Abstract
The physiological context of virus-infected cells can markedly affect multiplication and spread of the virus progeny. During persistent infection, the virus exploits the host cell without disturbing its vital functions. However, microenvironmental hypoxia can uncouple this intimate relationship and escalate virus pathogenesis. Accumulating evidence suggests that hypoxia-inducible factor (HIF) modulates gene expression of the viruses that pass through a DNA stage, contain hypoxia-responsive promoter elements, and replicate in the nucleus. Here we show that hypoxia can influence the gene expression and transmission of the cytoplasmic RNA virus lymphocytic choriomeningitis virus (LCMV), which is a neglected human pathogen and teratogen. The MX strain of LCMV, which we used as a model, replicates in a persistent mode in human HeLa cells, fails to produce mature envelope glycoproteins, and spreads through cell-cell contacts in the absence of extracellular infectious virions. Both exposure of MX-infected HeLa cells to chronic hypoxia and gene transfer approaches led to increased virus RNA transcription and higher levels of the viral proteins via a HIF-dependent mechanism. Moreover, hypoxia enhanced the formation of infectious virions capable of transmitting LCMV by cell-free medium. This LCMV “reactivation” might have health-compromising consequences in hypoxia-associated situations, such as fetal development and ischemia-related pathologies.
INTRODUCTION
The prototypic arenavirus lymphocytic choriomeningitis virus (LCMV) provides an important model for investigations of the mechanisms of viral persistence and pathogenesis. Studies using this model led to major advances in virology and immunology that apply universally to other viral and microbial infections of humans (5, 7, 43, 45). Even though LCMV infections are mostly asymptomatic and often remain unnoticed, compelling evidence indicates that LCMV is a neglected human pathogen of clinical significance, especially in cases of congenital infections leading to an increased risk of spontaneous abortion or central nervous system (CNS) disorders and chorioretinitis (3, 4, 17, 44). Moreover, LCMV poses a special threat to immunocompromised individuals, as tragically illustrated by recent cases of transplant-associated infections by LCMV with fatal outcomes in the United States (13) and Australia (25). LCMV has a bisegmented single-stranded RNA genome and a life cycle confined to the cell cytoplasm. The genome consists of a small segment (S) (3.4 kb) and a large segment (L) (7.2 kb). Each genomic segment uses an ambisense coding strategy to direct the synthesis of two polypeptides from two opposite open reading frames separated by an intergenic region. The S segment encodes a major viral protein nucleoprotein (NP) and a glycoprotein precursor (GPC), which is posttranslationally cleaved into peripheral glycoprotein 1 (GP1) and transmembrane glycoprotein 2 (GP2). The L segment encodes a viral RNA-dependent RNA polymerase (L) and a small regulatory RING domain-containing Z protein (Z) (6, 42). Studies using reverse genetic approaches identified NP and L as the minimal viral trans-acting factors required for RNA synthesis, both for transcription and replication (23).
The MX strain of LCMV, which served as a model in this study, was originally identified in the human MaTu cell line, which was presumably derived from a mammary tumor as described earlier, and was later transferred to HeLa cells (21, 26, 31). Sequence analysis of the coding regions confirmed that MX represents a distinct strain of LCMV (14, 31, 37). The biological features of LCMV MX exhibited several similarities with those of other persistent LCMV strains. It is a noncytolytic virus that does not form distinct virions, and its transmission to uninfected cells is mediated by cell-cell contact or by cell extract but not by filtered medium of the infected cells (31). Recently we showed that MX NP binds and stabilizes keratin 1, a part of an intermediate filament network, and thereby facilitates LCMV transmission via cell-cell contacts (22). LCMV MX-infected cells accumulate high cytoplasmic levels of NP and Z and full-length as well as deleted viral RNAs (14, 31, 37). In comparison with other LCMV strains, MX has a more restricted host range (31).
Despite a long-lasting interest in LCMV biology, the contribution of physiological factors to its multiplication and spread has not been consistently investigated. Nevertheless, based on the paradigms of other viruses, the outcome of viral infections can be significantly affected by components of tissue physiology and cellular microenvironment, including low oxygen supply, i.e., hypoxia, which occurs in many pathophysiological situations that might be present in infected humans. Molecular and cellular responses to hypoxia play key adaptive roles during embryonic development and in different diseases, such as anemia, pneumonia, diabetes, cardiovascular disorders, brain ischemia, inflammation, and cancer (24, 33). Proper oxygen availability is crucial for cellular metabolism and survival, and when it is insufficient, cells induce transcriptional reprogramming governed by hypoxia-inducible factor (HIF). HIF is composed of an oxygen-regulated α subunit (predominantly type 1 or 2) interacting with a constitutive β subunit to form a functional heterodimer, which activates target genes via binding to hypoxia-responsive elements (HRE) in their regulatory regions. HIF-induced genes are implicated in many different processes, including angiogenesis, vascular tonus, cell proliferation, apoptosis, extracellular matrix remodeling, glycolysis, pH regulation, etc. (24, 34). There is also growing evidence that hypoxia and HIF affect the host-virus interaction and hence the pathogenesis of the viruses that either posses a DNA genome or pass through a DNA stage, contain hypoxia-responsive elements in their promoters, and need the nucleus for their replication. For example, hypoxia enhances gene expression of human B19 erythrovirus (9, 29) and replication of oncolytic herpes simplex virus (1) and induces lytic replication of Epstein-Barr virus (18, 41) and Kaposi sarcoma-associated herpesvirus (KSHV) (12, 15), but it suppresses replication of the oncolytic parvovirus minute virus of mice (35), adenovirus (36), Moloney murine leukemia virus (M-MuLV) (30), and HIV-1 (11, 41).
To our knowledge, this work provides the first evidence that hypoxia enhances gene expression, production of extracellular infectious virions, and in vitro transmission of an RNA virus replicating in the cytoplasm. We demonstrated that exposure of cells persistently infected with LCMV to hypoxia resulted in activated expression of all virus genes and enhanced generation of infectious extracellular virus progeny. We also showed that this phenomenon depends on the HIF transcription factor. Our findings suggest that reduced oxygenation modulates LCMV replication and the outcome of infection and therefore might play a role in human pathologies linked with hypoxia.
MATERIALS AND METHODS
Cell culture and persistent LCMV infection.
Lymphocytic choriomeningitis virus strain MX was continuously propagated in persistently infected HeLa cervical carcinoma cells (designated HeLa-MX cells). The infection was established by infected cell extract, and cells were grown as described earlier (31, 38). The HeLa-Arm cell line persistently infected with LCMV strain Armstrong was generated and propagated as described previously (22). Noninfected HeLa cells cultured in parallel were used as a control. The cells were grown in Dulbecco modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum, 2 mM l-glutamine (Lonza, Verviers, Belgium), and 160 μg/ml gentamicin (Lek, Ljubljana, Slovenia) in a humidified air atmosphere at 37°C in the presence of 5% CO2. For hypoxic treatment, cells were incubated within a hypoxic workstation (Ruskinn Technology, Bridgend, United Kingdom) in a mixture of gases (2% O2, 5% CO2, 2% H2, and 91% N2) at 37°C for 48 h. Hypoxia was also induced chemically with 1 mM dimethyloxalylglycine (DMOG), an inhibitor of prolyl hydroxylases (PHDs) (Frontier Scientific, Logan, UT).
Antibodies and plasmids.
Mouse monoclonal antibody M87 was produced by the procedure described previously for similar NP-specific antibodies (26). Mouse monoclonal antibody MJ3, specific for LCMV Z, was generated using the hybridoma technique following immunization with two doses of 5 × 106 HeLa-MX cells and a booster of 100 μg glutathione S-transferase (GST)-Z protein bound to glutathione-Sepharose 4B. Anti-GP1 affinity-purified rabbit polyclonal antibody was raised against a peptide mapping within a region of the LCMV MX GP1 (amino acids 205 to 218) by GenScript USA Inc. (Piscataway, NJ). Antiactin goat polyclonal antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-HIF-1α mouse monoclonal antibody was purchased from BD Transduction Laboratories (San Jose, CA). Secondary anti-mouse peroxidase-conjugated antibody was from Sevapharma (Prague, Czech Republic), and anti-goat peroxidase-conjugated antibody was from Dako (Glostrup, Denmark). Alexa Fluor 488 goat anti-mouse IgG antibody was purchased from Invitrogen (Carlsbad, CA). Human HIF-1α and mouse HIF-2α cDNAs in plasmid pcDNA3.1/Neo were kindly provided by Patrick Maxwell, Imperial College of Science, Technology and Medicine, London, United Kingdom. Empty pcDNA3.1 plasmid (Invitrogen) was used as a negative control for mock transfection.
RNA isolation and RT.
Total cellular RNA was extracted with InstaPure reagent (Eurogentec, Seraing, Belgium) from HeLa, HeLa-MX, and HeLa-Arm cells either cultured in hypoxic versus normoxic conditions for 48 h or treated with DMOG for 24 h. RNA was also isolated from the corresponding cell culture media with the NucleoSpin RNA II kit (Macherey-Nagel, Duren, Germany). Reverse transcription (RT) was performed with M-MuLV reverse transcriptase (Finnzymes, Espoo, Finland) using random heptameric primers.
Standard and quantitative real-time PCR analysis.
PCRs were performed with GoTaq Flexi DNA polymerase (Promega, Madison, WI) in an automatic DNA thermal cycler (Eppendorf AG, Hamburg, Germany) using LCMV-MX gene-specific primers and primers for β-actin that served as internal standards (Table 1). PCR products were separated on 1.5% agarose gels and visualized by ethidium bromide staining.
Table 1.
Primers used for RT-PCR analysis
| Gene (strain) | Forward primer | Reverse primer |
|---|---|---|
| NP (MX) | GATCAGAAACAGTTCAAACAGGACT | GTCCCACACTTTGTCTTCATACTCT |
| GP (MX) | AACCAGTGCAGAACTTTTAGAGGTA | GCAAGTCTTCTAGTGAGGAACTTTG |
| L (MX) | AGCTGCTGTCTCGTTGTATAGAAAT | ATACATGCCAACTTGTTAGTGTCCT |
| Z (MX) | CCTGTGAGAGTACAGAGACAAACCT | GATATCTTCAGCTTGGTTGGTAATG |
| NP (ARM) | GATCAAAAACAATTCAAGCAAGATT | GTCCCACACTTTGTCTTCATACTCC |
| GP (ARM) | AGCCAGTGTAGAACCTTCAGAGGTA | GCTAGTCTCCTAGTGAAGAACTTAG |
| L (ARM) | AGTTGCTGTCACGCTGCATTGAAAT | ATGCATGCCAATTTGTTAGTGTCCT |
| Z (ARM) | TCGTGAGGCTGTCAGAAGTGGACCT | GATATCTTCAATCTGGTTGGTAATG |
| HIF-1α | GCTTGGTGCTGATTTGTGAACC | GCATCCTGTACTGTCCTGTGGTG |
| HIF-2α | GAAAACATCAGCAAGTTCATGG | GTGGGATGGGTGCTGGAT |
| CA9 | CCGAGCGACGCAGCCTTTGA | GGCTCCAGTCTCGGCTACCT |
| GLUT-1 | CTCCTTTCTCCAGCCAGCAATG | CCAGCAGAACGGGTGGCCATAG |
| VEGF | CTTGCTGCTCTACCTCCACCAT | CACACAGGATGGCTTGAAGATG |
| β-Actin | CCAACCGCGAGAAGATGA | GATCTTCATGAGGTAGTCAGT |
Quantitative real-time PCR was performed on a StepOne real-time PCR system (Applied Biosystems, Foster City, CA.) using POWER SYBR green PCR master mix (Applied Biosystems) and primers specific for LCMV genes (MX and Arm, respectively) as well as for HIF-1α, HIF-2α, vascular endothelial growth factor (VEGF), carbonic anhydrase 9 (CA9), and glucose transporter 1 (GLUT-1) (Table 1). Relative quantification was performed using β-actin as an endogenous control and expressed as fold induction relative to normoxic conditions or vehicle treatment. Results were analyzed with a two-tailed unpaired t test (Student test) with a P value of <0.05 considered significant.
RLM-RACE.
Selective amplification of 5′-capped transcripts of LCMV MX was carried out using the GeneRacer kit according to instructions of the manufacturer (Invitrogen, Life Technologies). LCMV MX gene-specific primers employed in RNA ligase-mediated rapid amplification of 5′ cDNA ends (RLM-RACE) on RNA isolated from normoxic and hypoxic HeLa-MX cells are listed in Table 2. β-Actin was employed as internal standard and control of RLM quality using the primers included in the kit. The resulting PCR fragments were run on 1.5% agarose gels, and their specificity was verified by sequencing and by reamplification with independent gene-specific primers. The intensity of bands corresponding to individual PCR products was evaluated with GeneTools software from Syngene. The amount of gene-specific PCR products was semiquantitatively expressed as the ratio of the intensity of each LCMV-specific band to the intensity of the corresponding β-actin internal standard. Commercial HeLa total RNA included in the kit was used for β-actin amplification as a control for the activities of calf intestinal alkaline phosphatase (CIP) and tobacco acid pyrophosphatase (TAP) enzymes.
Table 2.
Primers used for RLM-RACE
| Gene | 5′ RACE reverse primer | 5′ RACE nested reverse primer |
|---|---|---|
| NP | CAAGGTCGGCAGCGAGAGACATCA | AGAAGGCTAGTTGCGTCCTTGATG |
| GP | GGCTGAACATGCATTGGGCATTGT | TAGGAGAAGGAAGCTGACCAATGC |
| L | TCCTGGACACACAACTCCGGACTCTA | ACAGCCACTTTTGTCTGCACTGTC |
| Z | CTTCGTAGGGAGGTGGTGGGCTTG | AGTTCAGTGGACCGAGATAGGTGGT |
Western blot analysis.
One million HeLa and HeLa-MX cells were plated in petri dishes, left to attach overnight, and then incubated for 48 h in normoxia or hypoxia. Cells were disrupted in lysis buffer containing 50 mM Tris (pH 7.5), 150 mM NaCl, 1% Triton X-100, 0.1% sodium deoxycholate, and 1× complete protease inhibitor cocktail (Roche, Mannheim, Germany) in phosphate-buffered saline (PBS), and total protein concentrations were determined by bicinchoninic acid assay (Pierce, Rockford, IL) according to the manufacturer's instructions. Total protein extracts (100 μg/lane) were separated by SDS-PAGE under reducing conditions and blotted onto polyvinylidene fluoride membranes (Immobilon; Millipore, Billerica, MA). Membranes were treated for 1 h in blocking buffer (5% [wt/vol] nonfat dry milk in PBS containing 0,2% Nonidet P-40) and then incubated with specific antibodies against NP, Z, GP1, HIF-1α, or actin for 1 h and washed 4 times for 10 min with washing buffer (PBS containing 0,2% Nonidet P-40), followed by incubation with appropriate secondary antibody conjugated with horseradish peroxidase for 1 h. After an additional washing step, all immunoblots were developed with the ECL detection system.
LCMV MX infectivity assay.
Culture medium from HeLa-MX cells maintained in hypoxia or normoxia for 48 h was filtered through a 0.2-μm filter and used for infection of HeLa cells. Briefly, HeLa cells were plated at low density 16 h before use. At the time of infection, the medium was removed from the recipient cells and replaced by 2 ml of filtered medium from HeLa-MX cells. After 24 h of incubation at 37°C in normoxia, the virus inoculum was removed and fresh medium was added. The cells were cultivated as described above and subsequently passaged twice a week. Spread of infection was monitored by RT-PCR analysis and immunofluorescence detection of LCMV nucleoprotein in recipient cells.
Immunofluorescence analysis.
Cells grown on glass coverslips were fixed in ice-cold methanol at −20°C for 5 min. Nonspecific binding was blocked by incubation with PBS containing 3% bovine serum albumin (BSA) for 1 h at room temperature. Subsequently, the cells were incubated with M87 antibody in undiluted hybridoma medium for 1 h at room temperature, washed five times in PBS with 0.2% Tween 20 (Sigma) for 5 min, incubated with Alexa Fluor 488 goat anti-mouse IgG antibody diluted 1:1,000 in PBS containing 1% BSA for 45 min at room temperature, and washed as before. Finally, the cells were mounted on slides in fluorescent mounting medium (Calbiochem, Darmstadt, Germany), analyzed with a Leica DM4500B microscope, and photographed with a Leica DFC480 camera.
Transient transfection.
The HeLa-MX cells were plated in 35-mm-diameter petri dishes to reach approximately 70% monolayer density on the next day. Transfection was performed with 4 μg of HIF-1α or HIF-2α expression vector using a GenePorterII reagent (Genlantis, San Diego, CA). Empty pcDNA3.1 was used to adjust the total DNA content in the control sample. The day after transfection, cells were trypsinized and plated in duplicate in 6-well plates. They were allowed to attach for 24 h and then maintained in normoxia for an additional 24 h. Expression of viral genes relative to that in pcDNA3.1-transfected cells was assessed at 72 h after transfection using real-time RT-PCR. Efficiency of transfection was analyzed by flow cytometry using the green fluorescent protein (GFP)-expressing plasmid pEGFPN1.
RNA interference.
RNA duplexes targeting HIF-1α (Hs_HIF1A_5 HP validated small interfering RNA [siRNA]) and HIF-2α (Hs_EPAS1_5 HP validated siRNA) and negative-control siRNA were purchased from Qiagen (Qiagen AB, Solna, Sweden). HeLa-MX cells were transfected with 10 nM siRNA using GeneSilencer siRNA transfection reagent (Genlantis) according to the manufacturer's instructions. After 24 h of transfection, the medium was changed and cells were transferred to hypoxia for an additional 48 h. Total cellular RNA was then extracted and subjected to real-time RT-PCR analysis as described above.
RESULTS
Hypoxia enhances expression of LCMV genes.
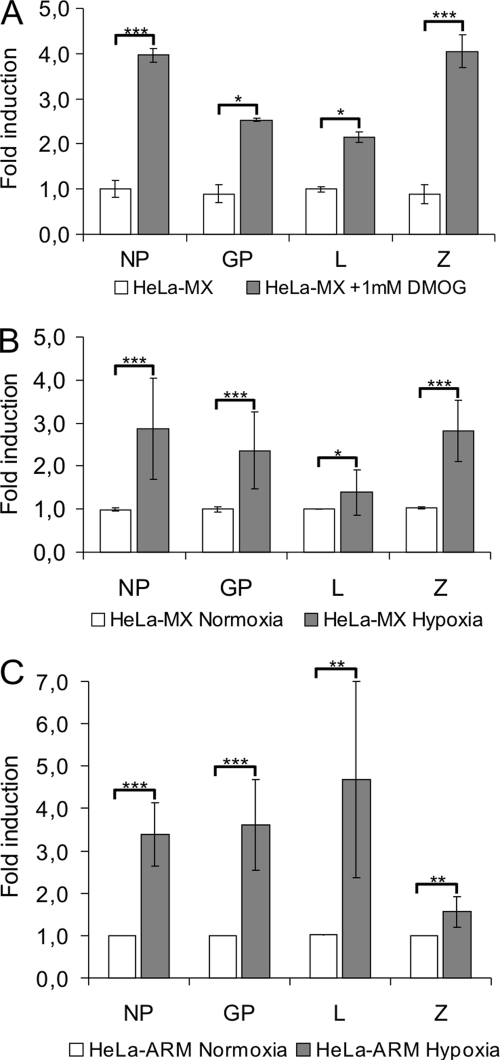
To examine whether hypoxia influences expression of LCMV genes, HeLa cells persistently infected with the MX strain of LCMV were treated with DMOG, a chemical mimic of hypoxia (practically anoxia). Real-time PCR analysis revealed that the levels of RNAs corresponding to all four viral genes were significantly (2 to 4 times) increased in DMOG-treated cells compared to their nontreated counterparts (Fig. 1A). The HeLa-MX cells were then incubated for 48 h in moderate hypoxia (2% O2) and normoxia (21% O2) to better reflect the physiological conditions of chronic hypoxia. A significant increase in expression of the viral NP, Z, and GP genes and only a slight, but statistically significant, increase in the L polymerase transcription level was demonstrated in hypoxic HeLa-MX cells compared to the normoxic controls (Fig. 1B). Similar results were obtained with HeLa cells persistently infected with LCMV strain Armstrong, which showed marked elevation in expression of the NP, GP, and L genes and only a minor increase of Z gene levels (Fig. 1C). This might be connected with the fact that the basal normoxic Z RNA levels related to NP levels are higher in Arm-infected cells than in MX-infected cells (data not shown) and thus the Arm response to hypoxia is less dependent on Z induction.
Fig. 1.
Hypoxia upregulates gene expression of LCMV MX. (A) DMOG enhances expression of viral genes. HeLa-MX cells were incubated for 24 h with or without 1 mM DMOG (a hypoxia-mimicking agent) in normoxia. Expression of LCMV genes was determined by quantitative RT-PCR. Values represent means from two separate experiments, each done in triplicates, with error bars denoting standard deviations. *, P < 0.05; ***, P < 0.001 (DMOG versus no DMOG). (B) HeLa-MX cells were cultured under normoxic or hypoxic conditions for 48 h and analyzed by quantitative RT-PCR. For each gene, the fold induction in hypoxia was determined in comparison with the normoxic control. Values represent means from three separate experiments, each done in quadruplicates. Error bars denote the standard deviations. *, P < 0.05; ***, P < 0.001 (hypoxia versus normoxia). (C) HeLa-Arm cells were cultured under normoxic or hypoxic conditions for 48 h, subjected to quantitative RT-PCR, and evaluated as for panel B. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
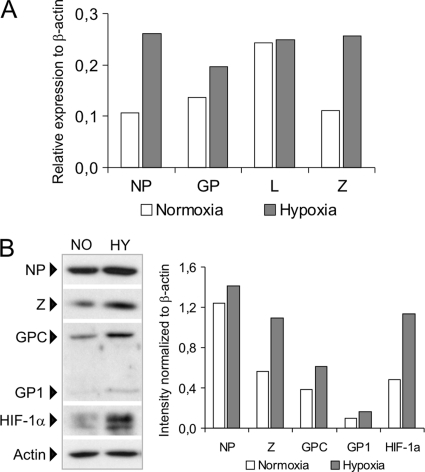
In order to prove that hypoxia influences virus genes at the transcriptional level, we performed RNA ligase-mediated rapid amplification of 5′ cDNA ends (RLM-RACE) using the GeneRacer method, which allows for selective amplification of the 5′-capped transcripts and eliminates noncapped genomic/antigenomic LCMV RNA templates. The semiquantitatively evaluated results of the RLM-RACE carried out on total RNA isolated from hypoxic (2% O2) versus normoxic HeLa-MX cells (Fig. 2A) were principally in accord with the above-described RT PCR data (Fig. 1B), suggesting that hypoxia affects the virus transcription. Furthermore, Western blot analysis revealed that hypoxia, monitored through detection of HIF-1α protein, the stability of which increases with decreasing oxygen availability, resulted in elevated amounts of both NP and Z proteins (Fig. 2B). Importantly, hypoxic HeLa-MX cells showed higher levels of the GPC precursor of the virus glycoproteins and correspondingly increased levels of GP1 glycoprotein, which is usually absent or at very low levels in persistently infected cells but expressed well during acute or productive chronic infection (Fig. 2B).
Fig. 2.
Hypoxia affects LCMV transcription and protein levels. (A) RLM-RACE analysis was performed on RNA isolated from hypoxic and normoxic HeLa-MX cells. Capped RNA transcripts were determined by semiquantitative RT-PCR using a β-actin internal standard. Data show fold induction of viral mRNA levels in hypoxia as in Fig. 1. (B, left) Immunoblot analysis of the indicated viral proteins with specific antibodies using whole-cell extracts prepared from HeLa-MX cells cultured for 48 h under normoxia (NO) or hypoxia (HY). The signal obtained with an antiactin antibody was used as a control for loading and transfer efficiency. Detection of HIF-1α served as a control for the induction of a cellular response to hypoxia. One representative of at least three independent experiments with similar results is shown. (B, right) The intensity of protein bands was evaluated with ImageJ 1.34s software. The relative quantity of proteins was calculated as the ratio of the intensity of each band to the intensity of the related actin internal standard.
Hypoxia induces production of infectious extracellular LCMV virions.
Previous studies have shown that LCMV MX is a noncytolytic virus that principally does not form distinct virions and that its transmission to uninfected cells is mediated by cell-cell contact or by cellular extract but not by cell-free culture medium (22, 31). Since the viral RNA and protein levels were increased in cells exposed to hypoxic stress and this was accompanied by induction of GP1, a protein typical of productive infections, we investigated whether LCMV MX may also be capable of generating functional viral particles and releasing them into the culture medium. We first looked at the levels of extracellular virus RNA using virus gene-specific RT-PCR amplifications on total RNA isolated from the filtered media of hypoxic and normoxic HeLa-MX cells. As shown in Fig. 3A, hypoxia caused a remarkable increase in the levels of the extracellular virus RNA, namely, of that coding for NP, GP, and Z protein, and also a slight but visible increase in the L RNA level.
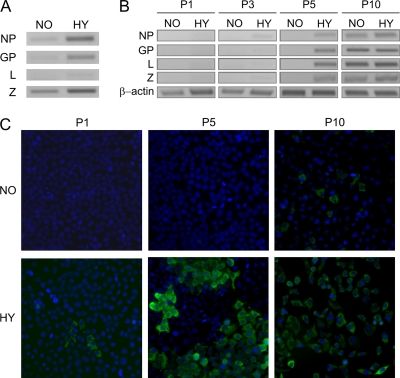
Fig. 3.
Hypoxia stimulates extracellular transmission of LCMV. Filtered medium from HeLa-MX cells that were cultured under normoxia or hypoxia for 48 h was used for infection of noninfected HeLa cells. The cells were then cultivated under normoxic conditions and subsequently passaged. (A) The presence of the LCMV genome in the medium from HeLa-MX cells cultured under normoxia (NO) or hypoxia (HY) was detected by the RT-PCR method. (B) The spread of infection in HeLa cells infected with the indicated medium was monitored by RT-PCR analysis in the first 5 passages and then in the 10th passage. (C) The progress of infection was also monitored by immunofluorescence detection of viral nucleoprotein in recipient cells. Magnification, ×20. The experiment was repeated three times, each time showing similar results. P, passage.
We then used the filtered media from hypoxic and normoxic HeLa-MX cells as inocula in noninfected HeLa cells. This was important to see whether the virus RNA was encompassed within infectious virus particles. The presumed virions were allowed to adsorb for 24 h, and then the inoculum was removed, fresh medium was added, and recipient cells were cultured and subsequently passaged (the whole procedure was done under normoxic conditions). Spread of infection was evaluated by RT-PCR detection of virus genes in isolated total cellular RNA and by immunofluorescence detection of LCMV nucleoprotein in monolayers of recipient cells. The progress of infection was monitored in 10 passages.
The HeLa cells inoculated with the medium from hypoxic HeLa-MX cells showed the first marks of LCMV replication as soon as in the first passage. RT-PCR analysis revealed a time-dependent increase in LCMV gene transcription levels that was consistent with the progressive dissemination of the virus in the cell population (Fig. 3B). Similarly, we could detect a few infected cells already in the first passage of hypoxic medium-inoculated culture using immunofluorescence for the viral nucleoprotein (Fig. 3C), and the number of infected NP-containing cells gradually increased over time. On the other hand, cells infected with the medium from HeLa-MX cells grown under normoxia did not show signs of infection until the fifth passage (Fig. 3B and C). It is noteworthy that both viral RNA and NP antigen were detectable in these cells between passages 5 and 10, depending on the particular conditions of transmission (actual inoculum and density of cells). This suggests that a limited number of virus particles were released into the culture medium of persistently infected cells grown under normoxic conditions, possibly as a result of pericellular hypoxia generated due to increasing density of growing cell monolayer.
Hypoxic upregulation of LCMV gene expression depends on HIF.
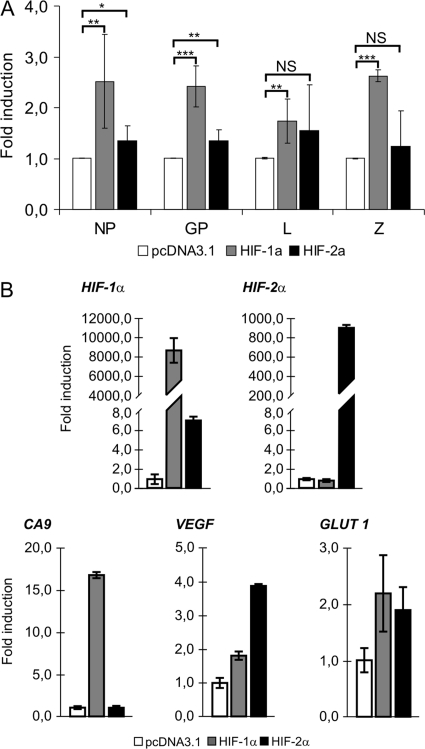
Our results demonstrated that hypoxia enhances LCMV MX gene expression, replication, and virus production. Since the cellular response to hypoxic stress is mediated principally by hypoxia-inducible factors, we explored whether the HIF-1 and/or HIF-2 transcriptional factor is involved in the observed viral “reactivation.” HeLa-MX cells were transfected transiently with HIF-1α and HIF-2α expression vectors and maintained under normoxia. The levels of viral genes relative to those in mock-transfected cells were assessed at 72 h after transfection using quantitative RT-PCR. While the transfection with HIF-2α resulted in only a modest effect on the viral gene transcription, transfection with HIF-1α led to significantly increased levels of viral transcripts encoding NP, GP, Z protein, and L polymerase (Fig. 4A). The transfection efficiencies determined by GFP cotransfection were similar for HIF-1α (41.5% of cells) and HIF-2α (39.9% of cells). HIF-1α and HIF-2α transfected in HeLa-MX cells displayed different mRNA levels (with HIF-1α being about 10 times more abundant), but their transactivation capabilities were comparable, as suggested by transcriptional upregulation of HIF target genes, including those coding for carbonic anhydrase 9 (CA9) (the preferential transcriptional target of HIF-1α), vascular endothelial growth factor (VEGF) (the preferential HIF-2α target), and glucose transporter 1 (GLUT-1) (Fig. 4B). Thus, the lower response of LCMV genes to HIF-2α could be related to its lower expression level and possibly also to the recipient cells, since this protein is of differential importance in various cell types.
Fig. 4.
Effect of HIF-α subunit transfection on LCMV MX gene expression under normoxia. (A) HeLa-MX cells were transfected transiently with HIF-1α or HIF-2α expression vector and maintained under normoxia. Empty pcDNA3.1 was used to adjust the total DNA content in the control sample. Expression of viral genes was assessed 72 h after transfection using quantitative RT-PCR. Fold induction was determined in comparison with the values from pcDNA3.1-transfected cells. Values represent means from three separate experiments, each done in triplicates. Error bars denote the standard deviations. *, P < 0.05; **, P < 0.01; ***, P < 0.001; NS (not significant), P > 0.05. (B) Transfected HeLa-MX cells were analyzed by quantitative RT-PCR to determine expression levels of HIF-1α and HIF-2α subunits and their transcriptional targets. Data were analyzed and illustrated as described above.
These findings were further supported by RNA interference-mediated knockdown of endogenous HIF-1α and HIF-2α. Hypoxic HeLa-MX cells transfected with the specific siRNAs clearly showed reduced levels of viral transcription compared to cells transfected with the control siRNAs (Fig. 5A). The effect of HIF-1α knockdown was more dramatic than that of HIF-2α in relationship to expression of the viral NP and GP genes, but the effects for the L and Z genes were similar. Again, we used HIF transcriptional targets CA9 and GLUT-1 to demonstrate similar suppression of both HIF-1α and HIF-2α mRNA levels. Thus, it seems plausible that HIF-1α plays a dominant role in hypoxic regulation of LCMV gene expression at least in this cell type, but a role of HIF-2α in other cell types cannot be excluded.
Fig. 5.
Effect of HIF-α subunit suppression on LCMV MX gene expression under hypoxia. (A) HeLa-MX cells were transfected with the specific HIF-1α, HIF-2α, and control siRNAs and after 24 h were transferred to hypoxia for additional 48 h. Total RNA was then extracted and subjected to real-time RT-PCR analysis to determine expression of viral genes. Fold induction was determined in comparison with the values from cells transfected with control siRNA. All values represent means from triplicate determinations in one representative experiment out of two, with error bars denoting standard deviations. (B) HeLa-MX cells transfected with siRNA were analyzed by quantitative RT-PCR to determine the effect of HIF-1α and HIF-2α suppression on their transcriptional targets. Data were analyzed and illustrated as described above.
DISCUSSION
Hypoxia represents a physiological stress that has a dramatic influence on cell metabolism and behavior. It stabilizes and activates the HIF transcription factor, which controls the expression and activity of a variety of regulatory molecules, thereby establishing the adaptive cell phenotype. In the case of persistently infected cells, this process can also affect the virus and can significantly affect its life style. The virus can directly use the hypoxic machinery or exploit the cellular adaptations to proceed through its replicative and viral particle assembly phases and produce progeny capable of improved spread of infection. During the last decade, reactivation and replication of different viruses have been linked to the HIF-1 pathway, mostly through a direct transcriptional regulation of viral gene promoters. This mechanism is clearly relevant to viruses that either have a DNA genome containing “classical” promoters (such as herpesviruses) or pass through a DNA stage (such as retroviruses). It is well conceivable that HIF, as a transcription factor that operates primarily in the nucleus, comes into direct or indirect contact with the DNA genome of the virus that replicates in the same compartment.
Here we provide the first example of a hypoxia-induced “reactivation” and spread of a virus with an RNA genome, which replicates in the cytoplasm in a persistent mode without disrupting cell integrity. We show that hypoxia increases the expression of LCMV genes in a HIF-dependent manner. Hypoxic transactivation is also accompanied by production of infectious virus particles that are released to the medium as is typical for acute or productive chronic infections.
There are several possible proposals for the mechanism(s) behind this phenomenon. In a traditional paradigm, HIF binds a double-stranded DNA at a consensus hypoxia-responsive element (HRE) usually located in the gene promoter. However, LCMV has a bisegmented single-stranded RNA genome with an ambisense arrangement. Its transcription depends on virus L polymerase and NP and starts at conserved terminal sequences that serve as the viral promoter (27). Interestingly, the most terminal nucleotides, which are indispensable for efficient virus RNA transcription, contain an HRE-like motif, GCGTG. However, it is unclear whether HIF assembly/activation in the cytoplasm and its binding to the RNA HRE would be feasible and if so whether it could impact LCMV transcription.
HIF can also affect gene transcription via protein-protein interactions with other transcription factors (2). This was also shown to occur on viral promoters, namely, in the case of KSHV, where interaction between HIF-1α and Lana protein and their binding to the RTA gene promoter leads to a shift from latent to acute infection (8), a situation reminiscent of our finding for LCMV. It is imaginable that the HIF-α subunit might interact with NP or L in a similar manner, but the experimental proof remains to be acquired.
Still another possibility points to an effect of HIF on expression and/or activity of cellular factors that affect LCMV gene expression, such as eukaryotic translation initiation factor eIF4E, which influences cap-dependent translation and mRNA export (28, 40). LCMV Z protein interacts with eIF4E, reduces its affinity for the m7G cap, and thereby acts as an inhibitor of eIF4E function (10, 39). This interaction was proposed to inhibit the host protein synthesis (10). Interestingly, hypoxia exerts a similar “shutoff” translation effect by eIF4E sequestration through induced expression of the 4E-BP1 inhibitor and the 4E-T transporter (19). Thus, it is tempting to speculate that hypoxia in conjunction with Z protein could enhance the sequestration of eIF4E and provide a signal for more efficient virus replication.
Finally, hypoxia could affect the processing of the LCMV GPC, as suggested by our finding that the peripheral GP1 envelope protein is elevated in hypoxic cells compared to their normoxic counterparts. GP1 is produced by cleavage of the GPC, and its expression is downregulated during persistent infection (20). We previously showed that HeLa-MX cells contain an aberrant GPC-carrying RNA subpopulation with a large deletion in the central part of the GPC gene, which is required for proper processing of the encoded precursor glycoprotein (37). Hypoxia may favor production of correct full-length genomic RNA, thus allowing for generation of functional mature glycoproteins. This idea is compatible with increased expression of GPC and mature GP1 under hypoxia. Further extensive experimentation is needed to clarify whether and to what extent the proposed mechanisms could contribute to phenomenon of hypoxic LCMV “reactivation.”
Our findings may also have implications for virus pathogenesis. It is conceivable that LCMV infection can coincide with the presence of physiological or pathological hypoxia that can aggravate its clinical outcome. Such a situation typically occurs during embryonic development. Indeed, a large body of evidence indicates that the early placental environment is hypoxic (16) and that HIF plays a critical role in correct tissue morphogenesis and organogenesis (32). Asymptomatically infected mothers can transmit LCMV to the developing embryo, where hypoxia can trigger its productive replication, leading to abortion or teratogenesis. Indeed, sporadic data suggest that infection with LCMV during the first trimester of pregnancy is associated with an increased risk of spontaneous abortion (3, 4); however, the true prevalence of congenital LCMV infection is unknown, partly because it mimics toxoplasmosis or cytomegalovirus infection (44). Thus, our present work prompts further studies toward understanding possible links among LCMV, hypoxia, and embryogenesis.
This “reactivation” mechanism might also contribute to fatal outcomes for transplant recipients of asymptomatically infected donor organs, where hypoxia typically occurring in transplanted tissues could exacerbate LCMV replication in immunosuppressed receivers.
In conclusion, we show here that hypoxia enhances LCMV expression and spread in vitro using a HIF-dependent pathway. We propose that this might represent a mechanism for altered virus pathogenesis in vivo in physiological and/or pathological situations that include hypoxia and might provide an explanation for associated complications with unknown etiology. Thus, this work represents a basis for future rational studies of the role of LCMV in human diseases including hypoxic components.
ACKNOWLEDGMENTS
This project was supported by grants from the Scientific Grant Agency of the Ministry of Education of the Slovak Republic and the Slovak Academy of Sciences (VEGA 2/0134/08 and VEGA 2/0128/11) and by the Research and Development Operational Program funded by the ERDF (project PV-INF-PAT, ITMS 26240220032).
Footnotes
Published ahead of print on 28 September 2011.
REFERENCES
- 1. Aghi M. K., Liu T. C., Rabkin S., Martuza R. L. 2009. Hypoxia enhances the replication of oncolytic herpes simplex virus. Mol. Ther. 17:51–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aprelikova O., Wood M., Tackett S., Chandramouli G. V., Barrett J. C. 2006. Role of ETS transcription factors in the hypoxia-inducible factor-2 target gene selection. Cancer Res. 66:5641–5647 [DOI] [PubMed] [Google Scholar]
- 3. Barton L. L., Mets M. B. 1999. Lymphocytic choriomeningitis virus: pediatric pathogen and fetal teratogen. Pediatr. Infect. Dis. J. 18:540–541 [DOI] [PubMed] [Google Scholar]
- 4. Barton L. L., Mets M. B., Beauchamp C. L. 2002. Lymphocytic choriomeningitis virus: emerging fetal teratogen. Am. J. Obstet. Gynecol. 187:1715–1716 [DOI] [PubMed] [Google Scholar]
- 5. Borrow P., Oldstone M. B. A. 1997. Lymphocytic choriomeningitis virus, p. 593–627. In Nathanson N., Ahmed R., Gonzalez-Scarano F., Griffin D. E., Holmes K. V., Murphy F. A., Robinson H. L. (ed.), Viral pathogenesis. Lippincott-Raven, Philadelphia, PA. [Google Scholar]
- 6. Buchmeier M. J., de La Torre J. C., Peters C. J. 2007. Arenaviridae: the viruses and their replication, p. 1793–1826. In Knipe D. M., Howley P. M. (ed.), Fields virology, 5th ed., vol. II. Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 7. Buchmeier M. J., Zajac A. J. 1999. Lymphocytic choriomeningitis virus, p. 575–605.In Ahmed R., Chen I. S. Y. (ed.), Persistent viral infections. John Wiley & Sons, Chichester, West Sussex, United Kingdom. [Google Scholar]
- 8. Cai Q., et al. 2006. Kaposi's sarcoma-associated herpesvirus latent protein LANA interacts with HIF-1 alpha to upregulate RTA expression during hypoxia: latency control under low-oxygen conditions. J. Virol. 80:7965–7975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Caillet-Fauquet P., Draps M. L., Di Giambattista M., de Launoit Y., Laub R. 2004. Hypoxia enables B19 erythrovirus to yield abundant infectious progeny in a pluripotent erythroid cell line. J. Virol. Methods 121:145–153 [DOI] [PubMed] [Google Scholar]
- 10. Campbell Dwyer E. J., Lai H., MacDonald R. C., Salvato M. S., Borden K. L. 2000. The lymphocytic choriomeningitis virus RING protein Z associates with eukaryotic initiation factor 4E and selectively represses translation in a RING-dependent manner. J. Virol. 74:3293–3300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Charles S., et al. 2009. Regulation of HIV-1 transcription at 3% versus 21% oxygen concentration. J. Cell. Physiol. 221:469–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Davis D. A., et al. 2001. Hypoxia induces lytic replication of Kaposi sarcoma-associated herpesvirus. Blood 97:3244–3250 [DOI] [PubMed] [Google Scholar]
- 13. Fischer S. A., et al. 2006. Transmission of lymphocytic choriomeningitis virus by organ transplantation. N. Engl. J. Med. 354:2235–2249 [DOI] [PubMed] [Google Scholar]
- 14. Gibadulinova A., et al. 1998. Sequence and characterisation of the Z gene encoding ring finger protein of the lymphocytic choriomeningitis virus MX strain. Acta Virol. 42:369–374 [PubMed] [Google Scholar]
- 15. Haque M., Davis D. A., Wang V., Widmer I., Yarchoan R. 2003. Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) contains hypoxia response elements: relevance to lytic induction by hypoxia. J. Virol. 77:6761–6768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jaffe R., Jauniaux E., Hustin J. 1997. Maternal circulation in the first-trimester human placenta—myth or reality? Am. J. Obstet. Gynecol. 176:695–705 [DOI] [PubMed] [Google Scholar]
- 17. Jamieson D. J., Kourtis A. P., Bell M., Rasmussen S. A. 2006. Lymphocytic choriomeningitis virus: an emerging obstetric pathogen? Am. J. Obstet. Gynecol. 194:1532–1536 [DOI] [PubMed] [Google Scholar]
- 18. Jiang J. H., et al. 2006. Hypoxia can contribute to the induction of the Epstein-Barr virus (EBV) lytic cycle. J. Clin. Virol. 37:98–103 [DOI] [PubMed] [Google Scholar]
- 19. Koritzinsky M., et al. 2006. Gene expression during acute and prolonged hypoxia is regulated by distinct mechanisms of translational control. EMBO J. 25:1114–1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kunz S., Edelmann K. H., C. de la Torre J., Gorney R., Oldstone M. B. 2003. Mechanisms for lymphocytic choriomeningitis virus glycoprotein cleavage, transport, and incorporation into virions. Virology 314:168–178 [DOI] [PubMed] [Google Scholar]
- 21. Labudova M., Tomaskova J., Kaluzova M., Pastorek J., Pastorekova S. 2006. Lymphocytic choriomeningitis virus mx strain does not induce the expression of tumor-associated carbonic anhydrase IX in persistently infected HeLa cells. Acta Virol. 50:53–58 [PubMed] [Google Scholar]
- 22. Labudova M., Tomaskova J., Skultety L., Pastorek J., Pastorekova S. 2009. The nucleoprotein of lymphocytic choriomeningitis virus facilitates spread of persistent infection through stabilization of the keratin network. J. Virol. 83:7842–7849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lee K. J., Novella I. S., Teng M. N., Oldstone M. B., de La Torre J. C. 2000. NP and L proteins of lymphocytic choriomeningitis virus (LCMV) are sufficient for efficient transcription and replication of LCMV genomic RNA analogs. J. Virol. 74:3470–3477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lendahl U., Lee K. L., Yang H., Poellinger L. 2009. Generating specificity and diversity in the transcriptional response to hypoxia. Nat. Rev. Genet. 10:821–832 [DOI] [PubMed] [Google Scholar]
- 25. Palacios G., et al. 2008. A new arenavirus in a cluster of fatal transplant-associated diseases. N. Engl. J. Med. 358:991–998 [DOI] [PubMed] [Google Scholar]
- 26. Pastorekova S., Zavadova Z., Kostal M., Babusikova O., Zavada J. 1992. A novel quasi-viral agent, MaTu, is a two-component system. Virology 187:620–626 [DOI] [PubMed] [Google Scholar]
- 27. Perez M., C. de la Torre J. 2003. Characterization of the genomic promoter of the prototypic arenavirus lymphocytic choriomeningitis virus. J. Virol. 77:1184–1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pestova T. V., et al. 2001. Molecular mechanisms of translation initiation in eukaryotes. Proc. Natl. Acad. Sci. U. S. A. 98:7029–7036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pillet S., et al. 2004. Hypoxia enhances human B19 erythrovirus gene expression in primary erythroid cells. Virology 327:1–7 [DOI] [PubMed] [Google Scholar]
- 30. Puppo M., Bosco M. C., Federico M., Pastorino S., Varesio L. 2007. Hypoxia inhibits Moloney murine leukemia virus expression in activated macrophages. J. Leukoc. Biol. 81:528–538 [DOI] [PubMed] [Google Scholar]
- 31. Reiserova L., et al. 1999. Identification of MaTu-MX agent as a new strain of lymphocytic choriomeningitis virus (LCMV) and serological indication of horizontal spread of LCMV in human population. Virology 257:73–83 [DOI] [PubMed] [Google Scholar]
- 32. Semenza G. L. 2001. HIF-1 and mechanisms of hypoxia sensing. Curr. Opin. Cell Biol. 13:167–171 [DOI] [PubMed] [Google Scholar]
- 33. Semenza G. L. 2009. Regulation of oxygen homeostasis by hypoxia-inducible factor 1. Physiology (Bethesda) 24:97–106 [DOI] [PubMed] [Google Scholar]
- 34. Semenza G. L. 2003. Targeting HIF-1 for cancer therapy. Nat. Rev. Cancer 3:721–732 [DOI] [PubMed] [Google Scholar]
- 35. Servais C., et al. 2006. Hypoxic-response elements in the oncolytic parvovirus minute virus of mice do not allow for increased vector production at low oxygen concentration. J. Gen. Virol. 87:1197–1201 [DOI] [PubMed] [Google Scholar]
- 36. Shen B. H., Bauzon M., Hermiston T. W. 2006. The effect of hypoxia on the uptake, replication and lytic potential of group B adenovirus type 3 (Ad3) and type 11p (Ad11p). Gene Ther. 13:986–990 [DOI] [PubMed] [Google Scholar]
- 37. Tomaskova J., Labudova M., Kopacek J., Pastorekova S., Pastorek J. 2008. Molecular characterization of the genes coding for glycoprotein and L protein of lymphocytic choriomeningitis virus strain MX. Virus Genes 37:31–38 [DOI] [PubMed] [Google Scholar]
- 38. van der Zeijst B. A., et al. 1983. Persistent infection of some standard cell lines by lymphocytic choriomeningitis virus: transmission of infection by an intracellular agent. J. Virol. 48:249–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Volpon L., Osborne M. J., Capul A. A., C. de la Torre J., Borden K. L. 2010. Structural characterization of the Z RING-eIF4E complex reveals a distinct mode of control for eIF4E. Proc. Natl. Acad. Sci. U. S. A. 107:5441–5446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. von der Haar T., Gross J. D., Wagner G., McCarthy J. E. 2004. The mRNA cap-binding protein eIF4E in post-transcriptional gene expression. Nat. Struct. Mol. Biol. 11:503–511 [DOI] [PubMed] [Google Scholar]
- 41. Washington A. T., Singh G., Aiyar A. 2010. Diametrically opposed effects of hypoxia and oxidative stress on two viral transactivators. Virol. J. 7:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Welsh R. 2008. Lymphocytic choriomeningitis virus: general features, p. 238–243. In Mahy B. W. J., van Regenmortel M. H. V. (ed.), Encyclopedia of virology, 3rd ed. Academic Press, Amsterdam, The Netherlands. [Google Scholar]
- 43. Welsh R. M. 2000. Lymphocytic choriomeningitis virus as a model for the study of cellular immunology, p. 289–312. In Cunningham M. W., Fujinami R. S. (ed.), Effects of microbes on the immune system. Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 44. Wright R., et al. 1997. Congenital lymphocytic choriomeningitis virus syndrome: a disease that mimics congenital toxoplasmosis or cytomegalovirus infection. Pediatrics 100:E9. [DOI] [PubMed] [Google Scholar]
- 45. Zinkernagel R. M., Doherty P. C. 1977. Major transplantation antigens, viruses, and specificity of surveillance T cells. Contemp. Top. Immunobiol 7:179–220 [DOI] [PubMed] [Google Scholar]