Abstract
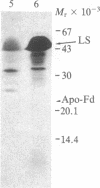
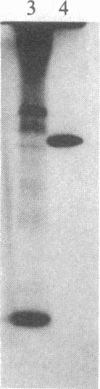
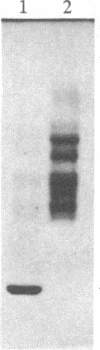
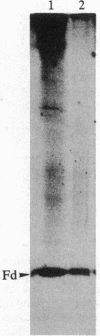
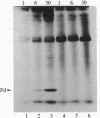
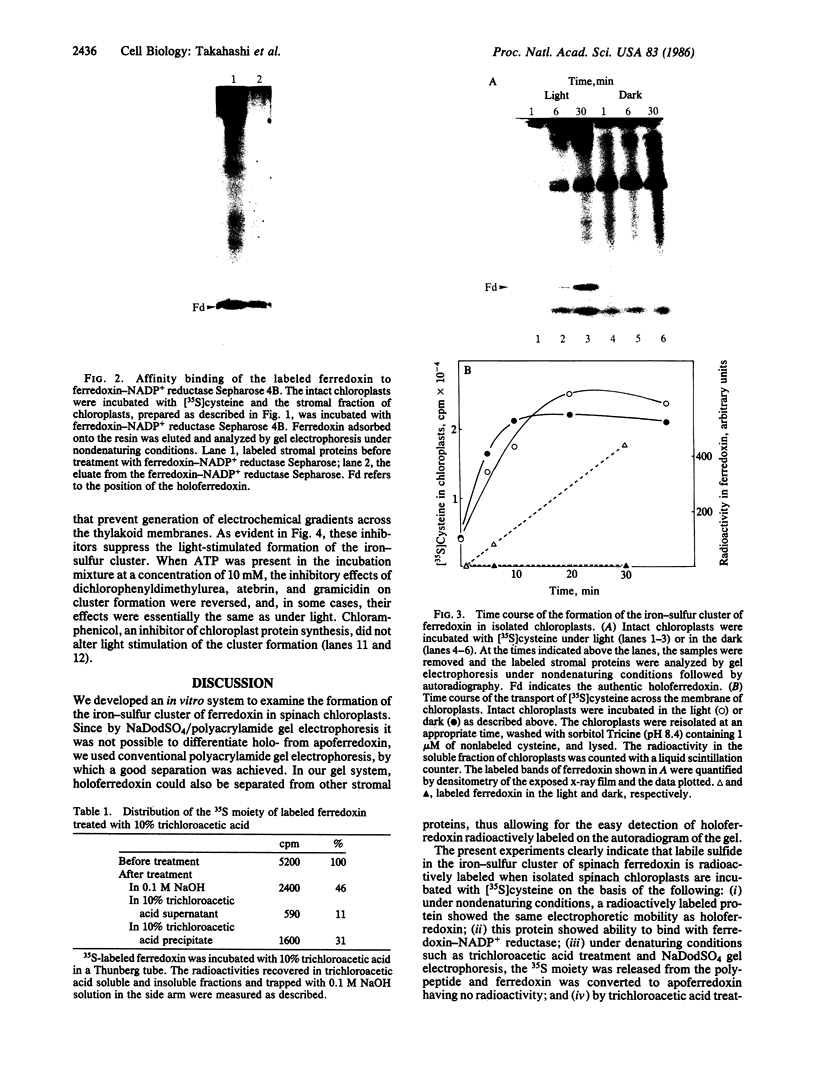
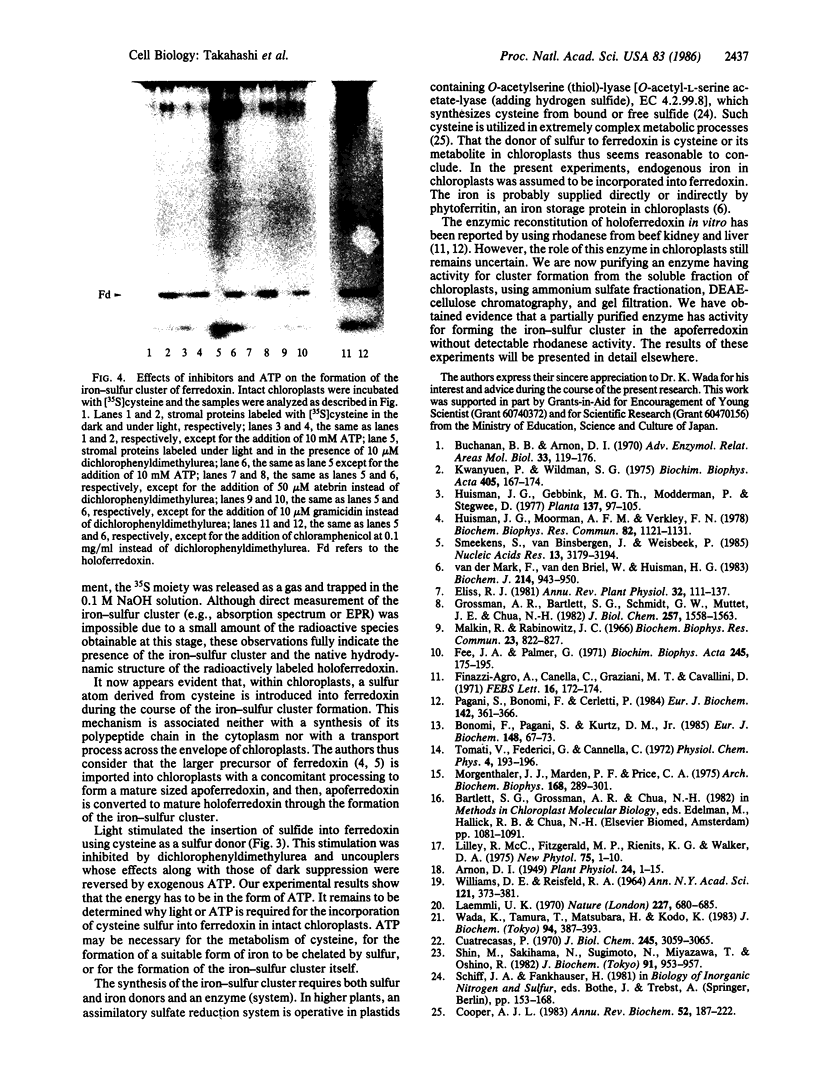
The formation of the iron-sulfur cluster of ferredoxin was examined in vitro by incubating isolated chloroplasts with [35S]cysteine. The ferredoxin molecule was radioactively labeled in chloroplasts without synthesis of its polypeptide and comigrated with holoferredoxin during polyacrylamide gel electrophoresis under nondenaturing conditions. When the labeled ferredoxin was denatured by the addition of trichloroacetic acid, radioactive acid-labile sulfide in the cluster was released from the polypeptide as a gas and trapped in a 0.1 M NaOH solution. These results indicate that the sulfur atom derived from cysteine was incorporated into ferredoxin through formation of the iron-sulfur cluster. This process was stimulated by light and inhibited by the electron transport inhibitor, dichlorophenyldimethylurea, and the uncouplers, atebrin and gramicidin, but not by the protein synthesis inhibitor, chloramphenicol. These inhibitory effects were reversed by the addition of ATP to the incubation mixture. Formation of the iron-sulfur cluster of ferredoxin in chloroplasts is thus dependent on ATP.
Keywords: cysteine, sulfide, iron-sulfur protein, light, ATP
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agrò A. F., Cannella C., Graziani M. T., Cavallini D. A possible role for rhodanese: The formation of 'labile' sulfur from thiosulfate. FEBS Lett. 1971 Aug 15;16(3):172–174. doi: 10.1016/0014-5793(71)80124-2. [DOI] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonomi F., Pagani S., Kurtz D. M., Jr Enzymic synthesis of the 4Fe-4S clusters of Clostridium pasteurianum ferredoxin. Eur J Biochem. 1985 Apr 1;148(1):67–73. doi: 10.1111/j.1432-1033.1985.tb08808.x. [DOI] [PubMed] [Google Scholar]
- Buchanan B. B., Arnon D. I. Ferredoxins: chemistry and function in photosynthesis, nitrogen fixation, and fermentative metabolism. Adv Enzymol Relat Areas Mol Biol. 1970;33:119–176. doi: 10.1002/9780470122785.ch3. [DOI] [PubMed] [Google Scholar]
- Cooper A. J. Biochemistry of sulfur-containing amino acids. Annu Rev Biochem. 1983;52:187–222. doi: 10.1146/annurev.bi.52.070183.001155. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Protein purification by affinity chromatography. Derivatizations of agarose and polyacrylamide beads. J Biol Chem. 1970 Jun;245(12):3059–3065. [PubMed] [Google Scholar]
- Fee J. A., Palmer G. The properties of parsley ferredoxin and its selenium-containing homolog. Biochim Biophys Acta. 1971 Aug 6;245(1):175–195. doi: 10.1016/0005-2728(71)90020-x. [DOI] [PubMed] [Google Scholar]
- Grossman A. R., Bartlett S. G., Schmidt G. W., Mullet J. E., Chua N. H. Optimal conditions for post-translational uptake of proteins by isolated chloroplasts. In vitro synthesis and transport of plastocyanin, ferredoxin-NADP+ oxidoreductase, and fructose-1,6-bisphosphatase. J Biol Chem. 1982 Feb 10;257(3):1558–1563. [PubMed] [Google Scholar]
- Huisman J. G., Moorman A. F., Verkley F. N. In vitro synthesis of chloroplast ferredoxin as a high molecular weight precursor in a cell-free protein synthesizing system from wheat germs. Biochem Biophys Res Commun. 1978 Jun 29;82(4):1121–1131. doi: 10.1016/0006-291x(78)90303-0. [DOI] [PubMed] [Google Scholar]
- Kwanyuen P., Wildman S. G. Nuclear DNA codes for Nicotiana ferredoxin. Biochim Biophys Acta. 1975 Sep 9;405(1):167–174. doi: 10.1016/0005-2795(75)90327-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Malkin R., Rabinowitz J. C. The reconstitution of clostridial ferredoxin. Biochem Biophys Res Commun. 1966 Jun 21;23(6):822–827. doi: 10.1016/0006-291x(66)90561-4. [DOI] [PubMed] [Google Scholar]
- Morgenthaler J. J., Marsden M. P., Price C. A. Factors affecting the separation of photosynthetically competent chloroplasts in gradients of silica sols. Arch Biochem Biophys. 1975 May;168(1):289–301. doi: 10.1016/0003-9861(75)90253-2. [DOI] [PubMed] [Google Scholar]
- Pagani S., Bonomi F., Cerletti P. Enzymic synthesis of the iron-sulfur cluster of spinach ferredoxin. Eur J Biochem. 1984 Jul 16;142(2):361–366. doi: 10.1111/j.1432-1033.1984.tb08295.x. [DOI] [PubMed] [Google Scholar]
- Shin M., Sakihama N., Sugimoto N., Miyazawa T., Oshino R. Immobilized ferredoxin-NADP+ reductase: preparation and properties. J Biochem. 1982 Mar;91(3):953–957. doi: 10.1093/oxfordjournals.jbchem.a133785. [DOI] [PubMed] [Google Scholar]
- Smeekens S., van Binsbergen J., Weisbeek P. The plant ferredoxin precursor: nucleotide sequence of a full length cDNA clone. Nucleic Acids Res. 1985 May 10;13(9):3179–3194. doi: 10.1093/nar/13.9.3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomati U., Federici G., Cannella C. Rhodanese activity in chloroplasts. Physiol Chem Phys. 1972;4(2):193–196. [PubMed] [Google Scholar]
- WILLIAMS D. E., REISFELD R. A. DISC ELECTROPHORESIS IN POLYACRYLAMIDE GELS: EXTENSION TO NEW CONDITIONS OF PH AND BUFFER. Ann N Y Acad Sci. 1964 Dec 28;121:373–381. doi: 10.1111/j.1749-6632.1964.tb14210.x. [DOI] [PubMed] [Google Scholar]
- Wada K., Tamura T., Matsubara H., Kodo K. Spirulina ferredoxin-NADP+ reductase. Further characterization with an improved preparation. J Biochem. 1983 Aug;94(2):387–393. doi: 10.1093/oxfordjournals.jbchem.a134367. [DOI] [PubMed] [Google Scholar]
- van der Mark F., van den Briel W., Huisman H. G. Phytoferritin is synthesized in vitro as a high-molecular-weight precursor. Studies on the synthesis and the uptake in vitro of the precursors of ferritin and ferredoxin by intact chloroplasts. Biochem J. 1983 Sep 15;214(3):943–950. doi: 10.1042/bj2140943. [DOI] [PMC free article] [PubMed] [Google Scholar]