Abstract
Objective:
Oral anticoagulation therapy (OAT) with warfarin increases mortality and disability after intracerebral hemorrhage (ICH), the result of increased ICH volume and risk of hematoma expansion. We investigated whether OAT also influences risk of development of intraventricular hemorrhage (IVH), the volume of IVH and IVH expansion, and whether IVH is a substantive mediator of the overall effect of OAT on ICH outcome.
Methods:
We performed a retrospective analysis of a prospectively collected single-center cohort of 1,879 consecutive ICH cases (796 lobar, 865 deep, 153 cerebellar, 15 multiple location, 50 primary IVH) from 1999 to 2009. ICH and IVH volumes at presentation, as well as hematoma expansion (>33% or >6 mL increase) and IVH expansion (>2 mL increase), were determined using established semiautomated methods. Outcome was assessed at 90 days using either the modified Rankin Scale or Glasgow Outcome Scale.
Results:
Warfarin use was associated with IVH risk, IVH volume at presentation, and IVH expansion in both lobar and deep ICH (all p < 0.05) in a dose-response relationship with international normalized ratio. Warfarin was associated with poor outcome in both lobar and deep ICH (p < 0.01), and >95% of this effect was accounted for by baseline ICH and IVH volumes, as well as ICH and IVH expansion.
Conclusion:
Warfarin increases IVH volume and risk of IVH expansion in lobar and deep ICH. These findings (along with effects on ICH volume and expansion) likely represent the mechanisms by which anticoagulation worsens ICH functional outcome.
Baseline hematoma volume and intraventricular hemorrhage (IVH) are potent predictors of outcome after spontaneous intracerebral hemorrhage (ICH).1–3 Hematoma enlargement and IVH expansion following presentation also worsen mortality and disability.4,5
ICH in the setting of oral anticoagulation therapy (OAT) has almost twice the mortality compared to non-warfarin-related ICH.6 The mechanisms underlying the effect of warfarin on ICH outcome are still not completely understood.7 While the underlying coagulopathy clearly plays a role in worsening the severity of OAT-ICH, patients on OAT often have higher rates of comorbidities, which may themselves influence ICH outcome. Hematoma expansion has been consistently found to be more frequent in OAT-ICH, and there is suggestive evidence that warfarin use is associated with larger ICH volume at presentation.8–10
We investigated whether OAT is associated with increased risk of developing IVH, as well as with increased IVH volume at presentation and rates of IVH expansion over time. Next, we sought to determine whether ICH and IVH volumes (both follow-up and baseline) can fully account for the effect of warfarin on ICH outcome.
METHODS
Patient selection and data collection.
Subjects were drawn from an ongoing longitudinal cohort study of primary intracerebral hemorrhage (ICH) as previously described.11 Briefly, enrolled cases were consecutive patients age ≥18 years presenting to the Massachusetts General Hospital (MGH) Emergency Department between January 1, 1999, and December 31, 2009, with a diagnosis of spontaneous primary ICH. Exclusion criteria included presence of trauma, brain tumor, hemorrhagic transformation of a cerebral infarction, vascular malformation, or any other cause of secondary ICH. All CT scans at presentation as well as follow-up CT scans obtained within 48 hours of symptom onset were analyzed.
Clinical data were recorded at the time of index presentation by stroke neurologists as part of routine clinical care. Collected data included information on demographics, previous medical history, Glasgow Coma Scale score (GCS), and pre-ICH medication use. International normalized ratio (INR) was determined as part of routine laboratory testing within 12 hours of admission. Subjects without an INR measurement obtained before reversal of OAT were excluded from analysis.
Standard protocol approvals, registrations, and patient consents.
This study was performed with approval of the MGH institutional review board and all subjects or their surrogates provided written informed consent prior to participation.
Patient follow-up.
Patients and their caregivers were interviewed by trained study staff over the telephone at 3 months post-ICH to assess functional outcome using either the modified Rankin Scale (mRS) or the Glasgow Outcome Scale (GOS).12 The use or discontinuation of medications after discharge (including warfarin, antiplatelet agents, and statins) was specifically assessed in this interview.
Neuroimaging analyses.
All admission CT scans were reviewed by stroke neurologists blinded to clinical data to determine ICH location and identify IVH presence. ICH isolated to the cortex (with or without involvement of subcortical white matter) was defined as lobar, while ICH selectively involving the internal capsule, thalamus, basal ganglia, or brainstem was defined as deep. Hemorrhages isolated to the cerebellum were defined as cerebellar ICH. IVH in the absence of a definite parenchymal hematoma on admission CT scan was defined as primary IVH. All subjects with ICH with evidence of one or more bleeds involving multiple locations simultaneously were classified as mixed location ICH.8 Disagreement regarding ICH location was resolved by consensus of study neurologists and neuroradiologists.
ICH and IVH volumes (both at admission and follow-up) were calculated as previously described using a semiautomated quantitative method with excellent interrater concordance.8
Outcomes and definitions.
Age at time of ICH was analyzed as a continuous variable. Pre-ICH medications (including warfarin use) were analyzed as binary variables. We repeated all analyses by substituting warfarin use with admission INR as a predictor variable; INR was analyzed as a ordinal variable, based on previously used cutoffs: <1.2, 1.2–2.0, 2.0–3.0, >3.0.10 We compared each INR level with the reference group (INR <1.2), and performed trend tests across categories to identify consistent associations with increasing INR. GCS was divided according to the following cutpoints: 15, 11–14, and 3–10 (roughly corresponding to GCS tertiles in our dataset). Consistent with prior studies, favorable functional outcome was defined as either 90-day mRS 0–2 (n = 1,493, ∼80%) or 90-day GOS 4–5 (n = 795, ∼42%).12 A subset of patients (n = 597, 32%) had both measures recorded, and we identified excellent concordance between measures (κ = 0.90) for identification of favorable outcome. We repeated all analyses after removal of patients with discordant outcome assessment (n = 41, ∼2%) and observed identical results (data not shown).
ICH and IVH volumes were analyzed as continuous variables (after log-transformation to achieve normality) when treated as dependent variables, and categorized based on 10-mL increases when treated as independent variables (expansion and outcome prediction analyses). IVH volumes were analyzed as dependent variables only when intraventricular extension of ICH was present, to preserve normality of distribution and ensure robustness of regression analyses. Hematoma expansion was defined as at least 33% or >6 mL increase in ICH volume on comparison of follow-up and admission CT scans.7 IVH expansion was defined as at least 2 mL increase between baseline and follow-up CT scans.5 Additional analyses of ICH and IVH expansion were performed using the absolute volumetric change (in cc) or the percent change as continuous variables and returned very similar results (data not shown).
Statistical methods.
Categorical variables were compared using Fisher exact test and continuous variables using the Mann-Whitney rank-sum or unpaired t tests as appropriate. To determine the influence of warfarin on functional outcome, hematoma (ICH) expansion, and IVH expansion, we used logistic regression. We used linear regression to correlate warfarin with ICH/IVH volumes. Candidate covariates for all multivariate models included all variables showing a trend in association with dependent variables in univariate analysis (p < 0.20), as well as variables showing trends toward differential distribution in warfarin users vs nonusers (p < 0.20). Bootstrap analysis (1,000 iterations) was subsequently used to compute multivariate effect sizes, confidence intervals (CIs), and p values (percentile method).
In order to investigate the impact of warfarin on functional outcome, and the intermediate effects on ICH and IVH volumetric data, we used mediation analysis to quantify the joint or independent contributions of pre-ICH characteristics and imaging data to ICH outcome.13 All variables achieving statistical significance in logistic regression (p < 0.05) were included in multivariate modeling for the purpose of effect size estimation. Effect sizes (both direct and mediated) were estimated via bootstrap (1,000 iterations). We also compared the contribution of IVH volume measures to prediction of ICH outcome by comparing area under the curve (AUC) from receiver operator characteristic (ROC) analyses for different multivariate models.
Because rates of IVH differ depending on location of ICH,14 we stratified all analyses by ICH location. All analyses were performed using R v 2.10.0 (The R Project for Statistical Computing). Significance tests were 2-tailed with significance threshold set at α = 0.05.
RESULTS
Cohort characteristics.
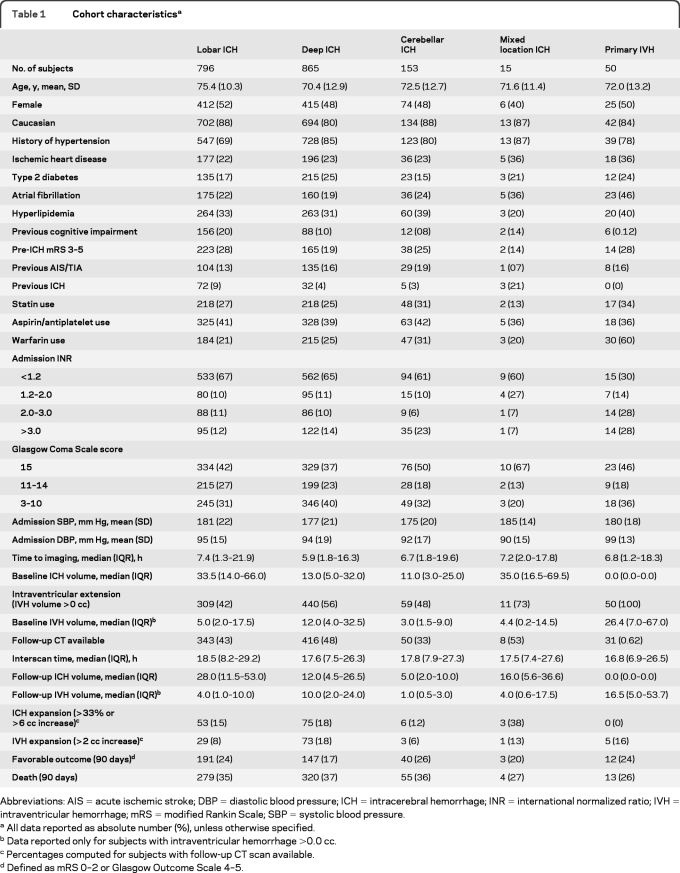
There were 1,956 individuals with primary ICH who presented during the study enrollment period. Of these, 77 were excluded (CT scan not available: n = 12; CT scan poor quality: n = 9; refused consent: n = 20; outcome determination not available: n = 15; admission INR not available: n = 21), yielding 1,879 eligible subjects (table 1). Excluded subjects did not differ from study participants (all p > 0.20) for all demographic, medical history, and medication characteristics.
Table 1.
Cohort characteristicsa
Abbreviations: AIS=acute ischemic stroke; DBP=diastolic blood pressure; ICH=intracerebral hemorrhage; INR=international normalized ratio; IVH=intraventricular hemorrhage; mRS=modified Rankin Scale; SBP=systolic blood pressure.
All data reported as absolute number (%), unless otherwise specified.
Data reported only for subjects with intraventricular hemorrhage >0.0 cc.
Percentages computed for subjects with follow-up CT scan available.
Defined as mRS 0–2 or Glasgow Outcome Scale 4–5.
Among eligible subjects, 819 (44%) had IVH on baseline CT scan, and 479 (26%) were on warfarin at time of ICH. Across ICH in all locations, warfarin use was more frequent (p = 0.008) in patients with IVH (n = 256, 27%) compared to those without (n = 198, 21%). IVH presence was more frequent in mixed location and deep ICH, as opposed to lobar and cerebellar ICH (both p < 0.001). Baseline IVH volumes were larger in patients with primary IVH, followed by deep ICH (both p < 0.001).
Follow-up CT scans were available for 848 subjects (45%): subjects without follow-up CT had lower GCS, higher baseline ICH and IVH volume and rates of warfarin use (all p < 0.05). IVH extension was more frequent in primary IVH and deep ICH compared to ICH in all other locations (both p < 0.05).
IVH.
Lobar ICH.
Of 796 individuals with lobar ICH, 309 (42%) presented with accompanying IVH (table 1). Baseline ICH volume, use of an antiplatelet agent or warfarin were associated with the development of IVH (p < 0.05). In multivariate analyses, only baseline ICH volume independently predicted IVH (p < 0.001). However, multivariate analysis including admission INR as a categorical variable (rather than warfarin use as binary) revealed an association between INR >3.0 and IVH (odds ratio [OR] = 2.14, 95% CI 1.08–4.25, p = 0.021).
Deep ICH.
Among 865 individuals with deep ICH, 440 (56%) had accompanying IVH at presentation (table 1). History of previous ICH, warfarin use, and baseline ICH volume were each associated with IVH (p < 0.05). In multivariate analysis, baseline ICH volume (p < 0.001) and warfarin (OR 1.38, 95% CI 1.07–1.08, p = 0.013) were independently associated with risk of IVH. We also identified associations between IVH and INR >3.0 (OR 1.63, 95% CI 1.12–2.36, p = 0.010).
Cerebellar and mixed ICH.
IVH was present in 59 cerebellar (48%) and 11 mixed location ICH (73%). Only baseline ICH volume was predictive of IVH in both univariate and multivariate analysis for these 2 groups (both p < 0.01). We found no association between IVH and either warfarin or admission INR (all p > 0.20). We therefore did not perform additional analyses in these subgroups.
Baseline IVH volume.
Lobar ICH.
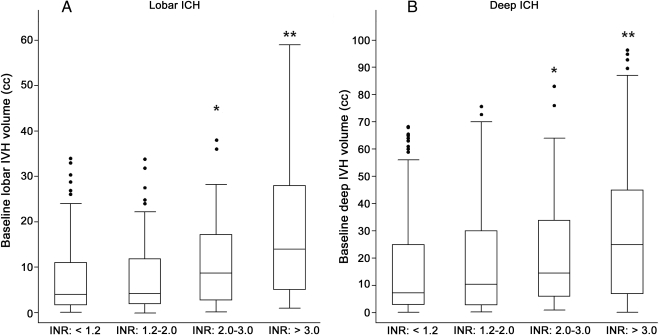
Among 309 lobar ICH with IVH at baseline, OAT and ICH volume were associated with increasing IVH volume (p < 0.001). In multivariate analysis both baseline ICH volume (p = 0.002) and warfarin (β 0.067, p < 0.001) independently increased IVH volume. We estimated that warfarin increased baseline IVH volume by approximately 1.3 mL based on multivariate regression coefficients. Analyses of INR are presented in figure 1A.
Figure 1. International normalized ratio (INR) and baseline intraventricular hemorrhage (IVH) volume.
IVH volumes for lobar ICH (A) and deep ICH (B) are presented and analyzed only when IVH present (IVH volume >0.0 mL). All analyses adjusted for baseline ICH volume. Lobar ICH: p value for comparison across categories (trend-test) = 0.008, deep ICH: p value for comparison across categories (trend-test) = 0.01. * p < 0.05 for comparison with INR <2.0, ** p < 0.01 for comparison with INR <2.0.
Deep ICH.
Among 440 deep ICH subjects with IVH warfarin and baseline ICH volume correlated with higher IVH volume (both p < 0.005). Multivariate analyses identified baseline ICH volume (p = 0.008) and warfarin (β 0.067, p = 0.004) as independent predictors. We estimated IVH volumes to be on average 2.2 mL larger in OAT users, based on multivariate modeling. Analyses of INR are presented in figure 1B.
Primary IVH.
Due to limited sample size (n = 50, table 1), we were not able to identify predictors of baseline primary IVH volume (all p > 0.20 in multivariate analysis). Warfarin was not associated with IVH volume (p > 0.20 in both univariate and multivariate analysis). We therefore did not perform additional analysis in this group.
IVH expansion.
Lobar ICH.
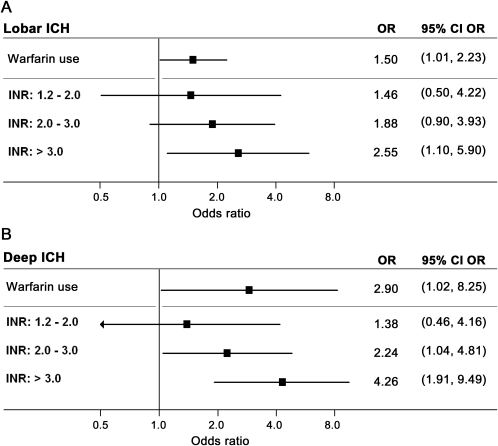
IVH expansion occurred in 29/309 (8%) patients (table 1). In univariate analysis warfarin, history of hypertension, admission systolic blood pressure, baseline ICH volume, baseline IVH volume, and ICH expansion were all associated with IVH expansion (p < 0.01). Multivariate modeling identified baseline ICH volume, baseline IVH volume, ICH expansion (all p < 0.007), and warfarin (OR 1.50, 95% CI 1.01–2.24, p = 0.046) as predictors of IVH expansion. Analyses of INR are presented in figure 2A.
Figure 2. International normalized ratio (INR) and intraventricular hemorrhage (IVH) expansion in lobar and deep intracerebral hemorrhage (ICH).
(A) IVH expansion risk for warfarin users (compared to nonusers) and by INR (compared to INR <1.2) for lobar ICH. p Value for comparison across categories (trend-test) = 0.011. (B) IVH expansion risk for warfarin users (compared to nonusers) and by INR (compared to INR <1.2) for deep ICH. p Value for comparison across categories (trend-test) = 0.006. All multivariate analyses adjusted for baseline ICH volume, baseline IVH volume, and ICH expansion. CI = confidence interval; OR = odds ratio.
Deep ICH.
Follow-up CT analyses identified IVH expansion in 73/440 (18%) subjects (table 1). Warfarin, baseline ICH volume, baseline IVH volume, and ICH expansion were associated with IVH expansion (p < 0.005), while history of hypertension, admission systolic blood pressure, and hyperlipidemia showed trends to association (p < 0.20). In multivariate analysis, baseline ICH volume, baseline IVH volume, ICH expansion (all p < 0.001), and warfarin (OR 2.90, 95% CI 1.02–8.25, p = 0.045) independently predicted subsequent IVH expansion. Analyses of INR are presented in figure 2B.
Functional outcome.
Lobar ICH.
Univariate and multivariate models of functional outcome that included only pre-ICH clinical and demographic data (table e-1 on the Neurology® Web site at www.neurology.org) confirmed that warfarin was associated with decreased probability of favorable outcome (OR 0.36, 95% CI 0.18–0.75, p = 0.006). Inclusion of baseline ICH and IVH data (both p < 0.0001 for association with functional outcome) reduced the strength of the association between OAT and outcome (OR 0.38, 95% CI 0.16–0.87, p = 0.022). Addition of ICH and IVH expansion data completely removed the association between OAT and mRS (OR 0.72, 95% CI 0.26–1.96, p = 0.52). We calculated the contribution of OAT as well as imaging parameters to 90-day outcome (figure e-1) and found that ∼97% of the effect of warfarin was explained by baseline and follow-up ICH and IVH volume data. Separate inclusion of ICH and IVH volume (both at baseline and at follow-up) resulted in the highest predictive performance (AUC = 0.92) of all models in ROC analysis (table e-2).
Deep ICH.
Modeling functional outcome using only pre-ICH clinical and demographic data (table e-3) confirmed an association between warfarin and 90-day unfavorable outcome (OR 0.22, 95% CI 0.07–0.77, p = 0.017). As for lobar ICH, subsequent introduction of baseline and expansion ICH and IVH data canceled the association between warfarin and outcome (OR 0.98, 95% CI 0.32–3.0, p = 0.97). Once again, ∼95% of the observed effect of warfarin on outcome was accounted for by ICH and IVH baseline volumes and expansion (figure e-2). As for lobar ICH, we observed the highest predictive performance for outcome (AUC = 0.91) when IVH and IVH volume and expansion were each included in regression analyses (table e-2).
DISCUSSION
Our results demonstrate that warfarin worsens severity of IVH in the setting of ICH, by increasing risk of its development, as well as its volume at presentation, and risk of subsequent IVH expansion. These data demonstrate that the effect of OAT on IVH plays a substantial role in mediating the impact of warfarin on ICH outcome. Indeed, inclusion of IVH measurements in predictive models of ICH outcome results in significant increase in predictive performance.
Our data suggest that the effect of warfarin on ICH outcome is almost entirely mediated by ICH volume, IVH volume, hematoma expansion, and IVH expansion. We build on previous studies, which identified a role for warfarin in worsening severity of IVH.9,15 The large sample size of our study allowed us to demonstrate a dose-response relationship between intensity of (i.e., INR) and IVH volume as well as demonstrate effects specific to ICH location. Taken together, these findings will allow for more detailed modeling of ICH outcome, particularly in the design of clinical trials. The most effective interventions will have to reduce both ICH and IVH expansion.
Persistence of association between warfarin and IVH after adjustment for ICH volume suggests that both increased pressure from the parenchymal hematoma and perturbation of normal hemostasis contribute to IVH formation and expansion. Warfarin's effect on IVH extension appears to be independent of hematoma growth, a reflection of the dynamic relationship between ICH and IVH. These data support the hypothesis that increased pressure due to hematoma growth does not cease to influence IVH progression after ventricular rupture.14
We used mediation analyses to estimate the independent contribution of warfarin (regardless of intermediate mechanisms) to 90-day outcome, and found that almost the entirety of this effect was mediated by 1) baseline ICH volume; 2) baseline IVH volume; 3) risk of ICH expansion; and 4) risk of IVH expansion. The specific biological phenomena associated with hematoma size and ventricular involvement remain only partially understood, and might include development of hydrocephalus, decreased consciousness, and intraventricular inflammation.16 Our data do not allow us to further address these issues, which are of great relevance in devising effective therapeutic solutions. We do, however, present evidence suggesting that future research should focus on CT volumetric data as important markers of ICH severity, particularly for OAT-ICH.
Our study has limitations. We performed a retrospective analysis of prospectively collected data, thus limiting our ability to standardize study protocol and procedures. A change in study design during the course of the cohort study forced us to use a heterogeneous definition of favorable outcome. Fortunately, this is more likely to have biased our analyses toward a negative finding, as we identified no correlation between GOS vs mRS availability and either ICH location or OAT use. We also observed good correlation between scales in a substantial subset of patients with both scores recorded, further supporting the robustness of our findings. Our study is also subject to unmeasured confounding by indication. Clinical care teams determined whether and when to perform follow-up CT scans, and warfarin-exposed patients did have lower rates of follow-up CT scanning. This imbalance is likely to have, if anything, biased our analysis toward the null hypothesis of no effect of warfarin on IVH expansion. Similarly, the approach to reversal of anticoagulation was at the discretion of the medical team caring for the patient. In order to avoid any bias introduced as a result, we could therefore only study admission INR and were unable to assess the role, if any, of anticoagulation reversal. Finally, despite the large sample size of our study cohort, we had limited statistical power for analyses of the subjects with cerebellar and mixed location ICH, as well as primary IVH.
Supplementary Material
ACKNOWLEDGMENT
The authors thank the clinical coordinators and research staff of the Hemorrhagic Stroke Research Group, Department of Neurology, and the nurses and staff of the Neuroscience Intensive Care Unit, Massachusetts General Hospital.
Glossary
GLOSSARY
- AUC
area under the curve
- CI
confidence interval
- GCS
Glasgow Coma Scale
- GOS
Glasgow Outcome Scale
- ICH
intracerebral hemorrhage
- INR
international normalized ratio
- IVH
intraventricular hemorrhage
- MGH
Massachusetts General Hospital
- mRS
modified Rankin Scale
- OAT
oral anticoagulation therapy
- OR
odds ratio
- ROC
receiver operator characteristic
Footnotes
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
Dr. Biffi: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, statistical analysis. T.W.K. Battey: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, acquisition of data, statistical analysis. A.M. Ayres: drafting/revising the manuscript, acquisition of data. L. Cortellini: drafting/revising the manuscript, study concept or design, acquisition of data, study supervision. K. Schwab: study concept or design, analysis or interpretation of data, acquisition of data, study supervision, obtaining funding. A.J. Gilson: analysis or interpretation of data, acquisition of data, study supervision. Dr. Rost: drafting/revising the manuscript, analysis or interpretation of data, acquisition of data. Dr. Viswanathan: drafting/revising the manuscript. Dr. Goldstein: drafting/revising the manuscript, study concept or design, acquisition of data. Dr. Greenberg: drafting/revising the manuscript, obtaining funding. Dr. Rosand: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, acquisition of data, study supervision.
DISCLOSURE
Dr. Biffi receives research support from the American Heart Association Bugher Foundation. T.W.K. Battey reports no disclosures. A.M. Ayres receives research support from the NIH. L. Cortellini receives research support from the NIH/NINDS. K.M. Schwab receives research support from the NIH (NINDS, NIA). A.J. Gilson reports no disclosures. Dr. Rost serves as Associate Editor for Frontiers in Hospitalist Neurology and Assistant Editor for Stroke and receives research support from the NIH/NINDS, the National Stroke Association, and the American Heart Association–Bugher Foundation. Dr. Viswanathan has served as consultant for Athena Diagnostics, Inc. and receives research support from the NIH/NIA. Dr. Goldstein serves on a scientific advisory board for and received funding for travel and consulting honoraria from CSL Behring; serves as an Associate Editor for Academic Emergency Medicine and on the editorial board of the International Journal of Emergency Medicine; and receives research support from CSL Behring and the NIH/NINDS. Dr. Greenberg serves on a data safety monitoring board for the NIH/NINDS; has received speaker honoraria from Esteve, Medtronics, Inc., and Pfizer Inc; serves on the editorial boards of Neurology®, Stroke, Cerebrovascular Disease, and the Journal of Alzheimer's Disease and Other Dementias; has served as a consultant for Roche, Janssen Alzheimer Immunotherapy, and Bristol-Myers Squibb; and has received/receives research support from the NIH and the Alzheimer's Association. Dr. Rosand receives research support from the NIH (NINDS, NHLBI), the American Heart Association–Bugher Foundation, and the MGH Deane Institute for Integrative Research in Atrial Fibrillation and Stroke.
REFERENCES
- 1. van Asch CJ, Luitse MJ, Rinkel GJ, van der Tweel I, Algra A, Klijn CJ. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol 2010; 9: 167–176 [DOI] [PubMed] [Google Scholar]
- 2. Broderick JP, Brott TG, Duldner JE, Tomsick T, Huster G. Volume of intracerebral hemorrhage: a powerful and easy-to-use predictor of 30-day mortality. Stroke 1993; 24: 987–993 [DOI] [PubMed] [Google Scholar]
- 3. Hanley DF. Intraventricular hemorrhage: severity factor and treatment target in spontaneous intracerebral hemorrhage. Stroke 2009; 40: 1533–1538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dowlatshahi D, Demchuk AM, Flaherty ML, Ali M, Lyden PL, Smith EE. on behalf of the VISTA Collaboration Defining hematoma expansion in intracerebral hemorrhage: relationship with patient outcomes. Neurology Epub 2011 Feb 23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Steiner T, Schneider D, Mayer S, et al. Dynamics of intraventricular hemorrhage in patients with spontaneous intracerebral hemorrhage: risk factors, clinical impact, and effect of hemostatic therapy with recombinant activated factor VII. Neurosurgery 2006; 59: 767–773 [DOI] [PubMed] [Google Scholar]
- 6. Rosand J, Eckman MH, Knudsen KA, Singer DE, Greenberg SM. The effect of warfarin and intensity of anticoagulation on outcome of intracerebral hemorrhage. Arch Intern Med 2004; 164: 880–884 [DOI] [PubMed] [Google Scholar]
- 7. Steiner T, Bösel J. Options to restrict hematoma expansion after spontaneous intracerebral hemorrhage. Stroke 2010; 41: 402–409 [DOI] [PubMed] [Google Scholar]
- 8. Flibotte JJ, Hagan N, O'Donnell J, Greenberg SM, Rosand J. Warfarin, hematoma expansion, and outcome of intracerebral hemorrhage. Neurology 2004; 63: 1059–1064 [DOI] [PubMed] [Google Scholar]
- 9. Zubkov AY, Mandrekar JN, Claassen DO, Manno EM, Wijdicks EF, Rabinstein AA. Predictors of outcome in warfarin-related intracerebral hemorrhage. Arch Neurol 2008; 65: 1320–1325 [DOI] [PubMed] [Google Scholar]
- 10. Flaherty ML, Tao H, Haverbusch M, et al. Warfarin use leads to larger intracerebral hematomas. Neurology 2008; 71: 1084–1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. FitzMaurice E, Wendell L, Snider R, et al. Effect of statins on intracerebral hemorrhage outcome and recurrence. Stroke 2008; 39: 2151–2154 [DOI] [PubMed] [Google Scholar]
- 12. Thompson BB, Béjot Y, Caso V, et al. Prior antiplatelet therapy and outcome following intracerebral hemorrhage: a systematic review. Neurology 2010; 75: 1333–1342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kaufman JS, Maclehose RF, Kaufman S. A further critique of the analytic strategy of adjusting for covariates to identify biologic mediation. Epidemiol Perspect Innov 2004; 1: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hallevi H, Albright KC, Aronowski J, et al. Intraventricular hemorrhage: anatomic relationships and clinical implications. Neurology 2008; 70: 848–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zubkov A, Claassen DO, Rabinstein AA. Warfarin-associated intraventricular hemorrhage. Neurol Res 2007; 29: 661–663 [DOI] [PubMed] [Google Scholar]
- 16. Bhattathiri PS, Gregson B, Prasad KS, Mendelow AD. Intraventricular hemorrhage and hydrocephalus after spontaneous intracerebral hemorrhage: results from the STICH trial. Acta Neurochir Suppl 2006; 96: 65–68 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.