Abstract
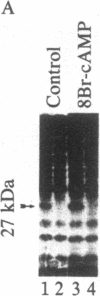
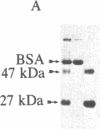
Membrane-permeant cAMP derivatives (dibutyryl- and 8-bromo-cAMP) increase gap-junctional conductance within minutes when applied to voltage-clamped pairs of rat hepatocytes. Glucagon also increases junctional conductances, but the response has a more rapid onset and is more rapidly reversible. The glucagon effect can be prevented by intracellular injection of the protein inhibitor of the cAMP-dependent protein kinase (Walsh inhibitor), indicating that the catalytic subunit of cAMP-dependent protein kinase is directly involved. The 27-kDa major gap junction polypeptide is phosphorylated when liver cells dissociated into small groups are incubated with 32P. Addition of 8-bromo-cAMP to cells increases the incorporation of 32P into the 27-kDa junctional protein. Serine is the amino acid residue that is phosphorylated. When isolated liver gap junctions are incubated in the presence of catalytic subunit of the cAMP-dependent protein kinase, the 27-kDa gap junction polypeptide is phosphorylated with low stoichiometry on serine. The rapid increases in gap junctional conductance caused by agents that elevate cAMP and phosphorylation of the gap junction protein by cAMP-dependent protein kinase suggest that cAMP-dependent phosphorylation of the gap junction channel modulates the conductance of liver gap junctions.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams W. B., Levitan I. B. Intracellular injection of protein kinase inhibitor blocks the serotonin-induced increase in K+ conductance in Aplysia neuron R15. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3877–3880. doi: 10.1073/pnas.79.12.3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander M. C., Kowaloff E. M., Witters L. A., Dennihy D. T., Avruch J. Purification of a hepatic 123,000-dalton hormone-stimulated 32P-peptide and its identification as ATP-citrate lyase. J Biol Chem. 1979 Aug 25;254(16):8052–8056. [PubMed] [Google Scholar]
- Azarnia R., Dahl G., Loewenstein W. R. Cell junction and cycle AMP: III. Promotion of junctional membrane permeability and junctional membrane particles in a junction-deficient cell type. J Membr Biol. 1981;63(1-2):133–146. doi: 10.1007/BF01969454. [DOI] [PubMed] [Google Scholar]
- Berry M. N., Friend D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969 Dec;43(3):506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellucci V. F., Kandel E. R., Schwartz J. H., Wilson F. D., Nairn A. C., Greengard P. Intracellular injection of t he catalytic subunit of cyclic AMP-dependent protein kinase simulates facilitation of transmitter release underlying behavioral sensitization in Aplysia. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7492–7496. doi: 10.1073/pnas.77.12.7492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellucci V. F., Nairn A., Greengard P., Schwartz J. H., Kandel E. R. Inhibitor of adenosine 3':5'-monophosphate-dependent protein kinase blocks presynaptic facilitation in Aplysia. J Neurosci. 1982 Dec;2(12):1673–1681. doi: 10.1523/JNEUROSCI.02-12-01673.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Mello W. C. Effect of intracellular injection of cAMP on the electrical coupling of mammalian cardiac cells. Biochem Biophys Res Commun. 1984 Mar 30;119(3):1001–1007. doi: 10.1016/0006-291x(84)90873-8. [DOI] [PubMed] [Google Scholar]
- Exton J. H. Mechanisms involved in effects of catecholamines on liver carbohydrate metabolism. Biochem Pharmacol. 1979 Aug 1;28(15):2237–2240. doi: 10.1016/0006-2952(79)90684-1. [DOI] [PubMed] [Google Scholar]
- Finbow M., Yancey S. B., Johnson R., Revel J. P. Independent lines of evidence suggesting a major gap junctional protein with a molecular weight of 26,000. Proc Natl Acad Sci U S A. 1980 Feb;77(2):970–974. doi: 10.1073/pnas.77.2.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagg-Newton J. L., Dahl G., Loewenstein W. R. Cell junction and cyclic AMP: 1. Upregulation of junctional membrane permeability and junctional membrane particles by administration of cyclic nucleotide or phosphodiesterase inhibitor. J Membr Biol. 1981;63(1-2):105–121. doi: 10.1007/BF01969452. [DOI] [PubMed] [Google Scholar]
- Garrison J. C., Borland M. K. Regulation of mitochondrial pyruvate carboxylation and gluconeogenesis in rat hepatocytes via an alpha-adrenergic, adenosine 3':5'-monophosphate-independent mechanism. J Biol Chem. 1979 Feb 25;254(4):1129–1133. [PubMed] [Google Scholar]
- Garrison J. C., Borland M. K. Regulation of mitochondrial pyruvate carboxylation and gluconeogenesis in rat hepatocytes via an alpha-adrenergic, adenosine 3':5'-monophosphate-independent mechanism. J Biol Chem. 1979 Feb 25;254(4):1129–1133. [PubMed] [Google Scholar]
- Hax W. M., van Venrooij G. E., Vossenberg J. B. Cell communication: a cyclic AMP mediated phenomenon. J Membr Biol. 1974;19(3):253–266. doi: 10.1007/BF01869981. [DOI] [PubMed] [Google Scholar]
- Hertzberg E. L. A detergent-independent procedure for the isolation of gap junctions from rat liver. J Biol Chem. 1984 Aug 10;259(15):9936–9943. [PubMed] [Google Scholar]
- Hertzberg E. L. Biochemical and immunological approaches to the study of gap junctional communication. In Vitro. 1980 Dec;16(12):1057–1067. doi: 10.1007/BF02619256. [DOI] [PubMed] [Google Scholar]
- Hertzberg E. L., Spray D. C., Bennett M. V. Reduction of gap junctional conductance by microinjection of antibodies against the 27-kDa liver gap junction polypeptide. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2412–2416. doi: 10.1073/pnas.82.8.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huganir R. L., Greengard P. cAMP-dependent protein kinase phosphorylates the nicotinic acetylcholine receptor. Proc Natl Acad Sci U S A. 1983 Feb;80(4):1130–1134. doi: 10.1073/pnas.80.4.1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttner W. B., DeGennaro L. J., Greengard P. Differential phosphorylation of multiple sites in purified protein I by cyclic AMP-dependent and calcium-dependent protein kinases. J Biol Chem. 1981 Feb 10;256(3):1482–1488. [PubMed] [Google Scholar]
- Kaczmarek L. K., Jennings K. R., Strumwasser F., Nairn A. C., Walter U., Wilson F. D., Greengard P. Microinjection of catalytic subunit of cyclic AMP-dependent protein kinase enhances calcium action potentials of bag cell neurons in cell culture. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7487–7491. doi: 10.1073/pnas.77.12.7487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagimoto T., Uyeda K. Hormone-stimulated phosphorylation of liver phosphofructokinase in vivo. J Biol Chem. 1979 Jul 10;254(13):5584–5587. [PubMed] [Google Scholar]
- Koch K. W., Kaupp U. B. Cyclic GMP directly regulates a cation conductance in membranes of bovine rods by a cooperative mechanism. J Biol Chem. 1985 Jun 10;260(11):6788–6800. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lawrence T. S., Beers W. H., Gilula N. B. Transmission of hormonal stimulation by cell-to-cell communication. Nature. 1978 Apr 6;272(5653):501–506. doi: 10.1038/272501a0. [DOI] [PubMed] [Google Scholar]
- Ljungström O., Ekman P. Glucagon-induced phosphorylation of pyruvate kinase (type L) in rat liver slices. Biochem Biophys Res Commun. 1977 Oct 24;78(4):1147–1155. doi: 10.1016/0006-291x(77)91413-9. [DOI] [PubMed] [Google Scholar]
- Louis C. F., Johnson R., Johnson K., Turnquist J. Characterization of the bovine lens plasma membrane substrates for cAMP-dependent protein kinase. Eur J Biochem. 1985 Jul 15;150(2):279–286. doi: 10.1111/j.1432-1033.1985.tb09018.x. [DOI] [PubMed] [Google Scholar]
- Osterrieder W., Brum G., Hescheler J., Trautwein W., Flockerzi V., Hofmann F. Injection of subunits of cyclic AMP-dependent protein kinase into cardiac myocytes modulates Ca2+ current. Nature. 1982 Aug 5;298(5874):576–578. doi: 10.1038/298576a0. [DOI] [PubMed] [Google Scholar]
- Piccolino M., Neyton J., Gerschenfeld H. M. Decrease of gap junction permeability induced by dopamine and cyclic adenosine 3':5'-monophosphate in horizontal cells of turtle retina. J Neurosci. 1984 Oct;4(10):2477–2488. doi: 10.1523/JNEUROSCI.04-10-02477.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitts J. D., Simms J. W. Permeability of junctions between animal cells. Intercellular transfer of nucleotides but not of macromolecules. Exp Cell Res. 1977 Jan;104(1):153–163. doi: 10.1016/0014-4827(77)90078-7. [DOI] [PubMed] [Google Scholar]
- Radu A., Dahl G., Loewenstein W. R. Hormonal regulation of cell junction permeability: upregulation by catecholamine and prostaglandin E1. J Membr Biol. 1982;70(3):239–251. doi: 10.1007/BF01870566. [DOI] [PubMed] [Google Scholar]
- Rodbell M., Lin M. C., Salomon Y., Londos C., Harwood J. P., Martin B. R., Rendell M., Berman M. Role of adenine and guanine nucleotides in the activity and response of adenylate cyclase systems to hormones: evidence for multisite transition states. Adv Cyclic Nucleotide Res. 1975;5:3–29. [PubMed] [Google Scholar]
- Sonne O., Gliemann J. Receptor binding of glucagon and adenosine 3',5'-monophosphate accumulation in isolated rat fat cells. Biochim Biophys Acta. 1977 Sep 29;499(2):259–272. doi: 10.1016/0304-4165(77)90008-3. [DOI] [PubMed] [Google Scholar]
- Spray D. C., Bennett M. V. Physiology and pharmacology of gap junctions. Annu Rev Physiol. 1985;47:281–303. doi: 10.1146/annurev.ph.47.030185.001433. [DOI] [PubMed] [Google Scholar]
- Spray D. C., Harris A. L., Bennett M. V. Equilibrium properties of a voltage-dependent junctional conductance. J Gen Physiol. 1981 Jan;77(1):77–93. doi: 10.1085/jgp.77.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teranishi T., Negishi K., Kato S. Dopamine modulates S-potential amplitude and dye-coupling between external horizontal cells in carp retina. Nature. 1983 Jan 20;301(5897):243–246. doi: 10.1038/301243a0. [DOI] [PubMed] [Google Scholar]
- White R. L., Spray D. C., Campos de Carvalho A. C., Wittenberg B. A., Bennett M. V. Some electrical and pharmacological properties of gap junctions between adult ventricular myocytes. Am J Physiol. 1985 Nov;249(5 Pt 1):C447–C455. doi: 10.1152/ajpcell.1985.249.5.C447. [DOI] [PubMed] [Google Scholar]
- Wiener E. C., Loewenstein W. R. Correction of cell-cell communication defect by introduction of a protein kinase into mutant cells. 1983 Sep 29-Oct 5Nature. 305(5933):433–435. doi: 10.1038/305433a0. [DOI] [PubMed] [Google Scholar]
- Witters L. A., Kowaloff E. M., Avruch J. Glucagon regulation of protein phosphorylation. Identification of acetyl coenzyme A carboxylase as a substrate. J Biol Chem. 1979 Jan 25;254(2):245–248. [PubMed] [Google Scholar]