection limit of the SIVmnd quantification assays was 250 RNA copies/ml.
Lymphocyte studies and flow cytometry.
Four-color flow cytometric analysis was performed in whole-blood samples according to standard procedures using a panel of monoclonal antibodies (MAbs) that were originally designed to detect human molecules but that we and others have shown to be cross-reactive with mandrills (30, 31, 37, 41–43, 60). The antibodies (Abs) used were as follows: anti-CD4-phycoerythrin (PE) or -allophycocyanin (APC) (clone SK3), anti-CD8-APC (clone SK1), anti-CD25-PE (clone 2A3), and anti-CD28-PE (clone L293) (all from BD Biosciences); Ki-67–fluorescein isothiocyanate (FITC) (clone B56), anti-CD3-PE (clone SP34-2), anti-CD69-CyChrome (clone FN50), anti-CD95-CyChrome (clone DX2), and anti-HLA-DR-CyChrome (clone G46-6) (all from BD Pharmingen); anti-Foxp3 (clone PCH-101; eBioscience); and anti-CD127-PE (clone R34.34; Beckman Coulter). Samples assessed for Ki-67 were first surface stained with the appropriate Abs and then fixed and permeabilized using a CytoFix/CytoPerm kit (BD Pharmingen) and stained intracellularly with Ki-67. Flow cytometric acquisition and analysis of samples were performed on at least 100,000 events on a FACSCalibur flow cytometer driven by the CellQuest software package (BD Biosciences). Analysis of the acquired data was performed using FlowJo software (Tree Star).
Cytokine production.
MND peripheral blood mononuclear cells (PBMCs) were isolated using density gradient centrifugation according to standard procedures and resuspended to 1 × 106 cells/ml in complete RPMI 1640 medium (RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum, 100 U/ml penicillin G, 100 μg/ml streptomycin sulfate, and 1.7 mM sodium glutamate). Cells were then incubated for 6 h at 37°C in medium containing Staphylococcus enterotoxin B (final concentration, 1 mg/ml; Sigma-Aldrich) or phorbol myristate acetate (PMA) plus A23187 (positive controls), medium alone (negative control), or SIV peptide pools in the presence of brefeldin A (Sigma-Aldrich). The pools consisted of 15-mer peptides overlapping by 11 amino acids and spanning the entire sequence of the Gag, Pol, and Env SIVmac proteins. The numbers of peptides per pool were as follows: for Gag, 3 pools of 41 peptides; for Env, 2 pools of 54 peptides and 2 pools of 55 peptides; and for Pol, 2 pools of 52 peptides and 3 pools of 53 peptides. Following incubation, the cells were washed once in phosphate-buffered saline (PBS) containing 1% bovine serum albumin and 0.1% sodium azide and then surface stained with directly conjugated MAbs against CD3, CD4, CD8, CD28, and CD95 for 20 min in the dark at room temperature, followed by fixation and permeabilization using the CytoFix/CytoPerm kit (BD Pharmingen). After permeabilization, the cells were washed twice in the supplied kit buffer and then stained intracellularly with anti-human gamma interferon (IFN-γ)-APC (clone B27), anti-human tumor necrosis factor alpha (TNF-α)-PE (clone MAB11), and anti-interleukin-2 (IL-2)–FITC (clone MQI-17H12) MAbs (all from BD Biosciences) for 20 min in the dark at room temperature. All used MAbs were previously shown to be cross-reactive with MND molecules. Following staining, the cells were washed a final time and then fixed in PBS containing 1% paraformaldehyde. The fixed cells were stored at 4°C until the time of fluorescence-activated cells sorter analysis. In all experiments, at least 100,000 T cells were acquired.
In vitro T cell apoptosis.
The level of spontaneous and activation-induced apoptosis in peripheral blood lymphocytes was determined in freshly isolated PBMCs (baseline) and after a 48-h incubation either with no stimulus (spontaneous apoptosis) or with concanavalin A (ConA; activation-induced apoptosis). The rate of apoptotic cells was determined by multicolor flow cytometry in both CD4+ and CD8+ T cells as the percentage of cells reactive to annexin V.
Statistical analysis.
Correlation analyses within the same group of animals for two different parameters were performed using Spearman's rank correlation test with α equal to 0.05. The Mann-Whitney U test (two-tailed; α = 0.05) was used to analyze the difference between the median percentage of specific immunological markers between SIV-infected and uninfected SMs. These statistical analyses were performed using GraphPad Prism (version 4.0b) software (GraphPad Software). Where multiple tests were performed and these were significant, we employed the Bonferroni correction to conservatively use a lower level for significance. To check if any differences in CD4+ T cell counts across SIV types are still significant when controlling for the age and sex of each animal, we used general linear models. Essentially, this corresponds to performing multiple regression analyses of the change of CD4+ T cell counts across viral infections, taking into account simultaneously the age and the sex of the animals. These analyses were done using R software (Comprehensive R Archive Network; CRAN).
RESULTS
SIVmnd-infected MNDs remain healthy, despite long-term, high-level virus replication.
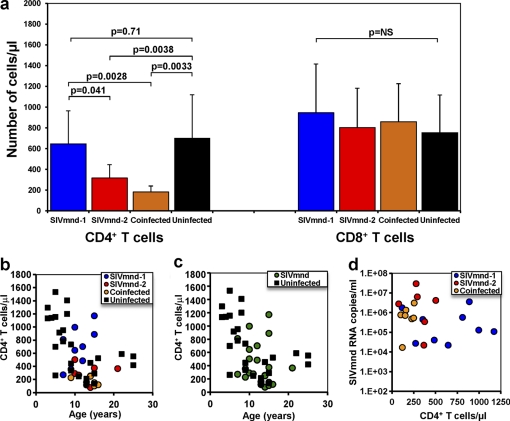
To characterize the impact of SIV infection on immune system composition and function in SIVmnd-infected MNDs, we performed a cross-sectional comparison of 23 SIV-infected and 25 uninfected MNDs. SIV-infected animals were estimated to have been infected for periods ranging from 1 to 21 years. The durations of infection ranged from 1 to 5 years for SIVmnd-1-infected animals, 3 to 21 years for SIVmnd-2-infected animals, and 4 to 5 years for SIVmnd-1 and SIVmnd-2-coinfected MNDs. All animals included in this study were healthy and free of any clinical signs of immunodeficiency, opportunistic infections, neoplasia, wasting syndromes, or neurological diseases. We measured the levels of virus replication in all infected MNDs and found no statistically significant difference between SIVmnd-1-infected (5.3078 ± 0.7947 log SIVmnd-1 RNA copies/ml), SIVmnd-2-infected (6.2062 ± 1.1043 log SIVmnd-2 RNA copies/ml), and coinfected (5.7175 ± 0.7093 log SIVmnd-1 and SIVmnd-2 RNA copies/ml) MNDs (Fig. 1). As such, this observation confirms the fact that SIV infections are by and large nonpathogenic in natural hosts, including both SIVmnd-1- and SIVmnd-2-infected mandrills, despite robust virus replication. Note, however, that viral load quantification revealed a trend toward higher levels of viral replication in SIVmnd-2-infected MNDs, which might be reflective of a higher replicative fitness of SIVmnd-2 (Fig. 1). This idea is also supported by the observation that in all SIVmnd-1 and SIVmnd-2-coinfected MNDs, SIVmnd-2 accounted for the bulk of viral replication (data not shown).
Fig. 1.
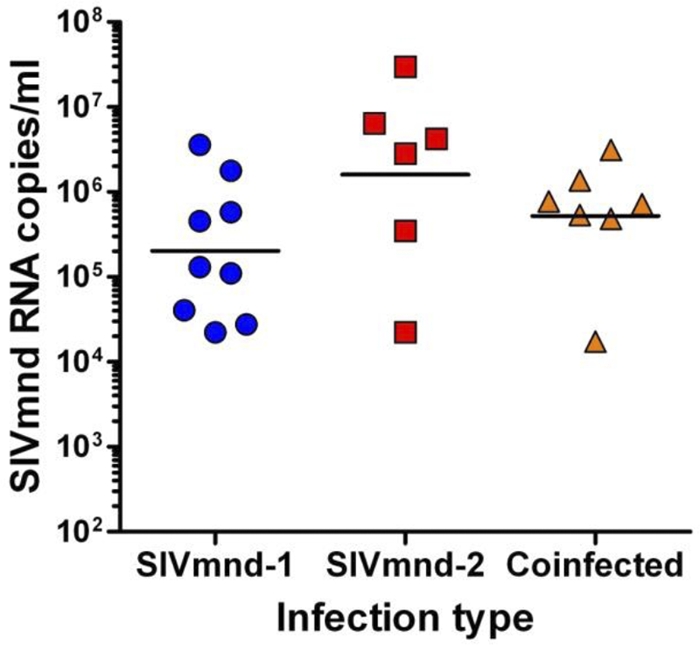
Plasma viral loads in SIVmnd-1-infected, SIVmnd-2-infected, and SIVmnd-1 and SIVmnd-2-coinfected mandrills. No significant difference was observed between the three groups. In coinfected mandrills, SIVmnd-2, the major replicative form, is responsible for the bulk of viral replication. Horizontal lines represent geometric means.
SIVmnd-2-infected MNDs showed a trend toward lower CD4+ T cell counts than SIVmnd-1-infected animals.
To assess the long-term immunological impact of SIVmnd infection, we first measured CD4+ T cell counts in both SIVmnd-infected and uninfected animals. CD4+ T cell counts were lower in SIV-infected MNDs than uninfected MNDs (418 ± 310 CD4+ T cells/μl versus 702 ± 417 CD4+ T cells/μl; P = 0.011; data not shown). Importantly, we found that CD4+ T cell counts were correlated with the infection type and that, in fact, CD4+ T cell depletion was present only in SIVmnd-2-infected MNDs (316 ± 129 CD4+ T cells/μl; P = 0.038) and in MNDs coinfected with SIVmnd-1 and SIVmnd-2 (181 ± 58 CD4+ T cells/μl; P = 0.0033) and not in MNDs infected with SIVmnd-1 only (598 ± 311 CD4+ T cells/μl), whose CD4+ T cell counts were not significantly different from those of uninfected MNDs (Fig. 2a).
Fig. 2.
CD4+ T cell counts in SIVmnd-infected mandrills. (a) Comparison of CD4+ T cell and CD8+ T cell counts (cells/μl) in SIVmnd-1-infected, SIVmnd-2-infected, SIVmnd-1 and SIVmnd-2-coinfected, and uninfected mandrills (P values were determined by the Mann-Whitney U test). Boxes, mean CD4+ T cell counts; bars, standard deviation; p = NS, the P value of the correlation coefficient was >0.05 (Spearman rank correlation test), i.e., not significant (NS). (b) Lack of correlation between CD4+ T cell counts (cells/μl) and ages of the animals (years) in the same cohort of mandrills. (c) Detailed analysis of CD4+ T cells counts in relation to the animal ages and genders for SIVmnd-1-infected, SIVmnd-2-infected, and SIVmnd-1 and SIVmnd-2-coinfected mandrills. (d) Lack of correlation between CD4+ T cell counts (cells/μl) and viral loads in the SIVmnd-infected animals.
We also noted that, in SIV-uninfected MNDs, CD4+ T cell counts are inversely correlated with the age of the animals (Fig. 2b), thus bringing up the possibility that the lower levels of CD4+ T cells of SIVmnd-2-infected MNDs are related to age rather than being a consequence of SIV infection. However, the average age was similar for SIVmnd-infected and uninfected animals (11.8 ± 2.8 years versus 11.7 ± 6.3 years) (Fig. 2b). Also, there were no significant differences in the ages of SIVmnd-1-infected (10.1 ± 3.9 years), SIVmnd-2-infected (11.8 ± 2.2 years), and coinfected (13.6 ± 2.0 years) MNDs. Next, we conducted more detailed multiple regression analyses (using general linear models) for the CD4+ T cell counts, taking into account simultaneously the age and the gender of the animals. In this analysis, the combined impact of age and gender on the CD4+ T cell counts resulted in the loss of significance for the difference in CD4+ T cell counts between SIVmnd-1- and SIVmnd-2-infected MNDs (Fig. 2c). However, a clear trend toward lower CD4+ T cell counts in mandrills infected with SIVmnd-2 than in those infected with SIVmnd-1 persisted even when data were adjusted for age and gender (Fig. 2c). Therefore, we concluded that SIVmnd infection is associated with a mild to moderate CD4+ T cell depletion that appears to be somewhat more pronounced in SIVmnd-2-infected animals.
Lack of correlation between viral replication, duration of infection, and CD4+ T cell counts in SIV-infected MNDs.
We next sought to better define whether or not, during SIVmnd infection of MNDs, there is a tendency toward significant CD4+ T cell depletion over time. In an attempt to reduce any confounding factor, we conducted our analysis without including an outlier SIVmnd-2-infected animal (21 years of infection, 76 CD4+ T cells/μl). We examined the remaining MNDs that were classified to be SIVmnd-1 infected (average infection duration, 4.7 ± 1.2 years), SIVmnd-2 infected (average infection duration, 4.2 ± 0.5 years), and SIVmnd-1 and SIVmnd-2 coinfected (average infection duration, 5.7 ± 1.4 years). We found no correlation between the duration of infection and the levels of CD4+ T cells in infected MNDs (data not shown). Furthermore, no correlation could be established between CD4+ T cell counts and the levels of viral replication (Fig. 2d). Overall, these results indicate that the duration of infection and the level of virus replication are unlikely to be key determinants of the CD4+ T cell count in natural SIV infection, a finding again in agreement with previous reports from other models (39, 61).
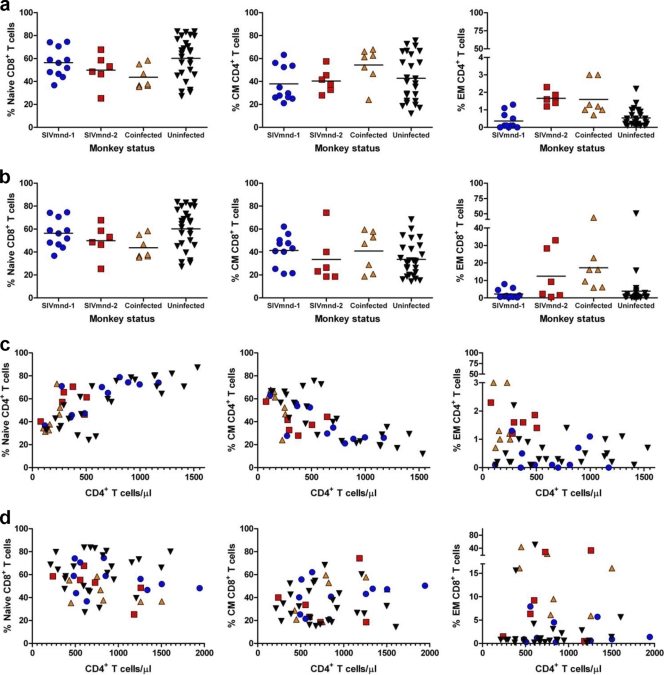
Characterization of the subsets of TN, TM, and TE CD4+ and CD8+ T cells in MNDs.
Previous studies have shown that a trend toward lower CD4+ T cell counts was associated with increased expression of markers of immune activation in both SIV-infected SMs and SIV-infected AGMs (38, 56, 61). To better define the relationship between CD4+ T cell counts and immune activation during natural SIV infection of MNDs, we sought to determine whether, in these animals, there is a correlation between the level of CD4+ T cells and the relative proportions of naive (TN), memory (TM), and effector (TE) subsets of CD4+ and CD8+ T cells. To this end, we phenotypically characterized the subsets of TN, TM, and TE CD4+ and CD8+ T cells in MNDs by adopting the same principles and techniques that have been used to define these T cell subsets in RMs (48), SMs (61), and AGMs (39). We therefore reasoned that the naive MND T cells are comprised of the CD28+ CD95− population and that the nonnaive T cells belong to either the CD28+ CD95+ (memory T cell) or the CD28− CD95+ (effector T cell) subset. While the functional characterization of these subsets in MNDs has been limited by logistical challenges, such as the inability to conduct flow cytometric experiments with more than four-color staining, we noted that the fraction of cells expressing the naive phenotype declined with age in both SIV-infected and uninfected MNDs (data not shown). As previously reported for AGMs and SMs (39, 61), the circulating CD8+ T cells include a large fraction of TE cells in MNDs, but only a small percentage (typically, <5%) of circulating CD4+ T cells displays the TE phenotype (Fig. 3a and b). A summary of the relative proportions of CD3+ CD4+ and CD3+ CD8+ TN, TM, and TE cells among lymphocytes isolated from peripheral blood of SIVmnd-infected and uninfected MNDs is shown in Fig. 3c and d. Despite a trend toward higher fractions of TE cells and lower fractions of TN cells in SIVmnd-2-infected animals, no statistically significant difference in the relative proportion of these three CD4+ T cell subsets was observed between SIV-infected and uninfected MNDs (Fig. 3a).
Fig. 3.
Assessment of CD4+ and CD8+ T cell subsets in SIV-infected and uninfected mandrills in peripheral blood. Summary of the relative proportion of CD3+ CD4+ (a) and CD3+ CD8+ (b) naive, memory (TM), and effector (TE) cells in lymphocytes isolated from peripheral blood. The correlation between the absolute number (cells/μl) of CD3+ CD4+ (c) and CD3+ CD8+ (d) naive, memory, and effector cells in SIVmnd-infected and uninfected mandrills demonstrates that in mandrills infected with SIVmnd-2 and coinfected with SIVmnd types 1 and 2 there is an expansion of effector cells. Lines in panels a and b represent average values.
The trend toward lower CD4+ T cell counts in SIVmnd-2-infected and SIVmnd-1 and SIVmnd-2-coinfected MNDs is associated with an expansion of effector-like CD8+ T cells.
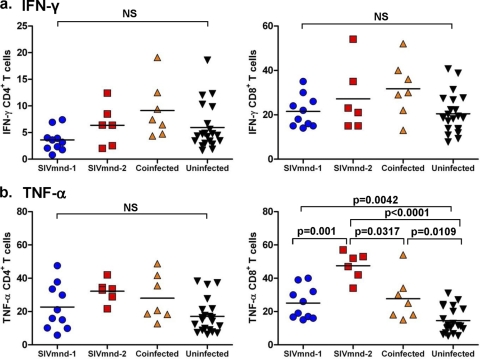
In a previous study, we showed that, during chronic HIV infection, the level of CD4+ T cell depletion is inversely correlated with an expansion of CD8+ CD127− T cells that express phenotypic and functional features of effector cells (32). We therefore sought to determine whether SIVmnd infection of MNDs (and particularly SIVmnd-2 infection) is associated with specific changes in the relative proportions of circulating CD8+ TN, TM, and TE cells. As shown in Fig. 3b and d, comparison of the percentages and absolute numbers of these three T cell subsets in our cohorts of 23 SIVmnd-infected and 25 uninfected animals revealed that SIVmnd-2-infected and SIVmnd-1 and SIVmnd-2-coinfected MNDs, but not SIVmnd-1-infected MNDs, experienced an expansion of the fraction of CD8+ TE cells. The magnitude of this expansion, however, was less prominent than that observed in HIV-infected individuals (32). We did not find a significant difference in the relative frequency of circulating TN and/or TM cells within either the CD4+ or the CD8+ T cell populations when comparing the cells from the various groups of SIV-infected and uninfected animals (Fig. 3c and d). To further investigate the effect of SIV infection on the function of CD8+ T cells of mandrills, we next measured in our cohorts of SIVmnd-1-infected, SIVmnd-2-infected, and coinfected MNDs the fraction of CD8+ T cells that produced either IFN-γ or TNF-α in response to PMA and ionomycin stimulation. Interestingly, we found a significant increase in the percentage of TNF-α-producing (but not IFN-γ-producing) CD8+ T cells in SIVmnd-2-infected mandrills (Fig. 4) (P = 0.0005 in comparison with SIVmnd-1-infected mandrills, which is significant even when we apply the Bonferroni correction for significance α of 0.0083, due to the six tests computed). Taken together, these data suggest that expansion of TNF-α-producing, effector-like CD8+ T cells is associated with lower CD4+ T cell counts in SIVmnd-2-infected MNDs.
Fig. 4.
Cytokine production by CD4+ (left) and CD8+ (right) T cells. (a) No significant difference in IFN-γ production was observed between SIVmnd-1-infected, SIVmnd-2-infected, or SIVmnd-1 and SIVmnd-2-coinfected mandrills compared to SIV-uninfected mandrills. (b) Production of TNF-α by CD4+ T cells was not significantly different between the different groups; however, significantly higher levels of TNF-α were produced by CD8+ T cells from infected mandrills than uninfected mandrills, with the highest levels being observed in SIVmnd-2-infected mandrills. Lines represent average values.
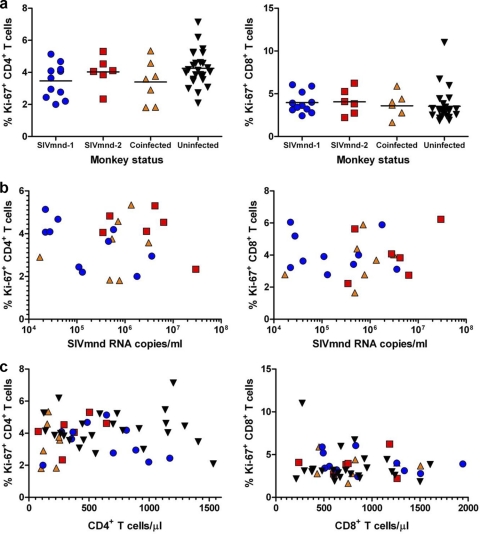
No correlation between CD4+ T cell counts and levels of T cell activation and proliferation in SIVmnd-infected MNDs.
HIV infection is associated with an increase in the fraction of T cells expressing markers of activation and proliferation (58), whose extent correlates directly with the level of viral replication and inversely with CD4+ T cell counts (50). Previous studies conducted in both AGMs and SMs indicated that the levels of T cell activation and proliferation do not significantly increase during chronic SIV infection in natural hosts (28, 39, 41, 56). In this study, we investigated whether any difference in the percentage of activated/proliferating T cells may be associated with the different impact of SIVmnd-1 and SIVmnd-2 infections on CD4+ T cell counts in MNDs. As shown in Fig. 5a, no discernible difference in the levels of CD4+ and CD8+ T cell proliferation was observed between the animals infected with SIVmnd-1, SIVmnd-2, or both SIVmnd-1 and SIVmnd-2 or between the SIVmnd-infected and uninfected MNDs. We also observed no differences in the percentages of either CD4+ or CD8+ T cells expressing the activation marker HLA-DR (data not shown), although the biological significance of this molecule in mandrills is unknown. Of note, no correlation between the fractions of Ki-67-positive (Ki-67+) T cells and the levels of viral replication (Fig. 5b) or the absolute CD4+ or CD8+ T cell counts (Fig. 5c) was observed.
Fig. 5.
Assessment of T cell proliferation (Ki-67) in SIVmnd-infected mandrills. No significant increase of proliferating CD4+ (left) and CD8+ (right) T cells was observed in SIVmnd-1-infected, SIVmnd-2-infected, or SIVmnd-1 and SIVmnd-2-coinfected mandrills compared to SIV-uninfected mandrills (a). Levels of T cell proliferation did not correlate with either viral loads (b) or CD4+ (left) or CD8+ (right) T cell counts (c). Lines in panel a represent average values.
Collectively, these data confirmed the results of studies conducted in SMs and AGMs reporting that the prevailing levels of CD4+ T cell proliferation do not seem to be a direct reflection of a higher viral antigenic load or CD4+ T cell counts (35, 47, 55).
SIVmnd infection of MNDs is not associated with increased T cell apoptosis.
Excessive bystander apoptosis of uninfected T cells is manifest in pathogenic HIV and SIV infections and is thought to be a major contributor to the progressive T cell depletion observed in AIDS (24). Such increased levels of lymphocyte apoptosis are related to the HIV-associated hyperimmune activation and may favor disease progression by accelerating the loss of CD4+ T cells and impairing T cell regeneration (24). Of note, several previous studies reported no significant increases in the levels of CD4+ T cell apoptosis during the chronic phase of natural SIV infections of AGMs and SMs, in stark contrast to the findings for RMs, in which a dramatic increase in CD4+ T cell apoptosis occurs during chronic pathogenic SIV infection (11, 39, 56). To investigate whether SIVmnd infection of MNDs results in increased levels of T cell apoptosis, we studied the expression of the apoptosis-related marker annexin V in PBMCs and LNs from SIV-infected and uninfected animals. No significant increases in the levels of baseline annexin V expression were observed in either SIVmnd-1-infected, SIVmnd-2-infected, or SIVmnd-1 and SIVmnd-2-coinfected MNDs compared to uninfected MND (data not shown). Moreover, there were no significant differences in annexin V expression between MNDs infected with SIVmnd-1, MNDs infected with SIVmnd-2, and MNDs coinfected with the two viruses. We next measured the level of spontaneous and activation-induced apoptosis in ex vivo cultures of PBMCs obtained from SIVmnd-infected and uninfected MNDs. The frequency of apoptotic CD4+ and CD8+ T cells was determined by enumeration of cells reactive with annexin V and/or 7-amino- actinomycin D (7-AAD) in freshly isolated PBMCs and in PBMCs following a 48-h incubation either with no stimulus (spontaneous) or with ConA stimulation (Fig. 6). No differences in the levels of baseline, spontaneous, or activation-induced lymphocyte apoptosis in either CD4+ or CD8+ T cells were observed between SIV-infected and uninfected MNDs or between SIVmnd-1-infected, SIVmnd-2-infected, and SIVmnd-1 and SIVmnd-2-coinfected MNDs (Fig. 6). Overall, these data are consistent with previously generated data in SMs and AGMs (11, 39, 56) and confirm that SIV infections of natural hosts are associated with low levels of apoptosis in the chronic phase of infection.
Fig. 6.
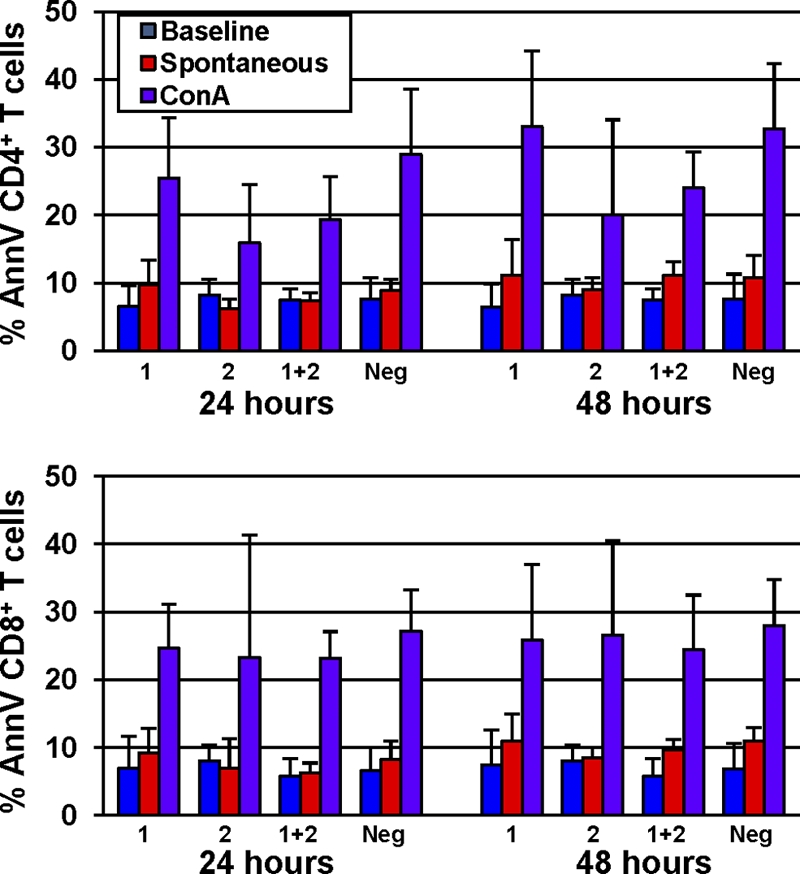
Assessment of spontaneous and activation-induced in vitro T cell apoptosis in SIV-infected and uninfected mandrills using the staining assay for 7-AAD and annexin V (AnnV). Apoptosis was measured at baseline and after 24 and 48 h in the presence or absence of ConA. Similarly to what was observed in SMs and AGMs, SIV-infected MNDs reveal levels of T cell apoptosis comparable to those of uninfected animals. Low levels of baseline and spontaneous (after 48 h without any stimulus) T cell apoptosis were observed in both SIV-infected and uninfected mandrills. No statistically significant differences in the rates of baseline, spontaneous, and activation-induced (following 48 h of ConA stimulation) apoptosis, measured as rates of annexin V-positive cells, in either CD4+ or CD8+ T cells were observed between SIV-infected and uninfected MNDs. Boxes, mean for annexin V-positive cells; bars, standard deviation; 1, SIVmnd-1; 2, SIVmnd-2; 1 + 2, coinfection; Neg, SIVmnd negative.
DISCUSSION
Over the past decade there has been increasing attention to the field of SIV infection in natural host species, such as SMs and AGMs. The most intriguing feature of these natural infections is the general lack of disease progression, despite levels of viremia that are as high as or even higher than those observed in HIV-infected humans and normal-progressor SIVmac-infected RMs. Defining the mechanisms underlying the control of progression to AIDS in natural hosts is a key challenge for current AIDS research, and it is believed that a better understanding of these mechanisms may reveal important molecular and cellular determinants of CD4+ T cell depletion and disease progression that define pathogenic HIV/SIV infections, thus paving the way for novel approaches to prevent and treat HIV infection in humans. Key features of SIV infection in SMs and AGMs include (i) acute infection characterized by a vigorous but transient inflammatory response to the virus, followed by low levels of immune activation throughout the chronic phase of infection (7, 28, 39); (ii) preservation of healthy levels of circulating and lymph node-based CD4+ T cells in ∼80 to 90% of animals (30, 31, 36, 40, 41, 54); (iii) significant early depletion of mucosal CD4+ T cells but relative preservation of the Th17 subset (12, 19, 39); (iv) stable or recovering levels of mucosal CD4+ T cells and absence of microbial translocation during chronic infection (19, 39); and (v) chronic infection characterized by a short in vivo life span of productively infected cells (18, 44). The observation that adaptive immune responses to SIV only partially control virus replication in SIV-infected natural hosts (13, 14, 52, 71) indicates that these animals avoid immunodeficiency by mechanisms that are ostensibly different from those described in HIV-infected long-term nonprogressors/elite controllers (LTNP/EC), in whom virus replication is extremely low (35, 47).
In this work, we focused on a relatively understudied natural host species, the mandrill (MND), and conducted a clinical, virological, and immunological cross-sectional survey of all the 23 naturally and experimentally SIVmnd-infected MNDs, as well as 25 uninfected MNDs, housed at CIRMF, Gabon, which is home to the only colony of captive MNDs in the world. Previous studies of this colony allowed (i) the discovery of the two SIVmnd viruses (59, 63, 64), which makes MNDs the only natural SIV host species known to harbor two different viruses, and (ii) the development of a mandrill model for AIDS research (30, 31, 43, 60). This study confirmed several findings from previous experiments conducted in smaller groups of animals and with limited follow-up postinfection (i.e., absence of disease progression, high levels of viremia, a mild to moderate CD4+ T cell depletion, and generally low levels of immune activation) (30, 31, 41, 43). Of note, these features are also present in natural SIV infections of SMs and AGMs (39, 56), thus suggesting that the mechanisms underlying the host-virus adaptation that results in the control of disease progression in natural hosts are, at least in part, shared between the different species. In particular, the observation that chronically SIV-infected MNDs display limited immune activation provides further evidence supporting the hypothesis that this phenomenon plays a central role in maintaining the AIDS-free status of natural SIV hosts.
Interestingly, when we focused our attention on the impact of SIV infection on CD4+ T cell populations of MNDs, the current study revealed a trend toward a more severe CD4+ T cell depletion in SIVmnd-2-infected (and SIVmnd-1 and SIVmnd-2-coinfected) mandrills. This difference in CD4+ T cell counts was statistically different when this marker was examined in a univariate statistical analysis and when age alone was added in the multivariate analysis. The fact that infection with SIVmnd-1 and SIVmnd-2 viruses was not gender matched is likely responsible for the lack of significance of these results when gender is also considered in the statistical analysis. As such, we could not unequivocally conclude that SIVmnd-2 infection is more pathogenic than SIVmnd-1 infection in MNDs on the basis of the results for this relatively small group of non-gender-matched infected animals, and further studies would be needed to reach that conclusion. However, the differences observed in Fig. 2 are, in fact, consistent, in our view, with the possibility that SIVmnd-2 infection has, on average, a more dramatic impact on CD4+ T cell counts than other SIV infections of natural hosts. In our previous studies, we did not observe this phenomenon, probably because of a relatively short follow-up, which might have masked this immunological effect of SIVmnd-2 infection (30, 31, 43). Of note, the trend toward lower CD4+ T cell counts in SIVmnd-2-infected MNDs was not associated with increased morbidity or mortality. Altogether, these observations underscore the possibility that, while natural SIV infections are typically nonpathogenic, in some cases they may slowly progress toward a significant CD4+ T cell depletion and immunodeficiency, as observed in a few African nonhuman primates infected with SIV (reviewed in reference 45).
Another novel observation is that, in SIVmnd-infected MNDs, there is no correlation between CD4+ T cell counts and the levels of SIV replication. Similar to SIV-infected SMs and AGMs and in contrast to HIV-infected humans (50), SIV-infected MNDs also show no inverse correlation between CD4+ T cell counts and the level of CD4+ T cell proliferation. This finding suggests that the prevailing level of CD4+ T cell depletion and/or proliferation may have a different biological significance in natural hosts than in HIV-infected individuals. In this view, the level of CD4+ T cell proliferation would mainly reflect, in humans, the level of immune activation in response to viral replication (20), while in natural SIV hosts, this parameter may reflect homeostatic mechanisms aimed at compensating for virus-induced cell loss (34). This interpretation is also supported by previous studies reporting that, in SMs and AGMs, the suppression of viral replication with antiretroviral therapy is not immediately followed by a decline in the fraction of Ki-67+ T cells (18, 44), as reported in HIV-infected patients (20).
In addition to the potential effect of gender, the mechanisms that may be responsible for the observed differences in CD4+ T cell counts after SIVmnd-2 and SIVmnd-1 infections of MNDs remain unclear, as the nature of this study was per force retrospective and descriptive for logistical reasons. The fact that SIVmnd-2-infected animals show also a trend toward higher viremia is consistent with the possibility that SIVmnd-2 has increased replicative fitness for MND cells than SIVmnd-1. Interestingly, SIVmnd-2 was always the prevalent circulating virus in SIVmnd-1 and SIVmnd-2-coinfected MNDs (data not shown). It should be noted, however, that no correlation between viral loads and CD4+ T cell counts was found in SIVmnd-2-infected MNDs. Another possibility is that SIVmnd-2 is intrinsically able to elicit higher levels of immune activation. While this hypothesis is possibly corroborated by the observation that SIVmnd-2-infected animals show some signs of increased CD8+ T cell activation (i.e., increased levels of CD28− CD95+ TE and TNF-α-producing cells), no consistent pattern of generalized immune activation and higher apoptosis was present in these MNDs. Finally, the observation that, in two different hosts, MNDs and RMs, the outcomes of infection with the two types of SIVmnd are opposite, with SIVmnd-2 possibly being more pathogenic than SIVmnd-1 in mandrills and SIVmnd-1 being more pathogenic than SIVmnd-2 in RMs (60), demonstrates that the pathogenicity of SIVs in different hosts is unpredictable and can be correctly assessed only in animal studies.
In conclusion, this study provides the first detailed virological and immunological analysis of long-term SIVmnd infection in MNDs. This analysis showed that SIVmnd-infected animals share a number of features with SIV-infected AGMs and SMs and delineated some potential differences in the impacts of SIVmnd-1 and SIVmnd-2 infection on the host immune function.
ACKNOWLEDGMENTS
We thank the staff of the CIRMF Primate Center.
This work was funded in part by the Centre International de Recherches Medicales de Franceville (CIRMF), Gabon. CIRMF is supported by the government of Gabon, Total-Elf, Gabon, and the Ministere de la Cooperation Francaise. C.A., I.P., and G.S. are supported by grants RO1 AI065325 (C.A.), RO1 AI064066 (I.P.), and RO1 AI066998 (G.S.) from the National Institutes of Health.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Published ahead of print on 28 September 2011.
REFERENCES
- 1. Apetrei C., et al. 2010. Pattern of SIVagm infection in patas monkeys suggests that host adaptation to simian immunodeficiency virus infection may result in resistance to infection and virus extinction..J. Infect. Dis. 202(Suppl. 3):S371–S376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Apetrei C., et al. 2004. Direct inoculation of simian immunodeficiency virus from sooty mangabeys in black mangabeys (Lophocebus aterrimus): first evidence of AIDS in a heterologous African species and different pathologic outcomes of experimental infection. J. Virol. 78:11506–11518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bailes E., et al. 2003. Hybrid origin of SIV in chimpanzees. Science 300:1713. [DOI] [PubMed] [Google Scholar]
- 4. Beaumier C. M., et al. 2009. CD4 downregulation by memory CD4+ T cells in vivo renders African green monkeys resistant to progressive SIVagm infection. Nat. Med. 15:879–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Beer B. E., et al. 1999. Simian immunodeficiency virus (SIV) from sun-tailed monkeys (Cercopithecus solatus): evidence for host-dependent evolution of SIV within the C. lhoesti superspecies. J. Virol. 73:7734–7744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Beer B. E., et al. 2001. Characterization of novel simian immunodeficiency viruses from red-capped mangabeys from Nigeria (SIVrcmNG409 and -NG411). J. Virol. 75:12014–12027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bosinger S. E., et al. 2009. Global genomic analysis reveals rapid control of a robust innate response in SIV-infected sooty mangabeys.J. Clin. Invest. 119:3556–3572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brenchley J. M., Silvestri G., Douek D. C. 2010. Nonprogressive and progressive primate immunodeficiency lentivirus infections. Immunity 32:737–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Clewley J. P., Lewis J. C., Brown D. W., Gadsby E. L. 1998. A novel simian immunodeficiency virus (SIVdrl) pol sequence from the drill monkey, Mandrillus leucophaeus. J. Virol. 72:10305–10309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dunham R., et al. 2006. The AIDS-resistance of naturally SIV-infected sooty mangabeys is independent of cellular immunity to the virus. Blood 108:209–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Estaquier J., et al. 1994. Programmed cell death and AIDS: significance of T-cell apoptosis in pathogenic and nonpathogenic primate lentiviral infections. Proc. Natl. Acad. Sci. U. S. A. 91:9431–9435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Favre D., et al. 2009. Critical loss of the balance between Th17 and T regulatory cell populations in pathogenic SIV infection. PLoS Pathog. 5:e1000295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gaufin T., et al. 2009. Effect of B cell depletion on viral replication and clinical outcome of SIV infection in a natural host. J. Virol. 83:10347–10357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gaufin T., et al. 2010. Experimental depletion of CD8+ cells in acutely SIVagm-infected African green monkeys results in increased viral replication. Retrovirology 7:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Georges-Courbot M. C., et al. 1998. Natural infection of a household pet red-capped mangabey (Cercocebus torquatus torquatus) with a new simian immunodeficiency virus. J. Virol. 72:600–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Goldstein S., et al. 2006. Comparison of simian immunodeficiency virus SIVagmVer replication and CD4+ T cell dynamics in vervet and sabaeus African green monkeys. J. Virol. 80:4868–4877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Goldstein S., et al. 2005. Plateau levels of viremia correlate with the degree of CD4+ T cell loss in simian immunodeficiency virus SIVagm-infected pigtailed macaques: variable pathogenicity of natural SIVagm isolates. J. Virol. 79:5153–5162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gordon S., et al. 2008. Short-lived infected cells support the bulk of virus replication in naturally SIV-infected sooty mangabeys: implications for AIDS pathogenesis. J. Virol. 82:3725–3735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gordon S. N., et al. 2007. Severe depletion of mucosal CD4+ T cells in AIDS-free simian immunodeficiency virus-infected sooty mangabeys. J. Immunol. 179:3026–3034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hazenberg M. D., et al. 2000. T cell division in human immunodeficiency virus (HIV)-1 infection is mainly due to immune activation: a longitudinal analysis in patients before and during highly active antiretroviral therapy (HAART). Blood 95:249–255 [PubMed] [Google Scholar]
- 21. Hirsch V. M., et al. 1999. Characterization of a novel simian immunodeficiency virus (SIV) from L'Hoest monkeys (Cercopithecus l'hoesti): implications for the origins of SIVmnd and other primate lentiviruses. J. Virol. 73:1036–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hirsch V. M., et al. 1995. Induction of AIDS by simian immunodeficiency virus from an African green monkey: species-specific variation in pathogenicity correlates with the extent of in vivo replication. J. Virol. 69:955–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hu J., et al. 2003. Characterization and comparison of recombinant simian immunodeficiency virus from drill (Mandrillus leucophaeus) and mandrill (Mandrillus sphinx) isolates. J. Virol. 77:4867–4880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hurtrel B., et al. 2005. Apoptosis in SIV infection. Cell Death Differ. 12(Suppl. 1):979–990 [DOI] [PubMed] [Google Scholar]
- 25. Kaur A., et al. 2001. Emergence of cytotoxic T lymphocyte escape mutations in nonpathogenic simian immunodeficiency virus infection. Eur. J. Immunol. 31:3207–3217 [DOI] [PubMed] [Google Scholar]
- 26. Kaur A., et al. 2000. Identification of multiple simian immunodeficiency virus (SIV)-specific CTL epitopes in sooty mangabeys with natural and experimentally acquired SIV infection. J. Immunol. 164:934–943 [DOI] [PubMed] [Google Scholar]
- 27. Keele B. F., et al. 2006. Chimpanzee reservoirs of pandemic and nonpandemic HIV-1. Science 313:523–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kornfeld C., et al. 2005. Antiinflammatory profiles during primary SIV infection in African green monkeys are associated with protection against AIDS.J. Clin. Invest. 115:1082–1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McClure H. M., et al. 1989. Spectrum of disease in macaque monkeys chronically infected with SIV/SMM. Vet. Immunol. Immunopathol. 21:13–24 [DOI] [PubMed] [Google Scholar]
- 30. Onanga R., et al. 2002. High levels of viral replication contrast with only transient changes in CD4+ and CD8+ cell numbers during the early phase of experimental infection with simian immunodeficiency virus SIVmnd-1 in Mandrillus sphinx. J. Virol. 76:10256–10263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Onanga R., et al. 2006. Primary simian immunodeficiency virus SIVmnd-2 infection in mandrills (Mandrillus sphinx). J. Virol. 80:3303–3309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Paiardini M., et al. 2005. Loss of CD127 expression defines an expansion of effector CD8+ T cells in HIV-infected individuals. J. Immunol. 174:2900–2909 [DOI] [PubMed] [Google Scholar]
- 33. Paiardini M., et al. 2011. Low levels of SIV infection in sooty mangabey central memory CD4+ T cells are associated with limited CCR5 expression. Nat. Med. 17:830–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Paiardini M., et al. 2009. T cell phenotypic and functional changes associated with social subordination and gene polymorphisms in the serotonin reuptake transporter in female rhesus monkeys. Brain Behav. Immun. 23:286–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pandrea I., Apetrei C. 2010. Where the wild things are: pathogenesis of SIV infection in African nonhuman primate hosts. Curr. HIV/AIDS Rep. 7:28–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pandrea I., et al. 2006. Simian immunodeficiency virus (SIV) SIVagm.sab infection of Caribbean African green monkeys: new model of the study of SIV pathogenesis in natural hosts.J. Virol. 80:4858–4867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pandrea I., et al. 2007. Paucity of CD4+CCR5+ T cells is a typical feature of natural SIV hosts. Blood 109:1069–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pandrea I., et al. 2008. Experimentally-induced immune activation in natural hosts of SIV induces significant increases in viral replication and CD4+ T cell depletion. J. Immunol. 181:6687–6691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pandrea I., et al. 2007. Acute loss of intestinal CD4+ T cells is not predictive of SIV virulence. J. Immunol. 179:3035–3046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pandrea I., et al. 2005. Impact of viral factors on very early in vivo replication profiles in SIVagm-infected African green monkeys. J. Virol. 79:6249–6259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pandrea I., et al. 2003. High levels of SIVmnd-1 replication in chronically infected Mandrillus sphinx. Virology 317:119–127 [DOI] [PubMed] [Google Scholar]
- 42. Pandrea I., et al. 2001. Chronic SIV infection ultimately causes immunodeficiency in African non-human primates. AIDS 15:2461–2462 [DOI] [PubMed] [Google Scholar]
- 43. Pandrea I., et al. 2008. Paucity of CD4+CCR5+ T cells may prevent breastfeeding transmission of SIV in natural non-human primate hosts.J. Virol. 82:5501–5509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pandrea I., et al. 2008. Simian immunodeficiency virus SIVagm dynamics in African green monkeys. J. Virol. 82:3713–3724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pandrea I., Silvestri G., Apetrei C. 2009. AIDS in African nonhuman primate hosts of SIVs: a new paradigm of SIV infection. Curr. HIV Res. 6:57–72 [DOI] [PubMed] [Google Scholar]
- 46. Pandrea I., et al. 2006. Simian immunodeficiency viruses replication dynamics in African non-human primate hosts: common patterns and species-specific differences. J. Med. Primatol. 35:194–201 [DOI] [PubMed] [Google Scholar]
- 47. Pandrea I., Sodora D. L., Silvestri G., Apetrei C. 2008. Into the wild: simian immunodeficiency virus (SIV) infection in natural hosts. Trends Immunol. 29:419–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pitcher C. J., et al. 2002. Development and homeostasis of T cell memory in rhesus macaque. J. Immunol. 168:29–43 [DOI] [PubMed] [Google Scholar]
- 49. Plantier J. C., et al. 2009. A new human immunodeficiency virus derived from gorillas. Nat. Med. 15:871–872 [DOI] [PubMed] [Google Scholar]
- 50. Sachsenberg N., et al. 1998. Turnover of CD4+ and CD8+ T lymphocytes in HIV-1 infection as measured by Ki-67 antigen. J. Exp. Med. 187:1295–1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Santiago M. L., et al. 2005. Simian immunodeficiency virus infection in free-ranging sooty mangabeys (Cercocebus atys atys) from the Tai Forest, Cote d'Ivoire: implications for the origin of epidemic human immunodeficiency virus type 2. J. Virol. 79:12515–12527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Schmitz J. E., et al. 2009. Inhibition of adaptive immune responses leads to a fatal clinical outcome in SIV-infected pigtailed macaques but not vervet African green monkeys. PLoS Pathog. 5:e1000691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Silvestri G. 2005. Naturally SIV-infected sooty mangabeys: are we closer to understanding why they do not develop AIDS? J. Med. Primatol. 34:243–252 [DOI] [PubMed] [Google Scholar]
- 54. Silvestri G., et al. 2005. Divergent host responses during primary simian immunodeficiency virus SIVsm infection of natural sooty mangabey and nonnatural rhesus macaque hosts. J. Virol. 79:4043–4054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Silvestri G., Paiardini M., Pandrea I., Lederman M. M., Sodora D. L. 2007. Understanding the benign nature of SIV infection in natural hosts.J. Clin. Invest. 117:3148–3154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Silvestri G., et al. 2003. Nonpathogenic SIV infection of sooty mangabeys is characterized by limited bystander immunopathology despite chronic high-level viremia. Immunity 18:441–452 [DOI] [PubMed] [Google Scholar]
- 57. Sodora D. L., et al. 2009. Towards an AIDS vaccine: lessons from natural SIV infections of African nonhuman primate hosts. Nat. Med. 15:861–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sodora D. L., Silvestri G. 2008. Immune activation and AIDS pathogenesis. AIDS 22:439–446 [DOI] [PubMed] [Google Scholar]
- 59. Souquiere S., et al. 2001. Wild Mandrillus sphinx are carriers of two types of lentivirus. J. Virol. 75:7086–7096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Souquière S., et al. 2009. SIVmnd-1 and SIVmnd-2 have different pathogenic potentials in Rhesus macaques upon experimental cross-species transmission. J. Gen. Virol. 90:488–499 [DOI] [PubMed] [Google Scholar]
- 61. Sumpter B., et al. 2007. Correlates of preserved CD4+ T cell homeostasis during natural, nonpathogenic simian immunodeficiency virus infection of sooty mangabeys: implications for AIDS pathogenesis. J. Immunol. 178:1680–1691 [DOI] [PubMed] [Google Scholar]
- 62. Takehisa J., et al. 2001. Natural infection of wild-born mandrills (Mandrillus sphinx) with two different types of simian immunodeficiency virus. AIDS Res. Hum. Retroviruses 17:1143–1154 [DOI] [PubMed] [Google Scholar]
- 63. Tsujimoto H., et al. 1988. Isolation and characterization of simian immunodeficiency virus from mandrills in Africa and its relationship to other human and simian immunodeficiency viruses. J. Virol. 62:4044–4050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Tsujimoto H., et al. 1989. Sequence of a novel simian immunodeficiency virus from a wild-caught African mandrill. Nature 341:539–541 [DOI] [PubMed] [Google Scholar]
- 65. VandeWoude S., Apetrei C. 2006. Going wild: lessons from T-lymphotropic naturally occurring lentiviruses. Clin. Microbiol. Rev. 19:728–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Van Heuverswyn F., et al. 2006. Human immunodeficiency viruses: SIV infection in wild gorillas. Nature 444:164. [DOI] [PubMed] [Google Scholar]
- 67. Vinton C., et al. 2011. CD4-like immunological function by CD4− T cells in multiple natural hosts of simian immunodeficiency virus. J. Virol. 85:8702–8708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wang Z., Metcalf B., Ribeiro R. M., McClure H., Kaur A. 2006. Th-1-type cytotoxic CD8+ T lymphocyte responses to simian immunodeficiency virus (SIV) are a consistent feature of natural SIV infection in sooty mangabeys. J. Virol. 80:2771–2783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Worobey M., et al. 2010. Island biogeography reveals the deep history of SIV. Science 330:1487. [DOI] [PubMed] [Google Scholar]
- 70. Zahn R. C., et al. 2008. Simian immunodeficiency virus (SIV)-specific CD8+ T cell responses in chronically SIVagm-infected vervet African green monkeys. J. Virol. 82:11577–11588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zahn R. C., et al. 2010. Suppression of adaptive immune responses during primary SIV infection of sabaeus African green monkeys delays partial containment of viremia but does not induce disease. Blood 115:3070–3078 [DOI] [PMC free article] [PubMed] [Google Scholar]