Abstract
In blood, the accumulation of terminally differentiated (TD) T cells during HIV infection is associated with CD4 T cell loss and HIV disease progression. Here, we investigated the maintenance and functional characteristics of memory T cells at the cervix. We found that CD4 T cell depletion at the cervix mirrors CD4 depletion in blood. In all women, depletion of CD4 T cells at the cervix was associated with significant reductions in CD45RA− CCR7+ (central memory [CM]) T cells and the accumulation of CD45RA+ CCR7− (TD T cells). We determined whether inflammation in the genital tract was associated with the local differentiation of T cells at the cervix. In uninfected women, genital tract inflammation was associated with the accumulation of CD45RA− CCR7+ CM CD4 T cells and reduced frequencies of CD45RA+ CCR7− TD cells at the cervix. This finding may reflect the fact that, in the absence of HIV infection, TD T cells may be slowly lost in the presence of genital inflammation, while CD45RA− CCR7+ CM T cells are recruited to replenish the diminishing CD4 T cell pool. Following global stimulation with phorbol myristate acetate (PMA)-ionomycin, we noted a significant interleukin 2 (IL-2) deficit in both cervical and blood CD4 T cells from HIV-infected women compared to uninfected women, while gamma interferon (IFN-γ) production was similar, irrespective of HIV status. Few HIV-infected women had detectable IFN-γ and IL-2 HIV-specific T cell responses at the cervix, and these responses were significantly lower in magnitude than the corresponding responses in blood. These data suggest that CD4 depletion was associated with the accumulation of terminally differentiated T cell phenotypes at the cervical mucosa defective in their ability to produce IL-2. CD4 depletion and compromised immunity at the cervix may be accompanied by progressive decline of central memory-like T cells and development of T cells toward terminally differentiated phenotypes.
INTRODUCTION
Most pathogens infect humans through mucosal surfaces, and the maintenance of memory T cells at these exposed effector sites is important as the first line of defense against pathogenic invasion (17, 26, 30). While the mucosal surfaces of the female genital tract serve as the major portal of entry for human immunodeficiency virus (HIV) during heterosexual HIV transmission, the mucosal surface of the gut serves as the predominant site of viral replication and CD4 T cell depletion (18, 24, 27). The female genital tract is a tertiary effector site that lacks organized lymphoid structures (41, 52), and immune cells residing here are recruited in response to an inflammatory signal (22, 29, 31) in an integrin-dependent manner (7, 16). The presence of T cells with the ability to respond rapidly at mucosal epithelial surfaces is essential, as these cells allow for rapid containment of invading pathogens at the local entry sites and prevent systemic spreading (6).
HIV infection is a chronic viral infection that has been associated with gradual exhaustion of the T cell memory pool (10, 11). Throughout the course of HIV infection, there are alterations in the phenotypic and maturational characteristics of T cells, reflected in the accumulation of terminally differentiated (TD) T cells during late stage disease (3). A better understanding of this process of T cell differentiation and maturation and its role in viral control is important for our understanding of T cell-mediated immunity. Studies of the maturational status of immune cells present in the female genital tract may give important insight into events associated with HIV transmission (39).
T cells can be divided into distinct memory subsets based on the expression of the chemokine (C-C motif) receptor 7 (CCR7), CD62L, CD27, CD28, and CD45RA, and they differ in their homing capacity and ability to proliferate and produce cytokines in response to stimuli (1, 32, 42, 45). Compared with naive T cells (N cells) cells, memory T cells divide more rapidly, express adhesion molecules that facilitate extravasation to tissues, and express the low-molecular-weight isoform of CD45 (CD45RO) (8). The ontogeny of memory T cells is still being debated, with different studies proposing a linear differentiation pathway of T cells and others suggesting a complex differentiation pathway (1–3, 5, 19, 23, 42, 43, 46, 51). T cell subpopulations can be grouped further into “early” and “intermediate effectors” and “terminally differentiated” subsets based on their position along a linear pathway of longevity and expression of CD127 on long-lived T cells and CD57 on short-lived T cells: naive cells (CD45RA+ CCR7+) → “early” central memory (CM; CD45RA− CCR7+) → “intermediate effector memory” (EM; CD45RA− CCR7−)→ “late” terminally differentiated cells (TD; CD45RA+ CCR7−) (18, 31, 34).
Studies of acute HIV and simian immunodeficiency virus (SIV) infections have shown that CD4 EM cells are rapidly depleted at mucosal effector sites (gut-associated lymphoid tissue [GALT] and lungs), while the CM pool is largely preserved (14, 33, 36, 37). About 30% to 60% of these mucosal CD4 EM cells are destroyed in the first few days of HIV infection (24, 27). During chronic HIV infection, several studies have shown that maintenance of EM numbers is dependent on the preservation of CM cells for their homeostasis and that this CM-dependent EM production progressively declines during chronic infection (25, 33). The frequency of CD4 CM cells in GALT was found to be associated with better mucosal CD4 T cell restoration (9, 44, 48). Despite replenishing of the EM pool by CM cells, the frequency of CD4 cells remains low at the mucosal sites during chronic HIV infection compared to uninfected individuals (9, 44).
Previous studies have demonstrated that the differentiation status of T cells is associated with the rate of HIV disease progression (4, 20, 34, 40, 47). Burgers et al. (4) showed that higher frequencies of early CD8 memory cells during early HIV infection were associated with subsequently lower viral set points. Papagno et al. (34) reported an inverse correlation between CD4 T cell counts and the percentage of highly differentiated CD27− cells in the whole CD8+ T cell population of HIV-1-infected individuals during chronic infection. Further, individuals who are able to better control HIV infection have a preserved CM pool, (40, 47), while individuals with a reduced CM CD4 pool had increased plasma HIV load (20).
HIV and SIV entry is confined to intermediate and short-lived CD4 T cells compared to naive and central memory CD4 T cells, and this is believed to be due to CCR5 expression levels (14). Since HIV targeting of CD4 T cells is confined to certain memory phenotypes, the differentiation status of T cells plays an important role in HIV pathogenesis. Previously, we showed that the majority of T cells in the female genital tract had an effector memory phenotype and that HIV infection was associated with significantly reduced CD4 T cell proportions (31). In this study, we investigated maintenance and functional characteristics of memory T cells at the cervical mucosa. Understanding the influence of persistent mucosal CD4 depletion and the surrounding inflammatory cervicovaginal milieu on the differentiation status of cervical T cells present at the site of both HIV acquisition and transmission will be important in the design of effective therapies to block new infections.
MATERIALS AND METHODS
Description of individuals included in the study.
Fifty-nine chronically HIV-infected and 67 uninfected women attending the Emphilisweni community clinic in Gugulethu, Cape Town, South Africa, were enrolled in this study. Women who were menstruating at the time of sampling, who were postmenopausal, or who had undergone a hysterectomy were excluded from the study. In South Africa, syndromic management is the national strategy used in the treatment of sexually transmitted infections (STIs) (28, 49). This approach uses clinical algorithms based on a woman's clinical signs or symptoms of an STI for nurses in primary health care facilities to treat. No laboratory confirmation of STIs is performed unless specifically requested. In line with South African national policy, syndromic management of sexually transmitted infections that does not take into account asymptomatic infections was followed in this study. This was done by excluding women who had abnormal vaginal discharge, visible ulcers, or genital warts and those that reported symptoms and clinical signs. Although data on the contraceptive methods used were not collected for the women enrolled in this study, the public sector family planning clinic associated with our study site reported ∼60% of women from their clinic use either 3 monthly injectable Depo Provera or Petogen injections. The study was approved by the Research Ethics Committee of the University of Cape Town, South Africa, and informed written consent was obtained from all volunteers who participated in the study.
Collection and processing of cervical specimens and blood samples.
Cervical mucosal mononuclear cells (MMCs) were collected using a Digene cervical sampler as described previously (35). Briefly, a Digene cervical cytobrush was inserted into the endocervical os (preferentially sampling from the transformation zone), rotated 360°, and immediately placed in 3 ml of cold transport medium (RPMI 1640 medium supplemented with 5 mM glutamine, amphotericin B, penicillin, streptomycin, and 10% fetal calf serum [FCS]). Cervical samples that had visible red blood cell contamination were discarded. Cervical cells were isolated within 4 h of collection by gently rotating the cytobrush against the sides of the tube to dislodge cells. Transport medium was then flushed through the cytobrush bristles 30 times using a sterile plastic Pasteur pipette to dislodge all cervix-derived cells. The cell suspension was transferred to a sterile 15-ml centrifuge tube, and the cells were pelleted at 1,200 rpm (280 × g) for 10 min using a Heraeus Megafuge 1.0 R centrifuge. The supernatant fraction was stored at −80°C until analysis for inflammatory cytokines and HIV shedding. Cervical MMCs were counted by an automated Guava cell counter (Guava Technologies) (31). Cervical mononuclear cells were adjusted to a concentration of ∼1 × 106 cells per ml and allowed to rest for 16 h at 37°C and 5% CO2 prior to staining for flow cytometry. Cervical cytobrush samples were excluded from further analysis by multiparameter flow cytometry if they yielded ≤104 CD3 T cells per cytobrush.
Blood samples from HIV-infected and uninfected women were collected by standard venipuncture and placed in sterile acid-citrate-dextrose (ACD) anticoagulant Vacutainer tubes (Becton Dickenson). Peripheral blood mononuclear cells (PBMCs) were isolated using Ficoll-Hypaque (Sigma) density gradient centrifugation using Leucosep tubes (Lasec). Plasma samples were aliquoted into cryovials and stored at −80°C for later measurement of plasma HIV RNA levels. PBMCs were counted using an automated Guava cell counter and analyzed using Cytosoft software (Guava Technologies). The viable cell concentration was adjusted to 2 × 106 cells/ml and allowed to rest for 16 h at 37°C and 5% CO2.
Flow cytometry.
The phenotypes of T cells in 26 samples were determined by staining with T cell phenotypic markers (CD3, CD8, and CD4), memory markers (CD45RA and CCR7), and the viability marker Vivid, while 90 samples were stimulated and stained with T cell phenotypic markers (CD3 and CD8), memory markers (CD45RA and CCR7), and intracellular cytokines (interleukin 2 [IL-2] and gamma interferon [IFN-γ]). The magnitudes of the T cell responses were not measured in women who were on antiretroviral therapy. Cervical and blood-derived T cells in women who had not been exposed to antiretroviral therapy (naive) were evaluated for intracellular IFN-γ and IL-2 production in response to stimulation with HIV Gag peptides and phorbol myristate acetate (PMA)-ionomycin. Cervical cells (∼0.1 × 106 to 1 × 106 cells/ml) and PBMCs (1 × 106 cells/ml) were stimulated for 6 h ex vivo at 37°C and 5% CO2 with (i) HIV subtype C (Du422) Gag peptides (122 peptides [15-mer peptides overlapping by 10 amino acids]; each peptide was present at a final concentration of 1 μg/ml; peptides kindly provided by the NIH AIDS Reagent Repository) or (ii) PMA-ionomycin (each at a concentration of 10 μg/ml; Sigma-Aldrich) or left unstimulated (negative control). Brefeldin A (10 μg/ml; Sigma, St. Louis, MO) was added after the first hour. After 6 h, stimulated cervical and blood-derived T cells were stained for markers of T cell differentiation (CD45RA and CCR7) and intracellular cytokines (IFN-γ and IL-2). Stimulated cells were initially washed twice with 2 ml of phosphate-buffered saline (PBS) (5 min, 300 × g, 1,300 rpm, room temperature). The cells were stained with pretitrated anti-CD8 antibody labeled with peridinin chlorophyll protein (PerCP) and Cy5.5 (anti-CD8-PerCP-Cy5.5) (BD Biosciences), anti-CCR7 antibody labeled with allophycocyanin (APC) (anti-CCR7-APC) (R&D Systems Inc., Minneapolis, MN), and anti-CD45RA antibody labeled with Cy7 and phycoerythrin (PE) (anti-CD45RA-Cy7.PE) (BD Biosciences) for 1 h at 4°C. The cells were washed with Perm/Wash buffer (2 ml; BD Biosciences) and permeabilized in Cytofix/CytoPerm (BD Biosciences) for 20 min at 4°C. The cells were then stained with anti-CD3 antibody labeled with pacific blue (anti-CD3-pacific blue) (BD Biosciences), anti-IFN-γ labeled with Alexa Fluor 700 (anti-IFN-γ-Alexa Fluor 700), and anti-IL-2 antibody labeled with fluorescein isothiocyanate (FITC) (anti-IL-2-FITC) for 1 h at 4°C. The cells were finally washed and fixed with Cell Fix (BD Biosciences), and cell fluorescence was measured using an LSRII flow cytometer (BD Biosciences, San Jose, CA). FlowJo version 8.5.3 (Tree Star, Inc., Ashland, OR) was used to set compensation and for data analysis. Fluorescence minus one (FMO) controls were used to set gates.
Measurement of cytokine concentrations in genital secretions.
The concentrations of IL-1β and IL-6 in cervical supernatants were determined using Quantikine high-sensitivity enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems Inc., Minneapolis, MN) according to the manufacturer's instructions. The limits of detection of IL-1β and IL-6 assays were ≥1 pg/ml and 0.7 pg/ml, respectively. All standards were run in duplicate, while the cytokine levels in cervical supernatants were measured once. ELISA plates were read on a VersaMax ELISA microplate reader, and data were analyzed using SoftMax Pro Ver. 4.3.1 software (Molecular Devices). Samples in which cytokine concentrations were below the level of detection of the ELISA were reported as zero.
Measurement of viral loads in cervical supernatants and plasma samples.
Viral loads were measured in cervical supernatants and plasma samples by the Nuclisens Easyq HIV-1 version 1.2 assay, which was performed by the National Health Service (NHLS) Diagnostic Virology Laboratory (Groote Schuur Hospital, Cape Town, South Africa). The detection limit of this assay was 50 HIV RNA copies/ml.
Statistical analysis.
Where indicated, Mann-Whitney U tests were used for nonparametric comparisons, while Spearman rank tests were used to test for correlations using GraphPad Prism version 5.0. All tests were two-tailed, and P values of ≤0.05 were considered significant.
RESULTS
Clinical description of study participants.
A total of 59 HIV-infected and 67 uninfected women were enrolled in this study to investigate the maturational status of T cells collected by cervical cytobrushing from the female genital tract. Of the cervical cytobrush samples obtained, 20/67 (29.9%) from uninfected women and 20/59 (33.9%) from HIV-infected women yielded CD3+ cell counts of ≤100 and were excluded for flow cytometric analysis. The frequency of cervical specimen exclusions due to low cell numbers did not differ significantly in HIV-infected and uninfected women.
As a result, cervical cytobrushes from 39/59 (66.1%) HIV-infected and 47/67 (70.1%) uninfected women were included in this study (Table 1). Of the 39 HIV-infected women, 14 were on antiretroviral therapy at the time of the study. The median CD4 T cell count of HIV-infected women was 382 cells/μl (interquartile range [IQR], 262 to 529 cells/μl), and the median plasma viral load was 2,700 RNA copies/ml (IQR, 50 to 39,250 RNA copies/ml). Only 30/39 HIV-infected women had genital tract samples available for viral load measurement (HIV shedding). Of these women, 9/30 (30%) HIV-infected women were shedding HIV in their genital secretions, and the median viral load detected in cervical secretions of those shedding virus was 2,000 RNA copies/ml (IQR, 370 to 2,700 RNA copies/ml), although this was a diluted sample. There was a significant positive association between plasma viral load and the amount of HIV being shed in genital secretions (ρ = 0.69; P = 0.02).
Table 1.
Clinical characteristics of HIV-infected and uninfected women
| Characteristic | Valuea for: |
|
|---|---|---|
| HIV-infected women | Uninfected women | |
| No. of women | 39 | 47 |
| Age, yr [median (IQR)] | 36.0 (29–39) | 38.0 (30–46) |
| No. of women on antiretroviral therapy (%) | 14 (36) | ND |
| Absolute blood CD4 count, cells/μl [median (IQR)] | 382 (262–529) | ND |
| CD3+ T cells per single cervical cytobrush [median (IQR)] | 125,600 (76,000–209,200) | 126,800 (59,800–201,600) |
| Cervical viral load, RNA copies/ml [median (IQR)] | 70 (70–330) | ND |
| Plasma viral load, RNA copies/ml [median (IQR)] | 2,700 (50–39,250) | ND |
| No. of women with detectable plasma viral load (%) | 19/34b (56) | ND |
| Viral load in women with detectable levels, RNA copies/ml [median (IQR)] | 31,000 (5,000–150,000) | ND |
| Range of plasma viral load in women with detectable levels (RNA copies/ml) | 180–760,000 | ND |
| No. of women with detectable cervical viral load (%) | 9/30c (30) | ND |
| Cervical viral load in women with detectable levels, RNA copies/ml [median (IQR)] | 2,000 (370–2,700) | ND |
| Range of cervical viral load in women with detectable levels (RNA copies/ml) | 110–9,300 | ND |
ND, not determined.
Plasma samples were available for viral load assessment from only 34 of the 39 HIV-infected women.
Cervical samples for evaluation of cervical viral loads were available from only 30 of the 39 HIV-infected women.
Depletion of CD4 T cells in the cervical compartment mirrors CD4 depletion in the blood of HIV-infected women.
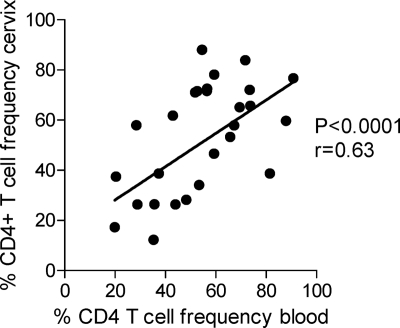
Gut-associated mucosal surfaces are major sites of CD4 T cell depletion during HIV infection (24, 27). However, we found that the extent of CD4 depletion in cervical cytobrush samples was significantly associated with CD4 T cell depletion in blood (ρ = 0.63; P = <0.0001) (Fig. 1). The finding that CD4 T cell depletion in the female genital tract mirrors that in the blood compartment may indicate that there are no strict physical barriers between the female genital tract and blood with infected cells of specific phenotypes trafficking freely between compartments and that CD4 T cells in the cervical compartment may be dependent on CD4 T cells in the peripheral blood for their replenishment.
Fig. 1.
Association between cervical and blood CD4 T cell percentages in HIV-infected women (n = 31). The Spearman rank test was used to test the association. P values of ≤0.05 were considered significant.
Relationship between T cell differentiation and CD4 depletion at the cervix.
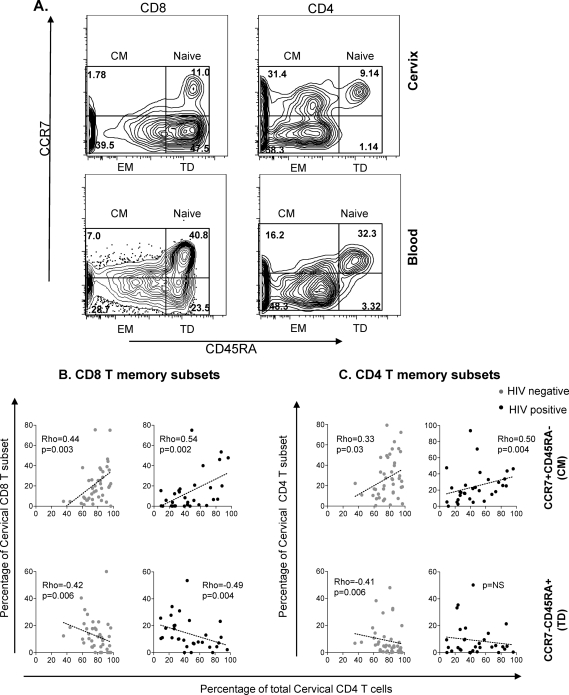
The differentiation status of cervical cytobrush- and blood-derived T cells was defined on the basis of differential staining with CD45RA and CCR7 (Fig. 2 A). On the basis of these phenotypic markers, T cell subsets were either defined as: (i) naive T cells (CD45RA+ CCR7+), (ii) central memory cells (CM cells; CD45RA− CCR7+), (iii) effector memory cells (EM cells; CD45RA− CCR7−), or (iv) terminally differentiated effectors (TD cells; CD45RA+ CCR7−).
Fig. 2.
Association between cervical CD4 frequencies and frequency of cervical memory T cell subsets. (A) Representative plots showing the gating strategy used to define memory subsets of cervical and blood CD8 and CD4 T cells based on expression of CD45RA and CCR7. T cells were defined as CD45RA− CCR7+ CM, CD45RA− CCR7− EM, and CD45RA+ CCR7− TD cells. FlowJo version 8.5.3 was used to set compensation and for analysis. Fluorescence minus one (FMO) controls were used to set gates. (B and C) Relationship between cervical CD4 frequencies and distribution of CD8 (B) and CD4 (C) memory T subsets at the cervix. CD8 and CD4 memory subsets were separated into 2 subsets of CD45RA− CCR7+ CM cells and CD45RA− CCR7− EM cells according to their stages of differentiation. Each dot represents the proportion for an individual woman. Black dots represent HIV-positive women (n = 31), and gray dots represent HIV-negative women (n = 42). Dotted lines represent best fits as predicted by linear regression. The Spearman rank test was used to test the correlation. P values of ≤0.05 were considered significant. NS, not significant.
When the relationship between the proportions of memory T cell subsets and cervical CD4 T cell frequency was evaluated, we found that reduced CD4 frequencies at the cervix were associated with significantly decreased CD45RA− CCR7+ CM CD8 T cell frequencies and correspondingly increased CD45RA+ CCR7− TD CD8 T cell frequencies (Fig. 2B). Similarly, the frequencies of CD45RA− CCR7+ CM CD4 T cells were significantly reduced at the cervixes of women with the most extensive CD4 depletion at the cervix (Fig. 2C). These results were still significant even when HIV-positive women who were on antiretroviral therapy were separated from those who were not on antiretroviral therapy (data not shown). These data suggest that women with the lowest frequencies of CD4 T cells in their cervixes (indicative of CD4 T cell depletion) are likely to have reduced frequencies of CD45RA− CCR7+ CM cells.
Differentiation status of cervical cytobrush T cells and HIV shedding.
We observed no relationship between plasma viral load or cervical HIV shedding (indicated by viral load in cervical secretions) and the differentiation status of T cells at the cervix and in blood (data not shown), suggesting that HIV viremia does not directly drive differentiation of T cells at the cervix.
Genital inflammation and cervical T cell differentiation.
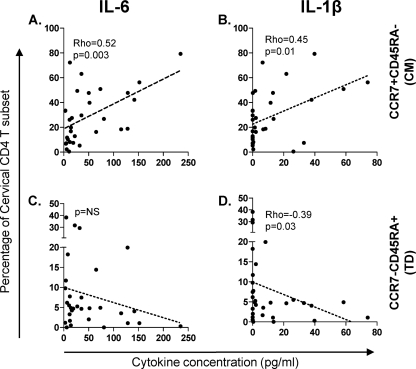
Previous in vitro studies have shown that T cell proliferation in the presence of certain inflammatory or regulatory cytokines (tumor necrosis factor alpha [TNF-α], IL-6, IL-10, and IL-12) has an impact on T cell differentiation (12). To investigate the relationship between in vivo genital tract inflammation and the differentiation status of cervical T cells, we compared the distribution of distinct memory subsets at the cervix with the concentrations of key inflammatory cytokines (IL-6 and IL-1β) detected in genital secretions. Irrespective of HIV status, we found no association between any of the inflammatory cytokines measured in this study and the differentiation status of CD8 T cell subsets (data not shown). In contrast, we found that IL-6 and IL-1β in genital secretions from uninfected women were positively associated with the accumulation of CD45RA− CCR7+ CM CD4 T cells at the cervix (Fig. 3 A and B) (P = 0.003 and ρ = 0.52 for IL-6; P = 0.01 and ρ = 0.45 for IL-1β). IL-1β was also significantly associated with reduced frequencies of CD45RA+ CCR7− TD CD4 cells at the cervix (Fig. 3D) (P = 0.03, ρ = −0.39). No relationship was observed between genital tract inflammation and T cell memory subset distribution in HIV-infected women (data not shown). It has previously been shown that there is a strong association between the use of hormonal contraceptive preparations and the numbers of cervicovaginal inflammatory cells (13). Given that injectable Depo Provera or Petogen were likely to be the predominant contraceptive choices in this cohort, our findings may be confounded by the influence of these contraceptives.
Fig. 3.
Relationship between the genital tract inflammatory cytokine milieu and T cell memory differentiation in uninfected women (not infected with HIV) (n = 31). (A and C) Association between genital IL-6 levels and frequencies of cervical CD45RA− CCR7+ CM CD4 T cells (A) and CD45RA+ CCR7− TD CD4 T cells (C). (B and D) Association between genital IL-β levels and frequencies of cervical CD45RA− CCR7+ CM CD4 T cells (B) and CD45RA+ CCR7− TD CD4 T cells (D). Dotted lines represent best fits as predicted by linear regression. The Spearman rank test was used to test the association. P values of ≤0.05 were considered significant.
Global cytokine deficits during HIV infection in T cells from the genital tract.
To compare the impact of HIV infection on global cytokine production by T cells from the cervix and blood, the ability of T cells to produce IFN-γ and IL-2 following stimulation with PMA-ionomycin was compared in HIV-infected women that had not been exposed to antiretroviral therapy and uninfected women (see Fig. 5). Although long-term in vitro stimulation of memory T cells has been reported to cause T cells to differentiate (∼36 h) (30), in vitro activation-induced T cell maturation is unlikely to have played a role in the present study, since the duration of stimulation was only 4 h, and the differentiation status of T cells stimulated with PMA-ionomycin was not different from those that were not stimulated (data not shown).
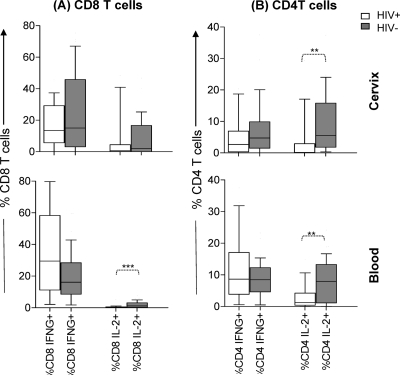
Fig. 5.
Comparison of the ability of CD8 (A) and CD4 (B) T cell subsets from cervical and blood samples from HIV-infected (n = 15) and uninfected (n = 39) women to produce IFN-γ and IL-2 after global stimulation with PMA-ionomycin. The magnitude of the response by CD45RA− CCR7+ CM, CD45RA− CCR7− EM, and CD45RA+ CCR7− TD cell subsets (based on the expression of phenotypic markers CD45RA and CCR7) were compared for each group using the Mann-Whitney U test, and P values of ≤0.05 were considered significant (*, P < 0.05; **, P < 0.005). All frequencies were adjusted for background by subtracting unstimulated cytokine production from PMA-ionomycin responses by each T cell subset.
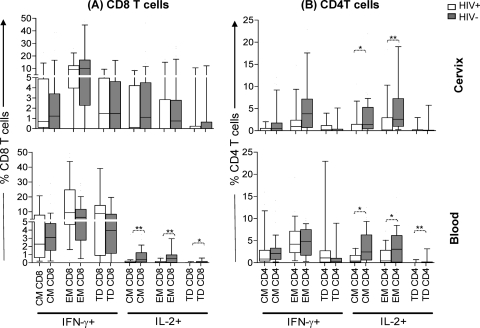
Both in the cervix and in blood, CD8 T cells secreted mainly IFN-γ following PMA-ionomycin stimulation irrespective of HIV status (Fig. 4 A). No significant differences were noted in the ability of genital tract CD8 T cells from HIV-infected and uninfected women to secrete IFN-γ or IL-2 (Fig. 4A). Although there was no significant difference in the ability of peripheral blood CD8 T cells from HIV-infected and uninfected women to secrete IFN-γ, IL-2 production by CD8 T cells in HIV-infected women was significantly lower than that in uninfected women (P = 0.0008) (Fig. 4A). In contrast, CD4 T cells in HIV-infected women had significantly lower IL-2 responses than uninfected women in both cervical mucosa and blood compartments (P = 0.003 for the cervix and P = 0.009 for blood) (Fig. 4B).
Fig. 4.
Comparison of the ability of T cells from the cervical and blood samples from HIV-infected (n = 15) and uninfected women (n = 39) to produce IFN-γ and IL-2 after global stimulation with PMA-ionomycin. The net frequencies of CD8 and CD4 T cells from the cervix and blood samples from HIV-positive (HIV+) versus HIV-negative (HIV−) women were compared. The data are shown as box-and-whisker plots, with the bottom of the box the 10th percentile, the top of the box the 90th percentile, and the median the middle of the box and the whiskers showing the minimum and maximum values. The responses were compared for each group using the Mann-Whitney U test, and P values of ≤0.05 were considered significant (**, P < 0.005; ***, P < 0.001).
Similar to previous studies (15, 42), IFN-γ was mainly secreted by CD45RA− CCR7− EM and CD45RA+ CCR7− TD cells, whereas IL-2 was mainly secreted by CD45RA− CCR7+ CM and CD45RA− CCR7− EM cells in both cervical and blood compartments (Fig. 5). The levels of IFN-γ production by all T cell subsets were similar for HIV-infected and uninfected women for both the cervical and blood compartments. At the cervix, both of the CD4 subsets that are known to produce IL-2 had significantly reduced levels of IL-2 production in HIV-infected women compared to uninfected women (P = 0.01 for CM and P = 0.004 for EM) (Fig. 5B). Terminally differentiated cells at the cervix did not produce any IL-2. In blood, however, the IL-2 deficit in HIV-infected women was observed in all T cell subsets (for CD8, P = 0.002 for CM cells, P = 0.001 for EM cells, and P = 0.02 for TD cells; for CD4, P = 0.01 for CM cells, P = 0.02 for EM cells, and P = 0.009 for TD cells) (Fig. 5B).
HIV-specific T cell responses at the cervixes of HIV-infected women were rare.
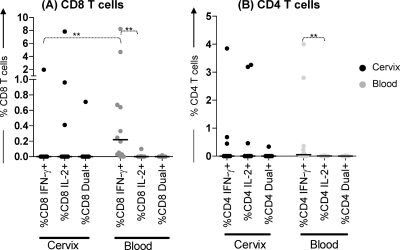
The differentiation status of HIV Gag-specific T cells in blood and at the cervix in HIV-infected women who had not been exposed to antiretroviral therapy was determined. Following stimulation with HIV Gag peptides, the magnitude and frequency of IFN-γ responses by CD8 T cell subsets in blood were significantly higher than those detected at the cervix where Gag-specific responses were largely absent (P = 0.0015) (Fig. 6). There was no significant difference in the overall magnitude of CD8 HIV-specific IL-2 responses between the two compartments, although more women appeared to have Gag-specific IL-2 responses by T cells at the cervix than in blood. The IFN-γ responses to HIV Gag peptides by CD4 T cells in both the blood and cervical compartments were similar (Fig. 6). In blood, Gag-responsive CD8 and CD4 T cells were found to belong to CD45RA− CCR7− EM and CD45RA+ CCR7− TD memory cell subsets and secreted mainly IFN-γ. In contrast, cervical CD8 T cells from only 1/12 HIV-infected women produced IFN-γ in response to stimulation with HIV Gag peptides, and the responding CD8 T cells were found to have the CD45RA+ CCR7− TD cell phenotype (data not shown).
Fig. 6.
Characterization of cytokine production profiles of HIV Gag-specific CD8 (A) and CD4 (B) T cell responses during chronic HIV infection. (A) Comparison of HIV-specific responses in cervical and blood CD8 T cells. (B) Comparison of HIV-specific responses in cervical and blood CD4 T cells. The frequency of IFN-γ secretion, IL-2 secretion, and secretion of both cytokines by T cell subsets in each of the two compartments was analyzed. Each dot represents the value for one individual, and the short black horizontal bars represent median responses. The frequencies of responses were compared for each group using the Mann-Whitney U test. Asterisks indicate significant differences between groups (P < 0.005 [**]). Net responses were calculated by subtracting unstimulated cytokine responses from responses measured following Gag stimulation.
DISCUSSION
This study investigated maintenance and functionality of T cells derived from the cervix. We showed that the extent of CD4 depletion seen systemically is reflected at the site of female-to-male sexual transmission of HIV and that depletion of CD4 T cells in the genital tract was associated with reduced frequencies of CD45RA− CCR7+ CM cells and accumulation of CD45RA+ CCR7− TD cells. Furthermore, during HIV infection, there is a significant reduction of IL-2-supplying cells with regenerative capacity. IL-6 and IL-1β in genital secretions from uninfected women were positively associated with accumulation of CD45RA− CCR7+ CM CD4 T cells at the cervix, while IL-1β was associated with significant reduction of CD45RA+ CCR7− TD CD4 T cells at the cervix. This suggests that CD4 T cells in the genital tracts of uninfected (non-HIV-infected) women may be maintained by recruitment of CD4 CD45RA− CCR7+ CM cells through an inflammatory gradient to replenish the diminishing CD45RA+ CCR7− TD cells.
Irrespective of HIV status, lower frequencies of CD4 T cells at the cervix was associated with reduced frequencies of CD45RA− CCR7+ CD8 and CD4 CM T cells and the accumulation of CD45RA+ CCR7− CD8 and CD4 TD T cells. These results may suggest that CD4 T cell depletion at the cervix is the result of accumulation of T cells that are differentiating toward a terminally differentiated phenotype and that these cells are destined to die of activation-induced apoptosis, regardless of the HIV status of the women.
It is likely that persistent strong antigenic stimuli, as is the case during chronic HIV infection, may cause expansion and differentiation of systemic T cells to CD45RA+ CCR7− TD cells that are no longer fit for development and survival (21, 50). To test this, we determined whether there was any association between HIV burden and differentiation status of T cells. There was no relationship between viral shedding in the genital tract and differentiation status of T cells at the mucosa. While the causal relationship between HIV load, T cell differentiation, and immune activation remains unclear, the findings from this study suggest that differentiation of T cells is not driven directly by HIV but rather by other immune factors such as immune activation in the presence of HIV. Although not evaluated simultaneously in this study, combined examination of activation and maturational status of T cells at the cervix would be valuable to understand the mechanisms that influence the differentiation and ultimate death of memory T cells.
We examined whether inflammation in the genital tract played a role in the local differentiation of T cells at the cervix. In uninfected women, genital tract inflammation was associated with the accumulation of CD45RA− CCR7+ CM CD4 T cells and reduced frequencies of CD45RA+ CCR7− TD cells at the cervix. This finding may reflect that, in the absence of HIV infection, TD T cells may be slowly lost through activation-induced death in the presence of genital inflammation, while CD45RA− CCR7+ CM T cells are recruited to replenish the diminishing CD4 T cell pool. Although CD45RA− CCR7+ CM T cells are not common at mucosal sites and their identification was surprising, CM cells that were identified in the mucosal effector sites in macaques have been proposed to be “reserve” antigen-experienced cells that are in a “quasiresting” state awaiting another exposure to the antigen of original stimulation (38). Despite evidence that inflammatory cytokines may alter the differentiation status of T cells in an antigen-independent manner (12, 42), the association between inflammatory cytokines in genital secretions and differentiation of individual T cell subsets isolated from the cervix was not evident in HIV-infected women in this study. This may be due to the fact that the antigenic burden in vivo, which may influence the activation and differentiation status of T cells, could not be taken into account in this study, particularly in HIV-infected women where HIV itself and other opportunistic genital tract infections may be present.
We found a significant reduction in the frequency of IL-2-producing CD4 and CD8 T cells in blood and CD4 T cells in the cervix in HIV-infected women compared to uninfected women following stimulation with PMA-ionomycin. These data suggest an altered response during HIV infection in which HIV may directly or indirectly trigger T cells to predominantly secrete IFN-γ rather than IL-2.
In conclusion, we describe the relationship between local CD4 depletion during HIV infection and the differentiation and function of T cells present in the cervical mucosa. CD4 depletion at the cervix was characterized by a reduction in CD45RA− CCR7+ CM T cells in the early stages of differentiation and an increase in CD45RA+ CCR7− TD T cells in the terminal stages of differentiation. The genital mucosa, like most other mucosal surfaces, is vulnerable to external pathogens. High levels of activation and progressively declining CD4 T cell numbers at mucosal sites during HIV infection might account for the increased incidence of bacterial vaginosis, fungal infections, and other sexually transmitted infections observed in HIV-infected women and could contribute to cervical dysfunction at this vulnerable mucosal site. These results also imply that mucosal memory T cells initially recruited from blood or recirculating memory T cells from other tertiary tissue may undergo local differentiation upon entering mucosal tissues due to local factors present in the mucosal microenvironment. These findings suggest that such mucosal factors can play an important role in determining the phenotype and fate of the responder cells.
ACKNOWLEDGMENTS
This study was supported by grants from the South African HIV/AIDS Research Platform (SHARP), Department of Science and Technology of South Africa, and the Medical Research Council of South Africa. P. Gumbi is funded by the Columbia University-Southern African Fogarty Program.
We thank all the women who kindly participated in the study, Ntombizonke Makhonza for collecting the specimens, and Darren Martin for reviewing the manuscript.
Footnotes
Published ahead of print on 12 October 2011.
REFERENCES
- 1. Ahmed R., Bevan M. J., Reiner S. L., Fearon D. T. 2009. The precursors of memory: models and controversies. Nat. Rev. Immunol. 9:662–668 [DOI] [PubMed] [Google Scholar]
- 2. Ahmed R., Gray D. 1996. Immunological memory and protective immunity: understanding their relation. Science 272:54–60 [DOI] [PubMed] [Google Scholar]
- 3. Appay V., Rowland-Jones S. L. 2004. Lessons from the study of T-cell differentiation in persistent human virus infection. Semin. Immunol. 16:205–212 [DOI] [PubMed] [Google Scholar]
- 4. Burgers W. A., et al. 2009. Association of HIV-specific and total CD8+ T memory phenotypes in subtype C HIV-1 infection with viral set point. J. Immunol. 182:4751–4761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Champagne P., Dumont A. R., Sekaly R. P. 2001. Learning to remember: generation and maintenance of T-cell memory. DNA Cell Biol. 20:745–760 [DOI] [PubMed] [Google Scholar]
- 6. Cheroutre H., Madakamutil L. 2005. Mucosal effector memory T cells: the other side of the coin. Cell. Mol. Life Sci. 62:2853–2866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cicala C., et al. 2009. The integrin alpha4beta7 forms a complex with cell-surface CD4 and defines a T-cell subset that is highly susceptible to infection by HIV-1. Proc. Natl. Acad. Sci. U. S. A. 106:20877–20882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Connors M., et al. 1997. HIV infection induces changes in CD4+ T-cell phenotype and depletions within the CD4+ T-cell repertoire that are not immediately restored by antiviral or immune-based therapies. Nat. Med. 3:533–540 [DOI] [PubMed] [Google Scholar]
- 9. Critchfield J. W., et al. 2008. Magnitude and complexity of rectal mucosa HIV-1-specific CD8+ T-cell responses during chronic infection reflect clinical status. PLoS One 3:e3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. El-Far M., et al. 2008. T-cell exhaustion in HIV infection. Curr. HIV/AIDS Rep. 5:13–19 [DOI] [PubMed] [Google Scholar]
- 11. Farrell A. 2006. Defeating T-cell fatigue in HIV. Nat. Med. 12:1124–1125 [DOI] [PubMed] [Google Scholar]
- 12. Geginat J., Sallusto F., Lanzavecchia A. 2001. Cytokine-driven proliferation and differentiation of human naive, central memory, and effector memory CD4(+) T cells. J. Exp. Med. 194:1711–1719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ghanem K. G., et al. 2005. Influence of sex hormones, HIV status, and concomitant sexually transmitted infection on cervicovaginal inflammation. J. Infect. Dis. 191:358–366 [DOI] [PubMed] [Google Scholar]
- 14. Grossman Z., Meier-Schellersheim M., Paul W. E., Picker L. J. 2006. Pathogenesis of HIV infection: what the virus spares is as important as what it destroys. Nat. Med. 12:289–295 [DOI] [PubMed] [Google Scholar]
- 15. Halwani R., et al. 2006. Generation and maintenance of human memory cells during viral infection. Springer Semin. Immunopathol. 28:197–208 [DOI] [PubMed] [Google Scholar]
- 16. Hawkins R. A., Rank R. G., Kelly K. A. 2000. Expression of mucosal homing receptor alpha4beta7 is associated with enhanced migration to the Chlamydia-infected murine genital mucosa in vivo. Infect. Immun. 68:5587–5594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hayday A., Theodoridis E., Ramsburg E., Shires J. 2001. Intraepithelial lymphocytes: exploring the third way in immunology. Nat. Immunol. 2:997–1003 [DOI] [PubMed] [Google Scholar]
- 18. Johnson R. P., Kaur A. 2005. HIV: viral blitzkrieg. Nature 434:1080–1081 [DOI] [PubMed] [Google Scholar]
- 19. Kaech S. M., Hemby S., Kersh E., Ahmed R. 2002. Molecular and functional profiling of memory CD8 T cell differentiation. Cell 111:837–851 [DOI] [PubMed] [Google Scholar]
- 20. Ladell K., et al. 2008. Central memory CD8+ T cells appear to have a shorter lifespan and reduced abundance as a function of HIV disease progression. J. Immunol. 180:7907–7918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lanzavecchia A., Sallusto F. 2005. Understanding the generation and function of memory T cell subsets. Curr. Opin. Immunol. 17:326–332 [DOI] [PubMed] [Google Scholar]
- 22. Lebre M. C., et al. 2005. Differential expression of inflammatory chemokines by Th1- and Th2-cell promoting dendritic cells: a role for different mature dendritic cell populations in attracting appropriate effector cells to peripheral sites of inflammation. Immunol. Cell Biol. 83:525–535 [DOI] [PubMed] [Google Scholar]
- 23. Lefrancois L., Marzo A. L. 2006. The descent of memory T-cell subsets. Nat. Rev. Immunol. 6:618–623 [DOI] [PubMed] [Google Scholar]
- 24. Li Q., et al. 2005. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature 434:1148–1152 [DOI] [PubMed] [Google Scholar]
- 25. Marzo A. L., Yagita H., Lefrancois L. 2007. Migration to nonlymphoid tissues results in functional conversion of central to effector memory CD8 T cells. J. Immunol. 179:36–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Masopust D., Vezys V., Wherry E. J., Barber D. L., Ahmed R. 2006. Gut microenvironment promotes differentiation of a unique memory CD8 T cell population. J. Immunol. 176:2079–2083 [DOI] [PubMed] [Google Scholar]
- 27. Mattapallil J. J., et al. 2005. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature 434:1093–1097 [DOI] [PubMed] [Google Scholar]
- 28. Mayaud P., Mabey D. 2004. Approaches to the control of sexually transmitted infections in developing countries: old problems and modern challenges. Sex. Transm. Infect. 80:174–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nagarajan U. M., et al. 2008. Type I interferon signaling exacerbates Chlamydia muridarum genital infection in a murine model. Infect. Immun. 76:4642–4648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nagler-Anderson C., Terhoust C., Bhan A. K., Podolsky D. K. 2001. Mucosal antigen presentation and the control of tolerance and immunity. Trends Immunol. 22:120–122 [DOI] [PubMed] [Google Scholar]
- 31. Nkwanyana N. N., et al. 2009. Impact of human immunodeficiency virus 1 infection and inflammation on the composition and yield of cervical mononuclear cells in the female genital tract. Immunology 128:e746–e757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Obar J. J., Lefrancois L. 2010. Early events governing memory CD8+ T-cell differentiation. Int. Immunol. 22:619–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Okoye A., et al. 2007. Progressive CD4+ central memory T cell decline results in CD4+ effector memory insufficiency and overt disease in chronic SIV infection. J. Exp. Med. 204:2171–2185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Papagno L., et al. 2004. Immune activation and CD8+ T-cell differentiation towards senescence in HIV-1 infection. PLoS Biol. 2:E20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Passmore J. A., et al. 2002. Single-cell cytokine analysis allows detection of cervical T-cell responses against human papillomavirus type 16 L1 in women infected with genital HPV. J. Med. Virol. 67:234–240 [DOI] [PubMed] [Google Scholar]
- 36. Picker L. J. 2006. Immunopathogenesis of acute AIDS virus infection. Curr. Opin. Immunol. 18:399–405 [DOI] [PubMed] [Google Scholar]
- 37. Picker L. J., et al. 2004. Insufficient production and tissue delivery of CD4+ memory T cells in rapidly progressive simian immunodeficiency virus infection. J. Exp. Med. 200:1299–1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Poonia B., et al. 2006. Chronic alcohol consumption results in higher simian immunodeficiency virus replication in mucosally inoculated rhesus macaques. AIDS Res. Hum. Retroviruses 22:589–594 [DOI] [PubMed] [Google Scholar]
- 39. Poonia B., Wang X., Veazey R. S. 2006. Distribution of simian immunodeficiency virus target cells in vaginal tissues of normal rhesus macaques: implications for virus transmission. J. Reprod. Immunol. 72:74–84 [DOI] [PubMed] [Google Scholar]
- 40. Potter S. J., et al. 2007. Preserved central memory and activated effector memory CD4+ T-cell subsets in human immunodeficiency virus controllers: an ANRS EP36 study. J. Virol. 81:13904–13915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Russell M. W., et al. 2000. Strategies of immunization against mucosal infections. Vaccine 19(Suppl. 1):S122–S127 [DOI] [PubMed] [Google Scholar]
- 42. Sallusto F., Lenig D., Forster R., Lipp M., Lanzavecchia A. 1999. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401:708–712 [DOI] [PubMed] [Google Scholar]
- 43. Seder R. A., Ahmed R. 2003. Similarities and differences in CD4+ and CD8+ effector and memory T cell generation. Nat. Immunol. 4:835–842 [DOI] [PubMed] [Google Scholar]
- 44. Tedla N., et al. 1999. Phenotypic and functional characterization of lymphocytes derived from normal and HIV-1-infected human lymph nodes. Clin. Exp. Immunol. 117:92–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tilton J. C., et al. 2007. Changes in paracrine interleukin-2 requirement, CCR7 expression, frequency, and cytokine secretion of human immunodeficiency virus-specific CD4+ T cells are a consequence of antigen load. J. Virol. 81:2713–2725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tussey L. G., et al. 2003. Antigen burden is major determinant of human immunodeficiency virus-specific CD8+ T cell maturation state: potential implications for therapeutic immunization. J. Infect. Dis. 187:364–374 [DOI] [PubMed] [Google Scholar]
- 47. van Grevenynghe J., et al. 2008. Transcription factor FOXO3a controls the persistence of memory CD4(+) T cells during HIV infection. Nat. Med. 14:266–274 [DOI] [PubMed] [Google Scholar]
- 48. Verhoeven D., Sankaran S., Silvey M., Dandekar S. 2008. Antiviral therapy during primary simian immunodeficiency virus infection fails to prevent acute loss of CD4+ T cells in gut mucosa but enhances their rapid restoration through central memory T cells. J. Virol. 82:4016–4027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Vuylsteke B. 2004. Current status of syndromic management of sexually transmitted infections in developing countries. Sex. Transm. Infect. 80:333–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wherry E. J., Barber D. L., Kaech S. M., Blattman J. N., Ahmed R. 2004. Antigen-independent memory CD8 T cells do not develop during chronic viral infection. Proc. Natl. Acad. Sci. U. S. A. 101:16004–16009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wherry E. J., et al. 2003. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat. Immunol. 4:225–234 [DOI] [PubMed] [Google Scholar]
- 52. Wu H. Y., Abdu S., Stinson D., Russell M. W. 2000. Generation of female genital tract antibody responses by local or central (common) mucosal immunization. Infect. Immun. 68:5539–5545 [DOI] [PMC free article] [PubMed] [Google Scholar]