Abstract
Inflammasomes are cytosolic protein complexes that stimulate the activation of caspase-1, which in turn induces the secretion of the inflammatory cytokines Interleukin-1β (IL-1β) and IL-18. Recent studies have indicated that the inflammasome known as the NOD-like-receptor-family, pyrin domain-containing 3 (NLRP3) inflammasome recognizes several RNA viruses, including the influenza and encephalomyocarditis viruses, whereas the retinoic acid-inducible gene I (RIG-I) inflammasome may detect vesicular stomatitis virus. We demonstrate that measles virus (MV) infection induces caspase-1-dependent IL-1β secretion in the human macrophage-like cell line THP-1. Gene knockdown experiments indicated that IL-1β secretion in MV-infected THP-1 cells was mediated by the NLRP3 inflammasome but not the RIG-I inflammasome. MV produces the nonstructural V protein, which has been shown to antagonize host innate immune responses. The recombinant MV lacking the V protein induced more IL-1β than the parental virus. THP-1 cells stably expressing the V protein suppressed NLRP3 inflammasome-mediated IL-1β secretion. Furthermore, coimmunoprecipitation assays revealed that the V protein interacts with NLRP3 through its carboxyl-terminal domain. NLRP3 was located in cytoplasmic granular structures in THP-1 cells stably expressing the V protein, but upon inflammasome activation, NLRP3 was redistributed to the perinuclear region, where it colocalized with the V protein. These results indicate that the V protein of MV suppresses NLRP3 inflammasome-mediated IL-1β secretion by directly or indirectly interacting with NLRP3.
INTRODUCTION
Measles is an acute contagious disease that remains a major cause of childhood mortality worldwide, especially in developing countries (6). Measles virus (MV), a member of the family Paramyxoviridae, is an enveloped virus with a nonsegmented single-stranded negative-sense RNA genome (15). Upon MV infection, cells produce alpha/beta interferon (IFN-α/β) following recognition of the virus by RNA helicases encoded by retinoic acid-inducible gene I (RIG-I) and melanoma differentiation-associated gene 5 (MDA5) (22, 43) or Toll-like receptor (TLR) 7 (51). MV encodes phosphoprotein (P) and V and C proteins, which can counteract the host IFN response (11, 34, 36, 54, 64). Among these viral proteins, the V protein is the most versatile antagonist. It blocks IFN-α/β signal transduction in infected cells (9, 11, 13, 36, 38, 47, 59, 64) and inhibits TLR7-mediated IFN-α production in human plasmacytoid dendritic cells (42). Furthermore, the MV V protein, like that of other paramyxoviruses, interacts with MDA5 and inhibits its function with respect to IFN induction (2). The N-terminal domain of the V protein (Vn), which is common to the P and V proteins, has been shown to interact with STAT1 (signal transducer and activator of transcription 1) and Jak1, which are involved in IFN signaling (9), whereas the unique cysteine-rich C-terminal domain of the V protein (Vc) was found to interact with MDA5 (39), STAT2 (47), and IκB kinase α (IKKα), which is involved in the activation of interferon-regulatory factor 7 through TLR7 (42). Furthermore, Vc interacts with the NF-κB subunit p65 (RelA) to suppress NF-κB activity (53). The mechanism by which the MV C protein acts as an IFN antagonist is not well understood, but it probably suppresses IFN induction by regulating viral RNA synthesis (34).
Secretion of the inflammatory cytokines interleukin-1β (IL-1β) and IL-18 is tightly regulated by the inflammasomes known as caspase-1-activating molecular complexes (32). Recognition of pathogens by pattern recognition receptors such as TLRs induces pro-IL-1β and pro-IL-18 in the cytoplasm. The activation of the inflammasomes leads to the conversion of procaspase-1 to caspase-1, which cleaves procytokines into mature IL-1β and IL-18, which are then secreted. The best-characterized inflammasome is the NOD-like receptor (NLR)-family pyrin domain-containing 3 (NLRP3, also known as Nalp3 or cryopyrin) inflammasome, which can be activated by a wide range of stimuli, such as endogenous danger signals from damaged cells, bacterial components, and environmental irritants (52).
Infection by RNA viruses such as influenza virus (1, 19, 20, 61), encephalomyocarditis virus (EMCV) (44, 45), and vesicular stomatitis virus (VSV) (45) also stimulates NLRP3, which then recruits the apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC) and procaspase-1, forming the NLRP3 inflammasome. How RNA viruses are recognized by NLRP3 is not well-known, but in the case of influenza virus, the proton-selective ion channel M2 protein, not genomic RNA, has been shown to stimulate the NLRP3 inflammasome pathway (20). One study reported that infection with VSV or transfection with 5′-triphosphate RNA activates the RIG-I inflammasome (44). Furthermore, AIM2 (absent in melanoma 2) binds to double-stranded DNA (dsDNA) and engages the adaptor protein ASC to form a caspase-1-activating inflammasome (7, 12, 18, 50). Experiments using AIM2-deficient mice revealed that AIM2 is essential in regulating caspase-1-dependent maturation of IL-1β and IL-18 in response to dsDNA, as well as DNA viruses such as vaccinia virus and mouse cytomegalovirus (49).
In the present study, we showed that MV induces secretion of IL-1β in human macrophage-like cells. By generating NLRP3 and RIG-I knockdown cells, we also demonstrated that MV activates the NLRP3 inflammasome, which results in caspase-1-mediated maturation of IL-1β. Furthermore, our results indicate that the MV V protein has the ability to interact with NLRP3 through its C-terminal domain, thereby inhibiting NRLP3 inflammasome-mediated IL-1β secretion.
MATERIALS AND METHODS
Cells and viruses.
Vero cells constitutively expressing human signaling lymphocyte activation molecule (SLAM; Vero/hSLAM cells) (37) and HEK293T cells were maintained in Dulbecco's modified Eagle's medium (DMEM; Wako Pure Chemical Industry) supplemented with 7.5% (vol/vol) fetal bovine serum (FBS). PLAT-gp cells (a gift from M. Shimojima and T. Kitamura) containing the retroviral gag and pol genes (33) were maintained in DMEM supplemented with 7.5% FBS and blasticidin (10 μg/ml; Invitrogen). Human monocytic THP-1 cells were cultured in RPMI 1640 medium (Wako Pure Chemical Industry) containing l-glutamine (2 mM; Nacalai Tesque), 2-mercaptoethanol (50 μM; Nacalai Tesque), and 10% (vol/vol) FBS. For macrophage differentiation, THP-1 cells were treated with phorbol 12-myristate 13-acetate (PMA) (0.5 μM; Sigma) at 37°C for 3 h. Cell surface expression of SLAM on THP-1 cells was examined by flow cytometry analysis using an anti-SLAM monoclonal antibody IPO-3 (Cayman Chemical). IC323-EGFP (17) and MV-ΔV (22) are enhanced green fluorescent protein (EGFP)-expressing recombinant viruses based on a wild-type IC-B strain of MV. MV-ΔV was generated by introducing four nucleotide substitutions into the region corresponding to the RNA-editing motif of the P gene of IC323-EGFP. All four substitutions were synonymous in the reading frame of the P protein. Viruses were titrated on Vero/hSLAM cells. UV inactivation was performed by exposing viruses to 1.0 J of UV light/cm2 with a Stratalinker UV cross-linker (Stratagene).
Plasmid constructions.
pCA7-Flag-MDA5 was generated by inserting Flag-tagged MDA5 from pEF-Flag-MDA5 (34) into the expression vector pCA7 (58). The cDNA for human NLRP3 was purchased from the National Institute of Technology and Evaluation, Biological Resource Center, Japan. The cDNAs encoding human ASC, procaspase-1, and pro-IL-1β were obtained by reverse transcription of total RNA from lipopolysaccharide (LPS)-treated THP-1 cells, followed by PCR using specific primers. These cDNAs were cloned into pCA7 (pCA7-NLRP3, pCA7-ASC, pCA7-procaspase-1, and pCA7-pro-IL-1β) or pCA7-Flag to produce Flag-tagged proteins (pCA7-Flag-NLRP3 and pCA7-Flag-ASC). pCAG-HA-IC-V and pCAG-HA-IC-Vn were generated by inserting the DNA fragments encoding the hemagglutinin (HA)-tagged full-length V protein from the IC-B strain of MV and the truncated V protein containing only the N-terminal 231 residues (36) into pCAGGS (35), respectively. pCA7-HA-orange-Vc, pCA7-HA-orange-Vc(C272R), pCA7-IC-V, and pCA7-EGFP were generated by inserting the DNA fragments encoding the HA-tagged kusabira orange-fused C-terminal 69 residues of the V protein (orange-Vc), orange-Vc with the C272R substitution [orange-Vc(C272R)], the full-length V protein, and EGFP into pCA7, respectively.
Knockdown of genes using shRNA.
Using the pRS-U6/puro vector (OriGene), plasmids pRS-shNLRP3, pRS-shRIG-I and pRS-shEGFP were constructed. They expressed short hairpin RNAs (shRNAs) targeting human NLRP3, human RIG-I, and EGFP mRNAs, respectively. Target sequences were designed using BLOCK-iT RNAi Designer (Invitrogen) or had been described previously (22, 63): 5′-GGA GAG ACC TTT ATG AGA AAG-3′ for NLRP3, 5′-GCC AGA ATG TTA GTG AGA ATT-3′ for RIG-I, and 5′-GGC ACA AGC TGG AGT ACA ACT-3′ for EGFP. To generate shRNA-expressing retroviruses, PLAT-gp cells in 10-cm dishes were transfected with 20 μg of each shRNA-expressing plasmid and 2 μg of pCVSV-G, which encodes the VSV G protein (55) using PEI-Max (Polysciences, Inc.). Culture medium was replaced with fresh medium 6 h later, and supernatants containing retroviruses were collected at 48 h posttransfection. To generate THP-1 cells constitutively expressing shRNA targeting NLRP3, RIG-I, and EGFP mRNAs, respectively, 4 × 105 THP-1 cells in 200 μl complete medium were centrifuged with each shRNA-expressing retrovirus (200 μl) containing Polybrene (10 μg/ml) at 370 × g at room temperature for 90 min. Then, 24 h later, the transduction was repeated to enhance virus infection, and cells were incubated for a further 24 h in the presence of Polybrene. Cells were cultured for 2 to 3 weeks in medium containing puromycin (0.5 μg/ml) to kill nontransduced cells.
Generation of cells stably expressing the MV V protein.
To generate the retrovirus expressing the MV V protein, the cDNA encoding the V protein of the IC-B strain of MV was cloned into pMXs-GFP (a gift from M. Shimojima and T. Kitamura) (27), producing pMX-V. PLAT-gp cells were transfected with 20 μg of pMX-V and 2 μg of pCVSV-G using PEI-Max, and the retrovirus was recovered. Cells in 24-well plates were centrifuged with 200 μl of medium containing the retrovirus expressing the MV V protein and Polybrene (10 μg/ml) at 370 × g for 90 min at room temperature. At 24 h after transduction, culture medium was replaced with complete RPMI 1640 medium containing puromycin (0.5 μg/ml). A single clone was isolated from puromycin-resistant clones that formed colonies in methylcellulose medium. As a control, cells stably expressing EGFP were also generated as above.
MV infection.
Cells were infected with MV at a multiplicity of infection (MOI) of 0.1 or influenza virus PR8 at an MOI of 2 for 2 h at 37°C, washed with phosphate-buffered saline (PBS), and cultured with complete RPMI medium for 24 to 48 h. To inhibit caspase-1 activation, cells were pretreated with 5 μM Ac-YVAD-CHO (Bachem) for 30 min. The cells were then infected with MV in the presence of Ac-YVAD-CHO for 2 h at 37°C, washed with PBS, and cultured with complete RPMI medium containing Ac-YVAD-CHO.
Measurement of IL-1β and pro-IL-1β.
Cell-free supernatants were collected at 24 to 48 h postinfection, at 24 h following transfection with poly(dA·dT) (Invitrogen) or at 24 h after stimulation with LPS plus ATP. The supernatants were analyzed for the presence of IL-1β using an enzyme-linked immunosorbent assay (ELISA) utilizing paired antibodies (eBiosciences) (20). To measure intracellular pro-IL-1β, cells were lysed by repeated cycles of freezing and thawing in PBS containing 2% FBS, and the lysates were analyzed by ELISA (44).
Reconstitution of the NLRP3 inflammasome in HEK293T cells.
HEK293T cells grown to approximately 60% confluence in 24-well plates were transfected with 15 ng of pCA7-NLRP3, 5 ng of pCA7-ASC, 5 ng of pCA7-procaspase-1, 150 ng of pCA7-pro-IL-1β, and either 600 ng of pCA7-IC-V or 600 ng of pCA7-EGFP using PEI-Max. Cell-free supernatants were collected 24 h after transfection and analyzed for IL-1β by ELISA. Under this condition, exclusion of any one of the expression plasmids encoding ASC, procaspase-1, or pro-IL-1β led to no production of IL-1β (data not shown).
Coimmunoprecipitation and Western blot analyses.
Subconfluent monolayers of HEK293T cells in six-well plates were transfected with 6 μg of pCA7-Flag-NLRP3, 2 μg of pCA7-Flag-ASC, or 6 μg of pCA7-Flag-MDA5 together with 2 μg of pCAG-HA-IC-V, 2 μg of pCAG-HA-IC-Vn, 2 μg of pCA7-HA-orange-Vc, or 2 μg of pCA7-HA-orange-Vc(C272R). At 48 h posttransfection, the cells were washed with PBS and lysed in 1 ml of coimmunoprecipitation buffer [50 mM Tris (pH 8.0), 280 mM NaCl, 0.5% NP-40, 0.2 mM EDTA, 2 mM EGTA, 10% glycerol, 1 mM dithiothreitol (DTT)] (29) containing protease inhibitors (Sigma). The lysates were centrifuged at 20,630 × g for 90 min at 4°C. A small amount (50 μl) of each supernatant was mixed with sodium dodecyl sulfate (SDS) loading buffer (50 mM Tris [pH 6.8], 100 mM DTT, 2% SDS, 0.1% bromophenol blue, 10% glycerol) and boiled for 5 min. The rest of the supernatant was incubated for 90 min at 4°C with protein A-Sepharose (GE Healthcare AB), which had been pretreated with an anti-Flag (F1804; Sigma) or anti-HA (sc-7392; Santa Cruz) antibody for 90 min at 4°C. Complexes with the Sepharose were obtained by centrifugation and washed three times with coimmunoprecipitation buffer. The polypeptides in the precipitated complexes were fractionated by SDS-polyacrylamide gel electrophoresis (PAGE) using 10 to 15% polyacrylamide gel and electroblotted onto polyvinylidene difluoride (PVDF) membranes (Hybond-P; Amersham Biosciences). The membranes were incubated with anti-Flag (F7425; Sigma) or anti-HA (561; Medical & Biological Laboratories Co.) antibody, followed by incubation with horseradish peroxidase-conjugated anti-rabbit IgG (Invitrogen) for detection of the Flag-tagged or HA-tagged proteins.
To detect the cleaved form of IL-1β, the supernatant was incubated for 90 min at 4°C with Dynabeads Pan Mouse IgG (Invitrogen), which had been pretreated with a mouse monoclonal antibody against human IL-1β (clone 8516.311; R&D Systems, Inc.) for 90 min at 4°C. Complexes with the beads were obtained using magnets and washed with 1× RIPA buffer (150 mM NaCl, 0.1% SDS, 1% deoxycholate, 1% Triton X-100, and 10 mM Tris [pH 7.4]) (29). For immunoblotting, a mouse anti-human IL-1β monoclonal antibody (MAB201; R&D) was used in Can Get Signal solution 1 (Toyobo) followed by a horseradish peroxidase-conjugated secondary antibody diluted in Can Get Signal solution 2. To detect human NLRP3, RIG-I, β-actin, and MV V protein, cells were lysed in 1× RIPA buffer. The lysates were subjected to SDS-PAGE under reducing conditions and subsequently blotted using the antibodies to the following proteins: for human NLRP3, AG-20B-0014-C100 (AdipoGen); for human RIG-I, 54285 (AnaSpec); for β-actin, sc-8432 (Santa Cruz); and for MV V protein, rabbit antiserum (34). The PVDF membranes were treated with Chemi-Lumi One Super (Nacalai Tesque) to elicit chemiluminescent signals, and the signals were detected and visualized using a VersaDoc 3000 imager (Bio-Rad).
Immunofluorescence staining.
Cells were fixed and permeabilized with PBS containing 2.5% formaldehyde and 0.5% Triton X-100. The fixed cells were washed with PBS and then incubated with antibodies against NLRP3 (AG-20B-0014-C100) and MV V protein for 1 h at room temperature, followed by incubation with Alexa Fluor 488-conjugated donkey anti-mouse IgG (heavy plus light chain [H+L]; Invitrogen) and Alexa Fluor 594-conjugated donkey anti-rabbit IgG (H+L; Molecular Probes) for 1 h at room temperature. The stained cells were observed using a confocal microscope (Radiance 2100; Bio-Rad).
Statistics.
Data were analyzed for statistical significance using a two-tailed Student t test. P values less than 0.05 were considered statistically significant.
RESULTS
MV induces IL-1β in PMA-stimulated THP-1 cells.
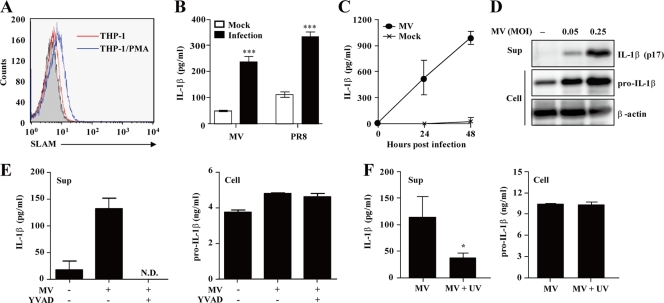
The human monocytic cell line THP-1 is differentiated into macrophage-like cells by stimulation with PMA (40, 62). THP-1 cells had little expression of SLAM, the principal cellular receptor for MV (60), but the SLAM expression level was elevated after stimulation with PMA (Fig. 1A). Infection of PMA-stimulated THP-1 cells (THP-1/PMA cells) with MV(IC323-EGFP) induced IL-1β secretion in the supernatant at 24 h postinfection, similar to that seen with the PR8 strain of influenza virus (1) (Fig. 1B). Secretion of IL-1β in MV-infected THP-1/PMA cells was increased in a time-dependent (Fig. 1C) and MOI-dependent (Fig. 1D) manner. Western blot analysis demonstrated that the p17 subunit, the mature processed form of IL-1β, was secreted in the supernatant (Fig. 1D). Furthermore, YVAD-CHO, a specific inhibitor of caspase-1, suppressed IL-1β production in MV-infected THP-1/PMA cells without affecting the amounts of pro-IL-1β in the cytosol (Fig. 1E). In order to examine whether viral replication is required to induce IL-1β, we infected THP-1/PMA cells with UV-treated or untreated MV. Infection with UV-treated MV failed to induce IL-1β secretion (Fig. 1F), indicating that viral particles or genomic RNA are not sufficient to stimulate IL-1β secretion. Together, these results indicate that infection with live MV causes caspase-1-dependent IL-1β secretion in THP-1/PMA cells.
Fig. 1.
Caspase-1-dependent IL-1β production by MV-infected THP-1 cells. (A) THP-1 and THP-1/PMA cells were stained with anti-SLAM antibody IPO-3 or mouse IgG1 control antibody (THP-1 cells, shaded histogram), followed by staining with fluorescein isothiocyanate-labeled anti-mouse IgG. (B and C) THP-1/PMA cells were infected with MV or influenza virus. Cell-free supernatants were collected at 24 h postinfection (B) or at the indicated time points (C) and analyzed for IL-1β using an ELISA. (D) Immunoblot analysis of the mature (p17) form of IL-1β in the supernatants (Sup) and pro-IL-1β in cell extracts (Cell) of the cells infected with MV at the indicated MOIs. (E) The effect of YVAD-CHO on IL-1β and pro-IL-1β production was determined in THP-1/PMA cells at 24 h after MV infection. N.D., not detected. (F) THP-1/PMA cells were inoculated with live or UV-inactivated MV. Cell-free supernatants and cell lysates were collected at 24 h postinfection and analyzed for IL-1β by ELISA. Values are means and standard deviations from triplicate samples. The data shown are representative of three experiments. *, P < 0.05; ***, P < 0.001.
MV activates the NLRP3 inflammasome.
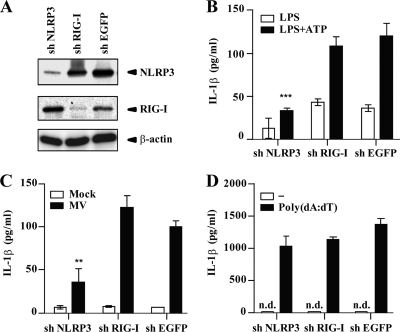
To evaluate individual contributions of NLRP3 and RIG-I inflammasomes to the induction of IL-1β after MV infection, we generated THP-1 cells stably expressing shRNA targeting human NLRP3 or RIG-I mRNA. Western blot analysis confirmed knockdown of NLRP3 and RIG-I in respective shRNA-expressing THP-1/PMA cells at 24 h after LPS stimulation (Fig. 2A). Expression levels of NLRP3 and RIG-I in knockdown cells were 16% and 18% (after normalization using expression levels of β-actin), compared with those in control cells, respectively. As expected, IL-1β secretion induced with LPS (an inducer of pro-IL-1β) plus ATP (an NLRP3 agonist), was significantly reduced in NLRP3 knockdown cells but not in RIG-I knockdown cells (Fig. 2B). NLRP3 knockdown but not RIG-I knockdown THP-1/PMA cells secreted a smaller amount of IL-1β in the supernatant at 24 h after MV infection than THP-1/PMA cells expressing a control shRNA (shEGFP) (Fig. 2C). Reduction of IL-1β production was not due to a general defect in the cytokine response of NLRP3 knockdown cells. After dsDNA-dependent activation of the AIM2 inflammasome, they produced a level of IL-1β comparable to that seen with control and RIG-I knockdown cells (Fig. 2D). These results indicate that the NLRP3 inflammasome, but not the RIG-I inflammasome, is involved in MV-induced IL-1β production in THP-1/PMA cells.
Fig. 2.
NLRP3 inflammasome activation by MV. (A) Expression levels of NLRP3 and RIG-I in PMA-stimulated THP-1 cells expressing shRNA targeting NLRP3, RIG-I, or EGFP mRNAs were examined by Western blot analysis at 24 h after LPS (1 μg/ml) stimulation. β-Actin was used as an internal control. (B to D) PMA-stimulated THP-1 cells expressing shRNA targeting mRNAs of NLRP3, RIG-I, or EGFP were treated with LPS (5 ng/ml) or LPS (5 ng/ml) plus ATP (5 mM) (B), MV (C), or poly(dA·dT) (400 ng/ml) (D). Cell-free supernatants were collected 24 h after treatment and analyzed for IL-1β by ELISA. n.d., not detected. Values are means and standard deviations from triplicate samples. The data shown are representative of at least three experiments. **, P < 0.01; ***, P < 0.001.
MV V protein inhibits MV-induced IL-1β secretion.
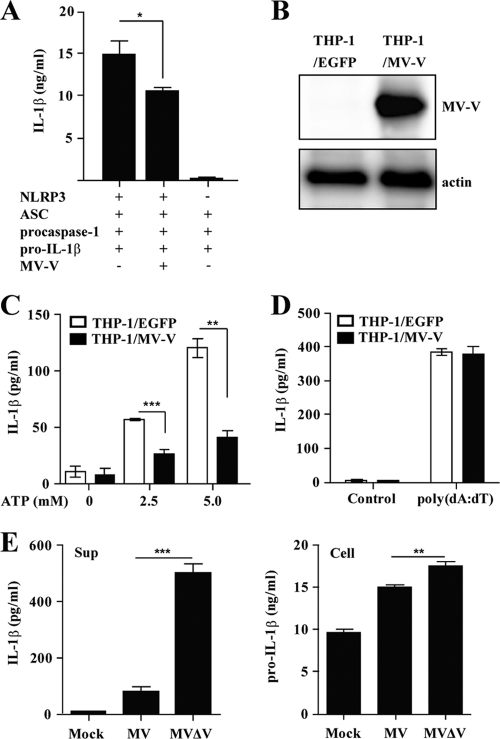
The MV V protein counteracts the IFN-α/β-induced antiviral activity through several different mechanisms (2, 9, 11, 38, 47, 59, 64). The inflammasomes, along with the IFN system, play an important role in innate immunity, and we suspected that the V protein may also possess the activity to block inflammasome activation. To test this hypothesis, two sets of experiments were performed. First, we examined the effect of the V protein on IL-1β secretion of HEK293T cells transiently transfected with plasmids encoding components of the NLRP3 inflammasome: NLRP3, ASC, procaspase-1, and pro-IL-1β (10). In this experiment, HEK293T cells were used, as they are human cells that can be transfected at a high efficiency. Furthermore, HEK293T cells are deficient in endogenous ASC and NLRP3 (5). Reconstitution of the NLRP3 inflammasome resulted in IL-1β secretion by HEK293T cells, in which NLRP3 was absolutely required (Fig. 3A). Inclusion of the MV V protein inhibited IL-1β secretion by ∼30%. Second, we established THP-1 cells stably expressing the MV V protein (THP-1/MV-V cells) (Fig. 3B). THP-1/MV-V/PMA cells secreted significantly smaller amounts of IL-1β in response to LPS plus ATP than control cells (THP-1/EGFP/PMA cells) (Fig. 3C), indicating that the V protein blocks NLRP3 inflammasome activation. In contrast, IL-1β production in response to poly(dA·dT) was comparable between THP-1/MV-V/PMA and control cells (Fig. 3D), indicating that the IL-1β secretion pathway, including the level of pro-IL-1β, is not generally affected in THP-1/MV-V/PMA cells. Thus, the inhibitory activity of the V protein appears to be specific for the NLRP3 inflammasome.
Fig. 3.
Inhibition of NLRP3 inflammasome-mediated IL-1β secretion by the MV V protein. (A) HEK293T cells were transfected with expression plasmids encoding NLRP3, ASC, procaspase-1, and pro-IL-1β, together with the expression plasmid encoding the MV V protein or control plasmid (−). Cell-free supernatants were collected 24 h after transfection, and analyzed for IL-1β by ELISA. (B) Expression of the V protein in THP-1 cells stably expressing the MV V protein or EGFP was examined by Western blot analysis. (C and D) PMA-stimulated THP-1 cells stably expressing the MV V protein or EGFP were stimulated with LPS (5 ng/ml) plus ATP (0, 2.5, or 5 mM) (C) or poly(dA·dT) (400 ng/ml) (D). (E) THP-1/PMA cells were infected with wild-type MV or MVΔV virus. Cell-free supernatants (Sup) and cell lysates (Cell) were collected at 24 h postinfection and analyzed for IL-1β and pro-IL-1β, respectively, by ELISA. Values are means and standard deviations from triplicate samples. The data shown are representative of three experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Next, we examined IL-1β production in THP-1/PMA cells infected with MV(IC323-EGFP) and its mutant recombinant virus lacking the V protein (MVΔV) (22). As expected, the amount of IL-1β in the supernatant at 24 h after MVΔV infection was about 5-fold higher than that after MV infection (Fig. 3E). The modest increase in the amount of intracellular pro-IL-1β in MVΔV-infected cells alone probably does not explain the increase in IL-1β secretion. Taken together, all these results indicate that the MV V protein blocks NLRP3 inflammasome activation.
MV V protein interacts with NLRP3.
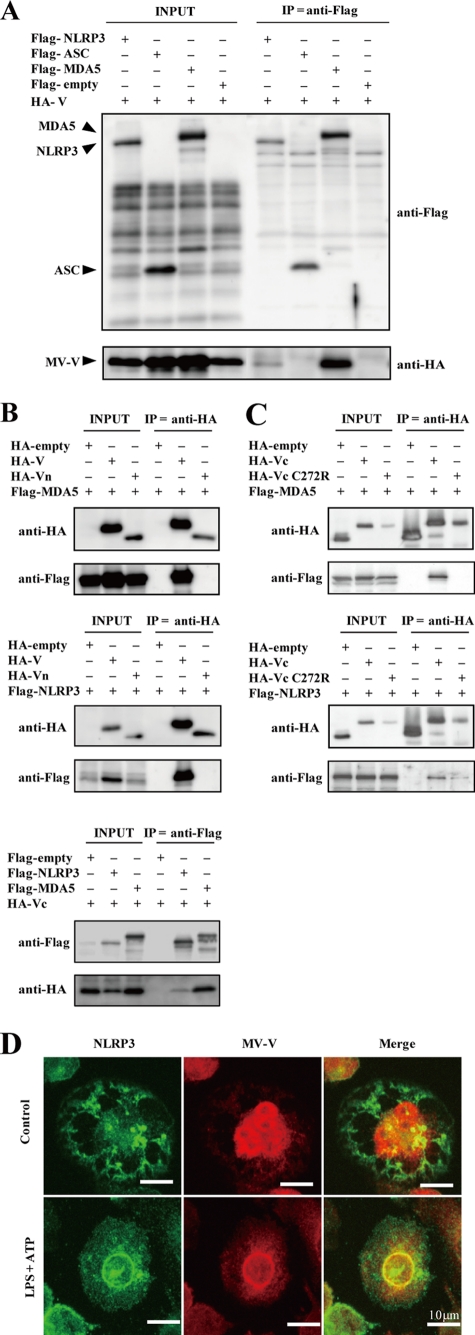
To gain insight into the mechanism by which the V protein inhibits NLRP3 inflammasome activation, coimmunoprecipitation experiments were performed using Flag- and HA-tagged proteins in HEK293T cells, which can be transfected at a high efficiency (Fig. 4A). The MV V protein has been shown to interact with MDA5 (2), and this interaction was used as a positive control. The V protein was found to coimmunoprecipitate with NLRP3 when HEK293T cells were transfected with plasmids encoding these two proteins (Fig. 4A). However, the V protein did not coimmunoprecipitate with ASC, a component of the NLRP3 inflammasome. To further dissect the interaction between the V protein and NLRP3, Vn and Vc were examined for their activity. Consistent with previous reports (2, 39), the full-length V protein and Vc, but not Vn, interacted with MDA5 (Fig. 4B, top and bottom). Similarly, the V protein and Vc, but not Vn, coimmunoprecipitated with NLRP3 (Fig. 4B, middle and bottom). Vc with the C272R substitution, which is unable to bind to MDA5 (57), was expressed at a reduced level compared with the intact Vc but nevertheless coimmunoprecipitated with NLRP3 (Fig. 4C), indicating that the site on the V protein used to interact with NLRP3 is different from that used to interact with MDA5.
Fig. 4.
Interaction of the MV V protein with NLRP3. (A) HEK293T cells were transfected with expression plasmids encoding HA-tagged V protein and either Flag-tagged NLPR3, ASC, MDA5, or empty vector. Proteins were immunoprecipitated 48 h later with anti-Flag antibody, followed by immunoblot analysis of total lysates (INPUT) and immunoprecipitates (IP) with indicated antibodies (anti-Flag or anti-HA). (B) V, Vn (top and middle), and Vc (bottom) were examined for their interaction with NLRP3 as described for panel A. (C) Vc and Vc with the C272R substitution (VcC272R) were examined for their interaction with NLRP3 as described for panel A. Vc and VcC272R were expressed as fusion proteins with kusabira orange, so that the control HA-empty plasmid directed the production of the kusabira orange peptide, which was detected with anti-HA-tag antibody. (D) PMA-stimulated THP-1/MV-V cells were treated with LPS (5 ng/ml) and ATP (5 mM) or left untreated for 6 h and examined for the localization of NLRP3 and MV V proteins. The data shown are representative of at least three experiments.
Next, we examined intracellular localizations of the V protein and NLRP3 in THP-1/MV-V/PMA cells. Under nonstimulatory conditions, most of the NLRP3 protein was found to be localized in granular cytoplasmic structures (Fig. 4D). Consistent with a previous report (38), the MV V protein was found in both the nucleus and cytoplasm. This pattern of localization greatly changed after NLRP3 inflammasome activation by LPS plus ATP. NLRP3 was relocated to the perinuclear region and colocalized with the V protein.
DISCUSSION
In the present study, we show that MV-infected human macrophage-like THP-1 cells secrete IL-1β through the activation of the NLRP3 inflammasome and that the MV V protein has the ability to interact with NLRP3 through its C-terminal domain, thereby inhibiting NRLP3 inflammasome-mediated IL-1β secretion.
A number of viruses induce the production of IL-1β in infected cells. Infection with RNA viruses such as influenza virus (1, 19, 20, 61) and EMCV (44, 45) has been shown to cause IL-1β secretion via NLRP3 inflammasome activation. Poeck et al. reported that VSV or 5′-triphosphate RNA triggers RIG-I inflammasome activation and IL-1β secretion (44); however, a recent report demonstrated that VSV activates the NLRP3 inflammasome independently of RIG-I and MDA5 (45). Our results also show that IL-1β secretion in MV-infected cells is mediated by the NLRP3 inflammasome but not the RIG-I inflammasome. Thus, the NLRP3 inflammasome appears to play a central role in caspase-1-dependent IL-1β secretion after infection with RNA viruses.
Although RIG-1 and AIM2 are known to recognize viral nucleic acids, how NLRP3 detects viruses has not been clearly shown. In our experiments, UV-inactivated MV did not induce IL-1β in infected cells, suggesting that newly produced MV transcripts, genomic RNA, and/or proteins are responsible for NLRP3 inflammasome activation. Because expression of the influenza virus M2 protein alone can cause NLRP3 inflammasome activation (20), the disturbance in the intracellular ionic milieu accompanying viral infection rather than viral nucleic acids may be important in this process.
Viruses usually possess strategies to counteract the IFN response (14, 25, 48). Several viruses are also known to encode proteins that interfere with IL-1β production. For example, myxoma and Shope fibroma viruses encode the pyrin domain-containing protein that interacts with ASC and inhibits inflammasome activation (23). Vaccinia, cowpox, and myxoma viruses encode the serpin-like protease inhibitor, which specifically binds to caspase-1 and inhibits pro-IL-1β cleavage (26, 28, 41). Furthermore, the influenza virus NS-1 protein prevents caspase-1 activation and IL-1β production by unknown mechanisms (56). Our data indicated that the MV V protein interacts with NLRP3 through its C-terminal domain and inhibits NLRP3 inflammasome-mediated IL-1β secretion. Because the MV V protein did not inhibit dsDNA-dependent AIM2 inflammasome activation, it must act specifically on NLRP3. A BLASTP (Basic Local Alignment Search Tool for Protein) search revealed that the MV V protein and NLRP3 do not have any homologous regions, indicating that the interaction is not dependent on the homologous conserved sequences.
Recently, IFN-α/β signaling was shown to inhibit NLRP1 and NLRP3 inflammasome activation via the STAT1 transcription factor (16). As NLRP3 inflammasome-dependent IL-1β maturation occurs in MV-infected THP-1 cells, MV(IC323-EGFP) may induce only marginal amounts of IFN-α/β insufficient to inhibit NLRP3 inflammasome activation. Alternatively, IFN signaling may be sufficiently suppressed by MV P, V, and/or C proteins. In that case, it might be expected that MVΔV fails to activate the NLRP3 inflammasome, because the V protein blocks STAT1 phosphorylation (9, 11, 59, 64), and its absence in MVΔV would lead to enhanced IFN signaling. However, infection with MVΔV led to increased IL-1β secretion (Fig. 3E). This is explained by our finding that the V protein inhibits NLRP3 inflammasome activation by interacting with NLRP3. The MV V protein comprises Vn and Vc and has many interacting partners in host cells. Vn interacts with STAT1 and Jak1 (9), whereas Vc does so with NLRP3, MDA5 (2), STAT2 (38, 47), IKKα (42), and NF-κB p65 (53). All these partner proteins are involved in antiviral responses, and the V protein interacts with these proteins differently, as demonstrated by the V protein with the C272R substitution. Based on the crystal structure of the parainfluenza virus 5 (PIV5) V protein (31), it was predicted that MV Vc also forms two loops that coordinate two zinc ions via one histidine and seven cysteine residues (8, 46). The first loop is likely formed by H232, C251, C276, and C279, and the second loop by the central cysteines (C255, C267, C269, and C272). The substitution of C251, C255, and C272 in the MV V protein has been reported to disrupt its interaction with MDA5 (46, 57), suggesting that both loops are important for the interaction of the MV V protein with MDA5. On the other hand, our results showed that a mutation in C272 does not affect the interaction of Vc with NLRP3. This may suggest that the intact first loop of Vc is sufficient for its interaction with NLRP3. Furthermore, two conserved tryptophan residues (W240 and W250), F246, and D248 have been shown to be critical for the interaction of the MV V protein with STAT2 (8, 47). Taken together, these data indicate that Vc is likely to interact with a variety of host proteins through its multiple interfaces.
The V protein inhibits NF-κB as well as MDA5, which is involved in the production of NF-κB and IFN-α/β. Importantly, NF-κB signaling leads to the upregulated transcription of pro-IL-1β (3). Thus, the MV V protein may inhibit NLRP3 inflammasome activation not only by interacting with NLRP3 but also by suppressing NF-κB p65 and MDA5. During the course of MV's coevolution with the host, the V protein may have acquired the ability to interact with and modulate various host proteins such that the virus has gained better fitness.
IL-1β-treated dendritic cells show an enhanced capability to stimulate T cells (30). Furthermore, proper inflammasome activation is found to be important in combating viral and bacterial infections (4, 19, 21, 24). These observations suggest that inhibition of inflammasome activation by the MV V protein may partly account for transient severe immunosuppression during measles (15). In vivo studies using SLAM transgenic or knock-in mice and recombinant viruses such as MVΔV would provide important insights into mechanisms underlying immunological alterations caused by MV.
ACKNOWLEDGMENTS
We thank the staff of the Research Support Center, Graduate School of Medical Sciences, Kyushu University for their technical support.
This work was supported by grants from the Ministry of Education, Culture, Sports, Science, and Technology, and the Ministry of Health, Labor, and Welfare of Japan, and the Kanae Foundation for the Promotion of Medical Science.
Footnotes
Published ahead of print on 12 October 2011.
REFERENCES
- 1. Allen I. C., et al. 2009. The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA. Immunity 30: 556–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Andrejeva J., et al. 2004. The V proteins of paramyxoviruses bind the IFN-inducible RNA helicase, mda-5, and inhibit its activation of the IFN-beta promoter. Proc. Natl. Acad. Sci. U. S. A. 101: 17264–17269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bauernfeind F., et al. 2011. Inflammasomes: current understanding and open questions. Cell. Mol. Life Sci. 68: 765–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brodsky I. E., Monack D. 2009. NLR-mediated control of inflammasome assembly in the host response against bacterial pathogens. Semin. Immunol. 21: 199–207 [DOI] [PubMed] [Google Scholar]
- 5. Bryan N. B., Dorfleutner A., Rojanasakul Y., Stehlik C. 2009. Activation of inflammasomes requires intracellular redistribution of the apoptotic speck-like protein containing a caspase recruitment domain. J. Immunol. 182: 3173–3182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bryce J., Boschi-Pinto C., Shibuya K., Black R. E. 2005. WHO estimates of the causes of death in children. Lancet 365: 1147–1152 [DOI] [PubMed] [Google Scholar]
- 7. Burckstummer T., et al. 2009. An orthogonal proteomic-genomic screen identifies AIM2 as a cytoplasmic DNA sensor for the inflammasome. Nat. Immunol. 10: 266–272 [DOI] [PubMed] [Google Scholar]
- 8. Caignard G., Bourai M., Jacob Y., Tangy F., Vidalain P. O. 2009. Inhibition of IFN-alpha/beta signaling by two discrete peptides within measles virus V protein that specifically bind STAT1 and STAT2. Virology 383: 112–120 [DOI] [PubMed] [Google Scholar]
- 9. Caignard G., et al. 2007. Measles virus V protein blocks Jak1-mediated phosphorylation of STAT1 to escape IFN-alpha/beta signaling. Virology 368: 351–362 [DOI] [PubMed] [Google Scholar]
- 10. Chuang Y. T., et al. 2011. Tumor suppressor death-associated protein kinase is required for full IL-1β production. Blood 117: 960–970 [DOI] [PubMed] [Google Scholar]
- 11. Devaux P., von Messling V., Songsungthong W., Springfeld C., Cattaneo R. 2007. Tyrosine 110 in the measles virus phosphoprotein is required to block STAT1 phosphorylation. Virology 360: 72–83 [DOI] [PubMed] [Google Scholar]
- 12. Fernandes-Alnemri T., Yu J. W., Datta P., Wu J., Alnemri E. S. 2009. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature 458: 509–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fontana J. M., Bankamp B., Bellini W. J., Rota P. A. 2008. Regulation of interferon signaling by the C and V proteins from attenuated and wild-type strains of measles virus. Virology 374: 71–81 [DOI] [PubMed] [Google Scholar]
- 14. Gotoh B., Komatsu T., Takeuchi K., Yokoo J. 2002. Paramyxovirus strategies for evading the interferon response. Rev. Med. Virol. 12: 337–357 [DOI] [PubMed] [Google Scholar]
- 15. Griffin D. E. 2007. Measles virus, p. 1551–1585 In Knipe D. M., Howley P. M., Griffin D. E., Lamb R. A., Martin M. A., Roizman B., Straus S. E. (ed.), Fields virology, 5th ed. Lippincott Williams & Wilkins, Philadelphia, Pa [Google Scholar]
- 16. Guarda G., et al. 2011. Type I interferon inhibits interleukin-1 production and inflammasome activation. Immunity 34: 213–223 [DOI] [PubMed] [Google Scholar]
- 17. Hashimoto K., et al. 2002. SLAM (CD150)-independent measles virus entry as revealed by recombinant virus expressing green fluorescent protein. J. Virol. 76: 6743–6749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hornung V., et al. 2009. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature 458: 514–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ichinohe T., Lee H. K., Ogura Y., Flavell R., Iwasaki A. 2009. Inflammasome recognition of influenza virus is essential for adaptive immune responses. J. Exp. Med. 206: 79–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ichinohe T., Pang I. K., Iwasaki A. 2010. Influenza virus activates inflammasomes via its intracellular M2 ion channel. Nat. Immunol. 11: 404–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ichinohe T., et al. 2011. Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc. Natl. Acad. Sci. U. S. A. 108: 5354–5359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ikegame S., et al. 2010. Both RIG-I and MDA5 RNA helicases contribute to the induction of alpha/beta interferon in measles virus-infected human cells. J. Virol. 84: 372–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Johnston J. B., et al. 2005. A poxvirus-encoded pyrin domain protein interacts with ASC-1 to inhibit host inflammatory and apoptotic responses to infection. Immunity 23: 587–598 [DOI] [PubMed] [Google Scholar]
- 24. Kanneganti T. D. 2010. Central roles of NLRs and inflammasomes in viral infection. Nat. Rev. Immunol. 10: 688–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Katze M. G., He Y., Gale M., Jr 2002. Viruses and interferon: a fight for supremacy. Nat. Rev. Immunol. 2: 675–687 [DOI] [PubMed] [Google Scholar]
- 26. Kettle S., et al. 1997. Vaccinia virus serpin B13R (SPI-2) inhibits interleukin-1β-converting enzyme and protects virus-infected cells from TNF- and Fas-mediated apoptosis, but does not prevent IL-1β-induced fever. J. Gen. Virol. 78 (Pt. 3): 677–685 [DOI] [PubMed] [Google Scholar]
- 27. Kitamura T., Morikawa Y. 2000. Isolation of T-cell antigens by retrovirus-mediated expression cloning. Methods Mol. Biol. 134: 143–152 [DOI] [PubMed] [Google Scholar]
- 28. Komiyama T., et al. 1994. Inhibition of interleukin-1 beta converting enzyme by the cowpox virus serpin CrmA. An example of cross-class inhibition. J. Biol. Chem. 269: 19331–19337 [PubMed] [Google Scholar]
- 29. Kraus T. A., Garza L., Horvath C. M. 2008. Enabled interferon signaling evasion in an immune-competent transgenic mouse model of parainfluenza virus 5 infection. Virology 371: 196–205 [DOI] [PubMed] [Google Scholar]
- 30. Kruse M., et al. 2001. Signaling lymphocytic activation molecule is expressed on mature CD83+ dendritic cells and is up-regulated by IL-1β. J. Immunol. 167: 1989–1995 [DOI] [PubMed] [Google Scholar]
- 31. Li T., Chen X., Garbutt K. C., Zhou P., Zheng N. 2006. Structure of DDB1 in complex with a paramyxovirus V protein: viral hijack of a propeller cluster in ubiquitin ligase. Cell 124: 105–117 [DOI] [PubMed] [Google Scholar]
- 32. Martinon F., Mayor A., Tschopp J. 2009. The inflammasomes: guardians of the body. Annu. Rev. Immunol. 27: 229–265 [DOI] [PubMed] [Google Scholar]
- 33. Morita S., Kojima T., Kitamura T. 2000. Plat-E: an efficient and stable system for transient packaging of retroviruses. Gene Ther. 7: 1063–1066 [DOI] [PubMed] [Google Scholar]
- 34. Nakatsu Y., et al. 2008. Measles virus circumvents the host interferon response by different actions of the C and V proteins. J. Virol. 82: 8296–8306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Niwa H., Yamamura K., Miyazaki J. 1991. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene 108: 193–199 [DOI] [PubMed] [Google Scholar]
- 36. Ohno S., Ono N., Takeda M., Takeuchi K., Yanagi Y. 2004. Dissection of measles virus V protein in relation to its ability to block alpha/beta interferon signal transduction. J. Gen. Virol. 85: 2991–2999 [DOI] [PubMed] [Google Scholar]
- 37. Ono N., et al. 2001. Measles viruses on throat swabs from measles patients use signaling lymphocytic activation molecule (CDw150) but not CD46 as a cellular receptor. J. Virol. 75: 4399–4401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Palosaari H., Parisien J. P., Rodriguez J. J., Ulane C. M., Horvath C. M. 2003. STAT protein interference and suppression of cytokine signal transduction by measles virus V protein. J. Virol. 77: 7635–7644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Parisien J. P., et al. 2009. A shared interface mediates paramyxovirus interference with antiviral RNA helicases MDA5 and LGP2. J. Virol. 83: 7252–7260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Park E. K., et al. 2007. Optimized THP-1 differentiation is required for the detection of responses to weak stimuli. Inflamm. Res. 56: 45–50 [DOI] [PubMed] [Google Scholar]
- 41. Petit F., et al. 1996. Characterization of a myxoma virus-encoded serpin-like protein with activity against interleukin-1 beta-converting enzyme. J. Virol. 70: 5860–5866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pfaller C. K., Conzelmann K. K. 2008. Measles virus V protein is a decoy substrate for IκB kinase alpha and prevents Toll-like receptor 7/9-mediated interferon induction. J. Virol. 82: 12365–12373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Plumet S., et al. 2007. Cytosolic 5′-triphosphate ended viral leader transcript of measles virus as activator of the RIG I-mediated interferon response. PLoS One 2: e279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Poeck H., et al. 2010. Recognition of RNA virus by RIG-I results in activation of CARD9 and inflammasome signaling for interleukin 1β production. Nat. Immunol. 11: 63–69 [DOI] [PubMed] [Google Scholar]
- 45. Rajan J. V., Rodriguez D., Miao E. A., Aderem A. 2011. The NLRP3 inflammasome detects encephalomyocarditis virus and vesicular stomatitis virus infection. J. Virol. 85: 4167–4172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ramachandran A., Horvath C. M. 2010. Dissociation of paramyxovirus interferon evasion activities: universal and virus-specific requirements for conserved V protein amino acids in MDA5 interference. J. Virol. 84: 11152–11163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ramachandran A., Parisien J. P., Horvath C. M. 2008. STAT2 is a primary target for measles virus V protein-mediated alpha/beta interferon signaling inhibition. J. Virol. 82: 8330–8338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Randall R. E., Goodbourn S. 2008. Interferons and viruses: an interplay between induction, signalling, antiviral responses and virus countermeasures. J. Gen. Virol. 89: 1–47 [DOI] [PubMed] [Google Scholar]
- 49. Rathinam V. A., et al. 2010. The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat. Immunol. 11: 395–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Roberts T. L., et al. 2009. HIN-200 proteins regulate caspase activation in response to foreign cytoplasmic DNA. Science 323: 1057–1060 [DOI] [PubMed] [Google Scholar]
- 51. Schlender J., et al. 2005. Inhibition of Toll-like receptor 7- and 9-mediated alpha/beta interferon production in human plasmacytoid dendritic cells by respiratory syncytial virus and measles virus. J. Virol. 79: 5507–5515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Schroder K., Zhou R., Tschopp J. 2010. The NLRP3 inflammasome: a sensor for metabolic danger? Science 327: 296–300 [DOI] [PubMed] [Google Scholar]
- 53. Schuhmann K. M., Pfaller C. K., Conzelmann K. K. 2011. The measles virus V protein binds to p65 (RelA) to suppress NF-κB activity. J. Virol. 85: 3162–3171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Shaffer J. A., Bellini W. J., Rota P. A. 2003. The C protein of measles virus inhibits the type I interferon response. Virology 315: 389–397 [DOI] [PubMed] [Google Scholar]
- 55. Shirogane Y., et al. 2010. Epithelial-mesenchymal transition abolishes the susceptibility of polarized epithelial cell lines to measles virus. J. Biol. Chem. 285: 20882–20890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Stasakova J., et al. 2005. Influenza A mutant viruses with altered NS1 protein function provoke caspase-1 activation in primary human macrophages, resulting in fast apoptosis and release of high levels of interleukins 1β and 18. J. Gen. Virol. 86: 185–195 [DOI] [PubMed] [Google Scholar]
- 57. Takaki H., et al. 2011. Strain-to-strain difference of V protein of measles virus affects MDA5-mediated IFN-beta-inducing potential. Mol. Immunol. 48: 497–504 [DOI] [PubMed] [Google Scholar]
- 58. Takeda M., et al. 2005. Long untranslated regions of the measles virus M and F genes control virus replication and cytopathogenicity. J. Virol. 79: 14346–14354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Takeuchi K., Kadota S. I., Takeda M., Miyajima N., Nagata K. 2003. Measles virus V protein blocks interferon (IFN)-alpha/beta but not IFN-gamma signaling by inhibiting STAT1 and STAT2 phosphorylation. FEBS Lett. 545: 177–182 [DOI] [PubMed] [Google Scholar]
- 60. Tatsuo H., Ono N., Tanaka K., Yanagi Y. 2000. SLAM (CDw150) is a cellular receptor for measles virus. Nature 406: 893–897 [DOI] [PubMed] [Google Scholar]
- 61. Thomas P. G., et al. 2009. The intracellular sensor NLRP3 mediates key innate and healing responses to influenza A virus via the regulation of caspase-1. Immunity 30: 566–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Tsuchiya S., et al. 1982. Induction of maturation in cultured human monocytic leukemia cells by a phorbol diester. Cancer Res. 42: 1530–1536 [PubMed] [Google Scholar]
- 63. Willingham S. B., et al. 2007. Microbial pathogen-induced necrotic cell death mediated by the inflammasome components CIAS1/cryopyrin/NLRP3 and ASC. Cell Host Microbe 2: 147–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Yokota S., et al. 2003. Measles virus suppresses interferon-alpha signaling pathway: suppression of Jak1 phosphorylation and association of viral accessory proteins, C and V, with interferon-alpha receptor complex. Virology 306: 135–146 [DOI] [PubMed] [Google Scholar]