Abstract
Hepatitis C virus (HCV) often leads to persistent infection. Interferon (IFN) and IFN-stimulated genes (ISGs) are amplified during HCV infection but fail to eliminate virus from the liver in a large number of infected patients. We have observed previously that HCV infection induces IFN-β production in immortalized human hepatocytes (IHH) as early as 24 h after infection, although virus replication is not inhibited. To gain insights on possible countermeasures of virus for the suppression of host antiviral response, the cellular transcriptional profiles of ISGs were examined after various treatments of IHH. The majority of ISGs were upregulated in IFN-treated IHH from the level for mock-treated cells. However, the comparison of ISG expression in IFN-treated IHH and IFN-pretreated, HCV genotype 2a-infected IHH indicated that virus infection suppresses the upregulation of a subset of effector molecules, including ISG56 and IFITM1. Similar results were observed for HCV-infected Huh7 cells. Subsequent study suggested that the exogenous expression of ISG56 or IFITM1 inhibits HCV replication in IHH or Huh7 cells, and the knockdown of these genes enhanced HCV replication. Further characterization revealed that the overexpression of these ISGs does not block HCV pseudotype entry into Huh7 cells. Taken together, our results demonstrated that ISG56 and IFITM1 serve as important molecules to restrict HCV infection, and they may have implications in the development of therapeutic modalities.
INTRODUCTION
Hepatitis C virus (HCV) is a hepatotropic Flavivirus in the Hepacivirus genus. The virus genome contains a linear, positive-strand RNA molecule of ∼9,500 nucleotides. The HCV genome encodes a polyprotein precursor of about 3,000 amino acids, which is cleaved by both viral and host proteases into structural (core, E1, E2, and p7) and nonstructural (NS2, NS3, NS4A, NS4B, NS5A, and NS5B) proteins. A number of HCV genomes have been cloned, and sequence divergences indicate several genotypes and a series of subtypes for the virus (19). An estimated 200 million people worldwide and 4 million people in the United States are infected with HCV. The majority of the chronically HCV-infected patients develop end-stage liver disease. Currently, the treatment is limited to a combination therapy of ribavirin and alpha interferon (IFN-α). This combination therapy is expensive and effective in ∼50% of HCV genotype 1-infected individuals (45), and the reason for this limited efficacy is not clear. Both viral and host factors may play roles that ultimately affect the level and extent of IFN-stimulated gene (ISG) expression and function induced during the course of IFN therapy (11, 12, 27, 28). Thus, there is an urgent need to identify novel antivirals or cellular molecules that can be used for therapy.
Viral proteins and nucleic acids are detected by pathogen recognition receptors (PRRs) as pathogen-associated molecular patterns (PAMPs). Subsequently, the signal transduction pathways are activated and trigger type I IFN and the production of other cytokines. Type I IFN then augments the expression of several ISGs and subverts virus replication by a variety of mechanisms (29, 32). IFN orchestrates a large number of genes of this antiviral response (14). However, many viruses deploy anti-IFN countermeasures, which for HCV are enacted primarily by the viral protein NS3/4A (5). To identify host factors that modify viral replication, we performed pathway-specific microarray of IFN-related genes. Our results identified 56 genes that were modulated between HCV-infected immortalized human hepatocytes (IHH) and IFN-pretreated, HCV-infected IHH. After validation, we focused on two IFN effector molecules, ISG56 (also known as IFIT1) and IFITM1, in determining antiviral effect in the current study. The overexpression of ISG56 is known to suppress HCV internal ribosome entry site (IRES)-mediated transcription, suggesting its potential as an anti-HCV molecule (43). On the other hand, IFITM family proteins are emerging as antiviral ISGs (2, 13, 24). Our results revealed that the exogenous expression of ISG56 or IFITM1 inhibits HCV replication and growth in infected hepatocytes.
MATERIALS AND METHODS
Cell lines and IFN-α.
The generation of immortalized human hepatocytes (IHH) by the transfection of the HCV core was described previously (30). Huh7, Huh7.5, and IHH were maintained in Dulbecco's modified Eagle's medium (DMEM) (Cambrex, Walkersville, MD) containing 10% fetal bovine serum (FBS), 100 U/ml of penicillin G, and 100 μg/ml of streptomycin at 37°C in a 5% CO2 incubator. The recombinant human IFN-α-2a was purchased from PBL InterferonSource (Piscataway, NJ).
Generation of cell culture-grown HCV and infection.
HCV genotype 2a (clone JFH1) was grown in Huh7.5 cells as previously described (16). For infection, cells were incubated with virus (multiplicity of infection [MOI], ∼0.5) in a minimum volume of medium. After 8 h of virus adsorption on hepatocytes, DMEM supplemented with 5% heat-inactivated FBS was added and cells were incubated for 96 h before analysis.
IFN PCR array.
Total RNA was extracted from cells under different conditions, and cDNA synthesis was performed using a random hexamer as described previously (18). The comparison of the relative expression of 84 genes whose expression is controlled by or involved in cell signaling mediated by IFN ligands and receptors was performed with a human interferon and receptor RT2 profiler PCR array (SuperArray Bioscience Corporation). For RNA quantitation, real-time PCR was conducted using SYBR green I (ABI Prism 7500; Applied Biosystems, CA). Three housekeeping genes (beta-2-microglobulin, hypoxanthine phosphoribosyltransferase 1, and ribosomal protein L13a) were used for normalization, and data were analyzed by the comparative threshold cycle method. The relative quantification of gene expression using the 2−ΔΔCT method was analyzed with RT2 profiler PCR array data analysis software (SuperArray Bioscience Corporation). The fold differences were calculated by comparing mock- or IFN-α-treated conditions to other conditions as described in Table S1 in the supplemental material.
Transfection.
Cells were transfected with 0.6 μg of a pCDNA3-Myc-tagged open reading frame (ORF) clone of human ISG56 and a pCMV6-Myc-DDK-tagged ORF clone of human IFITM1 (Origene) using Lipofectamine (Invitrogen, CA). The respective vector plasmid DNA was used as a control in parallel. For knockdown experiments, cells were transfected with control (scrambled) short interfering RNA (siRNA), IFIT1 (ISG56) siRNA, or IFITM1 siRNA (50 nM) with Lipofectamine 2000 (Invitrogen). Each commercially available siRNA used in this study was used in mixtures of three siRNAs, and the sequences were not disclosed by the supplier (Santa Cruz Biotechnology). siRNA-transfected cells were infected with HCV. A rescue experiment was performed by the introduction of ISG56 or IFITM1 plasmid DNA (∼1 μg) into siRNA-treated HCV-infected hepatocytes.
RNA quantitation.
Total RNA was isolated using TRIzol reagent (Invitrogen). cDNA was synthesized using random hexamer and a Superscript III reverse transcriptase kit (Invitrogen). Real-time PCR was performed on the cDNA for RNA quantitation using TaqMan universal PCR master mix (Applied Biosystems) and 6-carboxyfluorescein-minor groove binder (FAM-MGB) probe for IFIT1 (Hs00356631_g1), IFITM1 (Hs00705137_s1), IFITM2 (Hs00829485_sH), and HCV (AI6Q1GI). For the detection of the expression level of the endogenous control, FAM-MGB probe for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (TaqMan gene expression assay Hs99999905_m1; Applied Biosystems) was used. The fold changes of specific mRNA from cells under different conditions compared to levels for mock-infected cells are presented after normalizing data to those of GAPDH mRNA expression.
Immunofluorescence.
Cells were fixed with 3.7% formaldehyde for 20 min at room temperature and blocked with 3% bovine serum albumin for 1 h. Fixed cells were incubated with an IFITM1-specific rabbit polyclonal antibody (Santa Cruz Biotechnology) or HCV NS3 monoclonal antibody (Vision Biosystems) overnight. Cells were washed and incubated with anti-rabbit Ig conjugated with Alexa 594 and anti-mouse Ig conjugated with Alexa 488 (Molecular Probes, CA) secondary antibodies for 1 h at room temperature. Nuclei were stained with 4′,6′-diamidino-2-phenylindole (DAPI) (Invitrogen). Three-channel optical images were collected using the sequential scanning mode (405-, 488-, and 543-nm excitation and 450-, 522-, and 595-nm emission, respectively) of the Olympus FV1000 confocal system. Images were superimposed digitally for fine comparisons.
Western blot analysis.
Cells expressing empty vector control, ISG56, or IFITM1 were lysed in sample buffer, subjected to SDS-PAGE, and transferred onto a nitrocellulose membrane. The membrane was probed with a polyclonal antibody to ISG56 (Pierce, IL) or IFITM1 (Santa Cruz Biotechnology). The membrane was reprobed with a monoclonal antibody to actin (Santa Cruz Biotechnology) for comparisons of the protein loads. Proteins were visualized using enhanced chemiluminescence (ECL).
RESULTS
ISG status in HCV-infected hepatocytes.
Pathway-specific focused microarray was utilized to obtain a comprehensive view of the changes in IFN signaling after HCV infection. For this, IHH (∼105 cells) were treated with IFN-α (400 U) for 18 h or left untreated prior to infection with HCV genotype 2a. Mock-infected IHH were used in parallel as a control. Total RNA was isolated after 96 h, and a pathway-focused RT2 profiler PCR array was performed. The results were analyzed using PCR array data analysis software. HCV infection in IHH modestly upregulated or inhibited IFN signaling molecules (see Table S1 in the supplemental material). On the other hand, the majority of the genes were upregulated in cells treated with IFN-α compared to levels for mock-treated cells. Interestingly, most of the ISGs were downregulated when IFN-pretreated cells were infected with HCV. Twenty-eight out of 84 genes did not display a significant change. Garaigorta and Chisari (8) have shown that IFN-β- and IFN-α-induced ISG protein expression are attenuated in HCV-infected cells, and our results are in agreement with their observations. Several ISGs with known antiviral function were detected by our pathway-specific array. We focused on ISG56 and IFITM1 in this study because of their link to emerging antiviral function.
ISG56 belongs to a family of structurally related proteins that are induced by viral stresses. In humans, there are three other members of the ISG56 family, P60, P58, and P54 (35). Further, ISG56 protein families have been implicated in IFN antiviral actions against West Nile virus and lymphocytic choriomeningitis virus (LCMV) (42). A majority of cells, including IHH and Huh7, normally do not express ISG56 at a detectable level, but viral and other stresses rapidly and strongly induce the transcription of the ISG56 gene. We have previously observed a modest increase of ISG56 protein (∼3-fold) following HCV infection (17), and this may not be sufficient to inhibit viral replication. A similar observation was noted with Huh7 cells expressing an HCV subgenomic replicon (43).
IFITM family proteins also are induced by IFN (6). The antiviral function of IFITM proteins was acknowledged recently in studies with influenza A virus, West Nile virus, dengue virus, vesicular stomatitis virus (VSV), and HIV-1 (2, 15, 24), although little is known about their target specificity and mechanisms of action. IFITM1 has three other homologs in the human genome, IFITM2, IFITM3, and IFITM5 (22, 26). IFITM5 is solely expressed in osteoblasts and is involved in bone mineralization, unlike IFITM1, IFITM2, and IFITM3 (26). We did not detect a significant difference in IFITM2 expression in IFN-treated IHH and HCV-infected IHH (see Table S1 in the supplemental material). Further, Brass et al. (2) reported that HCV replication is not inhibited by the overexpression of IFITM3. We therefore pursued our current study of the role of IFN effector molecules ISG56 and IFITM1 on HCV replication.
HCV infection inhibits IFN-induced ISG56 and IFITM1 expression.
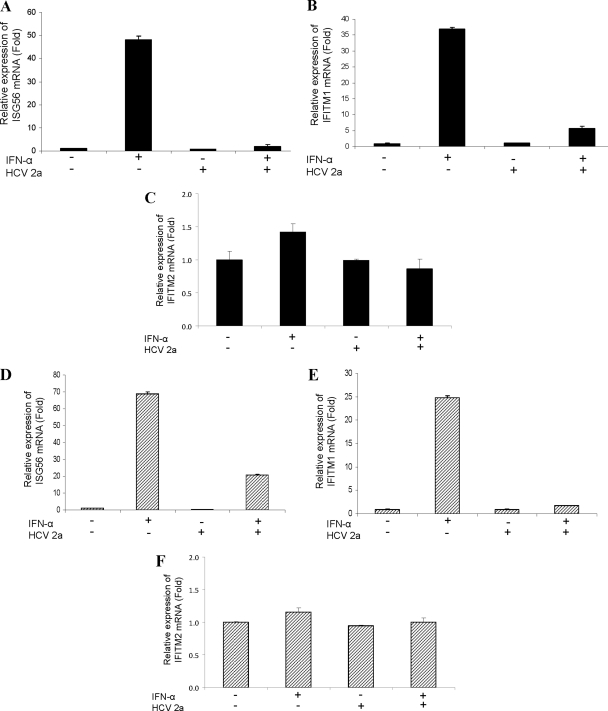
We next verified the role of HCV infection in IFN-induced ISGs. Real-time PCR analysis suggested that the IFN-α treatment of IHH significantly enhanced ISG56 or IFITM1 mRNA expression (Fig. 1A and B). On the other hand, IHH infected with HCV genotype 2a did not display a significant change of ISG56 or IFITM1 expression compared to that of mock-infected control hepatocytes. To our surprise, IHH pretreated with IFN-α and then infected with HCV genotype 2a displayed a significant inhibition of ISG56 (∼20-fold) or IFITM1 (∼5-fold) expression.
Fig. 1.
HCV infection inhibits IFN-α-induced ISG56 and IFITM1 expression. IHH were treated with IFN alone, infected with HCV, or pretreated with IFN and infected with HCV. Total RNA was isolated 96 h postinfection, and ISG56 (A), IFITM1 (B), or IFITM2 (C) mRNA was measured by real-time RT-PCR. GAPDH was used as an internal control for normalization. Huh7 cells were similarly treated, and the mRNA expression status of ISG56 (D), IFITM1 (E), or IFITM2 (F) is shown. The fold changes of mRNA are presented after normalizing data to a mock-infected control and arbitrarily set as 1. The results are presented as means from four independent experiments with standard errors.
The effect of IFN-α was further verified in the Huh7 cell line. Cells were either pretreated with IFN-α or mock treated before infection with HCV genotype 2a. The results demonstrated that the IFN-α treatment of Huh7 cells significantly enhanced ISG56 (Fig. 1D) or IFITM1 (Fig. 1E) mRNA expression. However, the HCV infection of Huh7 cells did not alter the status of these two ISGs. On the other hand, Huh7 cells pretreated with IFN-α and then infected with HCV genotype 2a displayed a significant inhibition of IFN-induced ISG56 (∼3.5-fold) or IFITM1 (∼10-fold) expression. The specificity of the observed effect on HCV replication machinery was verified by the expression of IFITM2 as an unaltered ISG observed in our initial microarray analyses. The results indicated that HCV infection in hepatocytes did not inhibit IFITM2 expression in the presence or absence of IFN-α (Fig. 1C and F). Taken together, these results suggest that HCV infection inhibits prior IFN-α-mediated upregulation of ISG56 and IFITM1 at the transcriptional level.
HCV infection inhibits IFN-induced IFITM1 expression.
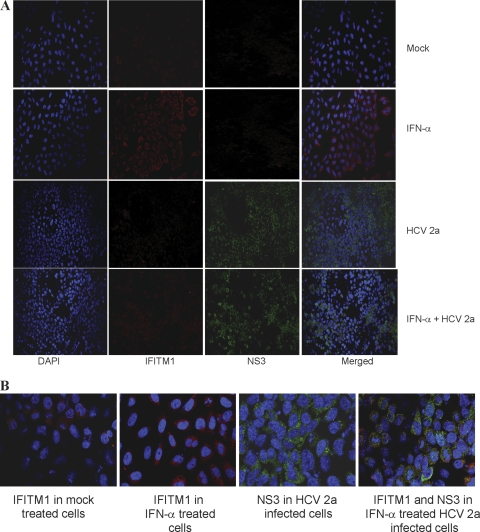
We have shown previously that HCV infection inhibits poly(I:C)-induced ISG56 expression (17). Here, we examined whether HCV infection inhibits IFITM1 protein expression in the presence or absence of IFN-α by immunofluorescence. IHH were treated with IFN-α, infected with HCV, or pretreated with IFN-α followed by HCV infection. Cells were fixed and stained with specific antibodies for the detection of HCV NS3 or IFITM1 protein expression. The IFN-α treatment of IHH induced IFITM1 expression (Fig. 2A). On the other hand, HCV infection inhibited IFN-α-induced IFITM1 expression. Similar IFITM1 expression was observed from Huh7 cells treated with IFN-α, infected with HCV genotype 2a, or pretreated with IFN-α followed by HCV infection (Fig. 2B).
Fig. 2.
Inhibition of IFN-α-induced IFITM1 expression by HCV. IHH were mock treated, treated with IFN as a positive control, infected with HCV genotype 2a, or pretreated with IFN and infected with HCV. (A) Cells were fixed after 96 h of infection and stained with DAPI (blue), IFITM1 antibody (red), and HCV NS3 antibody (green). Merged photographs are shown. (B) Huh7 cells were treated and stained as described for panel A. A merged photograph from a randomly selected field is shown.
Transient expression of ISG56 and IFITM1 inhibits HCV replication.
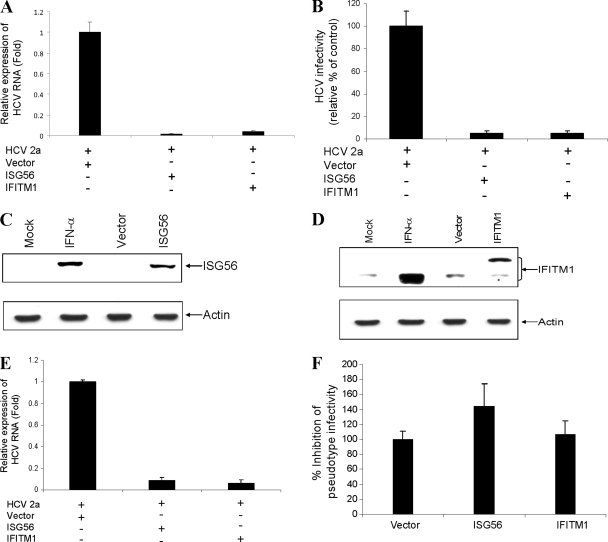
To determine the antiviral effect of ISG56 and IFITM1 on HCV genotype 2a replication, we transfected vector alone, ISG56, or IFITM1 plasmid DNA into HCV-infected hepatocytes. Total RNA was isolated on day 4 postinfection, and HCV replication was analyzed by reverse transcription-quantitative PCR (RT-qPCR) as described previously (31). The transient expression of ISG56 or IFITM1 in IHH resulted in a significant reduction of HCV replication compared to that of the vector control (Fig. 3A). To further assess the effect of ISG56 or IFITM1 on HCV growth, we determined the release of infectious HCV particles in cell culture medium as described recently (37). The HCV titer was significantly reduced in ISG56- or IFITM1-transfected IHH compared to that of vector-transfected control IHH (Fig. 3B).
Fig. 3.
Transient expression of ISG56 and IFITM1 inhibits HCV growth. IHH were infected with HCV genotype 2a and transfected with vector DNA, ISG56, or IFITM1 2 days postinfection. (A) HCV replication was measured after 48 h of transfection from intracellular RNA by RT-qPCR. The results are shown as means from four independent experiments with standard errors (P < 0.006 for ISG56 and P < 0.001 for IFITM1). (B) HCV infectivity was measured from culture supernatants of virus-infected IHH at serial dilutions and are expressed as a percentage of that in vector-transfected control cells (P < 0.0001). Western blot analysis was performed for the expression of ISG56 (C) or IFITM1 (D) in IHH transfected with the respective plasmid DNAs, empty vector, or mock-treated cells using specific antibodies. The blot was reprobed with an antibody to actin for comparisons of protein loads. (E) The transient expression of ISG56 or IFITM1 also suppresses HCV replication in Huh7 cells. The results are shown as means from three independent experiments with standard errors (P < 0.001 for ISG56 or IFITM1). Huh7 cells were stably transfected with vector, ISG56, and IFITM1. (F) Pooled stable cell lines were incubated with VSV/HCV pseudotype virus for 48 h, and plaque assay was performed to examine HCV entry. The results are shown as means from three independent experiments with standard errors.
Endogenous ISG56 protein expression normally is undetectable, and the IFITM1 expression level is very low in many cell types. We transfected ISG56 or IFITM1 plasmid DNA into IHH and examined the protein expression level using specific antibodies. IFN-α-treated cells induced both ISG56 (Fig. 3C) and IFITM1 (Fig. 3D) expression. Transient transfection displayed ISG56 expression, as expected. On the other hand, two protein bands were observed from the endogenous and exogenous expression of IFITM1. The slower-migrating band represented the IFITM1 expression together with the Myc-DDK tag (Fig. 3D).
We similarly examined the antiviral effect of these ISGs in Huh7 cells infected with JFH1. The transient expression of ISGs inhibited HCV replication in Huh7 cells (Fig. 3E), suggesting that these ISGs specifically restrict virus replication in Huh7 cells. To understand the mechanisms for antiviral action, these ISGs were tested for their ability to inhibit HCV entry. For this, Huh7 cells were stably transfected with ISG56, IFITM1, or empty vector and were selected following neomycin treatment. Pooled cell lines were incubated with VSV/HCV pseudotype virus (25) for 48 h before plaque count. A significant inhibition of VSV/HCV pseudotype entry was not observed in ISG56- or IFITM1-transfected Huh7 cells (Fig. 3F). On the other hand, a modest increase in VSV/HCV pseudotype plaque number in ISG56-transfected cells could be due to enhanced VSV replication, since the VSV titer increases in the presence of ISG56 (23). Further, IFITM3-overexpressing Huh7 cells did not block HIV/HCV pseudotype virus entry (2). We also have infected HCV genotype 2a in Huh7 cells stably transfected with ISG56 or IFITM1. HCV replication was similar between stable transfectants and control cells (data not shown). Taken together, our results suggested that the transient expression of ISG56 or IFITM1 reduces HCV RNA replication or generation of infectious virus particles from hepatocytes but does not interfere with HCV entry.
Transient expression of ISG56 or IFITM1 suppresses HCV subgenomic RNA replication.
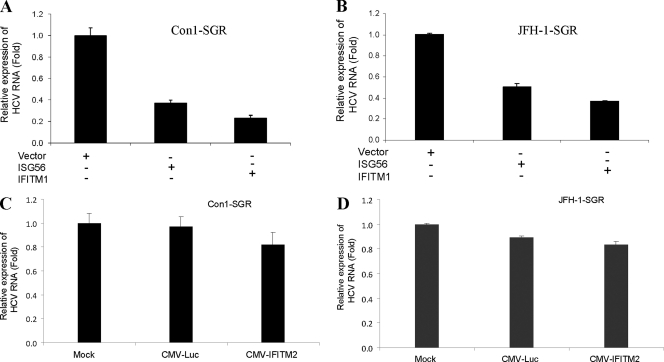
We next investigated the antiviral effect of ISG56 or IFITM1 on HCV replication using Huh7 cells harboring the subgenomic replicon Con1-SGR or JFH1-SGR (containing HCV NS3-NS5B genomic regions). Cells were transfected with empty vector as control, ISG56, or IFITM1 plasmid DNA. Total cellular RNA was isolated, and intracellular HCV RNA replication was measured by RT-qPCR. The exogenous expression of ISG56 or IFITM1 significantly inhibited the replication of Con1-SGR (Fig. 4A). A similar result was observed with the JFH1 subgenomic replicon (Fig. 4B). To further confirm that the inhibitory effect of ISG56 or IFITM1 was not competition or interference between cytomegalovirus (CMV)-driven protein and HCV RNA synthesis, we transfected a nonspecific gene, firefly luciferase, or IFITM2 driven by the CMV promoter in Huh7 cells harboring a subgenomic replicon. The inhibition of Con1-SGR or JFH1-SGR was not observed (Fig. 4C and D).
Fig. 4.
Transient expression of ISG56 or IFITM1 suppresses subgenomic HCV RNA replication. Huh7 cells harboring subgenomic replicon Con1 (A) or JFH1 (B) were transfected with vector DNA, ISG56, or IFITM1. Cellular RNA was extracted 48 h posttransfection, and viral RNA was analyzed by RT-qPCR. The results are shown as means from three independent experiments with standard errors (P < 0.003 for ISG56 and P < 0.001 for IFITM1). Huh7 cells harboring subgenomic replicon Con1 (C) or JFH1 (D) also were transfected with plasmid DNA containing CMV promoter-driven luciferase or IFITM2 as a control. Cellular RNA was extracted 48 h posttransfection, and viral RNA was analyzed by real-time RT-PCR. The results shown are from three independent experiments.
ISG56 binds the e subunit of the translation initiation factor eIF-3, causing the inhibition of translation initiation (33). Therefore, it is possible that ISG56 blocks HCV replication by inhibiting initial translation, and this result is in agreement with ISG56 as a translational control effector of the host response to HCV (38). Knowledge of the antiviral function of IFITM1 is emerging, and very little is known about how IFITM1 exerts its antiviral effect. Our data suggest that HCV infection per se does not inhibit IFITM1 status. HCV transcriptionally downregulates IFN-induced IFITM1 expression, although the mechanism of antiviral action and the target specificity of IFITM1 for the inhibition of HCV replication remain to be elucidated. Taken together, these results further suggested that ISG56 and IFITM1 inhibit HCV genome replication.
Knockdown of ISG56 or IFITM1 enhances HCV replication.
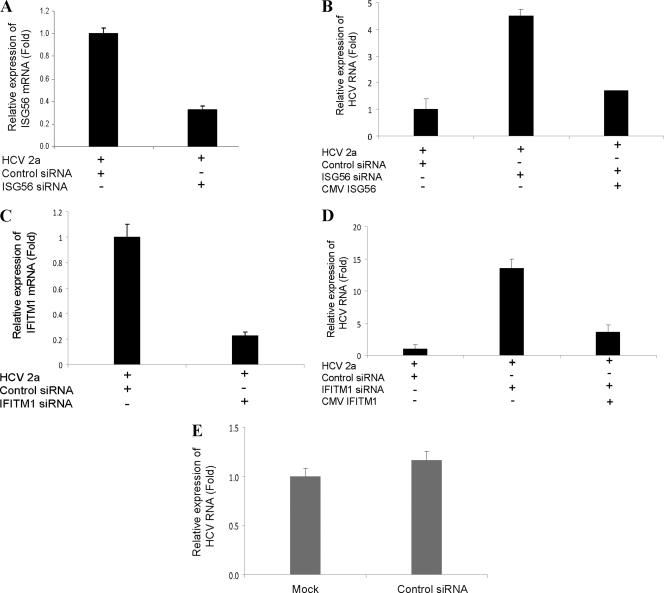
We next determined whether the knockdown of ISG56 or IFITM1 expression modulates HCV replication. For this, IHH were transfected with control (scrambled) siRNA, ISG56, or IFITM1 siRNA using Lipofectamine 2000 and subsequently were infected with HCV genotype 2a. Total RNA was isolated 96 h postinfection and analyzed for HCV replication by RT-qPCR. A significant inhibition of ISG56 (Fig. 5A) or IFITM1 (Fig. 5C) was observed following siRNA treatment. On the other hand, HCV replication was markedly enhanced in ISG56 (Fig. 5B) or IFITM1 (Fig. 5D) knockdown cells compared to that of control siRNA-transfected cells. We next examined whether the overexpression of ISG56 or IFITM1 in these knockdown cells can be rescued from the interference of siRNA. Our results suggested that the effect of siRNA could be rescued by the introduction of respective plasmid DNAs to evade interference (Fig. 5B and D). Additionally, we examined HCV replication in untreated or control siRNA-transfected cells. Similar HCV RNA replication was observed (Fig. 5E), suggesting that the control siRNA used in this study does not have a nonspecific inhibitory effect. Taken together, these results revealed that HCV replication is increased in ISG56 or IFITM1 knockdown cells.
Fig. 5.
Knockdown of ISG56 or IFITM1 enhances HCV RNA replication. IHH were transfected with control siRNA, ISG56 siRNA, or IFITM1 siRNA and subsequently infected with HCV genotype 2a. Total cellular RNA was extracted 96 h postinfection, and ISG56 (A), IFITM1 (C), and intracellular HCV RNA (B and D) were analyzed by RT-qPCR. The results are shown as means from three independent experiments with standard errors (P < 0.001). The introduction of plasmid DNA encoding ISG56 or IFITM1 reduced the siRNA-mediated interference of HCV replication (B and D). IHH also were transfected with control siRNA or were mock treated and then infected with HCV genotype 2a to ascertain nonspecific inhibitory effects. (E) Total RNA was extracted 96 h postinfection, and HCV RNA was analyzed by RT-qPCR. The results shown are from two independent experiments with three replicates.
DISCUSSION
In this study, we have observed that ISG56 or IFITM1 possesses antiviral function against HCV replication. ISG56 is the first of two identified ISGs belonging to the same family (21, 44). All members in this family contain multiple tetratricopeptide (TPR) motifs that are known to mediate protein-protein interactions through scaffolds formed among tandem TPR repeats (20). ISG56 is induced in response to type I IFNs, double-stranded RNAs, and viruses (10, 40), and it interacts with the translation initiation factor eIF-3, leading to the inhibition of protein synthesis (40). ISG56 also has been implicated in antiviral actions of IFNs against West Nile virus and LCMV (42), and it inhibits human papillomavirus DNA replication by binding to the viral protein E1 (41).
IFITM proteins are induced by type I and II interferons, and most cells express a basal level of one or more of these proteins (6). IFITM proteins restrict an early step in the life cycle of dengue virus and West Nile virus (2) and inhibit the genome replication of infectious influenza H1N1 virus, Marburg virus, and Ebola virus (13). The genome replication of infectious severe acute respiratory syndrome coronavirus (SARS-CoV) and entry mediated by the SARS-CoV spike (S) protein are restricted by IFITM proteins (13). Recently, IFITM1 has been shown to inhibit HIV replication as well as suppress virus production by interrupting Gag expression (24). In contrast, IFITM proteins do not inhibit the replication of murine leukemia virus (MLV). The entry processes of amphotropic MLV, Machupo virus (MACV), Lassa virus, and LCMV also are not inhibited by IFITM proteins. IFITM2 was not upregulated in IFN-treated IHH. We did not observe an inhibition of HCV entry process in IFITM1 or ISG56 protein-expressing cells using the VSV/HCV pseudotype. A similar observation was reported for IFITM3-overexpressing Huh7 cells with the HIV/HCV pseudotype (2). While our manuscript was in preparation, Schoggins et al. (36) reported that the overexpression of several ISGs displayed anti-HCV activity in Huh7 cells. We also have observed in our study that several of these ISG expressions were impaired in HCV-infected IHH. All ISGs with known antiviral function may not act in a similar fashion with each virus. For example, PKR is well recognized as an important effector of the antiviral response through its ability to arrest protein synthesis, and its importance is highlighted by a number of viral and cellular products that are able to abrogate or modulate its action (9). HCV NS5A and E2 proteins interact with PKR and are proposed to be viral inhibitors of the antiviral action of PKR (7, 39). On the other hand, the ability of HCV to activate PKR has been suggested to be advantageous for the virus during an IFN response (1, 8). Therefore, it is premature to make a conclusion based on the known function of a specific ISG, and further studies are necessary to understand the precise role of this effector molecule during early stages of HCV infection. Further, other ISGs also may be involved either alone or cooperatively in anti-HCV activity.
We have observed that interferon treatment following HCV infection is more effective in inducing ISG56 and IFITM1 expression. However, HCV infection suppresses ISG expression when cells are treated with IFN-α prior to infection. Perhaps HCV, when it senses the already-enhanced expression of ISGs, triggers the machineries required for evading host defense more effectively and then suppresses the expression of ISGs for the establishment of infection. In fact, earlier studies suggested that HCV-infected individuals with higher intrahepatic ISGs became nonresponders following IFN-based therapy (3, 4, 34). In conclusion, the results from this study revealed that (i) the introduction of ISG56 and IFITM1 into HCV-infected hepatocytes inhibits virus replication, and (ii) HCV infection can overcome the preexisting upregulation of these ISGs for establishing infection. It is possible that ISG56 exerts translational inhibitory effects for the impairment of virus replication. On the other hand, IFITM1 may interfere with the HCV replication complex, since we did not observe a blockade at the entry level. Our results therefore suggest an additional avenue in developing therapeutic modalities using antiviral ISGs for treatment against HCV infection.
Supplementary Material
ACKNOWLEDGMENTS
We thank Ralf Bartenschlager, Charlie Rice, Ganes C. Sen, and Takaji Wakita for providing reagents, Leonard Grosso for measuring HCV genome copy numbers, and Keith Meyer for technical assistance.
This work was supported by grants DK081817 and AI065535 (R.B.R.) and 5U54AI057160 (R.R.) to the Midwest Regional Center of Excellence for Biodefense and Emerging Infectious Diseases Research from the National Institutes of Health.
Footnotes
Supplemental material for this article may be found at http://jvi.asm.org/.
Published ahead of print on 5 October 2011.
REFERENCES
- 1. Arnaud N., et al. 2010. Hepatitis C virus controls interferon production through PKR activation. PLoS One 5: e10575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brass A. L., et al. 2009. IFITM proteins mediate the innate immune response to influenza A H1N1 virus, West Nile virus and dengue virus. Cell 139: 1243–1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen L., et al. 2005. Hepatic gene expression discriminates responders and nonresponders in treatment of chronic hepatitis C viral infection. Gastroenterology 128: 1437–1444 [DOI] [PubMed] [Google Scholar]
- 4. Feld J. J., et al. 2007. Hepatic gene expression during treatment with peginterferon and ribavirin: identifying molecular pathways for treatment response. Hepatology 46: 1548–1563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Foy E., et al. 2003. Regulation of interferon regulatory factor-3 by the hepatitis C virus serine protease. Science 300: 1145–1148 [DOI] [PubMed] [Google Scholar]
- 6. Friedman R. L., Manly S. P., McMahon M., Kerr I. M., Stark G. R. 1984. Transcriptional and posttranscriptional regulation of interferon-induced gene expression in human cells. Cell 38: 745–755 [DOI] [PubMed] [Google Scholar]
- 7. Gale M., Jr., et al. 1998. Control of PKR protein kinase by hepatitis C virus nonstructural 5A protein: molecular mechanisms of kinase regulation. Mol. Cell. Biol. 18: 5208–5218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Garaigorta U., Chisari F. V. 2009. Hepatitis C virus blocks interferon effector function by inducing protein kinase R phosphorylation. Cell Host Microbe 6: 513–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. García M. A., et al. 2006. Impact of protein kinase PKR in cell biology: from antiviral to antiproliferative action. Microbiol. Mol. Biol. Rev. 70: 1032–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Guo J., Peters K. L., Sen G. C. 2000. Induction of the human protein P56 by interferon, double-stranded RNA, or virus infection. Virology 267: 209–219 [DOI] [PubMed] [Google Scholar]
- 11. He Y., Katze M. G. 2002. To interfere and to anti-interfere: the interplay between hepatitis C virus and interferon. Viral Immunol. 15: 95–119 [DOI] [PubMed] [Google Scholar]
- 12. Heim M. H., Moradpour D., Blum H. E. 1999. Expression of hepatitis C virus proteins inhibits signal transduction through the Jak-STAT pathway. J. Virol. 73: 8469–8475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Huang I. C., et al. 2011. Distinct patterns of IFITM-mediated restriction of filoviruses, SARS coronavirus, and influenza A virus. PLoS Pathog. 7: e1001258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ishii K. J., Koyama S., Nakagawa A., Coban C., Akira S. 2008. Host innate immune receptors and beyond: making sense of microbial infections. Cell Host Microbe 3: 352–363 [DOI] [PubMed] [Google Scholar]
- 15. Jiang D., et al. 2010. Identification of five interferon-induced cellular proteins that inhibit West Nile virus and dengue virus infection. J. Virol. 84: 8332–8341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kanda T., et al. 2006. Generation of infectious hepatitis C virus in immortalized human hepatocytes. J. Virol. 80: 4633–4639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kanda T., Steele R., Ray R., Ray R. B. 2007. Hepatitis C virus infection induces the beta interferon signaling pathway in immortalized human hepatocytes. J. Virol. 81: 12375–12381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kanda T., Steele R., Ray R., Ray R. B. 2009. Inhibition of intrahepatic gamma interferon production by hepatitis C virus nonstructural protein 5A in transgenic mice. J. Virol. 83: 8463–8469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kuiken C., Simmonds P. 2009. Nomenclature and numbering of the hepatitis C virus. Methods Mol. Biol. 510: 33–53 [DOI] [PubMed] [Google Scholar]
- 20. Lamb J. R., Tugendreich S., Hieter P. 1995. Tetratrico peptide repeat interactions: to TPR or not to TPR? Trends Biochem. Sci. 20: 257–259 [DOI] [PubMed] [Google Scholar]
- 21. Levy D. N., Aldrovandi G. M., Kutsch O., Shaw G. M. 2004. Dynamics of HIV-1 recombination in its natural target cells. Proc. Natl. Acad. Sci. U. S. A. 101: 4204–4209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Levy D., Larner A., Chaudhuri A., Babiss L. E., Darnell J. E. 1986. Interferon-stimulated transcription: isolation of an inducible gene and identification of its regulatory region Proc. Natl. Acad. Sci. U. S. A. 83: 8929–8933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li Y., et al. 2009. ISG56 is a negative-feedback regulator of virus-triggered signaling and cellular antiviral response. Proc. Natl. Acad. Sci. U. S. A. 106: 7945–7950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lu J., et al. 2011. The IFITM proteins inhibit HIV-1 infection. J. Virol. 85: 2126–2137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Meyer K., Beyene A., Bowlin T. L., Basu A., Ray R. 2004. Coexpression of hepatitis C virus E1 and E2 chimeric envelope glycoproteins displays separable ligand sensitivity and increases pseudotype infectious titer. J. Virol. 78: 12838–12847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Moffatt P., et al. 2008. Bril: a novel bone-specific modulator of mineralization. J. Bone Miner. Res. 23: 1497–1508 [DOI] [PubMed] [Google Scholar]
- 27. Neumann A. U., et al. 1998. Hepatitis C viral dynamics in vivo and the antiviral efficacy of IFN-α therapy. Science 282: 103–107 [DOI] [PubMed] [Google Scholar]
- 28. Pflugheber J., et al. 2002. Regulation of PKR and IRF-1 during hepatitis C virus RNA replication. Proc. Natl. Acad. Sci. U. S. A. 99: 4650–4655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Randall R. E., Goodbourn S. 2008. Interferons and viruses: an interplay between induction, signalling, antiviral responses and virus countermeasures. J. Gen. Virol. 89: 1–47 [DOI] [PubMed] [Google Scholar]
- 30. Ray R. B., Meyer K., Ray R. 2000. Hepatitis C virus core protein promotes immortalization of primary human hepatocytes. Virology 271: 197–204 [DOI] [PubMed] [Google Scholar]
- 31. Raychoudhuri A., et al. 2010. Hepatitis C virus infection impairs IRF-7 translocation and alpha interferon synthesis in immortalized human hepatocytes. J. Virol. 84: 10991–10998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sadler A. J., Williams B. R. 2008. Interferon-inducible antiviral effectors. Nat. Rev. Immunol. 8: 559–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Saikia P., Fensterl V., Sen G. C. 2010. The inhibitory action of P56 on select functions of E1 mediates interferon's effect on human papillomavirus DNA replication. J. Virol. 84: 13036–13039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sarasin-Filipowicz M., et al. 2008. Interferon signaling and treatment outcome in chronic hepatitis C. Proc. Natl. Acad. Sci. U. S. A. 105: 7034–7039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sarkar S. N., Sen G. C. 2004. Novel functions of proteins encoded by viral stress-inducible genes. Pharmacol. Ther. 103: 245–259 [DOI] [PubMed] [Google Scholar]
- 36. Schoggins J. W., et al. 2011. A diverse range of gene products are effectors of the type I interferon antiviral response. Nature 472: 481–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shrivastava S., Raychoudhuri A., Steele R., Ray R., Ray R. B. 2011. Knockdown of autophagy enhances the innate immune response in hepatitis C virus-infected hepatocytes. Hepatology 53: 406–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sumpter R., Jr., et al. 2005. Regulating intracellular antiviral defense and permissiveness to hepatitis C virus RNA replication through a cellular RNA helicase, RIG-I. J. Virol. 79: 2689–2699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Taylor D. R., Shi S. T., Romano P. R., Barber G. N., Lai N. M. 1999. Inhibition of the interferon-inducible protein kinase PKR by HCV E2 protein. Science 285: 107–110 [DOI] [PubMed] [Google Scholar]
- 40. Terenzi F., Hui D. J., Merrick W. C., Sen G. C. 2006. Distinct induction patterns and functions of two closely related interferon-inducible human genes, ISG54 and ISG56. J. Biol. Chem. 281: 34064–34071 [DOI] [PubMed] [Google Scholar]
- 41. Terenzi F., Saikia P., Sen G. C. 2008. Interferon-inducible protein, P56, inhibits HPV DNA replication by binding to the viral protein E1. EMBO J. 27: 3311–3321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wacher C., et al. 2007. Coordinated regulation and widespread cellular expression of interferon-stimulated genes (ISG) ISG-49, ISG-54, and ISG-56 in the central nervous system after infection with distinct viruses. J. Virol. 81: 860–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wang C., et al. 2003. Alpha interferon induces distinct translational control programs to suppress hepatitis C virus RNA replication. J. Virol. 77: 3898–3912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wathelet M., et al. 1986. Molecular cloning, full-length sequence and preliminary characterization of a 56-kDa protein induced by human interferons. Eur. J. Biochem. 155: 11–17 [DOI] [PubMed] [Google Scholar]
- 45. Yang J. D., Roberts L. R. 2010. Hepatocellular carcinoma: a global view. Nat. Rev. Gastroenterol. Hepatol. 7: 448–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.