Abstract
Currently, we have limited understanding of how Toll-like receptor (TLR) engagement by microbial products influences the immune response during a concurrent virus infection. In this study, we established that dual TLR2 plus TLR3 (designated TLR2+3) stimulation alters the immunodominance hierarchies of lymphocytic choriomeningitis virus (LCMV) epitopes by reducing NP396-specific CD8+ T cell responses and shifting it to a subdominant position. The shift in immunodominance occurred due to a reduction in antigen uptake and the reduced cross-presentation of NP396, a major LCMV immunodominant epitope that is efficiently cross-presented. Moreover, the altered immunodominance was dependent on TLR stimulation occurring at the site of infection. Finally, as lipopolysaccharide failed to induce the same phenomenon, the data suggest that these findings are dependent not only on the dual engagement of the TRIF/MyD88 pathways but also on how TLR agonists activate antigen-presenting cells. Taken together, our data demonstrate a novel role for TLR ligands in regulating antiviral CD8+ T cell responses due to the regulation of the cross-presentation of cell-associated antigens.
INTRODUCTION
CD8+ T cells are important in clearing viral infections (4, 40). Despite the molecular structural complexity of most viruses, CD8+ T cells respond to a small subset of viral epitopes through a process termed immunodominance (44). This mechanism allows different viral epitopes that activate CD8+ T cells to various degrees to be organized into a hierarchy. Within this hierarchy, immunodominant epitopes will induce the expansion of a greater number of CD8+ T cells than subdominant ones (44). Immunodominance is influenced by complex factors, which include viral load, site of infection, and the kinetics of viral protein expression (24, 30, 39). In addition to this, T cell-related factors, which include T cell receptor (TCR) avidity and naïve CD8+ T cell precursor frequencies, also are important considerations (15, 17, 32).
Major histocompatibility complex class I (MHC-I) antigen presentation, in which peptide affinity to MHC-I molecules and the stability of peptide-MHC complexes are two major factors, is another key event that contributes to immunodominance (44). The presentation of MHC-I antigens occurs via two pathways: direct presentation and cross-presentation. Direct presentation is the process by which infected antigen-presenting cells (APCs) present peptides derived from proteins present in their own cytosol (4, 36), whereas cross-presentation occurs when professional APCs (pAPCs) present peptides derived from exogenous antigens obtained from other infected cells (4, 36).
Recently, a number of reports have suggested an association between immunodominance and cross-presentation. It has been demonstrated that subdominant epitopes are weakly cross-presented compared to immunodominant epitopes (21). In another study, cross-presentation was observed only for immunodominant epitopes (22). Moreover, using the lymphocytic choriomeningitis virus (LCMV) infection model, we observed better cross-presentation for LCMV-nucleoprotein 396 (NP396) than for LCMV-glycoprotein 33 (GP33); both epitopes are immunodominant after virus infection (2). However, the cross-priming of both epitopes was comparable in vivo due to the high GP33 T cell precursor frequency (2). Thus, certain viral epitopes need to be cross-presented to attain a high position in the immunodominance hierarchy (2, 21, 22). However, how this phenomenon is affected in the presence of microbial stimulation is unknown.
During infections, pAPCs employ various receptors to sense pathogen-associated molecular patterns, e.g., Toll-like receptors (TLRs) (6). The interaction of TLRs with their TLR ligands (TLR-L) affects the maturation and activation of pAPCs (13). Due to TLR activation, pAPCs express high levels of costimulatory molecules and secrete several cytokines depending on the TLR-L (7, 29). Previous reports that examined ovalbumin (OVA) antigens showed that TLR3-L engagement promotes cross-presentation (8, 28). However, other reports have shown that APC activated by exposure to TLR3-L do not cross-present subsequently encountered antigens (11, 41). Furthermore, if the activation of APCs persists in vivo, then cross-priming is impaired and virus-specific cytotoxic T lymphocyte (CTL) activities are hindered (41). This situation may be particularly relevant during secondary infections due to the presence of multiple TLR-L. Moreover, our group and others have demonstrated that combined TLR activation can induce immune cell activation different from that induced by a single TLR-L (29, 33, 45, 46). Despite the numerous reports examining TLR-L influence on immunity, their effects on immunodominance during virus infection have not been examined previously.
In this study, we report that coadministering TLR2-L and TLR3-L (designated TLR2+3-L) during LCMV infection significantly alters the CD8+ T cell immunodominance hierarchy by reducing NP396-specific CD8+ T cell responses, which allowed the subdominant epitope, GP276, to achieve a more dominant position. The mechanism accounting for this was associated with the reduced cross-presentation and cross-priming of the NP396 immunodominant epitope. Therefore, our data provide new insights into how TLR engagement can alter the primary immune response during virus infection.
MATERIALS AND METHODS
Mice, cells, and reagents.
The following TLR-L were purchased from Cedarlane (Hornby, Ontario, Canada): polyinosinic polycytidine acid (pIC) (TLR3-L) and Pam3CysSerLys4 (pam3csk4) (TLR2-L). C57BL/6 (H-2b) mice (6 to 8 weeks old) were purchased from JAX Labs (Bar Harbor, ME). Animal experiments were carried out in accordance with the guidelines of the Canadian Council of Animal Care. LCMV-WE originally was obtained from F. Lehmann-Grube (Hamburg, Germany) and was propagated and titrated as previously described (9). For in vivo virus titration, spleens were isolated on days 5 and 7 postinfection (p.i.) and homogenized in 1 ml Dulbecco's modified essential medium (DMEM), and supernatants were titrated onto MC57 monolayers by an immunofocus assay as previously described (30).
As antigen-presenting cells, BMA cells (a gift from K. Rock, University of Massachusetts Medical School, Worcester, MA) or bone marrow-derived dendritic cells (BMDC) (29) were used. BMDC preparations were described previously, and cells were used 7 days after culturing. HEK293 or HEK-NP was used as antigen donor cells as previously described (2, 5). All media were purchased from Invitrogen (Ontario, Canada).
NP396-specific CTLs were generated as previously described (1, 5). Briefly, mice were injected with 200 PFU LCMV-WE intravenously (i.v.). Four weeks postinjection, spleens were harvested and lymphocytes were purified by Ficoll-gradient centrifugation using lymphocyte separation medium (Fisher Scientific, Whitby, Ontario, Canada). Purified splenocytes then were restimulated with peptide-pulsed (10−7 M), γ-irradiated (4,500 rads) APCs at a ratio of 10:1 in the presence of 20 U/ml IL-2. On day 6, the cells were purified by Ficoll-gradient centrifugation again and resuspended in CTL medium for 2 days before testing in functional assays.
Isolation of intrahepatic lymphocytes.
To measure intrahepatic T cell activation ex vivo, cells were isolated as previously described (39). Briefly, livers were homogenized and incubated in 10 ml digestion medium composed of 0.25 mg of collagenase B (Boehringer Mannheim)/ml and 1 U of DNase (Sigma-Aldrich, Oakville, Ontario, Canada)/ml at 37°C for 45 min. The homogenate was pelleted, resuspended in 44% Percoll (Sigma), and underlaid with 56% Percoll. The homogenate then was centrifuged at 850 × g for 20 min. The intrahepatic lymphocytes were isolated from the interface, and red blood cells were lysed using lysis buffer (1.66% [wt/vol] NH4Cl).
Intracellular cytokine staining (ICS).
For the ex vivo analysis of T cell activation, gamma interferon (IFN-γ) production by CD8+ T cells was performed in peptide restimulation assays (30). Splenocytes were incubated with APCs (BMA cells) at an APC/responder ratio of 1:10 in the presence of brefeldin A (10 μg/ml). The APCs were loaded with the synthetic peptide NP396-404 (FQPQNGQFI), NP205-212 (YTVKYPNL), GP33-41 (KAVYNFATC), or GP276-286 (SGVENPGGYCL) or with an an irrelevant peptide control (SIINFEKL). The peptides (purity, >90%) were synthesized at CPC Scientific (San Jose, CA). T lymphocytes were stained with phycoerythrin (PE)-Cy5-conjugated, rat anti-mouse CD8α clone 53-6.7 (Cedarlane) at 4°C and then fixed with 1% paraformaldehyde before adding fluorescein isothiocyanate (FITC)-conjugated anti-IFN-γ antibody (0.1% saponin) clone XMG1.2 (Cedarlane) overnight at 4°C. Data were acquired by flow cytometry (FCM) (Epics XL-MCL) and analyzed by gating on the CD8+ cells using the Expo 32 software package (Beckman Coulter, Miami, FL).
To calculate the number of peptide-specific CD8+ T cells in the spleen, we counted total splenocytes and then made use of the percentages of cells that were double positive for both IFN-γ and CD8α after gating to calculate the number of epitope-specific CD8+ T cells. We enumerated the absolute number of CD8+ T cells in the spleen by counting total splenocytes after trypan blue exclusion for estimating live cells.
Tetramer staining.
Splenocytes (5 × 105/well) were stained using 0.5 to 1 μg of FITC-labeled NP396 and GP276 tetramers (obtained from the NIH tetramer facility) for 10 min at 37°C. Splenocytes then were stained with PE-Cy5-conjugated, rat anti-mouse CD8α clone 53-6.7 (Cedarlane) at 4°C for 30 min. Cells were washed twice, and data were acquired using FCM.
In vivo cytotoxic assays.
The in vivo killing assay was performed as previously described (9), with some modifications. Briefly, mice were injected with virus and TLR-L individually or in combination as in the immunodominance experiments. Five days postinjection, mice were injected with 1 × 107 control 0.5 μM carboxyfluorescein succinimidyl ester (CFSE)-labeled splenocytes and NP396-pulsed 1 × 107 2.5 μM CFSE-labeled splenocytes. After 16 h, spleens were harvested and analyzed by flow cytometry for the specific killing of NP396-labeled target cells. Percent killing was assessed using the following equation: specific killing = 100 − [(% peptide pulsed/% unpulsed in infected mice)/(% peptide pulsed/% unpulsed in naïve mice)] × 100).
Antigen presentation assays employing peptide-specific CD8+ T cells.
For measuring antigen presentation ex vivo, mice were injected subcutaneously (s.c.) with 500 PFU LCMV-WE along with pIC and pam3cysk4 individually or in combination. Twenty-four h postinjection, splenocytes were isolated after lysing red blood cells using 1.66% (wt/vol) ammonium chloride. Splenocytes were placed in plastic petri dishes for 2 h to allow for pAPC adherence. pAPCs were harvested using a cell scraper and incubated with CTL lines (APC/responder ratio of 1:1). Direct antigen presentation was measured using ICS.
For cross-priming assays, lysis and UV-irradiated (LYUV)-treated HEK-NP cells were used as antigen donor cells and were prepared as previously described (1, 5). HEK-NP cells are HEK cells transfected with LCMV-NP. As these cells do not express mouse MHC molecules and they are introduced as dead cells in the mice, the presentation of the NP epitopes to T cells can occur only if the epitopes access the cross-presentation pathway. Here, cells were induced to undergo death by LYUV treatment by subjecting cells to one round of freeze-thaw (liquid N2) followed by UVB radiation at an intensity of 200,000 μJ/cm2 for 10 min using a CL-1000 M UV cross-linker (Ultra-Violet Products Ltd., Cambridge, United Kingdom). To test for cross-priming, mice were injected with LYUV HEK-NP cells (7 × 106) and TLR-L. After 7 days, epitope-specific CTLs were quantified by performing ICS as previously described (2, 5).
Phagocytosis assays.
Mice were injected i.v. with 4 × 106 PKH2-labeled, LYUV-treated HEK cells with or without TLR-L. After 4 h, splenocytes were isolated and APCs were obtained by adherence to plastic petri dishes for 1 h. APCs were stained with anti-mouse CD11c-PE or CD11b-PE-Cy5 for 15 min, and uptake was measured using FCM. We gated on cells that were double positive for CD11c or CD11b, and PKH2 indicated the percent phagocytosis. Antigen uptake by untreated mice was assigned an arbitrary value of 100, and changes in uptake by TLR-L-treated mice were plotted relative to this value.
Statistical analyses.
Statistics were performed using the paired, two-tailed t tests, and differences in results between specified conditions were deemed significant when P < 0.05.
RESULTS
Presence of TLR2+3-L alters LCMV-specific CD8+ T cell immunodominance hierarchies during infections.
In an immunization protocol, combined TLR-L administration with an antigenic peptide was reported to enhance immune responses by inducing the clonal expansion of peptide-specific CD8+ T cells (45, 46). To further explore this phenomenon from a different perspective, we questioned if multiple TLR engagement during infection could influence CD8+ T cell immunodominance hierarchies.
To address this question, mice were injected s.c. with 500 PFU LCMV-WE along with 100 μg pIC, 20 μg pam3cysk4, or with both TLR-L. Spleens were harvested 8 or 12 days p.i., and epitope-specific CD8+ T cells were enumerated and their functions assessed via ICS assays (Fig. 1A and B). As expected, LCMV-specific CD8+ T cells exhibited the same immunodominance hierarchy in the spleen as that naïve mice at both 8 and 12 days p.i., i.e., GP33 and NP396 assumed the immunodominant position and GP276 and NP205 were subdominant (Fig. 1A and B) (30).
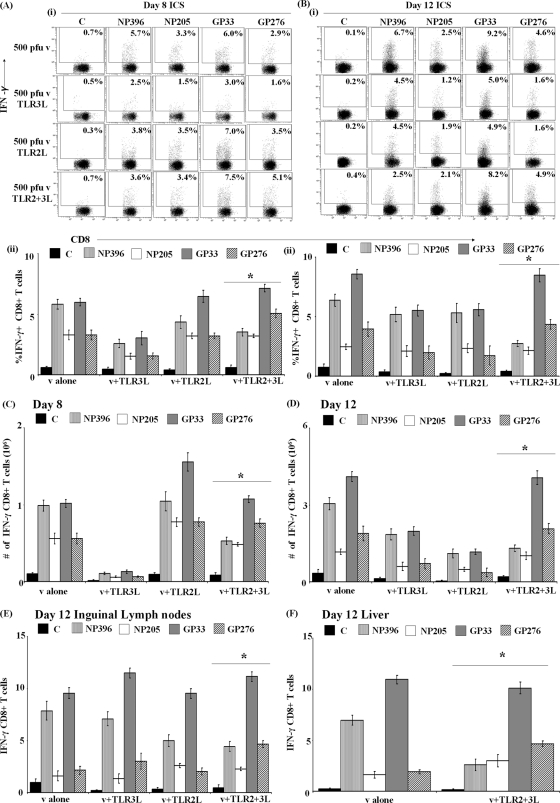
Fig. 1.
Immunodominance hierarchies of LCMV-specific CD8+ T cells are altered by TLR2+3-L administration. Mice were injected with 500 PFU LCMV s.c. with pIC (100 μg) and pam3cysk4 (20 μg) individually or in combination before quantifying T cell responses in the spleen at days 8 (A) and 12 (B). LCMV-specific CD8+ T cell responses were estimated for the NP396, NP205, GP33, or GP276 epitope (A and B) using ICS and measuring IFN-γ production after in vitro restimulation. Dot plots are representative of immunodominance profiles (i), and representative data of one experiment out of three independent trials ± standard deviations from triplicate animals in each condition are shown (ii). The controls (C) represent splenocytes from infected mice restimulated with BMA pulsed with irrelevant peptide. (C and D) T cell numbers specific for the NP396, NP205, GP33, or GP276 epitope were estimated from both the ICS data and the total number of T cells measured as described in Materials and Methods at 8 or 12 days p.i. LCMV-specific CD8+ T cell responses were estimated for the NP396, NP205, GP33, or GP276 epitope at day 12 using ICS and measuring IFN-γ production in the draining inguinal lymph nodes (E) and liver (F). For immunodominance analyses between TLR-L-treated and untreated mice, the change in the profile where NP396 becomes subdominant was depicted by an asterisk. v, virus.
When we injected mice with TLR3-L together with virus, we observed an immunodominance profile similar to that of mice injected with virus alone, albeit with a lower percentage of epitope-specific CD8+ T cells. With TLR2-L, we observed a slight shift in the hierarchy between the immunodominant epitopes, in which GP33 is the most immunodominant epitope, followed by NP396 in the β position. However, when we injected mice with TLR3-L and TLR2-L in combination, we observed a statistically significant 2-fold reduction in the percentage of NP396-specific CD8+ T cells, thereby changing the immunodominance hierarchy. Here, we observed that GP33 retains the α position, GP276 the β position, and NP396 and NP205 assumed the subdominant positions (Fig. 1A). Moreover, the altered hierarchy of these epitopes due to TLR3-L and TLR2-L was even more pronounced at day 12, indicating that this phenomenon was not restricted to a single time point of analysis (Fig. 1B).
We confirmed these data by estimating the number of epitope-specific T cells from the ICS data by calculating the percentages and translating back to the total number of splenocytes obtained as described in Materials and Methods. We performed these analyses to control for the variations in the number of splenocytes obtained in the spleen due to TLR administration. The data shown in Fig. 1C and D confirmed the immunodominance profiles obtained in Fig. 1A and B, in which combined TLR2+3-L administration reduced NP396-specific CD8+ T cells and shifted the immunodominance hierarchy.
Since administering TLR-L s.c. induces an accumulation of dendritic cells and enhanced CD8+ T cells in regional lymph nodes (20), we tested the immunodominance hierarchy in the draining inguinal lymph nodes. We performed our analyses on day 12 p.i., as this was the time point that revealed the most significant changes in the immunodominance of the LCMV epitopes tested. The data depicted in Fig. 1E demonstrate that the coadministration of TLR3-L and TLR2-L reduced NP396-specific CD8+ T cells in inguinal lymph nodes, again affecting the immunodominance hierarchy by day 12 (Fig. 1E) in a manner similar to that observed in the spleen.
We determined whether administering TLR-L s.c. alters the immunodominance hierarchy in nonlymphoid tissues, such as the liver, compared to that observed in the spleen and the draining lymph nodes. At day 12, we observed that administering both ligands reduces NP396-specific CD8+ T cells in the liver, shifting the immunodominance hierarchy (Fig. 1F). Taken together, the data demonstrate that the administration of combined TLR3+2L alters immunodominance hierarchies of LCMV epitopes by reducing the number of NP396-specific CD8+ T cells. Although NP396-specific CD8+ T cells were reduced due to the combined TLR-L treatment, we could not detect any significant reduction in the number of total CD8+ T cells compared to the level of the virus-alone condition (data not shown).
To further substantiate our findings, we quantified epitope-specific CD8+ T cells NP396 and GP276, whose positions in the hierarchy were changed by combined TLR administration, using tetramer analysis (Fig. 2A to C). The data depicted in Fig. 2A to C demonstrate that the coadministration of TLR3-L and TLR2-L reduced NP396-specific CD8+ T cells at day 12, suggesting that combined TLR administration reduced the expansion of NP396-specific CD8+ T cells and not the cells' ability to produce IFN-γ.
Fig. 2.
Tetramer analysis of altered immunodominance between NP396 and GP276. Mice were injected with 500 PFU LCMV s.c. as described in Materials and Methods, with or without pIC and pam3cysk4 individually or in combination. (A to C) LCMV-specific CD8+ T cell responses in the spleen were estimated at day 12 with tetramer staining for the NP396 or GP276 epitopes. (A) Dot plots show tetramer-positive CD8+ cells from a representative mouse. (B) Graphs summarize the data from three experiments (n = 3 mice in each trial). (C) Number of CD8+ T cells that are tetramer positive for NP396 or GP276 were estimated. Controls (C) represent infected spleens with CD8+ labeling to adjust for the compensation. Naïve splenocytes stained with the same tetramers gave similar background data (data not shown). For immunodominance analyses between TLR-L-treated and untreated mice, the condition where NP396 becomes subdominant is depicted by an asterisk.
Taken together, the data revealed that the administration of combined TLR3+2L alters immunodominance hierarchies of LCMV epitopes by reducing numbers of NP396-specific CD8+ T cells. Although NP396-specific CD8+ T cells were reduced due to the combined TLR-L treatment, we could not detect a reduction in the total number of CD8+ T cells compared to that of the virus-alone condition (data not shown).
Administration of dual TLR2+3-L in combination affects viral load in vivo.
As NP396-specific CD8+ T cells are most efficient at clearing LCMV infections (12), one would expect that reduced NP396-specific responses would result in a delay in virus clearance. To address this, we first measured virus clearance under these conditions. Mice were injected s.c. with 500 PFU LCMV-WE along with 100 μg pIC or 20 μg pam3cysk4 individually or in combination, and then LCMV was titrated in the spleen 5 or 7 days p.i. (Fig. 3A). At day 5, we did not observe any differences in LCMV titers when mice were injected with TLR-L in combination with virus compared to titers for mice injected with virus alone; however, at day 7, we observed reduced virus clearance when mice were injected with TLR3-L. Interestingly, we observed enhanced virus clearance when mice were injected with TLR2-L alone. In contrast, when TLR3-L was combined with TLR2-L during virus infection, it resulted in a slight but significant increase in the viral yield detected at day 7 compared to that of virus alone and a significant increase compared to that of the TLR2-L. Thus, the benefit gained in viral clearance with TLR2-L alone was totally reversed when the dual ligands were administered. By day 12, we could not detect any virus in any of the conditions (data not shown).
Fig. 3.
Presence of dual TLR2+3L during virus infection affects antiviral responses. (A) Virus titers were determined in spleens 5 or 7 days p.i. from mice infected with 500 PFU LCMV and pIC (100 μg) and pam3cysk4 (20 μg). n.d., not detected. Data shown are averages ± standard deviations and represent one of three experiments. Statistical analyses comparing TLR-L-treated to untreated mice are depicted in the corresponding columns, and P < 0.05 was considered significant. (B) For in vivo cytotoxicity assays, mice were injected with TLR-L and LCMV. Five days later, target syngeneic NP396-labeled splenocytes were used for the assay as described in Materials and Methods. Splenocytes were isolated 16 h later and analyzed for specific killing by comparing ratios of CFSE-labeled cells. Histograms are representative of in vivo killing (i), and representative data are from three independent trials ± standard deviations (ii).
We determined whether administering TLR2-L and TLR3-L during virus infection affected the quality of NP396-specific CD8+ T cells by measuring the cytolytic ability of NP396-specific effector CD8+ T cells in vivo. Mice were injected s.c. with 500 PFU LCMV-WE along with 100 μg pIC and 20 μg pam3cysk4 in combination, and 6 days later mice were injected i.v. with CFSE-labeled NP396-specific target splenocytes from syngeneic mice. After harvesting spleens 16 h later, we observed that coadministering TLR2+3-L resulted in the reduced efficiency of in vivo killing (49 versus 95%) compared to that for mice that were injected with virus alone (Fig. 3B). The reduced efficiency of in vivo killing in mice administered TLR2+3-L was observed as early as 5 days p.i.; however, by day 7, we could not detect differences in killing in vivo (data not shown). Taken together, these data demonstrate that administering TLR2+3-L can affect antiviral immunity in vivo by lowering the numbers of NP396-specific CD8+ T cells (Fig. 3).
Analyses of APC activation and LCMV-NP396 antigen presentation.
To determine whether the activation state of pAPC was affecting antigen presentation in our model, we injected mice as described above and isolated APC from spleens 24 h p.i., and we found increased CD86 expression when mice were injected with LCMV alone compared to that of naïve mice, indicating that virus infection enhanced costimulatory molecule expression (Fig. 4A). Moreover, the administration of TLR3-L, TLR2-L, or TLR2+3-L along with virus further increased CD86 expression compared to that of virus alone (Fig. 4A). However, we did not observe an additive increase when both TLR-L were injected in combination. Therefore, introducing TLR-L and virus through the s.c. route affects splenic APC within 24 h by increasing CD86 expression, which is indicative of a more activated pAPC phenotype.
Fig. 4.
Assessment of APC activation and LCMV-NP396 antigen presentation. Mice were injected with 500 PFU LCMV s.c. with or without pIC and pam3cysk4 as described in Materials and Methods. After 24 h, splenic APC were isolated by adherence for 1 h and assayed for CD86 surface molecule expression (A) and antigen presentation (B). (A) The graph shows mean fluorescence values from three independent experiments. (B) Antigen presentation was assessed using NP396-specific CTLs in an IFN-γ functional assay. The data shown are from one representative experiment of three, and error bars are averages ± standard deviations from three replicates. For statistical analyses, columns were depicted by an asterisk (P < 0.05) by comparing TLR-L to virus (v) alone.
We asked if TLR-L administration influences the presentation of NP396, as it appeared to be the epitope most affected by TLR engagement. Here, we injected mice as described above and measured antigen presentation by splenic APCs 24 h p.i. using NP396-specific CTLs (Fig. 4B). The results show that TLR2 engagement significantly enhanced the presentation of NP396 epitopes compared to that of virus alone. However, when TLR3-L and TLR2-L were administered together, it was clear that TLR3-L abrogated this enhanced NP396 presentation. It is interesting that the dual administration of TLR3-L and TLR2-L did not significantly alter the presentation of the NP396 epitope compared to that of virus alone, even though APC activation was higher in the latter (Fig. 4A).
Simultaneous TLR2 and TLR3 activation impairs LCMV-NP396 cross-priming in vivo.
A potential explanation for the shift in immunodominance is due to the altered cross-presentation of the NP396 epitope, since cross-presentation is important for certain epitopes to attain higher positions in the immunodominance hierarchies (2, 9, 21, 22). To test this hypothesis, we employed an in vivo cross-priming assay using HEK-NP cells as a source of exogenous antigens, which enables us to detect NP396 cross-presentation in vivo (2, 5). Here, mice were injected s.c. with 5 × 106 HEK-NP (2, 5) along with 100 μg pIC or 20 μg pam3cysk4 individually or in combination. After 7 days, we isolated splenocytes and cultured NP396-specific CD8+ T cells before testing them in a functional ICS assay (Fig. 5A and B). We observed that TLR3-L reduced the cross-priming of NP396. Furthermore, this reduction in NP396 cross-priming was more significant when mice were treated with TLR2+3-L. Therefore, the administration of TLR2+3-L results in impaired NP396 cross-priming, which may have influenced its position in the immunodominance hierarchy during infection.
Fig. 5.
Cross-priming of NP396 epitopes is impaired by combined TLR2 and TLR3 activation. (A and B) To assess cross-priming, mice were injected with 5 × 106 HEK-NP cells s.c. with or without pIC and pam3cysk4. After 7 days, splenocytes were isolated and NP396-specific T cells were cultured. Peptide-specific T cells were restimulated with the NP396 peptide and assessed for their capacity to produce IFN-γ. Dot plots represent cross-priming in one mouse. (B) Graphs summarize the data from three experiments (n = 3 mice in each trial). Statistical significance of the comparison of TLR-L-treated to untreated mice is designated by an asterisk, and that for the comparison of combined TLR-L to single TLR-L is designated by two asterisks (significance is P < 0.05).
Administration of TLR2+3-L downregulates cell-associated antigen uptake by APCs.
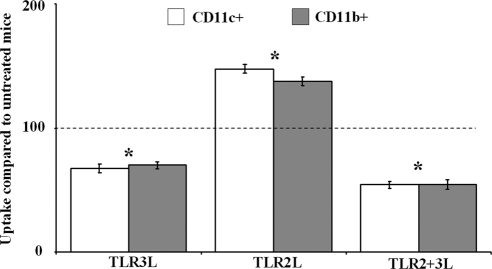
It has been reported previously that reduced phagocytosis as a result of pAPC activation can downregulate cross-presentation (41). Therefore, we determined whether combined TLR2+3L influences antigen uptake by injecting mice i.v. with 4 × 106 PKH2-labeled HEK cells along with 100 μg pIC or 20 μg pam3cysk4 individually or in combination (Fig. 6). After 6 h, splenocytes were isolated from the spleen and stained with anti-CD11c or anti-CD11b antibody, and uptake was measured using FCM after gating on double positive cells. In the experiments depicted in Fig. 5, antigen uptake by untreated mice was given an arbitrary value of 100, and changes in uptake by TLR-L-treated mice were plotted relative to this value. We observed that the administration of TLR3-L alone resulted in a decrease in phagocytosis, while administering TLR2-L increased phagocytosis. The increase in phagocytosis by TLR2-L again was significantly reversed in the TLR2+3L condition. This reduction was also a statistically significant decrease in the phagocytosis of cell-associated antigens compared to that of untreated cells, which may contribute to the decreased cross-presentation of the LCMV-NP396 epitope.
Fig. 6.
Combined TLR engagement downregulates phagocytic ability of APC. Mice were injected with pIC (100 μg) and pam3cysk4 (20 μg) individually or in combination, along with 4 × 106 PKH2-labeled HEK cells for 4 h. Splenic APC were isolated and stained with anti-CD11c or anti-CD11b and analyzed with FCM by gating on APC that have taken up HEK cells. Graphs summarize the data ± standard deviations from three experiments (n = 3 mice in each trial) and represent the relative percentage of phagocytosis compared to that of untreated mice, which were assigned an arbitrary value 100. For statistical analysis, significance was depicted by an asterisk in comparisons of TLR-L-treated and untreated cells (P < 0.05).
Analyses of the conditions favoring altered immunodominance hierarchy when TLR-L is present.
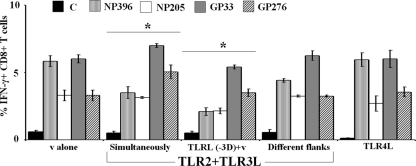
To test whether the altered immunodominance hierarchy is contingent on TLR-L being in the same environment as the virus infection, mice were injected with either TLR2+3L followed by LCMV infection 3 days later or were injected with TLR2+3L and LCMV simultaneously as in earlier experiments. When we analyzed immunodominance hierarchies of LCMV epitopes 8 days p.i., we observed similar downregulation of NP396-specific CD8+ T cells and a shift in the immunodominance hierarchy when mice were injected with TLR2+3L prior to virus infection (Fig. 7). Therefore, TLR-L can affect antiviral immunity if they were present recently in the milieu before the infection occurs.
Fig. 7.
Analyses of the conditions that favor changing immunodominance hierarchies during viral infection. Mice were injected with 500 PFU LCMV-WE and TLR2+3L with TLR4-L (10 μg) simultaneously or TLR2+3L 3 days prior to virus infection. For different flank conditions, mice were injected with virus in one flank and TLR2+3L in another flank. Eight days p.i., LCMV-specific CD8+ T cell responses were estimated using ICS. The data are representative of three experiments. For immunodominance analyses of TLR-L-treated and untreated mice, the change in the profile where NP396 becomes subdominant was depicted by an asterisk.
It is known that TLR2 and TLR3 signal through different pathways; TLR2 engages the MyD88 adaptor and TLR3 signals through TRIF (6). We asked whether lipopolysaccharide (LPS), which uses both signaling pathways, would shift immunodominance in a manner similar to that observed with TLR2+3L administration. Here, mice were injected s.c. with 500 PFU LCMV and 10 μg LPS, and immunodominance hierarchies were measured as described above 8 days p.i. The immunodominance profile observed with TLR4-L administration was unaltered, in that it was similar to that observed when mice were injected with virus alone, i.e., GP33 and NP396 assumed the immunodominant position and NP205 and GP276 were subdominant (Fig. 7). Therefore, the altered immunodominance hierarchies were specific to the dual administration of TLR2-L and TLR3-L.
We sought to determine whether administering TLR-L in a different site from that for LCMV would result in altered immunodominance. Here, mice were injected s.c. with 500 PFU LCMV in the left flank and TLR2+3L in the right flank. Eight days p.i., immunodominance hierarchies were measured in the spleen using ICS. We observed a slight downregulation in the percentage of NP396-specific CD8+ T cells; however, this did not result in a shift in immunodominance hierarchies where NP396 becomes subdominant to GP276 (Fig. 7). Therefore, the effects of dual TLR2+3L need to be close to the location where virus infection occurs to alter the immunodominance hierarchies, presumably because the pAPC activation will be occurring in this locale.
DISCUSSION
Recently, TLR11 was reported to regulate the CD4+ T cell immunodominance hierarchy, suggesting a novel mechanism by which TLR-L can influence adaptive immune responses (42). Moreover, combined TLR2+3-L stimulation in a peptide immunization model induced better protection against subsequent recombinant vaccinia virus infections that expressed the same peptide (45, 46). Here, we questioned if the presence of TLR-L during viral infections could influence CD8+ T responses against several epitopes from LCMV and if it can affect CD8+ T immunodominance hierarchies.
We employed ligands that activate TLR2/1 or TLR3 and signal through the MyD88-dependent pathway and the TRIF-dependent pathway, respectively (6). When we examined the effects of administering TLR-L individually, we observed that administering TLR3-L showed a delay in the expansion of CD8+ T cells specific for the LCMV epitopes at day 8; however, by day 12, CD8+ T cells reached high numbers. On the other hand, in the case of TLR2-L, CD8+ T cells expanded earlier and declined by day 12. The differences in CD8+ T cell profiles observed between days 8 and 12 could be due to changes in the migration and activation kinetics of CD8+ T cells. CD8+ T cell migration can be affected by factors which include cytokines and virus load (43). TLR2-L and TLR3-L can induce the release of different sets of cytokines (25, 34) that could contribute to the results we observed. Supporting this notion, the virus titration data indicate that mice injected with TLR2-L clear infection quicker than mice injected with TLR3-L, suggesting that administering TLR2-L aids in CD8+ T cells expanding earlier and clearing virus sooner.
We examined if combined TLR-L administration can influence LCMV-specific CD8+ T cell immunodominance hierarchies. Based on several reports examining CD8+ T cell responses (37, 45, 46), one would expect an overall boost in T cell responses when more than a single TLR-L is present. Surprisingly, we observed that dual TLR2 and TLR3 stimulation alters immunodominance hierarchies of LCMV epitopes due to reduced NP396-specific CD8+ T cell responses, causing the epitope to become subdominant. Furthermore, there was a shift of a subdominant epitope, GP276, into the β position in the immunodominance hierarchy of the epitopes examined. Thus, there was a newly formed hierarchy where LCMV-GP33 > GP276 > NP396 = NP205. Interestingly, the effect of dual TLR2 and TLR3 engagement on CD8+ T cell responses was limited to the NP396 epitope, as TLR2+3-L did not reduce the overall CD8+ T cell numbers recovered from the spleen.
In our study, we found that dual TLR2 and TLR3 stimulation had a deleterious, albeit small, effect on the cytolytic activities of NP396-specific CD8+ T cells at day 6 p.i., probably because of the smaller number of cells that had expanded at this point. This may be related to the affected virus load, since NP396-specific CD8+ T cells are important for virus clearance during LCMV infections (24). It is important to point out that if the NP396-specific CD8+ T cells reach a high enough number, and although they reached a lower position in the hierarchy, the efficient killing of targets was observed. This is probably due to the high efficiency of NP396-specific CD8+ T cells to lyse NP396-labeled target cells once they passed a certain threshold in their numbers. However, this situation might dramatically change if the host was suffering from a chronic infection with an overwhelming TLR2-L presence in the environment.
The reduced NP396-specific CD8+ T cells in the dual condition (TLR2-L and TLR3-L) in the presence of virus infection may have been associated with an overactivation of pAPC and therefore a reduced efficiency in cross-presentation. In this scenario, NP396-specific CD8+ T cell priming and activation would be adversely affected because of reduced cross-presentation during the priming stages. This proposal is based on the findings that the activation of CD8+ T cells specific for certain immunodominant epitopes need efficient cross-presentation to attain immunodominant status (2, 9, 21, 22). Importantly, as the other LCMV epitopes, which include NP205, GP33, and GP276, are not efficiently cross-presented (2), the number of CD8+ T cells specific for these epitopes would be minimally affected by combined TLR administration. Therefore, altering the pAPC ability to cross-present antigens via TLR activation could influence mainly the immunodominance of the NP396 epitope.
Interestingly, we also observed that TLR3 stimulation reduced the cross-priming of cell-associated antigens. These findings are supported by previous reports suggesting that exposure to TLR3-L alone induces the maturation of DC, which are in turn partially impaired in their capacity to cross-present antigens (11, 41).
In contrast to two recent findings (23, 31) where TLR2 stimulation either inhibited (31) or enhanced cross-presentation (23), we did not record a significant influence of TLR2-L in our model in vivo. Several reasons can account for these differences; for instance, it could be because the results are dependent on which pAPC population was studied. Harding's group examined cross-presentation using BMDCs, and Behrens' group used splenic DC subsets. Different DC subsets differ in their TLR expression profile (14, 19), therefore pAPCs may differ in their capacity to cross-present antigens in response to TLR stimulation. Second, TLR2 stimulation may differentially influence the outcome of cross-presentation based on the type of ligand used. For instance, Harding's group employed a TLR2/1-L (31) that is similar to the constructs of our study and in contrast to the TLR2/6-L construct used by Behrens' group (23). In support of this hypothesis, different outcomes due to TLR2/1 versus TLR2/6 activation have been reported recently (46), in addition to unpublished data from our laboratory that demonstrates that TLR2/1 and TLR2/6 differ in their downstream immune responses (data not shown). Another reason that accounts for the observations is linked to the possibility that TLR engagement differentially influences cross-presentation based on the form of antigen employed, as both of the reports discussed above (23, 31) used soluble OVA as a source of antigens and our study employed cell-associated antigens. This suggestion is supported by recent reports which showed that soluble antigen cross-presentation is enhanced by pIC (8, 16, 28), while studies employing cell-associated antigens observed a TLR3-induced reduction in cross-presentation (11, 41).
We observed that the cross-priming of NP396 epitopes was further downregulated due to combined TLR2 and TLR3 stimulation. These results correlate with our immunodominance findings, in which we observed that NP396-specific CD8+ T cells were further reduced under these conditions, leading to a shift in the immunodominance hierarchy. We confirmed that the direct presentation of NP396 epitopes was unaffected by combined TLR2+3 stimulation, as previously demonstrating in vitro by our group (29). This probably explains why we observed that NP396-specific CD8+ T cell responses were reduced but not completely obliterated. The reduced NP396-specific CD8+ T cell response after combined TLR2+3-L administration was accompanied by the increased expansion of GP276-specific CD8+ T cells, possibly explaining why we did not observe differences in the overall CD8+ T cell numbers. Interestingly, in contrast to our previous in vitro studies (29), we observed an increase in the presentation of NP396 epitopes on TLR2 stimulation, possibly because the TLR2-L used in these experiments were different.
As antigen uptake is needed for cross-presentation to occur (4), we investigated the influence of combined TLR2 and TLR3 stimulation on the phagocytosis of antigens. We observed that TLR3 engagement inhibited the phagocytosis of antigens by DC, while TLR2 stimulation had no effect. These observations are supported by earlier studies showing that TLR-L which signal through the TRIF-dependent pathway inhibit antigen uptake, and those TLR-L that are limited to signaling through the MyD88-dependent pathway have no effect on antigen uptake (38, 41). Moreover, we observed that combined TLR2 and TLR3 stimulation also showed inhibition in antigen uptake. However, we did not observe any statistically significant differences in antigen uptake when we compared TLR2+3 stimulation to TLR3 stimulation alone. It is possible that TLR-L influences cross-presentation through antigen uptake as well as by altering other parameters, such as phagosomal pH through NOX2 activation (3, 35). Increased NOX2 activity could exacerbate antigen degradation, which in turn reduces the amount of antigen available from cross-presentation (1, 27).
Another interesting observation we made in this study revealed that even if the TLR-L were administered a few days prior to virus infections, immunodominance was affected. This indicates that if the environment where virus entry occurred was previously in a state of activation due to the presence of TLR-L, one then could expect a significant influence on antiviral responses, which is an important concern for people suffering coinfections within a short time span. Finally, we could not recapitulate our findings when activating MyD88- and TRIF-dependent signaling pathways with LPS, which suggests that the nature of how TLR-L influence additional immune parameters such as cytokine production (26, 29) also is critical to how the immunodominance hierarchies are regulated. Moreover, unique cytokine profiles of individual TLR-L could influence immunodominance hierarchies, in addition to antigen presentation, by modifying APC or T cell migration patterns and T cell proliferation (10, 18, 43). Future work needs to be directed at clarifying the relative contribution of each of these factors to formulate conclusive answers regarding the role of TLR-L influence on CD8+ T cell immunodominance.
Interestingly, in contrast to NP396-specific CD8+ T cells, we did not observe any differences in the expansion of GP33-specific CD8+ T cells when mice were injected with dual TLR2-L and TLR3-L. This is likely related to the fact that even though GP33 is cross-presented (2, 22), it does so with lower efficiency than NP396 (2). Moreover, since GP33-specific CD8+ T cells are found at a high precursor frequency in vivo
(15), any reduction in GP33 cross-presentation is likely to have a less negative effect than that of NP396 on the activation and expansion of T cells.
In summary, this study established that combined TLR2 and TLR3 engagement alters the immunodominance hierarchy of virus-specific T cells. We elucidated that the shift in immunodominance was due to reduced antigen uptake and the cross-presentation of antigens, which affected an immunodominant epitope that usually is efficient at accessing cross-priming. Our data are significant because they defined a new function for TLR signaling in regulating the presentation of viral epitopes and how they affect CD8+ T cell immunodominance.
ACKNOWLEDGMENTS
This work was supported by grants from the NSERC to S.B. and from OGS to S.S.
We thank M. van den Broek, M. Groettrup, R. Zinkernagel, and K. Rock for providing antibodies and cell lines and the NIH tetramer facility for the production of tetramers.
We have no financial or commercial conflicts of interest.
Footnotes
Published ahead of print on 28 September 2011.
REFERENCES
- 1. Alatery A., et al. 2010. Cross, but not direct, presentation of cell-associated virus antigens by spleen macrophages is influenced by their differentiation state. Immunol. Cell Biol. 88:3–12 [DOI] [PubMed] [Google Scholar]
- 2. Alatery A., Tarrab E., Lamarre A., Basta S. 2010. The outcome of cross-priming during virus infection is not directly linked to the ability of the antigen to be cross-presented. Eur. J. Immunol. 40:2190–2199 [DOI] [PubMed] [Google Scholar]
- 3. Amigorena S., Savina A. 2010. Intracellular mechanisms of antigen cross presentation in dendritic cells. Curr. Opin. Immunol. 22:109–117 [DOI] [PubMed] [Google Scholar]
- 4. Basta S., Alatery A. 2007. The cross-priming pathway: a portrait of an intricate immune system. Scand. J. Immunol. 65:311–319 [DOI] [PubMed] [Google Scholar]
- 5. Basta S., Stoessel R., Basler M., van den Broek M., Groettrup M. 2005. Cross-presentation of the long-lived lymphocytic choriomeningitis virus nucleoprotein does not require neosynthesis and is enhanced via heat shock proteins. J. Immunol. 175:796–805 [DOI] [PubMed] [Google Scholar]
- 6. Brikos C., O'Neill L. A. 2008. Signalling of toll-like receptors. Handb. Exp. Pharmacol. 183:21–50 [DOI] [PubMed] [Google Scholar]
- 7. Cella M., et al. 1996. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J. Exp. Med. 184:747–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Datta S. K., Raz E. 2005. Induction of antigen cross-presentation by Toll-like receptors. Springer Semin. Immunopathol. 26:247–255 [DOI] [PubMed] [Google Scholar]
- 9. Dunbar E., Alatery A., Basta S. 2007. Cross-priming of a single viral protein from lymphocytic choriomeningitis virus alters immunodominance hierarchies of CD8+ T cells during subsequent viral infections. Viral Immunol. 20:585–598 [DOI] [PubMed] [Google Scholar]
- 10. Eberl G., et al. 1996. Immunodominance of cytotoxic T lymphocyte epitopes co-injected in vivo and modulation by interleukin-12. Eur. J. Immunol. 26:2709–2716 [DOI] [PubMed] [Google Scholar]
- 11. Frleta D., et al. 2009. Influenza virus and poly(I:C) inhibit MHC class I-restricted presentation of cell-associated antigens derived from infected dead cells captured by human dendritic cells. J. Immunol. 182:2766–2776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gallimore A., Dumrese T., Hengartner H., Zinkernagel R. M., Rammensee H.-G. 1998. Protective immunity does not correlate with the hierarchy of virus-specific cytotoxic T cell responses to naturally processed peptides. J. Exp. Med. 187:1647–1657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ishii K. J., Akira S. 2007. Toll or toll-free adjuvant path toward the optimal vaccine development. J. Clin. Immunol. 27:363–371 [DOI] [PubMed] [Google Scholar]
- 14. Jelinek I., et al. 2011. TLR3-specific double-stranded RNA oligonucleotide adjuvants induce dendritic cell cross-presentation, CTL responses, and antiviral protection. J. Immunol. 186:2422–2429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kotturi M. F., et al. 2008. Naive precursor frequencies and MHC binding rather than the degree of epitope diversity shape CD8+ T cell immunodominance. J. Immunol. 181:2124–2133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Le Bon A., et al. 2003. Cross-priming of CD8+ T cells stimulated by virus-induced type I interferon. Nat. Immunol. 4:1009–1015 [DOI] [PubMed] [Google Scholar]
- 17. Liu F., Whitton J. L., Slifka M. K. 2004. The rapidity with which virus-specific CD8+ T cells initiate IFN-gamma synthesis increases markedly over the course of infection and correlates with immunodominance. J. Immunol. 173:456–462 [DOI] [PubMed] [Google Scholar]
- 18. Melchionda F., et al. 2005. Adjuvant IL-7 or IL-15 overcomes immunodominance and improves survival of the CD8+ memory cell pool. J. Clin. Investig. 115:1177–1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Muzio M., et al. 2000. Differential expression and regulation of toll-like receptors (TLR) in human leukocytes: selective expression of TLR3 in dendritic cells. J. Immunol. 164:5998–6004 [DOI] [PubMed] [Google Scholar]
- 20. Oh J. Z., Kedl R. M. 2010. The capacity to induce cross-presentation dictates the success of a TLR7 agonist-conjugate vaccine for eliciting cellular immunity. J. Immunol. 185:4602–4608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Otahal P., et al. 2005. Inefficient cross-presentation limits the CD8+ T cell response to a subdominant tumor antigen epitope. J. Immunol. 175:700–712 [DOI] [PubMed] [Google Scholar]
- 22. Pavelic V., Matter M. S., Mumprecht S., Breyer I., Ochsenbein A. F. 2009. CTL induction by cross-priming is restricted to immunodominant epitopes. Eur. J. Immunol. 39:704–716 [DOI] [PubMed] [Google Scholar]
- 23. Prajeeth C. K., et al. 2010. The synthetic TLR2 agonist BPPcysMPEG leads to efficient cross-priming against co-administered and linked antigens. Eur. J. Immunol. 40:1272–1283 [DOI] [PubMed] [Google Scholar]
- 24. Probst H. C., et al. 2003. Immunodominance of an antiviral cytotoxic T cell response is shaped by the kinetics of viral protein expression. J. Immunol. 171:5415–5422 [DOI] [PubMed] [Google Scholar]
- 25. Re F., Strominger J. L. 2004. IL-10 released by concomitant TLR2 stimulation blocks the induction of a subset of Th1 cytokines that are specifically induced by TLR4 or TLR3 in human dendritic cells. J. Immunol. 173:7548–7555 [DOI] [PubMed] [Google Scholar]
- 26. Re F., Strominger J. L. 2001. Toll-like receptor 2 (TLR2) and TLR4 differentially activate human dendritic cells. J. Biol. Chem. 276:37692–37699 [DOI] [PubMed] [Google Scholar]
- 27. Savina A., et al. 2006. NOX2 controls phagosomal pH to regulate antigen processing during crosspresentation by dendritic cells. Cell 126:205–218 [DOI] [PubMed] [Google Scholar]
- 28. Schulz O., et al. 2005. Toll-like receptor 3 promotes cross-priming to virus-infected cells. Nature 433:887–892 [DOI] [PubMed] [Google Scholar]
- 29. Siddiqui S., Alatery A., Kus A., Basta S. 2011. TLR engagement prior to virus infection influences MHC-I antigen presentation in an epitope-dependent manner as a result of nitric oxide release. J. Leukoc. Biol. 89:457–468 [DOI] [PubMed] [Google Scholar]
- 30. Siddiqui S., Tarrab E., Lamarre A., Basta S. 2010. Altered immunodominance hierarchies of CD8+ T cells in the spleen after infection at different sites is contingent on high virus inoculum. Microbes Infect. 12:324–330 [DOI] [PubMed] [Google Scholar]
- 31. Simmons D. P., et al. 2010. Mycobacterium tuberculosis and TLR2 agonists inhibit induction of type I IFN and class I MHC antigen cross processing by TLR9. J. Immunol. 185:2405–2415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Trautmann L., et al. 2005. Selection of T cell clones expressing high-affinity public TCRs within human cytomegalovirus-specific CD8 T cell responses. J. Immunol. 175:6123–6132 [DOI] [PubMed] [Google Scholar]
- 33. Trinchieri G., Sher A. 2007. Cooperation of Toll-like receptor signals in innate immune defence. Nat. Rev. Immunol. 7:179–190 [DOI] [PubMed] [Google Scholar]
- 34. Vanhoutte F., et al. 2008. Toll-like receptor (TLR)2 and TLR3 synergy and cross-inhibition in murine myeloid dendritic cells. Immunol. Lett. 116:86–94 [DOI] [PubMed] [Google Scholar]
- 35. Vulcano M., et al. 2004. Toll receptor-mediated regulation of NADPH oxidase in human dendritic cells. J. Immunol. 173:5749–5756 [DOI] [PubMed] [Google Scholar]
- 36. Vyas J. M., Van der Veen A. G., Ploegh H. L. 2008. The known unknowns of antigen processing and presentation. Nat. Rev. Immunol. 8:607–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Warger T., et al. 2006. Synergistic activation of dendritic cells by combined Toll-like receptor ligation induces superior CTL responses in vivo. Blood 108:544–550 [DOI] [PubMed] [Google Scholar]
- 38. Weck M. M., et al. 2007. TLR ligands differentially affect uptake and presentation of cellular antigens. Blood 109:3890–3894 [DOI] [PubMed] [Google Scholar]
- 39. Wherry E. J., Blattman J. N., Murali-Krishna K., van der Most R., Ahmed R. 2003. Viral persistence alters CD8 T cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J. Virol. 77:4911–4927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wiesel M., Walton S., Richter K., Oxenius A. 2009. Virus-specific CD8 T cells: activation, differentiation and memory formation. Apmis 117:356–381 [DOI] [PubMed] [Google Scholar]
- 41. Wilson N. S., et al. 2006. Systemic activation of dendritic cells by Toll-like receptor ligands or malaria infection impairs cross-presentation and antiviral immunity. Nat. Immunol. 7:165–172 [DOI] [PubMed] [Google Scholar]
- 42. Yarovinsky F., Kanzler H., Hieny S., Coffman R. L., Sher A. 2006. Toll-like receptor recognition regulates immunodominance in an antimicrobial CD4+ T cell response. Immunity 25:655–664 [DOI] [PubMed] [Google Scholar]
- 43. Yewdell J. T., Bennink J. R. 1999. Immunodominance in major histocompatibility complex class I-restricted T lymphocyte responses. Annu. Rev. Immunol. 17:51–88 [DOI] [PubMed] [Google Scholar]
- 44. Yewdell J. W. 2006. Confronting complexity: real-world immunodominance in antiviral CD8+ T cell responses. Immunity 25:533–543 [DOI] [PubMed] [Google Scholar]
- 45. Zhu Q., et al. 2010. Using 3 TLR ligands as a combination adjuvant induces qualitative changes in T cell responses needed for antiviral protection in mice. J. Clin. Investig. 120:607–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhu Q., et al. 2008. Toll-like receptor ligands synergize through distinct dendritic cell pathways to induce T cell responses: implications for vaccines. Proc. Natl. Acad. Sci. U. S. A. 105:16260–16265 [DOI] [PMC free article] [PubMed] [Google Scholar]