Abstract
Epstein-Barr Virus (EBV) is an ubiquitous human herpesvirus which can lead to infectious mononucleosis and different cancers. In immunocompromised individuals, this virus is a major cause for morbidity and mortality. Transplant patients who did not encounter EBV prior to immunosuppression frequently develop EBV-associated malignancies, but a prophylactic EBV vaccination might reduce this risk considerably. Virus-like particles (VLPs) mimic the structure of the parental virus but lack the viral genome. Therefore, VLPs are considered safe and efficient vaccine candidates. We engineered a dedicated producer cell line for EBV-derived VLPs. This cell line contains a genetically modified EBV genome which is devoid of all potential viral oncogenes but provides viral proteins essential for the assembly and release of VLPs via the endosomal sorting complex required for transport (ESCRT). Human B cells readily take up EBV-based VLPs and present viral epitopes in association with HLA molecules to T cells. Consequently, EBV-based VLPs are highly immunogenic and elicit humoral and strong CD8+ and CD4+ T cell responses in vitro and in a preclinical murine model in vivo. Our findings suggest that VLP formulations might be attractive candidates to develop a safe and effective polyvalent vaccine against EBV.
INTRODUCTION
Epstein-Barr Virus (EBV) infects more than 90% of the human population worldwide and persists for life. Primary infection normally occurs during early childhood and is usually asymptomatic, but EBV causes a lymphoproliferative syndrome termed infectious mononucleosis (IM) in about 50% of adolescents and adults on their first contact with EBV (40). Although the disease is normally self-limiting, prolonged forms of IM (52) and chronic active EBV infection with a fatal outcome have been reported (30). IM also significantly increases the risk of developing Hodgkin's disease and other types of lymphoma (14) and is an independent risk factor for multiple sclerosis (51). In addition, EBV is causally associated with a heterogeneous group of malignancies, including undifferentiated nasopharyngeal carcinoma, gastric carcinoma, and various types of lymphoma (29). As a consequence, the International Agency for Research on Cancer (IARC) classifies the virus as a class I carcinogen (1).
Opportunistic infections represent severe complications in patients with HIV/AIDS and patients treated with immunosuppressive drugs. Transplant recipients who are immunologically naïve for EBV at the onset of iatrogenic immuno- suppression are at a particularly high risk of developing life-threatening EBV-positive posttransplant lymphoproliferative disease (PTLD). The exposure to immunosuppressive drugs impairs T cell immunity, and the patients are unable to effectively prime EBV-specific T cells, which control and prevent proliferation of EBV-infected B cells in vivo. Patients who are EBV seropositive at transplant have a much lower risk for PTLD (see reference 12 for a review), and the adoptive transfer of EBV-specific effector T cells can sometimes cure this life-threatening disease (18, 33), demonstrating the essential role of EBV-specific T cells in eliminating virally infected B cells and controlling infections with EBV.
These EBV-associated diseases provide a strong case for developing an EBV vaccine, which must be both safe and efficient in coping with subsequent EBV infections and preventing EBV-associated diseases (see reference 35 for a review). First trials in humans, showing a delay of wild-type EBV infection, were performed in the 1990s with a recombinant vaccinia virus expressing the major EBV membrane glycoprotein, gp350/220 (15). More recently, different prophylactic vaccines, based on peptide epitopes derived from EBNA3a (11) and gp350 (36, 47), aiming at seroconversion and the prevention of IM were tested in healthy volunteers. These vaccines induced EBV-neutralizing antibodies (36, 47) or CD8+-specific T cell responses (11) and protected healthy volunteers from developing IM but not from EBV infection per se. In a phase I clinical study in children awaiting kidney transplantation, adequate gp350 antibody responses were obtained, but insufficient B cell memory was achieved, probably due to the weak adjuvant used (38). Thus, it is currently not known whether this vaccine also prevents the development of posttransplant lymphoproliferative disorder.
Previously, we described HEK293 cell-based EBV packaging cell lines that harbor helper EBV genomes for the generation of EBV-based viral gene vectors (9, 19). Transient cotransfection of these cell lines with an expression plasmid encoding BZLF1, the viral transactivator of the lytic cycle (16), and appropriate vector plasmid DNA results in the induction of EBV's lytic phase and concomitant release of recombinant viral vectors with potential to efficiently deliver genes of interest to human B lymphocytes (17, 19). The first-generation packaging cell line, termed TR-2/293, contained a helper genome that lacked the packaging element TR essential for encapsidation and thus did not release detectable amounts of infectious helper EBV (9). However, it remained unclear whether particles from TR-2/293 are entirely free of viral DNA due to illegitimate recombination and thus retained transformation capacity (13). In order to meet these major safety issues, we generated a next-generation packaging cell line, termed 293-VII+, in that we additionally deleted or functionally inactivated six viral genes (EBNA2, LMP1, EBNA3A, -B, and -C, BZLF1) which contribute to the transformation of human B cell or virus synthesis (19). We show here that these packaging cell lines are also appropriate producer cells for virus-like particles (VLPs) which maintain key characteristics of EBV, such as B cell tropism and antigenicity. In the absence of packagable vector DNA and upon induction of the viral productive phase, the producer cells assemble and release VLPs via the endosomal sorting complex required for transport (ESCRT). VLPs are free of detectable amounts of viral DNA but equipped with glycoproteins and tegument proteins also present in infectious EBV virion particles (24). EBV-based VLPs exclusively bind to human B cells via the genuine viral surface receptor CD21, are highly immunogenic in vitro, and elicit multivalent EBV-specific neutralizing humoral and cellular immune responses in vivo.
In summary, we present the first prophylactic EBV vaccine based on VLPs that may help to reduce the risk for IM and EBV-associated malignant diseases.
MATERIALS AND METHODS
Construction of 293-VII+ cell line.
The construction of the first-generation packaging cell line, TR-2/293, and its successor, 293-VII+, and the BZLF1 expression plasmid p509 have been described elsewhere (9, 16, 19).
Generation and purification of VLPs from 293-VII+ cells and exosomes from HEK293 cells.
Three days after induction of the viral lytic cycle, cell-free culture supernatants containing VLPs from 293-VII+ cells or exosomes from HEK293 cells were collected and purified to yield high titers of purified VLPs or exosomes. Supernatants were subjected to sequential centrifugation steps (300 × g for 10 min, 5,000 × g for 10 min, 100,000 × g for 120 min). The pelleted VLPs or exosomes were washed and resuspended in 500 μl phosphate-buffered saline (PBS). Highly purified VLP preparations were obtained by floating VLPs into OptiPrep gradients. VLPs floated at densities of between 1.03 and 1.08, corresponding to 1.13 to 1.18 in a sucrose gradient. The VLP protein content was analyzed in a Lowry microassay (Bio-Rad, Munich, Germany). Expression plasmids for tsg101, vps4a, vps4b, and dominant-negative mutants thereof were kindly provided by E. Freed and J. Yasuda and are described elsewhere (10, 53). A PCR on VLPs from 293-VII+ cells and virions from EBV strain 2089-infected cells were performed using the BZLF1-specific primers 5′-CAGCAGCAGCAGTGGTGTTTG-3′ (forward) and 5′-AAGCCACCCGATTCTTGTATCG-3′ (backward).
Binding assays and flow cytometry.
Raji cells or primary B cells were cultivated with 300 μg of VLPs in a final volume of 2 ml for 2 days. Green fluorescent protein (GFP) fluorescence as a result of VLP binding to the cells was measured by flow cytometry. The inhibitory gp350-specific antibody 72A1 (43) was provided by E. Kremmer (Munich, Germany), and the inhibitory CD21 antibody FE8 was provided by W. Prodinger (Innsbruck, Austria). Other antibodies were purchased from Immunotools (Friesoyte, Germany). EBV-specific CD8+ T cells were quantified with uncoupled Pro5 pentamers for LMP2A (CLGGLLTMV, HLA A2 [28]; FLYALALLL, HLA A2 [28]), BRLF1 (YVLDHLIVV, HLA A2 [44]), BZLF1 (RAKFKQLL, HLA B8 [44]; EPLPQGQLTAY, HLA B35 [44]), and the cellular protein HER2 (KIFGSLAFL, HLA A2 [39]; the three underlined amino acids in each sequence define how the epitope is termed; e.g., the epitope FLYALALLL is referred to as “FLY” throughout), together with Pro5-phycoerythrin (PE) fluorotag (Proimmune). The PE-coupled HLA A2-restricted tetramer GLCTLVAML (Beckman Coulter) (48) was used for BMLF1-specific CD8+ T cells.
Stimulation of T cells.
Peripheral blood mononuclear cells (PBMCs) from EBV-seropositive donors were isolated and cultured in 24-well cell culture plates. CD19+ B cells and CD3+ T cells were isolated from PBMCs using CD19 and CD3 MicroBeads, respectively (Miltenyi, Bergisch-Gladbach, Germany). Where indicated, PBMCs or subfractions were lethally irradiated (50 Gy), loaded with 50 μg of 293-VII+ VLPs or exosomes from HEK293 cells, and used as stimulators. After 30 days and three rounds of stimulation, cells were analyzed by flow cytometry. Reactivation of EBV-specific T cells was revealed in a gamma interferon (IFN-γ) enzyme-linked immunospot (ELISPOT) assay, using autologous PBMCs loaded with VLPs as target. For the stimulation of the BNRF1-specific CD4+ T cell clone (32), 5 × 104 cells of an autologous lymphoblastoid B cell line (LCL) transformed with a mini-EBV (34) were loaded with VLPs from 293-VII+ cells or with exosomes from HEK293 cells and used as stimulators. An autologous LCL expressing BNRF1 was used as a positive control.
Immunization of mice.
BALB/c mice (n = 4) were immunized twice within a period of 14 days with 10 μg of VLPs in a volume of 200 μl PBS injected intraperitoneally. Control mice (n = 2) were immunized with the same amount of exosomes isolated from supernatants of HEK293 cells. Four weeks after the second immunization, sera and spleens were collected and analyzed. Neutralizing EBV-specific antibodies were detected with mouse sera diluted 1:10 prior to incubation with EBV 2089 stocks (8) for 30 min, and the EBV 2089 stocks were subsequently used to infect human primary B cells at a calculated multiplicity of infection (MOI) of 0.1. The fraction of infected, GFP-positive B cells was measured by flow cytometry 3 days after infection.
Inhibition studies.
Preparations of EBV 2089 (8) were preincubated with diluted (1:200) sera from immunized mice for 30 min at 37°C and then used at an MOI of 0.1 to infect primary human PBMCs. Two days later, the number of infected GFP-positive B cells was measured by flow cytometry as described previously (5). The inhibitory gp350-specific antibody 72A1 was gift of E. Kremmer; the inhibitory CD21 antibody (clone FE8) was purchased from Millipore (Schwabach, Germany).
ELISA and ELISPOT assay.
IFN-γ and granulocyte-macrophage colony-stimulating factor (GM-CSF) ELISAs and ELISPOT assays were performed according to the manufacturer's instructions (Mabtech). For the analysis of EBV-specific antibodies in immunized mice, sera were diluted 1:200 and incubated in a 96-well cluster plate with lysates of HEK293 cells (prepared in 1 mM EDTA, 150 mM NaCl, 0.1% SDS, 0.5% deoxycholate, 1% Triton X-100, 50 mM Tris) which had been transiently transfected with single-expression plasmids encoding EBV proteins, as indicated. Bound antibodies were detected with a peroxidase-conjugated anti-mouse IgG antibody. HEK293 cells transfected with expression plasmids for EBNA2 and the cytomegalovirus (CMV) protein pp65 were used as controls. EBV-specific T cells in mice were quantified with a murine IFN-γ ELISPOT Ready-SET-Go assay (eBioscience, San Diego, CA). Briefly, 5 × 105 lethally irradiated splenocytes were preincubated for 5 h with lysates of HEK293 cells that had been transiently transfected with expression plasmid for different EBV proteins. After extensive washing steps, 5 × 105 nonirradiated splenocytes were added and their activation was measured in an IFN-γ ELISPOT assay 48 h later.
Electron microscopy.
For negative staining, the samples were fixed with 1% glutaraldehyde in cacodylate buffer (pH 7.0). A drop of the sample was placed on a carbon-coated copper grid, freshly lyophilized by glow discharge. After incubation for 2 min, the drop was quickly removed with a Pasteur pipette and the grid was stained with 2% phosphotungstic acid and 0.05% glucose. Micrographs were taken with an EM 912 electron microscope (Zeiss, Oberkochen, Germany) equipped with an integrated Omega energy filter operated in the zero-loss mode.
RESULTS
293-VII+ cells release viral particles with B cell tropism that lack viral DNA.
Upon activation of EBV's lytic cycle (9) and in the absence of vector plasmid DNA, supernatants from the packaging cell line 293-VII+ conferred GFP fluorescence to human primary B cells (Fig. 1A). Because the EBV helper genome encodes a constitutively expressed gfp gene (19), we hypothesized that upon lytic induction and in the absence of vector plasmid DNA, EBV packaging cells presumably release particles with GFP protein as cargo, which is delivered to human B cells, turning them dimly green in flow cytometry. In contrast, infectious gfp-positive stocks of the wild-type 2089 strain of EBV (8) induced de novo synthesis of GFP protein upon infection and yielded an additional fraction of highly GFP-positive B cells (Fig. 1A). An immunoblot with preparations of particles from 293-VII+ cells purified on a density gradient revealed that these particles contained the EBV glycoproteins gp350, gp140, and gp125 (Fig. 1B). The gradient also demonstrated the cosedimentation of EBV proteins with the exosome markers hsp70 and tsg101 that are present in particles from 293-VII+ cells and HEK293-derived exosomes. In order to investigate whether particles from 293-VII+ cells have an EBV-like B cell tropism, we used human PBMCs. The results of these experiments demonstrated that the particles selectively interacted with CD21+ B cells but not with any other cell type (Fig. 1C, right). Exosomes from uninduced 293-VII+ cells conferred a weak but global GFP fluorescence to all cells compared to exosomes released from GFP-negative parental HEK293 cells (Fig. 1C, left and middle). Because EBV's B cell tropism is mainly owed to gp350/220 (BLLF1) (23), we next wanted to elucidate whether this is also the case for particles from 293-VII+ cells. We incubated human B cells with these particles either alone or in the presence of inhibitory gp350 or CD21 antibodies. Both antibodies interfered with particle binding, indicating that the particles mainly bind to B cells via gp350 (Fig. 1D). We also performed confocal microscopy on single human B cells incubated with particles from 293-VII+ cells. Three-dimensional reconstruction of confocal images revealed the colocalization of gp350/220 with CD21 on the cell surface, indicative of a frank interaction of these molecules (Fig. 1E). The particles obtained from lytically induced 293-VII+ cells were generated in the absence of packagable vector plasmid DNA, but they might contain EBV DNA due to an illegitimate encapsidation (13). To test whether particles from 293-VII+ cells contain viral DNA or RNA, we performed PCR and reverse transcription-PCR analysis, which did not detect any viral DNA, in contrast to the detection of infectious recombinant EBV virions released by the EBV 2089-infected cell line (8). Intriguingly, particles from both induced 293-VII+ cells and EBV 2089-infected cells contained detectable amounts of mRNAs encoding the viral immediate-early genes BZLF1 and BRLF1 (Fig. 1F). Thus, lytically induced 293-VII+ EBV packaging cells release particles that specifically interact with B cells and carry different EBV proteins and RNAs but do not contain detectable amounts of viral DNA.
Fig. 1.
293-VII+ cells release VLPs with a B cell tropism that lack viral DNA. (A) B cells were isolated from human adenoids and incubated for 2 days with VLPs from 293-VII+ cells (bold line) or EBV 2089 (fine line). VLPs from 293-VII+ cells render cells faintly GFP positive, indicating a direct interaction. Incubation of B cells with EBV 2089 stocks results in a majority of cells which are dimly GFP positive and 12.5% strongly GFP-positive cells in which the gfp gene is expressed after viral transduction. (B) VLPs from 293-VII+ cells contain the exosome markers hsp70 and tsg101 and the EBV structural proteins BLLF1 (gp350/220), BNRF1 (gp140), and BALF4 (gp125). Lysates from VLPs from 293-VII+ cells and exosomes from HEK293 were spotted onto nitrocellulose and hybridized to the antibodies indicated. (C) VLPs released from induced 293-VII+ cells exclusively bind to human CD21-positive cells. PBMCs were treated either with GFP-negative exosomes from parental HEK293 cells (left dot blot), with exosomes spontaneously released from uninduced 293-VII+ cells (middle dot blot), or with VLPs from lytically induced 293-VII+ cells (right dot blot) and analyzed by flow cytometry. Exosomes from uninduced 293-VII+ cells bound weakly and unspecifically to PBMCs (inset). In contrast, VLPs have a CD21 tropism, as revealed by GFP-positive CD21+ cells (right dot blot). (D) VLPs interact with B cells via gp350. Human primary B cells were incubated overnight with VLPs either alone or in the presence of inhibitory gp350- or CD21-specific antibodies. Binding of VLPs to B cells was quantified by flow cytometry by measuring GFP. Both antibodies interfered with VLP binding. (E) VLPs from 293-VII+ cells were incubated with primary B cells, and the interaction was analyzed by laser scanning microscopy, revealing a colocalization of CD21 (green) and gp350 (red) in three-dimensional reconstruction images. Cells were counterstained with 4′,6-diamidino-2-phenylindole (blue). (F) BZLF1- and BRLF1-specific PCR analysis detected viral mRNA in virions isolated from induced EBV 2089-infected cells and in VLPs from induced 293-VII+ cells. In contrast, viral DNA was detectable in EBV 2089-infected cells only. WT, wild type; RNA, PCR performed without reverse transcription proved that the RNA preparation was free of contaminating DNA.
EBV release depends on ESCRT.
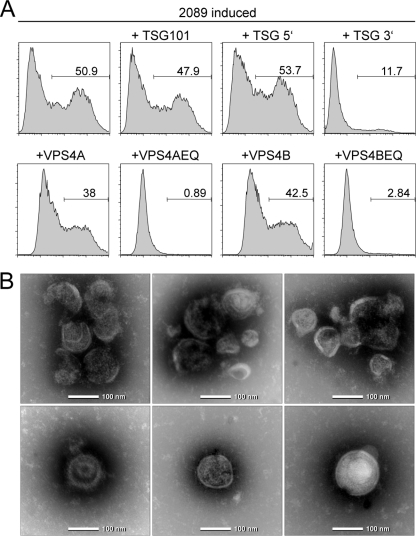
An independent set of data indicated that EBV's de novo synthesis and viral egress strictly depend on ESCRT. Small RNA viruses such as lentiviruses recruit ESCRT to viral budding sites by means of a dedicated structural viral peptide motif (54). Herpesviruses functionally rely on ESCRT as part of the viral cytoplasmic assembly line (50) or even incorporate the components into mature virions (37). As the ESCRT machinery is also essential for formation of multivesicular bodies (22), we argued that, upon lytic induction, the EBV packaging cells release endosomal vesicles or exosomes which carry GFP as cargo in conjunction with viral glycoproteins on their surface. The data in Fig. 2A demonstrate that ESCRT is involved in virion maturation and/or egress of EBV because dominant-negative mutants of the ESCRT proteins TSG101 and VPS4 efficiently blocked EBV release. Along this line, particles from the lytically induced EBV packaging cell lines could be equivalent to exosomes constantly produced by many cells, including HEK293 cells. We tested this assumption and prepared exosomes from parental HEK293 cells and 293-VII+ cells after induction of EBV's lytic phase by sequential rounds of fractionated sedimentations according to standard protocols. Clearly, hsp70 and the ESCRT component tsg101, both protein components of exosomes (see www.exocarta.org), were present in purified exosomes of HEK293 cells and lytically induced 293-VII+ cells (Fig. 1B). Viral glycoproteins characteristic of infectious virions were detectable in exosomal preparations from the 293-VII+ cells but not from parental HEK293 cells (Fig. 1B). As a final proof that the particles that we isolated from induced 293-VII+ cells were indeed of the size and shape of exosomes, we performed electron microscopy. We identified round particles of 100 to 150 nm in size, some of which contained capsid-like structures (Fig. 2B). For simplicity, we therefore term exosomal vesicles derived from lytically induced EBV packaging cell VLPs throughout this article, although their exact structure and biochemical composition are currently elusive.
Fig. 2.
EBV uses the exosome biogenesis pathway for viral egress. (A) HEK293 producer cells stably transfected with wild-type EBV 2089 (8) were transiently cotransfected with p509, an expression plasmid for BZLF1, to induce EBV's lytic cycle, together with expression plasmids encoding TSG101 or VSP4 proteins or dominant-negative mutants thereof. The supernatants were collected 3 days later and used for the infection of Raji cells. GFP-positive Raji cells were quantified by flow cytometry 3 days later. The numbers indicate the percentage of EBV-infected Raji cells. It became obvious that the dominant-negative mutants VPS4AEQ, VPS4BEQ, and TSG101 3′ completely blocked the release of infectious wild-type EBV 2089. (B) Electron microscopy revealed that purified VLPs from 293-VII+ cells are round and approximately 100 nm in diameter and morphologically resemble infectious EBV virions (27).
VLPs interact with and are taken up by human B cells.
The interaction of EBV gp350/220 with CD21 leads to the activation of B lymphocytes (31), rendering these cells immunogenic. Our data so far demonstrated that viral gp350/220 most likely mediates the B cell tropism of VLPs. To test whether the interaction of VLPs from 293-VII+ cells with primary PBMC B cells also led to their activation, we incubated primary B cells for 1 day with VLPs from 293-VII+ cells and then measured the surface levels of CD54 (intercellular adhesion molecule 1 [ICAM-1]), CD80 (B7.1), and CD86 (B7.2) by flow cytometry. This assay clearly revealed that VLPs induced the upregulation of these immune accessory molecules, indicating that VLP-treated B cells may serve as professional antigen-presenting cells (APCs) (Fig. 3A).
Fig. 3.
VLP-loaded B cells are efficient stimulators of autologous T cells. (A) Overnight incubation of CD19+ B cells purified from PBMCs with 293-VII+-derived VLPs induced the surface expression of the costimulatory molecules ICAM-1/CD54, B7.1/CD80, and B7.2/CD86 (black line) compared to B cells incubated with exosomes from HEK293 cells (gray region). (B) VLP-loaded B cells are potent stimulators of a BNRF1-specific CD4+ T cell clone. A mini-LCL line (34) was loaded with serial dilutions of VLPs obtained from lytically induced 293-VII+ cells. The loaded cells were used as stimulators for an autologous CD4+ T cell clone, which recognizes a BNRF1-specific epitope (32). Stimulation of the T cell clone with the mini-LCL line loaded with exosomes (exo) from HEK293 cells was used as a negative control, and an autologous LCL, which expresses BNRF1, was used as a positive control. (C) Reactivation of EBV-specific T lymphocytes with VLPs depends on CD19+ B cells. CD3+ T cells from a healthy donor who had been immunized with Twinrix 2 years previously were stimulated three times within a period of 14 days with autologous, lethally irradiated stimulator cells, as indicated, which had been loaded with HEK293 exosomes (exo), VLPs from 293-VII+ cells, or 30 μl Twinrix (corresponding to 22 ELISA units inactivated HAV and 0.6 μg HBV surface protein). The stimulator cells were either unfractionated PBMCs, marker-assisted congenic screening-sorted CD19+ B cells, or PBMCs from which the B cells were depleted (CD19−). After three rounds of stimulation, reactivation of T cells was assessed in an IFN-γ ELISPOT assay, using autologous PBMCs loaded with either VLPs, exosomes, or Twinrix as targets. VLP-induced T cell reactivation was strictly dependent on the presence of CD19+ B cells as stimulators. Shown are a scan of the ELISPOT assay and the calculated numbers.
Adhikary et al. have recently shown that particles derived from the first-generation EBV packaging cell line TR-2/293 interacted with B cells that then efficiently expanded an EBV-specific CD4+ T cell clone (2). Such a reactivation is indicative of internal cellular processing and presentation of viral antigens via HLA class II molecules following receptor-mediated uptake (3). In order to define whether VLPs obtained from 293-VII+ cells are engulfed by human B cells and have the potential to reactivate EBV-specific T cells, we incubated an LCL transformed with a mini-EBV (26) with VLPs from 293-VII+ cells. We then cocultivated the loaded LCL with an autologous CD4+ T cell clone specific for the EBV tegument protein BNRF1 not encoded in mini-EBVs (26). The experiments demonstrated that VLP-loaded B cells efficiently reactivated the CD4+ T cell clone, as measured by a GM-CSF ELISA (Fig. 3B). This set of experiments shows that VLPs from 293-VII+ cells specifically interact with and are engulfed by human B cells, which, in turn, are potent APCs and stimulators of EBV-specific CD4+ T cells, corroborating the findings of Adhikary et al. (2).
To address the contribution of B cells and other APCs, like monocytes and dendritic cells, to the observed T cell stimulations, we purified CD19+ and CD19− cells from PBMCs and incubated the fractionated mononuclear cells with VLPs from 293-VII+ cells or exosomes from HEK293 cells. Reactivation of T cells immediately after the third round of stimulation with VLP-loaded PBMCs was quantified in IFN-γ ELISPOT assays with CD19+ and CD19− PBMCs. The results clearly indicated that only CD19+ B cells are potent presenters of VLP-derived viral antigens (Fig. 3C). Hardly any T cells were identified with CD19− PBMCs as APCs, suggesting that only B cells activated the virus epitope-specific T cells, including HLA class I-restricted CD8+ T cells. In order to test the APC function of CD19+ B cells and CD19− PBMCs, we loaded the cells with the inactivated hepatitis A virus (HAV) and hepatitis B virus (HBV) surface protein contained in the marketed vaccine Twinrix (GlaxoSmithKline). HAV/HBV antigens were efficiently presented by CD19− PBMCs to T cells of an immunized healthy donor, demonstrating their function as professional APCs. These assays again revealed that VLPs from 293-VII+ cells preferentially interact with human B cells (Fig. 3C).
VLPs reactivate EBV-specific CD8+ T cells from seropositive hosts.
The efficacy of vaccines depends on the generation of a long-lasting immunological memory, which relies on both CD4+ and CD8+ T cells (4, 49). It was questionable whether VLPs of 293-VII+ cells can activate EBV-specific CD8+ T cells, a cell population that is mandatory for surveillance of EBV-infected cells in vivo (see reference 21 for a review). To test the capacity of VLPs to reactivate CD8+ memory T cells, we first incubated PBMCs with VLPs from 293-VII+ cells or exosomes from HEK293 cells overnight and then used them as targets for EBV-specific CD8+ T cell clones. An IFN-γ ELISA revealed a weak but distinct activation of T cells that were incubated with PBMCs that had been preincubated with VLPs (data not shown). In the next series of experiments, we stimulated PBMCs from EBV-seropositive donors with autologous, irradiated PBMCs preincubated with VLPs from 293-VII+ cells. After 28 days and three rounds of stimulation, a detailed flow cytometric analysis revealed that PBMCs loaded with VLPs from 293-VII+ cells induced the proliferation of autologous CD4+ T cells, in contrast to PBMCs loaded with exosomes from parental HEK293 cells or untreated PBMCs, which did not(Fig. 4A).
Fig. 4.
VLPs from 293-VII+ cells selectively expand EBV-specific CD4+ and CD8+ T cells. PBMCs from different EBV-positive donors were lethally irradiated, loaded with either VLPs from lytically induced 293-VII+ cells or exosomes from HEK293 cells, or left untreated and used as stimulators for autologous PBMCs. After 28 days and three rounds of stimulation, cells were analyzed by flow cytometry. (A) VLP- but not exosome-loaded (exo) or untreated (w/o) irradiated PBMCs expanded CD4+ T cells as described recently (2). (B) PBMCs loaded with VLPs reactivated and expanded EBV-specific CD8+ T cells, as revealed by staining with HLA B03/B08- or A02-restricted tetramers/pentamers to selected EBV protein epitopes. A tetramer (KIF) to the cellular protein Her2/neu served as a negative control. (C) VLP-loaded irradiated PBMCs reliably expanded EBV-specific CD8+ cells from four different donors up to 30-fold within 28 days, whereas the total number of CD8+ T cells dropped by about half compared to the initial CD8+ T cell numbers.
In order to determine whether VLPs also reactivated EBV-specific CD8+ T cells, we measured their initial frequency in donor PBMCs and compared it to the frequency of in vitro VLP-expanded CD8+ T cell populations for the specific recognition of known HLA class I-restricted EBV epitopes, which elicit strong CD8+ T cell immune responses in infected hosts (21). We chose the peptide epitopes CLG and FLY of the latent protein LMP2 and the peptide epitopes EPL/RAK, GLC, and YVL of the early lytic proteins BZLF1, BMLF1, and BRLF1, respectively, which are HLA B03/B08 or A02 restricted (33). Staining with HLA/peptide pentamers revealed a small but detectable fraction of CD8+ T cells in the initial donor PBMCs predominantly recognizing the early lytic epitopes (Fig. 3B). The fraction of epitope-specific CD8+ T cells expanded from about 0.15% to 0.25% to 1.1% to 2.7% in three rounds of VLP stimulation (Fig. 4B), and absolute numbers of epitope-specific CD8+ T cells increased up to approximately 10-fold (Fig. 4C).
VLPs elicit EBV-specific high-titer neutralizing antibodies and cellular immune responses in naïve BALB/c mice.
The results from the previous experiments demonstrated the potential of VLPs to stimulate EBV-specific recall immune responses. In a subsequent series of experiments, we evaluated whether VLPs can also induce EBV-specific immune responses in naïve mice in vivo. To that purpose, we immunized BALB/c mice (four animals) twice within a period of 14 days with 10 μg VLPs, whereas control mice (two animals) were immunized with the same amount of exosomes from HEK293 cells. Four weeks after the second immunization, the sera were analyzed for the presence of EBV-specific antibodies with an ELISA and protein lysates from HEK293 cells as antigens, in which the HEK293 cells had been transiently transfected with expression plasmids for single EBV proteins, as shown in Fig. 5A. To avoid background signals caused by antibodies raised against HEK293-derived proteins, sera were preincubated for 2 h with HEK293 lysates coated onto cell culture plates. As shown, sera from VLP-immunized mice but not mice immunized with exosomes from HEK293 cells revealed strong immunoreactivity against the selected viral proteins, which are components of virions of EBV (24). All antibodies were unambiguously detectable in sera from VLP-immunized mice at 1 to 200 dilutions (Fig. 5A). Intriguingly, VLP-immunized mice also developed high levels of antibodies against the transcription factor Zta, encoded by the BZLF1 gene. As controls, we measured the reactivity of the sera against lysates from HEK293 cells transfected with expression plasmids encoding CMV tegument protein pp65 and the EBV transactivator EBNA2. EBNA2 is translated from the BYRF1 gene, which is deleted in the EBV helper genome in 293-VII+ cells (19). As anticipated, we did not detect any humoral responses against these two proteins in the VLP-immunized mice, indicating the specificity of our assays.
Fig. 5.
VLPs elicit EBV-specific humoral and cellular immune responses in immunized mice. BALB/c mice were immunized twice with 10 μg VLPs from 293-VII+ cells (n = 4) or with the same amounts of exosomes (exo) from HEK293 cells (n = 2). Sera and splenocytes were analyzed 4 weeks after the last immunization. (A) In ELISAs, sera from VLP-immunized mice (black bars) but not from exosome-immunized mice (gray bars) contain antibodies specific to EBV proteins present in virions (24). OD, optical density. (B) Antibodies generated in mice immunized with VLPs from 293-VII+ cells neutralize infectious EBV. GFP-positive EBV 2089 stocks were preincubated for 30 min with sera from mice immunized with VLPs or exosomes and subsequently used to infect human primary B cells at a calculated multiplicity of infection of 0.1. Forty-eight hours later, the number of GFP-expressing infected cells was determined by flow cytometry. The neutralizing anti-gp350 antibody 72A1 was used as a positive control at two different concentrations. (C) VLPs from 293-VII+ cells activate EBV-specific T cells. The occurrence of EBV-specific T cells in mice immunized with VLPs (black bars) or exosomes (gray bars) was measured in a mouse-specific IFN-γ ELISPOT assay with irradiated splenocytes as APCs loaded with lysates from HEK293 cells which had been transiently transfected with expression plasmids encoding the indicated viral proteins. The CMV protein pp65 was used to control the specificity of the assays.
We wanted to learn whether sera from VLP-immunized animals contained EBV-specific neutralizing antibodies, which can inhibit cellular infection with EBV. We used the recombinant gfp-encoding EBV 2089, which confers GFP fluorescence to B cells as a quantitative measure of infection (8). Virus stocks of EBV 2089 were preincubated with mouse sera for 30 min and then used to infect primary human B cells at a calculated multiplicity of infection of 0.1. After 48 h, GFP-positive infected cells were quantified by flow cytometry. As shown in Fig. 5B, sera from VLP-immunized mice impaired infection with EBV, but sera from mice immunized with exosomes from HEK293 cells also had an inhibitory but weaker effect, probably due to the induction of antibodies against HEK293-derived proteins. Virus stocks of EBV 2089 are obtained from HEK293 producer cells (8), suggesting that sera from mice immunized with exosomes from HEK293 cells could also recognize EBV 2089 particles and sterically compromise the interaction of CD21 with gp350/22 on VLPs.
Next, we asked whether the immunization of mice led to the induction of EBV-specific cellular immune responses, which are essential for the immune surveillance of EBV (21). We prepared single cells from spleens of the VLP-immunized and control mice described above. Lethally irradiated splenocytes from individual mice were incubated with lysates obtained from HEK293 cells transiently transfected with single-expression plasmids encoding the viral genes shown in Fig. 4C and present in virions (24). To allow adsorption, proteolytic degradation, and presentation of the exogenously added lysates, the splenocytes were incubated for 5 h and subsequently washed to remove free lysate. The capacity of the cells to present antigen was assessed with 5 × 105 nonirradiated splenocytes, which were added as indicators. The activation of the indicator cells was determined in an IFN-γ ELISPOT assay after 24 h. As shown in Fig. 5C, splenocytes from VLP-immunized mice but not from control mice immunized with exosomes from HEK293 cells were clearly reactivated. Taken together, this experiment indicated that VLPs from 293-VII+ cells can induce an EBV-specific cellular immune response in naïve mice.
DISCUSSION
We describe here the development of a new vaccine candidate against EBV based on highly immunogenic VLP preparations derived from the producer cell line 293-VII+. Like wild-type EBV, these VLPs have an intrinsic B cell tropism and are effectively engulfed after interaction with the cellular receptor CD21. Subsequently, VLPs are presumably degraded and their peptides are presented to the immune system, stimulating strong humoral and cellular immune responses. EBV-based VLPs not only reactivate EBV-specific CD4+ and CD8+ T cells from EBV-seropositive individuals but also prime broad-spectrum cellular and humoral immune responses in mice. The VLP-mediated in vitro stimulation of EBV-specific CD8+ T cells is a surprising yet important result (Fig. 4B and C), since it is generally believed that exogenous engulfed proteins are exclusively presented via the major histocompatibility complex (MHC) class II pathway and human B cells were reported to fail to cross-present MHC class I-restricted epitopes derived from viral particles (25). We knew from independent experiments that VLPs, like virions from different herpesviruses, contain viral mRNAs (6, 7, 46), which are translated in newly infected cells and presented via MHC class I molecules (S. Jochum et al., submitted for publication). We therefore hypothesize that the activation of EBV-specific CD8+ T cells stems from viral RNAs encapsidated into VLPs and translated in infected cells. Encapsidated RNAs also provide an explanation for our observation that VLPs from 293-VII+ cells readily activated BRLF1 and BZLF1 epitope-specific CD8+ T cells (Fig. 4B), although we failed to detect these proteins in our EBV-based VLP preparations. Cellular immune responses against BRFL1 and BZLF1 are important because specific T cells against these two proteins are believed to control virus spread from lytically infected cells (see reference 21 for a review). VLPs also transport sufficient amounts of EBNA1 and LMP2 to induce specific immune responses (Fig. 5). Both proteins are potential therapeutic targets, as they are expressed in most EBV-associated malignant diseases, including PTLD. Despite the fact that EBNA1 and LMP2 have been described to have oncogenic potential, we do not consider this to be a problem for the in vivo application of VLPs, because VLPs lack EBV DNA and therefore have no transformation potential.
EBV VLPs induced neutralizing antibodies in immunized mice. Although neutralizing antibodies are essential for defense against various viruses, their role in humans with respect to protection from EBV diseases remains to be elucidated, although at least one group reported on different serum concentrations of gp350/220 antibodies in healthy individuals and patients with different EBV-related diseases (56). EBV VLPs are produced in human HEK293 cells and contain various viral and cellular proteins that are immunogenic in mice. We also observed the induction of HEK293-specific antibodies in immunized mice (Fig. 5A), probably because EBV VLPs are produced in HEK293 cells, which are foreign to mice. However, because HEK293 cells are of human origin, we consider the potential risk that EBV VLPs will induce autoimmune disease in humans to be minimal. In line with this, no evidence of an association between vaccination and autoimmune manifestations was found for the marketed smallpox vaccine that contains an attenuated varicella-zoster strain produced in human MRC-5 cells (45).
EBV-based VLPs primarily target B cells, which have been described to play a major role in T cell priming (41), are required for generating long-term T cell immunity, and augment antigen-specific T cell responses (55). In line with this, EBV VLP-mediated immune responses strongly depend on the presence of CD19+ B cells (Fig. 3C). It may further be speculated that EBV-based VLPs also interact with CD21+ follicular dendritic cells in vivo, further enhancing cellular immune responses (42). Next steps aim at optimizing the immunogenicity of the VLPs, e.g., by eliminating viral immunoevasins such as BNLF2a (20) and viral interleukin-10 (57). Also, efforts will be undertaken to allow the large-scale production of VLPs. VLPs provide an inherent advantage over soluble antigens, in that they are structurally identical to the wild-type virus but, in contrast to attenuated live vaccines, do not contain viral DNA, thus eliminating the risk of incidental infection and recombination events. VLPs elicit much broader EBV-specific immune responses than monovalent peptide formulations but, on the other side, are more complicated to produce in large quantities than peptide-based vaccines. Thus, whereas VLPs may be more efficient in preventing malignant EBV disease in immunocompromised patients, peptide-based vaccine formulations may constitute a more suitable standard vaccine to avoid IM in healthy individuals.
In summary, we show here that EBV-based VLPs are free of viral DNA and efficiently induce strong polyvalent neutralizing humoral and cellular immune responses in mice. In contrast to monovalent subunit vaccines, which are under development by several pharmaceutical companies, VLPs contain multiple and complex EBV antigens, including, for example, the latent protein LMP2a that is expressed in most EBV-associated tumors. We are aware that experiments with EBV in immunocompetent mice have their limitation, as murine B cells are not susceptible to EBV. As a consequence, VLP-induced immune responses in mice may differ from those in humans. In order to determine the potential of VLPs to protect vaccinated individuals from EBV-induced diseases, in vivo experiments should be performed in the future in primates susceptible to EBV.
ACKNOWLEDGMENTS
We acknowledge the help of M. Deutsch with laser scanning microscopy. We are indebted to J. Mautner for the BNRF1-specific CD4+ T cell clone, to E. Freed and J. Yasuda for vsp4 and tsg101 expression plasmids, and to E. Kremmer for the gp350-specific antibody.
This work was supported by the Deutsche Forschungsgemeinschaft (grant no. Ze 419/10-1), the Deutsche Krebshilfe (grant no. 107793), and the José Carreras Leukämie-Stiftung (grant no. DJCLS R 09/17).
Footnotes
Published ahead of print on 12 October 2011.
REFERENCES
- 1. Ablashi D. V., et al. 1997. Epstein-Barr virus and Kaposi's sarcoma herpesvirus/human herpesvirus 8, p. In (ed.), IARC monograph on the evaluation of carcinogenic risks to humans. International Association for Research on Cancer, Lyon, France [Google Scholar]
- 2. Adhikary D., Behrends U., Feederle R., Delecluse H. J., Mautner J. 2008. Standardized and highly efficient expansion of Epstein-Barr virus-specific CD4+ T cells by using virus-like particles. J. Virol. 82:3903–3911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Adhikary D., et al. 2006. Control of Epstein-Barr virus infection in vitro by T helper cells specific for virion glycoproteins. J. Exp. Med. 203:995–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ahlers J. D., Belyakov I. M. 2010. Memories that last forever: strategies for optimizing vaccine T-cell memory. Blood 115:1678–1689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Altmann M., Hammerschmidt W. 2005. Epstein-Barr virus provides a new paradigm: a requirement for the immediate inhibition of apoptosis. PLoS Biol. 3:e404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bechtel J., Grundhoff A., Ganem D. 2005. RNAs in the virion of Kaposi's sarcoma-associated herpesvirus. J. Virol. 79:10138–10146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bresnahan W. A., Shenk T. 2000. A subset of viral transcripts packaged within human cytomegalovirus particles. Science 288:2373–2376 [DOI] [PubMed] [Google Scholar]
- 8. Delecluse H. J., Hilsendegen T., Pich D., Zeidler R., Hammerschmidt W. 1998. Propagation and recovery of intact, infectious Epstein-Barr virus from prokaryotic to human cells. Proc. Natl. Acad. Sci. U. S. A. 95:8245–8250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Delecluse H. J., Pich D., Hilsendegen T., Baum C., Hammerschmidt W. 1999. A first-generation packaging cell line for Epstein-Barr virus-derived vectors. Proc. Natl. Acad. Sci. U. S. A. 96:5188–5193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Demirov D. G., Ono A., Orenstein J. M., Freed E. O. 2002. Overexpression of the N-terminal domain of TSG101 inhibits HIV-1 budding by blocking late domain function. Proc. Natl. Acad. Sci. U. S. A. 99:955–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Elliott S. L., et al. 2008. Phase I trial of a CD8+ T-cell peptide epitope-based vaccine for infectious mononucleosis. J. Virol. 82:1448–1457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Everly M. J., Bloom R. D., Tsai D. E., Trofe J. 2007. Posttransplant lymphoproliferative disorder. Ann. Pharmacother. 41:1850–1858 [DOI] [PubMed] [Google Scholar]
- 13. Feederle R., Shannon-Lowe C., Baldwin G., Delecluse H. J. 2005. Defective infectious particles and rare packaged genomes produced by cells carrying terminal-repeat-negative Epstein-Barr virus. J. Virol. 79:7641–7647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Goldacre M. J., Wotton C. J., Yeates D. G. 2009. Associations between infectious mononucleosis and cancer: record-linkage studies. Epidemiol. Infect. 137:672–680 [DOI] [PubMed] [Google Scholar]
- 15. Gu S. Y., et al. 1995. First EBV vaccine trial in humans using recombinant vaccinia virus expressing the major membrane antigen. Dev. Biol. Stand. 84:171–177 [PubMed] [Google Scholar]
- 16. Hammerschmidt W., Sugden B. 1988. Identification and characterization of oriLyt, a lytic origin of DNA replication of Epstein-Barr virus. Cell 55:427–433 [DOI] [PubMed] [Google Scholar]
- 17. Hellebrand E., et al. 2006. Epstein-Barr virus vector-mediated gene transfer into human B cells: potential for antitumor vaccination. Gene Ther. 13:150–162 [DOI] [PubMed] [Google Scholar]
- 18. Heslop H. E., et al. 2010. Long-term outcome of EBV-specific T-cell infusions to prevent or treat EBV-related lymphoproliferative disease in transplant recipients. Blood 115:925–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hettich E., et al. 2006. Genetic design of an optimized packaging cell line for gene vectors transducing human B cells. Gene Ther. 13:844–856 [DOI] [PubMed] [Google Scholar]
- 20. Hislop A. D., et al. 2007. A CD8+ T cell immune evasion protein specific to Epstein-Barr virus and its close relatives in Old World primates. J. Exp. Med. 204:1863–1873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hislop A. D., Taylor G. S., Sauce D., Rickinson A. B. 2007. Cellular responses to viral infection in humans: lessons from Epstein-Barr virus. Annu. Rev. Immunol. 25:587–617 [DOI] [PubMed] [Google Scholar]
- 22. Hurley J. H., Emr S. D. 2006. The ESCRT complexes: structure and mechanism of a membrane-trafficking network. Annu. Rev. Biophys. Biomol. Struct. 35:277–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Janz A., et al. 2000. Infectious Epstein-Barr virus lacking major glycoprotein BLLF1 (gp350/220) demonstrates the existence of additional viral ligands. J. Virol. 74:10142–10152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Johannsen E., et al. 2004. Proteins of purified Epstein-Barr virus. Proc. Natl. Acad. Sci. U. S. A. 101:16286–16291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Keller S. A., et al. 2009. Follicular and marginal zone B cells fail to cross-present MHC class I-restricted epitopes derived from viral particles. J. Immunol. 182:6261–6266 [DOI] [PubMed] [Google Scholar]
- 26. Kempkes B., Pich D., Zeidler R., Sugden B., Hammerschmidt W. 1995. Immortalization of human B lymphocytes by a plasmid containing 71 kilobase pairs of Epstein-Barr virus DNA. J. Virol. 69:231–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Klein F., et al. 1978. Large-scale production and concentration of infectious Epstein-Barr virus. Appl. Environ. Microbiol. 35:172–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lautscham G., et al. 2003. Identification of a TAP-independent, immunoproteasome-dependent CD8+ T-cell epitope in Epstein-Barr virus latent membrane protein 2. J. Virol. 77:2757–2761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lopes V., Young L. S., Murray P. G. 2003. Epstein-Barr virus-associated cancers: aetiology and treatment. Herpes 10:78–82 [PubMed] [Google Scholar]
- 30. Lu G., et al. 2009. Clinical analysis and follow-up study of chronic active Epstein-Barr virus infection in 53 pediatric cases. Chin. Med. J. (Engl.). 122:262–266 [PubMed] [Google Scholar]
- 31. Masucci M. G., et al. 1987. Activation of B lymphocytes by Epstein-Barr virus/CR2 receptor interaction. Eur. J. Immunol. 17:815–820 [DOI] [PubMed] [Google Scholar]
- 32. Mautner J., et al. 2004. Epstein-Barr virus nuclear antigen 1 evades direct immune recognition by CD4+ T helper cells. Eur. J. Immunol. 34:2500–2509 [DOI] [PubMed] [Google Scholar]
- 33. Moosmann A., et al. 2010. Effective and long-term control of EBV PTLD after transfer of peptide-selected T cells. Blood 115:2960–2970 [DOI] [PubMed] [Google Scholar]
- 34. Moosmann A., et al. 2002. B cells immortalized by a mini-Epstein-Barr virus encoding a foreign antigen efficiently reactivate specific cytotoxic T cells. Blood 100:1755–1764 [PubMed] [Google Scholar]
- 35. Morgan A. J., Khanna R. 2007. Epstein-Barr virus vaccines, p. 1292–1305. In Arvin A., et al. (ed.), Human herpesviruses: biology, therapy and immunoprophylaxis. Cambridge University Press, New York, NY. [PubMed] [Google Scholar]
- 36. Moutschen M., et al. 2007. Phase I/II studies to evaluate safety and immunogenicity of a recombinant gp350 Epstein-Barr virus vaccine in healthy adults. Vaccine 25:4697–4705 [DOI] [PubMed] [Google Scholar]
- 37. Pawliczek T., Crump C. M. 2009. Herpes simplex virus type 1 production requires a functional ESCRT-III complex but is independent of TSG101 and ALIX expression. J. Virol. 83:11254–11264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rees L., et al. 2009. A phase I trial of Epstein-Barr virus gp350 vaccine for children with chronic kidney disease awaiting transplantation. Transplantation 88:1025–1029 [DOI] [PubMed] [Google Scholar]
- 39. Renkvist N., Castelli C., Robbins P. F., Parmiani G. 2001. A listing of human tumor antigens recognized by T cells. Cancer Immunol. Immunother. 50:3–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rickinson A., Kieff E. 2007. Epstein-Barr virus, p. 2655–2700. In Knipe D. M., et al. (ed.), Fields virology, 5th ed. Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 41. Ron Y., Sprent J. 1987. T cell priming in vivo: a major role for B cells in presenting antigen to T cells in lymph nodes. J. Immunol. 138:2848–2856 [PubMed] [Google Scholar]
- 42. Roozendaal R., Carroll M. C. 2007. Complement receptors CD21 and CD35 in humoral immunity. Immunol. Rev. 219:157–166 [DOI] [PubMed] [Google Scholar]
- 43. Sairenji T., et al. 1988. Inhibition of Epstein-Barr virus (EBV) release from P3HR-1 and B95-8 cell lines by monoclonal antibodies to EBV membrane antigen gp350/220. J. Virol. 62:2614–2621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Saulquin X., et al. 2000. A global appraisal of immunodominant CD8 T cell responses to Epstein-Barr virus and cytomegalovirus by bulk screening. Eur. J. Immunol. 30:2531–2539 [DOI] [PubMed] [Google Scholar]
- 45. Schattner A. 2005. Consequence or coincidence? The occurrence, pathogenesis and significance of autoimmune manifestations after viral vaccines. Vaccine 23:3876–3886 [DOI] [PubMed] [Google Scholar]
- 46. Sciortino M. T., Suzuki M., Taddeo B., Roizman B. 2001. RNAs extracted from herpes simplex virus 1 virions: apparent selectivity of viral but not cellular RNAs packaged in virions. J. Virol. 75:8105–8116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sokal E. M., et al. 2007. Recombinant gp350 vaccine for infectious mononucleosis: a phase 2, randomized, double-blind, placebo-controlled trial to evaluate the safety, immunogenicity, and efficacy of an Epstein-Barr virus vaccine in healthy young adults. J. Infect. Dis. 196:1749–1753 [DOI] [PubMed] [Google Scholar]
- 48. Steven N. M., et al. 1997. Immediate early and early lytic cycle proteins are frequent targets of the Epstein-Barr virus-induced cytotoxic T cell response. J. Exp. Med. 185:1605–1617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Stevenson P. G., Simas J. P., Efstathiou S. 2009. Immune control of mammalian gamma-herpesviruses: lessons from murid herpesvirus-4. J. Gen. Virol. 90:2317–2330 [DOI] [PubMed] [Google Scholar]
- 50. Tandon R., AuCoin D. P., Mocarski E. S. 2009. Human cytomegalovirus exploits ESCRT machinery in the process of virion maturation. J. Virol. 83:10797–10807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Thacker E. L., Mirzaei F., Ascherio A. 2006. Infectious mononucleosis and risk for multiple sclerosis: a meta-analysis. Ann. Neurol. 59:499–503 [DOI] [PubMed] [Google Scholar]
- 52. Tobi M., et al. 1982. Prolonged atypical illness associated with serological evidence of persistent Epstein-Barr virus infection. Lancet i:61–64 [DOI] [PubMed] [Google Scholar]
- 53. Urata S., Noda T., Kawaoka Y., Yokosawa H., Yasuda J. 2006. Cellular factors required for Lassa virus budding. J. Virol. 80:4191–4195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. von Schwedler U. K., et al. 2003. The protein network of HIV budding. Cell 114:701–713 [DOI] [PubMed] [Google Scholar]
- 55. Whitmire J. K., et al. 2009. Requirement of B cells for generating CD4+ T cell memory. J. Immunol. 182:1868–1876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Xu J., et al. 1998. The Epstein-Barr virus (EBV) major envelope glycoprotein gp350/220-specific antibody reactivities in the sera of patients with different EBV-associated diseases. Int. J. Cancer 79:481–486 [DOI] [PubMed] [Google Scholar]
- 57. Zeidler R., et al. 1997. Downregulation of TAP1 in B lymphocytes by cellular and Epstein-Barr virus-encoded interleukin-10. Blood 90:2390–2397 [PubMed] [Google Scholar]