Abstract
Respiratory syncytial virus (RSV) is the most important cause of severe, lower respiratory tract infections in infants, and RSV infections have been associated with chronic wheezing and asthma during childhood. However, the mechanism of RSV-induced airway inflammation and airway hyperresponsiveness (AHR) is poorly understood. Furthermore, there are presently neither effective vaccines nor drugs available for the prevention or treatment of RSV infections. In this study, we investigated the effect of the plant extract resveratrol as a means of preventing airway inflammation and attenuating RSV-induced AHR. Our data showed that resveratrol reduced RSV lung titers and the number of infiltrating lymphocytes present in bronchoalveolar lavage fluid (BALF) and reduced inflammation. Furthermore, resveratrol attenuated airway responses to methacholine following RSV infection and significantly decreased gamma interferon (IFN-γ) levels in BALF of RSV-infected mice. Data presented in this report demonstrated that resveratrol controlled Toll-like receptor 3 (TLR3) expression, inhibited the TRIF signaling pathway, and induced M2 receptor expression following RSV infection. These data support a role for the use of resveratrol as a means of reducing IFN-γ levels associated with RSV-mediated airway inflammation and AHR, which may be mediated via TLR3 signaling.
INTRODUCTION
Respiratory syncytial virus (RSV) is the primary cause of lower respiratory tract infections resulting in hospitalization during the first year of life in most parts of the world (43). It is estimated that 50% of children are infected during the first year of life, and by 3 years of age, 100% have experienced at least one RSV infection. RSV infections do not elicit lifelong protective immunity; therefore, repeated infections are common. Previous studies demonstrated that RSV has been associated with severe respiratory illness not only in the elderly or in immunocompromised patients but also in healthy adults (2, 15). As a result, RSV is associated with significant morbidity and mortality. In the United States alone, about 100,000 hospital admissions were related to RSV infections, with estimated patient care costs exceeding over 300 million dollars annually (43). Unfortunately, vaccines with the capacity to elicit protective immunity against RSV infections are not available; that is, recently developed formulations not only have proven to be ineffective but also have led to vaccine-enhanced disease (29, 44). Currently, the only approved therapy for the treatment of active RSV infections is the aerosol delivery of the nucleotide analog ribavirin. However, this treatment option is questionable, since the beneficial effects associated with clinical outcomes remain unproven (1). However, the prophylactic treatment of premature infants with palivizumab, a monoclonal antibody against the RSV fusion (F) protein, significantly reduced wheezing and symptoms associated with RSV infections compared to controls. Unfortunately, this treatment option is not effective in treating acute RSV infections, and preliminary experiments demonstrated that this approach would not be cost-effective.
Resveratrol (trans-3,4,5-trihydroxystilbene), a natural polyphenol present in various fruits and vegetables and abundant in grapes, functions as a phytoalexin that protects against fungal infections (21). Previous studies demonstrated that resveratrol exhibited a wide range of biological and pharmacological activities, including anticarcinogenic and anti-inflammatory properties in addition to being beneficial to the maintenance of cardiovascular integrity (8, 28). In addition, various recent studies have confirmed that resveratrol exhibited cardioprotective and chemopreventive effects in mouse studies (10, 33). More recently, the anti-inflammatory effects of resveratrol have been associated with the inhibition of the transcription factor NF-κB (35), possibly by mediating the inhibition of Iκ-B kinase (26). NF-κB activation is required for the expression of many proinflammatory proteins, such as granulocyte-macrophage colony-stimulating factor (GM-CSF), interleukin-8 (IL-8), COX-2, and inducible nitric oxide synthase (iNOS) (6, 41). Therefore, the inhibition of NF-κB might reduce the expression levels of inflammation-associated genes, providing a target for anti-inflammatory glucocorticosteroid-based treatments (40). Moreover, Youn et al. demonstrated previously that resveratrol suppressed NF-κB activation and COX-2 expression by inhibiting TRIF signaling associated with the Toll-like receptor 3 (TLR3) and TLR4 MyD88-independent pathway by targeting TANK-binding kinase 1 and RIP1 in the TRIF complex (51). In recent years, several studies have demonstrated that resveratrol inhibited viral replication, e.g., influenza A virus, human cytomegalovirus, herpes simplex virus, and varicella-zoster virus, both in vitro and in vivo (11–14, 42).
RSV infections during infancy have been associated with chronic wheezing and asthma later in childhood. As a member of the type II interferon (IFN) family, the role of IFN-γ in airway hyperresponsiveness has been extensively studied. Yang et al. reported previously that IFN-γ contributed to the prolongation of airway hyperresponsiveness (AHR) in a BALB/c mouse asthma model (50), and IFN-γ has been shown to play an important role in RSV infection-associated airway inflammation and AHR.
RSV infections occur mainly in children under 2 years of age due to their immature immune systems (7, 48). Healthy BALB/c mice are not susceptible to RSV infections; however, preliminary experiments demonstrated that mice that were immunocompromised as a result of cyclophosphamide (CYP) treatment were susceptible to RSV infection (30). Therefore, in this study, we examined the effects of resveratrol treatment on immunocompromised mice to investigate its effects on RSV-induced airway inflammation and AHR. In this study, we investigated the effects of resveratrol on RSV replication and its anti-inflammatory and antihyperresponsive effects in a model of acute RSV infection. Furthermore, we studied the effects of IFN-γ on airway inflammation and AHR and found that resveratrol inhibited IFN-γ production via the TLR3 signaling pathway, which prevented both airway inflammation and AHR.
MATERIALS AND METHODS
Virus preparation and animal model.
A stock of human A2 strain RSV was obtained from the Viral Laboratory at Beijing Children's Hospital (Capital University of Medical Sciences, Beijing, China). The virus was grown on HEp2 cell monolayers by using Dulbecco's modified Eagle's medium (DMEM; Invitrogen, Carlsbad, CA) plus 5% fetal bovine serum (FBS; HyClone, Logan, UT) and titrated by using a plaque assay (36). Master and working stocks of RSV were prepared as described previously (18).
Specific-pathogen-free female BALB/c mice, 6 to 8 weeks old, were purchased from the Chongqing Medical University Animal Laboratory and housed in individually filtered cages. Cages, bedding, food, and water were sterilized before use. The room temperature was maintained at 23°C, and animals were maintained on a 12-h light/dark cycle. All animal procedures were carried out on sterile surfaces. Mice were treated with cyclophosphamide (CYP) as described previously (30). Briefly, CYP was administered in a single dose of 100 mg/kg of body weight, and 5 days later, mice were intranasally infected with 3.4 × 105 RSV PFU in a volume of 100 μl. Mock-infected mice were inoculated intranasally with the same amount of HEp-2 cell culture supernatant at the same time. At 1 h postinoculation, mice were injected intraperitoneally with either resveratrol (Sigma-Aldrich Corp., St. Louis, MO) at 30 mg/kg/day (30) or placebo (PBS [phosphate-buffered saline]) for 5 days. Airway hyperresponsiveness (AHR) was measured, and mice were sacrificed 5 days after treatment. All studies were performed with the approval of the Experimental Animal Committee of the Chongqing Medical University.
Measurement of AHR.
Twenty-four hours after the final resveratrol treatment, AHR was assessed in conscious, unrestrained mice by means of whole-body plethysmography (Emca instrument; Allmedicus, France). Each mouse was placed into a plastic chamber and exposed to aerosolized PBS followed by increasing concentrations of an aerosolized methacholine solution (3.125, 6.25, 12.5, 25, and 50 mg/ml; Sigma) in PBS for 3-min exposures. Bronchoconstriction was recorded for an additional 5 min after each dose of methacholine. The highest Penh value obtained during each methacholine challenge was expressed as a proportion of the basal Penh value seen in response to PBS challenge.
Inflammatory cell counts in BALF.
Mice were anesthetized with urethane (15 mg/10 g body weight intraperitoneally) 24 h after the last resveratrol treatment, and the abdominal cavity was opened. Tracheas were cannulated, bronchoalveolar lavage fluid (BALF) was collected following 3 lavage washes with 0.5 ml ice-cold PBS, and total cell numbers in BALF were determined. BALF was then centrifuged, supernatants were stored at −70°C prior to cytokine profile characterization, and the cell pellet was used for differential cell analysis by cytospin. Briefly, slides were fixed and stained with DiffQuik (Baxter Healthcare Corp., Miami, FL) for leukocyte differential analysis, and the number of monocytes, lymphocytes, neutrophils, and eosinophils in a total of 200 cells was determined per slide.
Determination of lung virus titers.
Mice were sacrificed at 3, 4, and 5 days post-resveratrol treatment, and lungs were removed to determine the effects of resveratrol on RSV replication by using a plaque assay described previously (36). The results were expressed as means ± standard deviations (SD).
Lung tissue histopathology.
Lung tissues were fixed in 10% (vol/vol) neutral buffered formalin for 24 h, embedded in paraffin, cut into 5-μm sections, and stained with hematoxylin and eosin (H&E; Sigma). Tissues were subsequently mounted and coverslipped by use of Dako mounting medium (Dakocytomation, Denmark, CA). The degree of airway inflammatory cell infiltration was scored in a double-blind fashion by two independent investigators (38). Peribronchiole and perivascular inflammation was evaluated by using a 0-to-5 scoring system, where 0 represents no cells, 1 represents a few cells, 2 represents a ring of cells 1 cell layer deep, 3 represents a ring of cells 2 to 4 cells deep, 4 represents a ring of cells 5 cells deep, and 5 represents a ring of cells more than 5 cells deep.
BALF cytokine measurements.
Cytokine concentrations in BALF were measured with commercial enzyme-linked immunosorbent assay (ELISA) kits according to the manufacturer's instructions. ELISA kits used for the measurement of IFN-γ, IL-4, IL-6, and IL-10 were purchased from Sizhengbai (Beijing, China), and the IL-17 detection ELISA was purchased from Bender.
Characterization of IFN-γ levels associated with airway inflammation and AHR.
To assess the effect of IFN-γ on airway inflammation and AHR, mice were treated with blocking anti-IFN-γ (R4-6A2; BD PharMingen, San Diego, CA) antibodies. Anti-IFN-γ antibody (30 μg) was administered by intraperitoneal injection on day 0 (1 h post-RSV infection) and on days 1 and 3 postinfection.
Measurement of TLR3 and M2 mRNA expression levels.
Total RNA from lung tissues was extracted by using a high-purity total RNA extraction kit (BioTeke, Beijing, China). After quantification, 4 μg of RNA was used to carry out reverse transcription (TaKaRa, Shiga, Japan). Real-time quantitative PCR (RT-PCR) was performed to detect the relative expression levels of TLR3 and muscarinic 2 receptor (M2R). Reaction conditions were as follows: 95°C for 10 min followed by 40 cycles at 95°C for 15 s and 72°C for 30 s. The TLR3 and M2R primers are described in Table 1.
Table 1.
Real-time quantitative PCR primers
| Gene | GenBank accession no. | Primer | Size (bp) |
|---|---|---|---|
| TLR3 | NM_126166 | Forward, 5′-ACCTTTCCGCCCTCTTCGTAAC-3′ | 93 |
| Reverse, 5′-TTCTCAAGACCCTCCAGCAAGTC-3′ | |||
| M2 | NM_203491 | Forward, 5′-TGGTTTGGCTATTACCAGTCCT-3′ | 136 |
| Reverse, 5′-CTGAAGGTGGCGGTTGACTT-3′ | |||
| β-Actin | NM_007393 | Forward, 5′-TGGCATTGTTACCAACTGGGAC-3′ | 132 |
| Reverse, 5′-TCACGGTTGGCCTTAGGGTTC-3′ |
Western blot analysis.
Total protein extracts from lung tissues were obtained by using a total protein extraction kit (KeyGEN, Nanjing, China), and protein concentrations were determined by using the BCA assay reagent (Bioteke) according to the manufacturer's protocol. The respective lung protein extracts containing equal quantities of protein were separated on an 8% SDS-PAGE (sodium dodecyl sulfate-polyacrylamide gel electrophoresis) gel and then transferred onto polyvinylidene difluoride (PVDF) membranes (Millipore, Billerica, MA). Membranes were probed with primary antibodies against TRIF (1:500; Abcam, Cambridge, MA) or β-actin (1:5,000; 4abio, Beijing, China). Alkaline phosphatase-conjugated goat anti-rabbit secondary antibody (1:10,000; Minneapolis) and goat anti-mouse antibody (1:10,000; MultiSciences, China) were used to detect the presence of the respective protein bands. Signals were quantified by use of Quantity One software (Bio-Rad, Hercules, CA) and normalized relative to β-actin.
Statistics.
All results are expressed as means ± SD. Analysis of variance (ANOVA) was used to determine the level of significant differences between all groups. Groups were compared by using the unpaired t test. Differences were considered significant at a P value of <0.05.
RESULTS
Resveratrol inhibits RSV replication in a murine infection model.
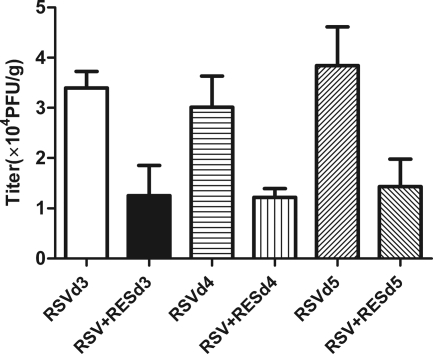
To evaluate the ability of resveratrol to suppress RSV replication in RSV-infected mice, lung tissues were collected at 3, 4, and 5 days post-resveratrol treatment. On days 3, 4, and 5 postinfection, RSV titers in untreated mice were 3.4 × 104 ± 0.33 × 104, 3.01 × 104 ± 0.62 × 104, and 3.845 × 104 ± 0.3146 × 104 PFU/g, respectively. However, titers in resveratrol-treated mice at the same time points were 1.25 × 104 ± 0.6 × 104, 1.21 × 104 ± 0.18 × 104, and 1.429 × 104 ± 0.2261 × 104 PFU/g, respectively, i.e., an average decrease of 61.6% (P < 0.01 for 3.4 × 104 ± 0.33 × 104 versus 1.25 × 104 ± 0.6 × 104, 3.01 × 104 ± 0.62 × 104 versus 1.21 × 104 ± 0.18 × 104, and 3.845 × 104 ± 0.3146 × 104 versus 1.429 × 104 ± 0.2261 × 104 PFU/g) (Fig. 1).
Fig. 1.
Effect of resveratrol on pulmonary virus titers. Lungs harvested from resveratrol (RES)-treated (30 mg/kg/day) and RSV-infected mice (n = 6/group) were individually homogenized, and virus titers were determined by use of a plaque assay. Data are expressed as PFU per gram of lung tissue. Values are expressed as means ± SD on day 3 (P < 0.01 for the resveratrol-treated group versus the RSV-infected group), day 4 (P < 0.05 for the resveratrol-treated group versus the RSV-infected group), and day 5 (P < 0.01 for the resveratrol-treated group versus the RSV-infected group) postinfection.
Resveratrol inhibited RSV-induced airway inflammation and AHR.
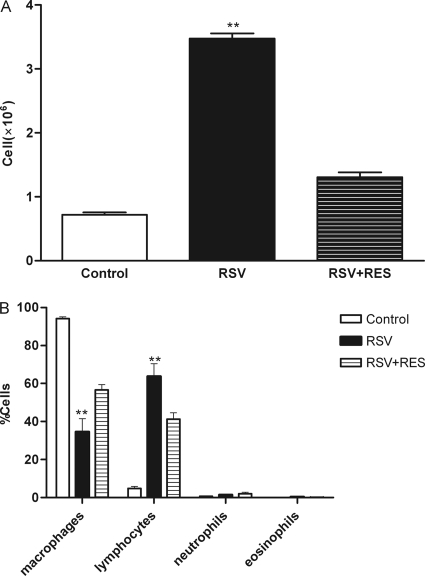
To evaluate the ability of resveratrol to suppress inflammatory cell infiltration into the lungs following RSV infection, cells present in the BALF were counted daily for 5 days. RSV infection resulted in a marked leukocyte influx into the BALF; i.e., BALF cell counts were 3.475 × 106 ± 0.231 × 106 cells/mouse, compared to 0.720 × 106 ± 0.105 × 106 cells/mouse for untreated controls. Resveratrol-treated mice had an average of 1.306 × 106 ± 0.221 × 106 cells/mouse, a 62.4% ± 15.1% decrease in total cell numbers (P < 0.01) and a 37.8% drop in lymphocyte numbers (P < 0.05) compared to RSV-infected mice (Fig. 2).
Fig. 2.
Effect of resveratrol on the recruitment of inflammatory cells recoverable from BALF. Cells were isolated by cytospin and stained with DiffQuik. Cell numbers were assessed by use of a hemocytometer. Control, PBS control; RSV, PBS-treated RSV-infected mice; RSV+RES, resveratrol (RES)-treated RSV-infected mice. Values are expressed as the means ± SD (n = 6/group). (A) Total cells present in the BALF of the respective treatment groups. (B) Differences in cell types between treatment groups. **, P < 0.01 versus control and resveratrol-treated mice.
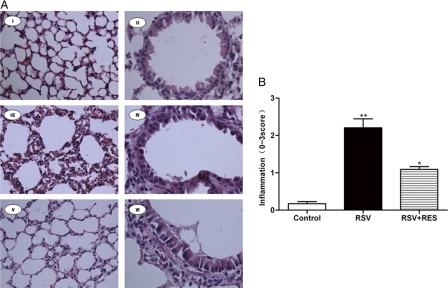
To estimate the anti-inflammatory or antiremodeling effects of resveratrol, lung tissues were harvested post-resveratrol treatment every day for 5 days. In RSV-infected mice, leukocytes were found to have infiltrated into the peribronchiole and perivascular connective tissue, with leukocytes being the primary infiltrating cell type. In resveratrol-treated and RSV-infected mice, however, both lymphocyte-rich infiltrates and tissue damage were significantly reduced (Fig. 3). Control mice had an average pathology score of 0.175 ± 0.02, and RSV-infected mice had an average score of 2.208 ± 0.23. However, the average score for RSV-infected mice receiving resveratrol was 1.09 ± 0.07 (P < 0.01 compared to RSV-infected mice and P < 0.05 compared to control mice).
Fig. 3.
Effect of resveratrol on the recruitment of leukocytes into lung tissues. (A) Histological examination of lung tissues performed at 5 days post-resveratrol treatment. Lung tissues were fixed, and 5-μm-thick sections were stained with H&E (magnifications, ×200 [I, III, and V] and ×400 [II, IV, and VI]). Panels I and II, control mice; panels III and IV, RSV-infected mice; panels V and VI, resveratrol-treated mice. (B) Lung tissue inflammatory cell infiltration scores. *, P < 0.05 versus the control group; **, P < 0.01 versus the control and resveratrol-treated mice.
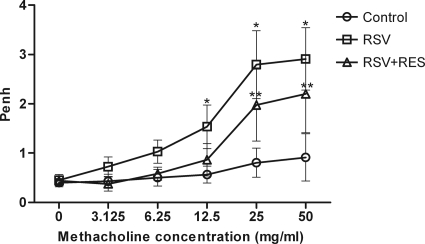
AHR was evaluated by the calculation of Penh values (enhanced pauses) 5 days after daily treatment with resveratrol. The Penh value for RSV-infected mice was significantly higher than the Penh value for animals in the PBS control group (P < 0.01) at methacholine concentrations of between 12.5 and 50.0 mg/ml. Resveratrol treatment, however, significantly reduced the Penh values compared to values obtained for mice in the RSV infection group at concentrations of between 12.5 and 50.0 mg/ml (P < 0.01). However, the Penh value for resveratrol-treated mice was higher than that for control group animals (P < 0.01) at methacholine concentrations of between 25.0 and 50.0 mg/ml. These results showed that RSV-induced AHR was decreased following resveratrol treatment; however, AHR presentation was not completely inhibited (Fig. 4).
Fig. 4.
Effect of resveratrol on RSV-induced AHR. AHR was measured at 5 days post-resveratrol treatment in mice treated with increasing methacholine concentrations (3.125 to 50.0 mg/ml) by plethysmography. Control, untreated mice; RSV, RSV-infected mice; RSV+RES, resveratrol (RES)-treated RSV-infected mice. Values are expressed as means ± SD (n = 8/group). *, P < 0.01 versus the control and resveratrol-treated RSV-infected groups; **, P < 0.01 versus the control group.
Resveratrol reduced RSV-induced IFN-γ production.
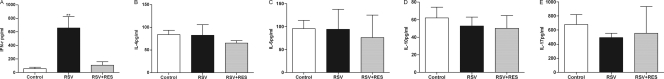
To evaluate the effects of resveratrol on BALF cytokine profile changes, a screening of cytokines present in the BALF was carried out. ELISA-based assays were used to determine the IFN-γ, IL-4, IL-6, IL-10, and IL-17 concentrations at 5 days post-RSV infection. This analysis demonstrated that RSV infection induced a significant increase in the IFN-γ BALF levels to 656.3 ± 170.9 pg/ml, compared to baseline concentrations of 56.66 ± 20.12 pg/ml in uninfected mice. Following resveratrol treatment, the IFN-γ levels in RSV-infected mice were significantly reduced by 547.7 ± 109.42 pg/ml compared to the RSV group (656.3 ± 170.9 pg/ml for RSV infection versus 108.6 ± 61.48 pg/ml for RSV infection and resveratrol treatment; P < 0.05) (Fig. 5A). There were no differences in the levels of IL-4, IL-6, IL-10, and IL-17 between groups (Fig. 5B and C to E). These results demonstrated that resveratrol specifically reduced IFN-γ levels in the BALF of RSV-infected mice without affecting the production of other cytokines.
Fig. 5.
Effect of resveratrol on BALF cytokine levels. BALF was collected at 5 days post-resveratrol treatment. Each sample was analyzed by an ELISA (n = 8/group). IFN-γ (A), IL-4 (B), IL-6 (C), IL-10 (D), and IL-17 (E) levels in BALF are shown. Control, untreated mice; RSV, RSV-infected mice; RSV+RES, resveratrol-treated mice. Values are expressed as means ± SD (n = 8/group). **, P < 0.01 versus the control and resveratrol-treated groups.
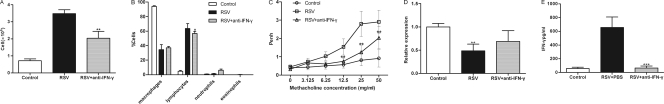
To further assess the effects of IFN-γ on airway inflammation and AHR during RSV infection, RSV-infected mice were treated with IFN-γ-neutralizing antibodies. As shown in Fig. 6A, the total number of cells in the BALF of RSV-infected mice treated with anti-IFN-γ antibody was 2.036 × 106 ± 0.409 × 106 cells/mouse, compared to 3.475 × 106 ± 0.231 × 106 cells/mouse for RSV-infected mice (P < 0.05 for 2.036 × 106 ± 0.409 × 106 cells/mouse for RSV infection versus 3.475 × 106 ± 0.231 × 106 cells/mouse for RSV infection and treatment with anti-IFN-γ antibody). A 7% decrease in lymphocyte levels was observed for RSV-infected mice following treatment with anti-IFN-γ antibody compared to RSV-infected mice (P < 0.05) (Fig. 6B).
Fig. 6.
Effect of IFN-γ on airway inflammation and hyperresponsiveness. (A) Total cells present in BALF. (B) Leukocyte percentages before and after anti-IFN-γ treatment. (C) Penh levels. (D) M2R mRNA levels. (E) IFN-γ levels after anti-IFN-γ treatment. Values are expressed as means ± SD (n = 8/group). *, P < 0.05 versus the RSV group; **, P < 0.001 versus the RSV group with between 12.5 and 50 mg/ml methacholine.
The effect of anti-IFN-γ treatment on AHR presentation was also assessed. This analysis demonstrated that RSV-infected mice treated with anti-IFN-γ antibody presented with reduced Penh values compared to those for RSV-infected mice at methacholine concentrations of between 12.5 and 50.0 mg/ml (P < 0.001) (Fig. 6C). There were no differences in muscarinic 2 receptor (M2R) expression levels between groups (Fig. 6D). These data suggested that IFN-γ could affect airway responses to methacholine.
Resveratrol inhibits TLR3 signaling and M2R expression.
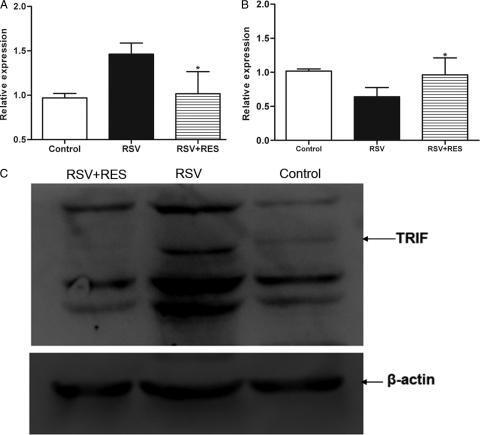
Previous studies indicated that RSV infections induced TLR3 expression in airway epithelial cells (19, 34) and that TLR3 signaling pathways are associated with IFN-γ production (39). Virus-induced IFN-γ production inhibited M2R expression associated with AHR presentation (27). Therefore, the effects of resveratrol on the TLR3 signaling pathway and its effects on M2R expression following RSV infection were examined. At 5 days postinfection, we detected significantly high TLR3 and reduced M2R mRNA expression levels following RSV infection compared to those of controls (P < 0.05) (Fig. 7A and B). Following resveratrol treatment, however, the TLR3 mRNA expression level was reduced and M2R mRNA levels were increased compared to levels observed for RSV-infected mice (P < 0.05) (Fig. 7A and B).
Fig. 7.
Effect of resveratrol on TLR3, TRIF, and M2 expression levels. Lung tissues were harvested at 5 days post-resveratrol treatment. (A) TLR3 gene expression in lung tissue. (B) M2R gene expression in lung tissue. Resveratrol decreased the TLR3 level and increased the M2 gene expression level. Each sample was analyzed by RT-PCR (n = 7/group). (C) Relative TRIF protein expression was inhibited by resveratrol treatment. Each sample was analyzed by Western blotting (n = 5/group). *, P < 0.05 versus the RSV group.
Western blot analysis further illustrated a resveratrol-mediated inhibition of TRIF expression in RSV-infected mice compared to mice infected with RSV (but not treated with resveratrol; P < 0.01) (Fig. 7C). These data indicated that resveratrol could reduce TLR3 mRNA levels, increase M2R mRNA expression levels, and inhibit TRIF expression following RSV infection.
DISCUSSION
Severe symptoms caused by RSV infections in infants are a major public health burden that has severe socioeconomic impacts worldwide. A number of clinical epidemiologic studies found that RSV exposure during early childhood can underpin the development of chronic asthma in later childhood. RSV appears to be intimately linked to the intensity of both physiologic and immunologic responses associated with asthmatic responses in many individuals. These exacerbated responses are associated with increased airway hyperresponsiveness and a significant increase in the number of leukocyte infiltrates into the lungs. The intensity of the inflammatory response is correlated directly to the expression of chemokines by virally infected pulmonary (structural) cells, resident immune cells, and infiltrating leukocytes.
RSV infections elicit the production of various chemokines and cytokines, including IL-6, IL-8, RANTES, and IFN-γ, in the lungs (5, 49). In this study, the level of IFN-γ production in BALF was significantly increased following RSV infection of mice; however, the expression profiles of IL-4, IL-6, IL-10, and IL-17 were unaltered following infection. IL-17 (a proinflammatory cytokine) was reported previously to mediate important roles associated with the presentation of airway hyperresponsiveness in asthma (4, 23). However, in the study described in this report, no changes in IL-17 expression levels were detected following RSV-induced AHR. This may be due to different mechanisms resulting in the induction of AHR (following RSV infections) and the development of allergic asthma.
In most cases, AHR is strongly associated with airway inflammation (24, 25), and most viral infections cause airway inflammation and hyperreactivity. Many studies have previously shown that viral infections increased vagal nerve-mediated bronchoconstriction in virus-infected animals (9, 16). This is caused by the loss of function of the inhibitory M2R present on parasympathetic neurons (16). These inhibitory M2R signals normally decrease the level of acetylcholine released onto M3 receptors located on airway smooth muscles (ASMs), resulting in a subsequent decrease in contraction and bronchoconstriction (17). Jacoby et al. (27) demonstrated previously that viral infections may have increased vagal nerve-mediated bronchoconstriction by either directly inhibiting M2R gene expression or causing the release of IFN-γ (which inhibits M2R gene expression).
Toll-like receptors (TLRs) represent an innate immune antigen recognition system designed to detect molecular patterns associated with the respective pathogens. The ligation of the respective TLRs with their ligands in turn activates immune responses resulting in pathogen clearance. These important innate immune molecules not only are necessary for the initiation of effective immunity but also have been implicated in the early activation of pathways necessary for cellular immune responses as a consequence of chemokines generated at the site of infection. A number of TLRs have been linked to viral infections, including TLR3 (recognition of double-stranded RNA [dsRNA]), TLR4 (binding to the RSV F protein) (20, 22), TLR7/8 (recognition of single-stranded RNA [ssRNA]), and TLR9 (recognition of unmethylated CpG). The replication of some viruses (like RSV) requires the generation of dsRNA that in turn can be recognized by TLR3, resulting in the activation of host immune responses. The activation of TLR3 in epithelial cells following exposure to RSV leads to significant levels of IL-8, RANTES, and IFN-inducible protein 10 (IP-10) production, which indicated that TLR3 activation mediated inflammatory cytokine and chemokine production and induced inflammation in RSV-infected epithelial cells (45). Rudd et al. found previously that the activation of TLR3 during immune response progression shaped the pulmonary immune environment, promoting a predominant Th1-type response that better contained the infection and resulted in diminished pathology (46). Interestingly, poly(I:C) internalized by ASM cells differentially regulated M2R and M3R expression levels and functioned by decreasing and increasing M2R and M3R expression levels, respectively, following interactions with TLR3 (37). This finding suggested that the differential expression of M2R and M3R represents a potential mechanism by which the recognition of viral dsRNA by TLR3 expressed on ASM cells may contribute to airway hyperreactivity. In this study, we found that the TLR3 gene expression level in lung tissues was increased post-RSV infection and that the M2R expression level was decreased. Based on the above-described data, we hypothesized that RSV-induced TLR3 expression reduced M2R expression levels by promoting IFN-γ production post-RSV infection. Here we demonstrated that resveratrol possessed anti-RSV properties by using a CYP-induced immunocompromised BALB/c mouse model of RSV infection. We further determined that resveratrol-mediated effects on airway inflammation and AHR (induced by RSV infection) were the result of reduced IFN-γ production that may be related to TLR3 signaling.
Resveratrol has been shown to be produced by various trees, a few flowering plants, peanuts, and grapes. The major properties of interest are the antioxidative, anti-inflammatory, and estrogenic effects as well as its anticancer and chemopreventative activities (3, 32). Recently, resveratrol was suggested to have many properties that could be beneficial for the treatment of several chronic inflammatory diseases, such as chronic obstructive pulmonary disease, arthritis, and asthma (26, 31, 47). Since resveratrol has potent antioxidative and anti-inflammatory properties, its antiviral effects have therefore been investigated. Recently, resveratrol was shown to be a potent antiviral molecule against various types of DNA and RNA viruses, including HSV-1 (herpes simplex virus 1), HSV-2, VZV (varicella-zoster virus), HCMV (human cytomegalovirus), and influenza A virus (11–14, 42). Here we demonstrated that resveratrol also possessed anti-RSV properties by using a CYP-induced immunocompromised BALB/c mouse model of RSV infection.
In our study, we found that resveratrol significantly reduced RSV replication in infected mouse lung tissues, attenuated pulmonary inflammation, significantly inhibited BALF inflammatory cell and lymphocyte infiltrates, attenuated the AHR response to methacholine following RSV infection, and dramatically reduced BALF IFN-γ levels without altering IL-4, IL-6, IL-10, and IL-17 expression profiles. In other studies, resveratrol was shown to reduce levels of IL-4, IL-5, and IgE that inhibited airway hyperresponsiveness in a mouse asthma model (31). This inconsistency with the data presented in this report was likely due to the use of different animal models. In addition, we directly assessed the role of IFN-γ in the presentation of AHR and airway inflammation by administering blocking anti-IFN-γ antibodies that downregulated IFN-γ expression levels by 95% (Fig. 6E). These studies demonstrated that the downregulation of the IFN-γ response correlated with decreased Penh values and a decrease in total BALF infiltrates. These data further confirmed that resveratrol reduced IFN-γ levels and attenuated AHR, similar to data described previously by Jacoby et al. (27). Interestingly, resveratrol treatment decreased TLR3 and M2R mRNA expression levels and inhibited the upregulation of TRIF expression following RSV infection. These results suggested that resveratrol treatment altered airway inflammation and AHR presentation associated with RSV infections via a TLR3 signaling pathway. This observation was consistent with findings described previously by Youn et al. (51), who reported that resveratrol suppressed NF-κB activation and COX-2 expression specifically by inhibiting TRIF signaling via TLR3 and TLR4 MyD88-independent signaling pathways by targeting TANK-binding kinase 1 and RIP1 (associated with the TRIF complex). We previously showed that resveratrol inhibited RSV replication in 9HTEo airway epithelial cells in vitro and reduced the expression levels of RSV infection-induced IL-6 and IL-8 associated with the suppression of the TRIF signaling pathway (data not shown). Results presented in this report showed that resveratrol attenuated airway inflammation and AHR by inhibiting TRIF expression that resulted in decreased IFN-γ production.
In summary, our findings provided evidence that resveratrol was a potent suppressor of RSV infection, inflammation, and the AHR response associated with RSV infection. These data suggested that resveratrol is an important lead compound for the development of a new family of pharmacological agents capable of suppressing IFN-γ-mediated inflammatory and airway hyperresponsive events associated with RSV infections. Our data further demonstrated that resveratrol treatment interfered with IFN-γ production by interfering with the TLR3 signaling pathway. The use of resveratrol as an antiviral agent presents some distinct advantages over other currently used antiviral and anti-inflammatory agents.
ACKNOWLEDGMENTS
We thank Jie Chen for providing advice throughout the course of this work. We also thank the Experimental Animal Center at Chongqing Medical University for providing the BALB/c mice.
This work was supported by the New Century Excellent Talents Program from the Education Ministry of China (NCET-06-0775), the Key Program of Chongqing Medical University (XBZD200808), the second Colleges and Universities Excellent Talents Program in Chongqing (2011.1-2012.12), and the National Natural Science Foundation of China (81170010).
We declare that there is no conflict of interest.
Footnotes
Published ahead of print on 21 September 2011.
REFERENCES
- 1. American Academy of Pediatrics Committee on Infectious Diseases and Committee of Fetus and Newborn. 1998. Prevention of respiratory syncytial virus infections: indications for the use of palivizumab and update on the use of RSV-IGIV. Pediatrics 102:1211–1216 [DOI] [PubMed] [Google Scholar]
- 2. Anonymous. 2004. Respiratory syncytial virus activity—United States, 2003-2004. MMWR Morb. Mortal. Wkly. Rep. 53:1159–1160 [PubMed] [Google Scholar]
- 3. Baatjes A. J., et al. 2002. Anti-allergic therapies: effects on eosinophil progenitors. Pharmacol. Ther. 95:63–72 [DOI] [PubMed] [Google Scholar]
- 4. Barczyk A., Pierzchala W., Sozanska E. 2003. Interleukin-17 in sputum correlates with airway hyperresponsiveness to methacholine. Respir. Med. 97:726–733 [DOI] [PubMed] [Google Scholar]
- 5. Bennett B. L., et al. 2007. Immunopathogenesis of respiratory syncytial virus bronchiolitis.J. Infect. Dis. 195:1532–1540 [DOI] [PubMed] [Google Scholar]
- 6. Blackwell T. S., Christman J. W. 1997. The role of nuclear factor-kappa B in cytokine gene regulation. Am. J. Respir. Cell Mol. Biol. 17:3–9 [DOI] [PubMed] [Google Scholar]
- 7. Boeck K. D. 1996. Respiratory syncytial virus bronchiolitis: clinical aspects and epidemiology. Monaldi Arch. Chest Dis. 51:210–213 [PubMed] [Google Scholar]
- 8. Bradamante S., Barenghi L., Villa A. 2004. Cardiovascular protective effects of resveratrol. Cardiovasc. Drug Rev. 22:169–188 [DOI] [PubMed] [Google Scholar]
- 9. Buckner C. K., Songsiridej V., Dick E. C., Busse W. W. 1985. In vivo and in vitro studies on the use of the guinea pig as a model for virus-provoked airway hyperreactivity. Am. Rev. Respir. Dis. 132:305–310 [DOI] [PubMed] [Google Scholar]
- 10. Burstein B., et al. 2007. Effects of resveratrol (trans-3,5,4′-trihydroxystilbene) treatment on cardiac remodeling following myocardial infarction. J. Pharmacol. Exp. Ther. 323:916–923 [DOI] [PubMed] [Google Scholar]
- 11. Docherty J. J., et al. 2005. Effect of resveratrol on herpes simplex virus vaginal infection in the mouse. Antiviral Res. 67:155–162 [DOI] [PubMed] [Google Scholar]
- 12. Docherty J. J., Sweet T. J., Bailey E., Faith S. A., Booth T. 2006. Resveratrol inhibition of varicella-zoster virus replication in vitro. Antiviral Res. 72:171–177 [DOI] [PubMed] [Google Scholar]
- 13. Evers D. L., Wang X., Huong S. M., Huang D. Y., Huang E. S. 2004. 3,4′,5-Trihydroxy-trans-stilbene (resveratrol) inhibits human cytomegalovirus replication and virus-induced cellular signaling. Antiviral Res. 63:85–95 [DOI] [PubMed] [Google Scholar]
- 14. Faith S. A., Sweet T. J., Bailey E., Booth T., Docherty J. J. 2006. Resveratrol suppresses nuclear factor-kappaB in herpes simplex virus infected cells. Antiviral Res. 72:242–251 [DOI] [PubMed] [Google Scholar]
- 15. Falsey A. R., Hennessey P. A., Formica M. A., Cox C., Walsh E. E. 2005. Respiratory syncytial virus infection in elderly and high-risk adults. N. Engl. J. Med. 352:1749–1759 [DOI] [PubMed] [Google Scholar]
- 16. Fryer A. D., Jacoby D. B. 1991. Parainfluenza virus infection damages inhibitory M2 muscarinic receptors on pulmonary parasympathetic nerves in the guinea-pig. Br. J. Pharmacol. 102:267–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fryer A. D., Maclagan J. 1984. Muscarinic inhibitory receptors in pulmonary parasympathetic nerves in the guinea-pig. Br. J. Pharmacol. 83:973–978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Graham B. S., Bunton L. A., Wright P. F., Karzon D. T. 1991. Role of T lymphocyte subsets in the pathogenesis of primary infection and rechallenge with respiratory syncytial virus in mice. J. Clin. Invest. 88:1026–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Groskreutz D. J., et al. 2006. Respiratory syncytial virus induces TLR3 protein and protein kinase R, leading to increased double-stranded RNA responsiveness in airway epithelial cells. J. Immunol. 176:1733–1740 [DOI] [PubMed] [Google Scholar]
- 20. Haeberle H. A., et al. 2002. Respiratory syncytial virus-induced activation of nuclear factor-kappaB in the lung involves alveolar macrophages and Toll-like receptor 4-dependent pathways.J. Infect. Dis. 186:1199–1206 [DOI] [PubMed] [Google Scholar]
- 21. Hain R., Bieseler B., Kindl H., Schroder G., Stocker R. 1990. Expression of a stilbene synthase gene in Nicotiana tabacum results in synthesis of the phytoalexin resveratrol. Plant Mol. Biol. 15:325–335 [DOI] [PubMed] [Google Scholar]
- 22. Haynes L. M., et al. 2001. Involvement of Toll-like receptor 4 in innate immunity to respiratory syncytial virus. J. Virol. 75:10730–10737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hellings P. W., et al. 2003. Interleukin-17 orchestrates the granulocyte influx into airways after allergen inhalation in a mouse model of allergic asthma. Am. J. Respir. Cell Mol. Biol. 28:42–50 [DOI] [PubMed] [Google Scholar]
- 24. Henderson W. R., Jr., et al. 1997. Blockade of CD49d (alpha4 integrin) on intrapulmonary but not circulating leukocytes inhibits airway inflammation and hyperresponsiveness in a mouse model of asthma. J. Clin. Invest. 100:3083–3092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Henderson W. R., Jr., Lu J., Poole K. M., Dietsch G. N., Chi E. Y. 2000. Recombinant human platelet-activating factor-acetylhydrolase inhibits airway inflammation and hyperreactivity in mouse asthma model. J. Immunol. 164:3360–3367 [DOI] [PubMed] [Google Scholar]
- 26. Holmes-McNary M., Baldwin A. S., Jr 2000. Chemopreventive properties of trans-resveratrol are associated with inhibition of activation of the IkappaB kinase. Cancer Res. 60:3477–3483 [PubMed] [Google Scholar]
- 27. Jacoby D. B., Xiao H. Q., Lee N. H., Chan-Li Y., Fryer A. D. 1998. Virus- and interferon-induced loss of inhibitory M2 muscarinic receptor function and gene expression in cultured airway parasympathetic neurons. J. Clin. Invest. 102:242–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jang M., et al. 1997. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 275:218–220 [DOI] [PubMed] [Google Scholar]
- 29. Kim H. W., et al. 1969. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am. J. Epidemiol. 89:422–434 [DOI] [PubMed] [Google Scholar]
- 30. Kong X., et al. 2005. An immunocompromised BALB/c mouse model for respiratory syncytial virus infection. Virol. J. 2:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lee M., et al. 2009. Anti-inflammatory and anti-asthmatic effects of resveratrol, a polyphenolic stilbene, in a mouse model of allergic asthma. Int. Immunopharmacol. 9:418–424 [DOI] [PubMed] [Google Scholar]
- 32. Leung D. Y., Szefler S. J. 1998. New insights into steroid resistant asthma. Pediatr. Allergy Immunol. 9:3–12 [DOI] [PubMed] [Google Scholar]
- 33. Lin J. F., et al. 2008. Resveratrol reduces infarct size and improves ventricular function after myocardial ischemia in rats. Life Sci. 83:313–317 [DOI] [PubMed] [Google Scholar]
- 34. Liu P., et al. 2007. Retinoic acid-inducible gene I mediates early antiviral response and Toll-like receptor 3 expression in respiratory syncytial virus-infected airway epithelial cells. J. Virol. 81:1401–1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Manna S. K., Mukhopadhyay A., Aggarwal B. B. 2000. Resveratrol suppresses TNF-induced activation of nuclear transcription factors NF-kappa B, activator protein-1, and apoptosis: potential role of reactive oxygen intermediates and lipid peroxidation. J. Immunol. 164:6509–6519 [DOI] [PubMed] [Google Scholar]
- 36. McKimm-Breschkin J. L. 2004. A simplified plaque assay for respiratory syncytial virus—direct visualization of plaques without immunostaining. J. Virol. Methods 120:113–117 [DOI] [PubMed] [Google Scholar]
- 37. Morishima H., Kajiwara K., Akiyama K., Yanagihara Y. 2008. Ligation of Toll-like receptor 3 differentially regulates M2 and M3 muscarinic receptor expression and function in human airway smooth muscle cells. Int. Arch. Allergy Immunol. 145:163–174 [DOI] [PubMed] [Google Scholar]
- 38. Myou S., et al. 2003. Blockade of inflammation and airway hyperresponsiveness in immune-sensitized mice by dominant-negative phosphoinositide 3-kinase-TAT. J. Exp. Med. 198:1573–1582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Negishi H., et al. 2008. A critical link between Toll-like receptor 3 and type II interferon signaling pathways in antiviral innate immunity. Proc. Natl. Acad. Sci. U. S. A. 105:20446–20451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Newton R. 2000. Molecular mechanisms of glucocorticoid action: what is important? Thorax 55:603–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Newton R., Kuitert L. M., Bergmann M., Adcock I. M., Barnes P. J. 1997. Evidence for involvement of NF-kappaB in the transcriptional control of COX-2 gene expression by IL-1beta. Biochem. Biophys. Res. Commun. 237:28–32 [DOI] [PubMed] [Google Scholar]
- 42. Palamara A. T., et al. 2005. Inhibition of influenza A virus replication by resveratrol.J. Infect. Dis. 191:1719–1729 [DOI] [PubMed] [Google Scholar]
- 43. Paramore L. C., Ciuryla V., Ciesla G., Liu L. 2004. Economic impact of respiratory syncytial virus-related illness in the US: an analysis of national databases. Pharmacoeconomics 22:275–284 [DOI] [PubMed] [Google Scholar]
- 44. Polack F. P., et al. 2002. A role for immune complexes in enhanced respiratory syncytial virus disease. J. Exp. Med. 196:859–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rudd B. D., Burstein E., Duckett C. S., Li X., Lukacs N. W. 2005. Differential role for TLR3 in respiratory syncytial virus-induced chemokine expression. J. Virol. 79:3350–3357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rudd B. D., et al. 2006. Deletion of TLR3 alters the pulmonary immune environment and mucus production during respiratory syncytial virus infection. J. Immunol. 176:1937–1942 [DOI] [PubMed] [Google Scholar]
- 47. Tsai S. H., Lin-Shiau S. Y., Lin J. K. 1999. Suppression of nitric oxide synthase and the down-regulation of the activation of NFkappaB in macrophages by resveratrol. Br. J. Pharmacol. 126:673–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Welliver R. C. 2003. Review of epidemiology and clinical risk factors for severe respiratory syncytial virus (RSV) infection. J. Pediatr. 143:S112–S117 [DOI] [PubMed] [Google Scholar]
- 49. Xie X. H., et al. 2009. Lipopolysaccharide induces IL-6 production in respiratory syncytial virus-infected airway epithelial cells through the Toll-like receptor 4 signaling pathway. Pediatr. Res. 65:156–162 [DOI] [PubMed] [Google Scholar]
- 50. Yang M., Kumar R. K., Foster P. S. 2010. Interferon-gamma and pulmonary macrophages contribute to the mechanisms underlying prolonged airway hyperresponsiveness. Clin. Exp. Allergy 40:163–173 [DOI] [PubMed] [Google Scholar]
- 51. Youn H. S., et al. 2005. Specific inhibition of MyD88-independent signaling pathways of TLR3 and TLR4 by resveratrol: molecular targets are TBK1 and RIP1 in TRIF complex. J. Immunol. 175:3339–3346 [DOI] [PubMed] [Google Scholar]