Abstract
Objective
To estimate the association between vitamin D deficiency and bacterial vaginosis (BV) among nonpregnant HIV-infected and uninfected women.
Methods
In a substudy of the Women's Interagency HIV Study, including women from Chicago and New York, the association between BV and vitamin D deficiency, demographics, and disease characteristics was tested using generalized estimating equations. Deficiency was defined as <20 ng/mL 25 (OH) vitamin D and insufficiency as >20 and ≤30 ng/mL. BV was defined by the Amsel criteria.
Results
Among 602 observations of nonpregnant women (480 HIV infected and 122 uninfected), BV was found in 19%. Vitamin D deficiency was found in 59.4%, and insufficiency was found in 24.4%. In multivariable analysis, black race was the most significant predictor of BV (adjusted odds ratio [AOR] 5.90, (95% confidence interval [CI] 2.52-13.8). Vitamin D deficiency was independently associated with BV among HIV-infected women (AOR 3.12, 95% CI 1.16-8.38) but not among HIV-uninfected women. There was a negative linear correlation between vitamin D concentration and prevalence of BV in HIV-infected women (r=−0.15, p=0.001).
Conclusions
Vitamin D deficiency was very common in this cohort and significantly associated with BV among HIV-infected women. These preliminary findings suggest that further epidemiologic and mechanistic exploration of the relationship between vitamin D and BV in HIV-infected women is warranted.
Introduction
Bacterial vaginosis (BV) is the most frequent cause of vaginitis in U.S. women.1 BV involves a shift in vaginal flora from a predominance of hydrogen peroxide-producing Lactobacillus species to a polymicrobial flora with a higher proportion of anaerobes and high pH. Although usually causing only discomfort, BV is associated with serious morbidities, including postsurgical gynecologic infection and among pregnant women, premature delivery.2 In addition, BV is associated with increased susceptibility to sexually transmitted infections (STI), including HIV, gonorrhea, Chlamydia infection, and herpes simplex virus.3–5
A number of demographic and behavioral factors have been found to be associated with the prevalence of BV, including age, race, number of sex partners, menopausal status, smoking, alcohol use, and use of hormonal contraception.6 It has long been observed that African American women have a higher incidence of BV than white women, and this observation has been found to be independent of behavioral factors, such as number of sex partners and douching. Investigators from the University of Pittsburgh observed an association between vitamin D deficiency and BV. Among 489 pregnant women, the authors found a strong association between BV and vitamin D concentration <50 nmol/mL, with a dose-response relationship. They postulated that this association could explain, in part, the racial disparity in the prevalence and incidence of BV.7 This finding was replicated in a small study of pregnant HIV-uninfected adolescents,8 and a large study from National Health and Nutrition Examination Survey (NHANES) confirmed a correlation between vitamin D deficiency and BV among pregnant women.9 For nonpregnant women, the NHANES investigators found no relationship between vitamin D deficiency and BV in the overall multivariate model. They found, however, that vitamin D deficiency moderated the association between smoking and BV; among vitamin D-deficient women, but not among women with sufficient vitamin D levels, smoking was associated with BV.
Among HIV-infected women, BV has been associated with increased genital shedding of HIV RNA and increased persistence of BV.10,11 We sought to explore the relationship between BV and vitamin D deficiency, as a theoretically modifiable risk factor, in nonpregnant HIV-infected and uninfected women participating in the Women's Interagency HIV Study (WIHS).
Materials and Methods
The WIHS is a longitudinal study of HIV-infected and uninfected at-risk women at six sites: Chicago, San Francisco Bay Area, Brooklyn and Bronx/Manhattan, New York, Washington, DC, and Los Angeles. Women are seen semiannually for an interview, physical examination, and collection of blood and genital specimens. Informed consent was obtained from all participants in accordance with the US Department of Health and Human Services guidelines and the institutional review boards of participating institutions. The cohort was designed to reflect the demographics of the HIV epidemic among women in the United States. Details of cohort recruitment, retention, and demographics are published elsewhere.12,13 We report here a cross-sectional substudy including 434 observations of women from the Chicago and 168 from the Bronx/Manhattan sites of the WIHS. Vitamin D measurements had been performed on 475 women in the context of two studies. The Chicago site performed a prevalence study, measuring vitamin D from the most recent semiannual winter visit for all Chicago participants with available serum (median date 2007, inter quartile range [IQR] 2007–2008, range 2002–2008). Vitamin D levels had been determined previously in the context of a metabolic substudy including women from Bronx/Manhattan and Chicago WIHS sites (median date 2003, IQR 2002–2003, range 2001–2004); the study included premenopausal women without diabetes who were not using corticosteroids, exogenous sex steroids, or bisphosphonates. Vitamin D levels from the Bronx/Manhattan site were determined from visits throughout the year. One hundred twenty seven Chicago women were included in both studies. The vitamin D measurements for these women were performed a median of 5 years apart (range 1–7 years). Pregnant women were excluded from the current study.
BV was defined by the Amsel criteria, determined routinely at each WIHS visit, with the presence of three of four of the following: abnormal vaginal discharge, amine odor with addition of 10% KOH to vaginal secretions, clue cells on wet mount of vaginal secretions, and pH of vaginal discharge>4.5.14 The BV diagnoses were made by experienced gynecologic clinicians (MDs, nurse practitioners, and physician assistants) trained in physical examination and microscopy. Certification for BV diagnosis in WIHS involves didactic then observation of physical examination and microscopy by an expert clinician/microscopist. At WIHS onset, a training DVD was also used to teach microscopy.
Vitamin D levels for the Chicago prevalence study were measured using the liquid chromatography, tandem mass spectrometry (LC-MS/MS) method. Specimens from the metabolic study were measured by high performance liquid chromatography (HPLC) using ultraviolet. Both methods are sensitive and specific for detecting both forms of 25-hydroxyvitamin D (OH)D (25(OH)D2 and 25(OH)D3), with a strong correlation between results obtained from the two methods: correlation coefficient 0.997 for 25(OH)D2 and 0.987 for 25(OH)D3 over a range of vitamin D values from 3.2 to 105 μg/L.15 Vitamin D deficiency was defined as 25(OH)D ≤20 ng/ml, vitamin D insufficiency was defined as levels >20 and ≤30 ng/mL, and sufficient vitamin D was defined as >30 ng/mL. A vitamin D level of 20 ng/mL is equivalent to 49.92 nmol/mL, the units used in the Bodnar study7 and many European studies of vitamin D; the conversion factor is 2.496.
Statistical methods
Data in the demographic table (Table 1) are for each individual woman and include data from the first visit for women who contributed two observations to the data. In all analyses, data for time-varying covariates were from the same visit as the vitamin D measurement and include presence of BV, age, CD4+ T lymphocyte count (CD4 count), HIV RNA level, use of highly active antiretroviral therapy (HAART), smoking and alcohol use (by self-report), menopausal status (defined by self-report of cessation of menstrual periods for >1 year), use of hormonal contraceptives, genital HPV infection (defined by detection of HPV DNA in cervicovaginal fluid using L1 consensus primer MY09/MY11/HMB01 PCR assay16), and number of sexual partners in the last 6 months. The Pearson correlation coefficient was used to measure correlation. To control for repeated measurements within women, the crude association between BV and covariates was assessed using generalized estimating equation (GEE) adjusted odds ratios (AORs) and 95% confidence intervals (CI). Multivariable GEE models were fit to assess the adjusted association between vitamin D and BV using ORs and 95% CI. All variables that were associated with BV in the univariate model and factors associated with BV in the literature were included in preliminary multivariate models. Variables that were significant in the final model or modified the association between vitamin D and BV by 10% were included in the final model. All analyses were performed in SAS software, version 9.2 (SAS Institute Inc, Cary NC).
Table 1.
Demographics of 475 HIV-Infected and Uninfected Women Included in Study
| Characteristic | Number (%) |
|---|---|
| HIV infected | 353 (74.3) |
| HIV uninfected | 122 (25.7) |
| Race/ethnicity | |
| Non-Hispanic white | 115 (24.2) |
| Non-Hispanic black | 313 (65.9) |
| Other | 47 (9.9) |
| Age <40 | 246 (51.8) |
| Vitamin D status | |
| Deficient (≤20 ng/mL) | 282 (59.4) |
| Insufficient (>20–30 ng/mL) | 116 (24.4) |
| Sufficient (>30 ng/mL) | 77 (16.2) |
| Postmenopausal | 58 (12.2) |
| Educational attainment | |
| Less than high school | 181 (38.1) |
| Completed high school | 132 (27.8) |
| Some college | 162 (34.1) |
| Current smoker | 266 (56.0) |
| Alcohol use | |
| Abstainer | 244 (51.4) |
| Light (<3 drinks/week) | 127 (26.7) |
| Moderate-heavy (≥3 drinks/week) | 104 (21.9) |
| Number of sexual partners in last 6 months | |
| None | 136 (28.7) |
| 1 | 280 (59.0) |
| 2 | 38 (8.0) |
| ≥3 | 21 (4.4) |
| Recent use of hormonal contraceptives | 33 (7.0) |
| Genital human papillomavirus | 176 (37.1) |
| Antibiotics in last 6 months | 37 (7.8) |
Results
Among 475 WIHS participants, 602 observations were made, including 480 among HIV-infected and 122 among uninfected women. Demographic, behavioral, and disease characteristics of the 475 women are presented in Table 1. Vitamin D deficiency was common; 59.4% of women had deficient levels (≤20 ng/mL) of vitamin D, and an additional 24.4% had levels defined as insufficient (>20–30 ng/mL). In a univariate analyses that included age, HIV serostatus, body mass index (BMI), socioeconomic status (SES), HIV therapy, hepatitis C status, CD4 count, HIV RNA level, and WIHS site (Chicago vs Bronx/Manhattan), only race was significantly associated with vitamin D deficiency; 267 of 396 (67.4%) observations among black women vs. 59 of 149 (39.6%) among white women (OR 3.15, CI 2.14-4.67) were consistent with vitamin D deficiency (data not shown).
BV was found in 19% of observations overall, 17.3 % among HIV-infected women and 25.4% among HIV-uninfected women (OR 0.62, CI 0.38-1.01, p=0.05). Table 2 shows the prevalence of vitamin D deficiency and correlates of BV for the 480 observations from HIV-infected women. Demographic and disease characteristics associated with BV by univariate analysis were vitamin D deficiency, vitamin D insufficiency, black race, age <40, higher number of sexual partners, premenopausal status, lower educational attainment, higher levels of alcohol use, lower CD4 count, and higher HIV RNA. The factors associated with BV in multivariable analysis included black race, having one or two sexual partners vs. sexual abstinence, and lower educational attainment. Vitamin D deficiency was significantly associated with BV in HIV-infected women (OR 3.12, CI 1.16-8.38). The analysis was performed for HIV-uninfected women only (Table 3). Only moderate-heavy alcohol use was significantly associated with BV; the influence of race could not be quantified because of sample size. Vitamin D deficiency was not associated with BV among the HIV-uninfected women.
Table 2.
Factors Associated with Bacterial Vaginosis Among 480 Observations of HIV-Infected Women
| |
|
GEE adjusted |
Multivariable |
||||
|---|---|---|---|---|---|---|---|
| Characteristic (n) | BV n (%) | OR | 95% CI | p | Adjusted OR | 95% CI | p |
| Race | |||||||
| Non-Hispanic white (121) | 7 (5.8) | 1.00 | 1.00 | ||||
| Non-Hispanic black (315) | 75 (23.81) | 4.94 | 2.19-11.17 | 0.0001 | 3.53 | 1.45-8.63 | 0.006 |
| Other (44) | 1 (2.27) | 0.41 | 0.05-3.44 | 0.41 | 0.5 | 0.39-3.22 | 0.36 |
| Vitamin D, ng/mL | |||||||
| >30 (86) | 5 (5.81) | 1.00 | 1.00 | ||||
| >20–30 (108) | 16 (14.81) | 2.56 | 1.06-6.15 | 0.04 | 2.20 | 0.79-6.17 | 0.13 |
| ≤20 (286) | 62 (21.68) | 4.11 | 1.79-9.49 | 0.001 | 3.12 | 1.16-8.38 | 0.02 |
| Sex partners in last 6 months | |||||||
| 0 (162) | 13 (8.02) | 1.00 | 1.00 | ||||
| 1 (272) | 56 (20.59) | 2.94 | 1.59-5.44 | 0.0006 | 2.30 | 1.1.13-4.67 | 0.02 |
| 2 (31) | 9 (29.03) | 4.29 | 1.79-10.31 | 0.001 | 3.65 | 1.33-10.04 | 0.01 |
| >2 (15) | 5 (33.33) | 4.70 | 1.20-18.41 | 0.026 | 3.32 | 0.77-14.36 | 0.11 |
| Age <40 years (206) | 46 (22.33) | 1.00 | 0.82 | 0.46-1.45 | 0.49 | ||
| Age ≥40 years (274) | 37 (13.50) | 0.61 | 0.37-0.99 | 0.04 | 1.00 | ||
| Current smoker (252) | 53 (21.03) | 1.55 | 0.93-2.57 | 0.09 | |||
| Alcohol consumption | |||||||
| Abstinent (267) | 41 (15.36) | 1.00 | 1.00 | ||||
| Light (<3 drinks/week) (128) | 21 (16.41) | 1.10 | 0.60-2.00 | 0.77 | 1.43 | 0.73-2.76 | 0.29 |
| Moderate-heavy (≥3 drinks/week) (85) | 21 (24.71) | 1.76 | 1.03-2.98 | 0.004 | 1.53 | 0.83-2.83 | 0.17 |
| Educational attainment | |||||||
| Less than high school (186) | 43 (23.12) | 3.69 | 1.80-7.55 | 0.0004 | 3.32 | 1.56-7.06 | 0.002 |
| Completed high school (125) | 28 (22.40) | 3.36 | 1.56-7.22 | 0.002 | 3.17 | 1.44-6.99 | 0.004 |
| Some college (169) | 12 (7.10) | 1.00 | 1.00 | ||||
| Current use of hormonal contraceptives (31) | 4 (12.90) | 0.70 | 0.25-1.94 | 0.50 | |||
| Use of antibiotics in last 6 months (31) | 5 (16.13) | 1.07 | 0.48-2.35 | 0.87 | |||
| Postmenopausal (89) | 6 (6.74) | 0.36 | 0.16-0.81 | 0.01 | 0.42 | 0.15-1.15 | 0.09 |
| HPV infection (215) | 38 (17.67) | 0.99 | 0.59-1.66 | 0.97 | |||
| HAART use | |||||||
| No (280) | 56 (20.0) | ||||||
| Yes (200) | 27 (13.5) | 0.68 | 0.45-1.05 | 0.08 | |||
| CD4 count | |||||||
| >500 (163) | 23 (14.1) | 1.00 | 1.0 | ||||
| 200–500 (213) | 34 (16.0) | 1.13 | 0.64-2.01 | 0.67 | 1.19 | 0.62-2.26 | 0.60 |
| <200 (104) | 26 (25.0) | 1.98 | 1.07-3.67 | 0.03 | 1.89 | 0.94-3.80 | 0.08 |
| Mean log HIV RNA | 8.42 vs 7.32 | 1.11a | 1.03-1.19 | 0.001 | |||
For linear values, statistics are t tests.
CI, confidence interval; GEE, generalized estimating equation; HAART, highly active antiretroviral therapy; HPV, human papillomavirus.
Table 3.
Factors Associated with Bacterial Vaginosis Among 122 Observations of HIV Uninfected Women
| |
|
GEE adjusted |
Multivariable |
||||
|---|---|---|---|---|---|---|---|
| Characteristic (n) | BV n (%) | OR | 95% CI | p | Adjusted OR | 95% CI | p |
| Race | |||||||
| Non-Hispanic white (28) | 0 (0) | 1.00 | |||||
| Non-Hispanic black (81) | 28 (34.57) | N/A | |||||
| Other (13) | 3 (23.08) | ||||||
| Vitamin D, ng/mL | |||||||
| >30 (14) | 3 (21.43) | 1.00 | 1.00 | ||||
| >20–30 (34) | 7 (20.59) | 0.95 | 0.21-4.36 | 0.95 | 0.39 | 0.07-2.18 | 0.29 |
| ≤20 (74) | 21 (28.38) | 1.45 | 0.37-5.73 | 0.59 | 1.05 | 0.22-5.10 | 0.95 |
| Sex partners in last 6 months | |||||||
| 0 (29) | 5 (17.24) | 1.00 | 1.00 | ||||
| 1 (74) | 20 (27.03) | 1.78 | 0.60-5.30 | 0.30 | 2.03 | 0.54-7.69 | 0.30 |
| 2 (11) | 3 (27.27) | 1.80 | 0.35-9.28 | 0.48 | 1.73 | 0.23-12.98 | 0.59 |
| >2 (8) | 3 (37.50) | 2.88 | 0.51-16.17 | 0.23 | 2.55 | 0.37-17.46 | 0.34 |
| Age<40 years (67) | 19 (28.36) | 1.00 | 0.53 | 0.18-1.51 | 0.23 | ||
| Age≥40 years (55) | 12 (21.82) | 0.71 | 0.31-1.62 | 0.41 | 1.00 | ||
| Current smoker (74) | 22 (29.73) | 1.83 | 0.76-4.42 | 0.18 | |||
| Alcohol consumption | |||||||
| Abstinent (53) | 10 (18.87) | 1.00 | 1.00 | ||||
| Light (<3 drinks/week) (34) | 7 (20.59) | 1.11 | 0.38-3.28 | 0.84 | 1.17 | 0.37-3.69 | 0.79 |
| Moderate-heavy (≥3 drinks/week) (35) | 14 (40.0) | 2.87 | 1.09-7.52 | 0.032 | 3.90 | 1.30-11.72 | 0.02 |
| Educational attainment | |||||||
| Less than high school (41) | 10 (24.39) | 1.13 | 0.41-3.07 | 0.81 | 1.32 | 0.43-4.08 | 0.63 |
| Completed high school (36) | 11 (30.56) | 1.54 | 0.57-4.18 | 0.40 | 2.23 | 0.77-6.48 | 0.14 |
| Some college (45) | 10 (22.22) | 1.00 | 1.00 | ||||
| Current use of hormonal contraceptives (8) | 1 (12.50) | 0.40 | 0.05-3.39 | 0.40 | |||
| Use of antibiotics in last 6 months (15) | 3 (20.0) | 0.71 | 0.19-2.68 | 0.61 | |||
| Postmenopausal (14) | 3 (21.43) | 0.78 | 0.20-2.80 | 0.72 | 2.03 | 0.31-13.48 | 0.46 |
| HPV infection (22) | 3 (13.64) | 0.41 | 0.11-1.48 | 0.17 | |||
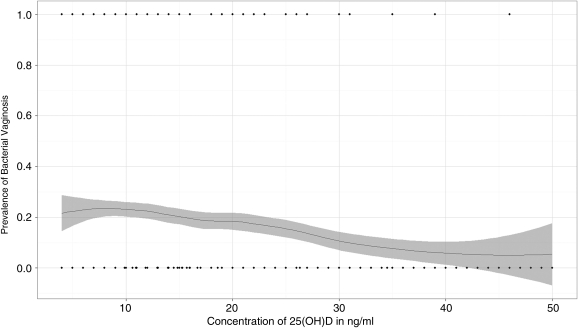
Vitamin D level showed a significant negative linear correlation with prevalence of BV among all women (p<0.001, r=−0.14) and among HIV-infected women (p=0.001, r=−0.15) (Fig. 1).
FIG. 1.
Correlation between prevalence of bacterial vaginosis and 25(OH) vitamin D concentration among HIV-infected women (r=−0.15, p=0.001).
Discussion
In this large cohort of HIV infected and uninfected women, we found that vitamin D deficiency was very common and independently associated with BV among HIV-infected women; 59% of women studied had vitamin D deficiency, and an additional 24% had insufficient levels of vitamin D.
HIV-infected women deficient in vitamin D were more than three times as likely by multivariable analysis to have BV compared to HIV-infected women with sufficient vitamin D levels. In HIV-uninfected women, we found no association between vitamin D deficiency and BV, although the number of observations was relatively small. The mechanism by which vitamin D deficiency may increase susceptibility to BV is not clear. The classic infections associated with vitamin D deficiency are mycobacterial diseases.17 The mechanism underlying this association is thought to be the role of vitamin D in regulating the production and function of innate antimicrobial defense molecules, such as cathelicidin, a neutrophil degradation product found in the female genital tract.18 Cathelidicin has antibacterial properties and may influence susceptibility to intracellular as well as extracellular pathogens; the molecule is found in granules in the neutrophils that are destined for exocytosis.19 Vitamin D receptors are found in many human tissues and have been found to be an important regulator of host immune response to bacterial infections, such as dental infections and lower respiratory tract infections.20 Vitamin D is also linked to cytokine expression in a number of disease states and in vitro model systems.21 In HIV infection specifically, our understanding of the effect of vitamin D on immune function is rapidly evolving but far from clear.22 Vitamin D deficiency was associated with lower CD4 count in one study of postmenopausal women,23 but other studies have not confirmed the association.24,25
Women with HIV were less likely to have BV, but among HIV-infected women, those with a lower CD4 count were more likely to have BV. These apparently contradictory findings are explained, we believe, by two separate phenomena. Women with HIV are more likely to be sexually abstinent and when they do have intercourse, are more likely to use condoms.26 However, HIV infection, particularly immunosuppression defined by a CD4 count <200, is associated with more persistent infection once BV occurs.11
This study is one of several reporting an association between BV and specific nutritional deficiencies or nutritional status. The current study and all such studies need to be interpreted with the recognition that nutritional deficiencies rarely occur in isolation and are often a reflection of poor health status overall, which in turn may reflect poverty and other social or economic adversity. BV has been found to be associated with iron deficiency among pregnant women,27 lower concentrations of vitamins A, C, E, and beta carotene among HIV-infected and uninfected women in the HIV Epidemiology Research Study (HERS) study,28 higher intake of dietary fat and lower intake of vitamin E, folate, and protein among nonpregnant women,29 and lower BMI in a cohort of nonpregnant women in India.30 We did not have diet history or levels of other nutrients available to aid in the interpretation of our data from the WIHS participants.
Vitamin D deficiency may partially explain the higher rates of BV observed in black women compared to white women. Racial discrepancies in the prevalence of BV have long been observed but are not fully explained and have been found to be independent of markers of SES, sexual practices, and other behaviors, such as smoking and douching.31 Several biologic differences between black and white women may be relevant to this racial disparity. Differences in genital cytokine expression have been found between black and white pregnant women with normal flora,32 and differences in coordinated regulation of the local genital immune response have also been reported.33 BV prevalence and incidence has been found to be associated with higher chronic stress levels,34 and there may be genetic differences across ethnicities in stress-related genes that affect BV prevalence.35 Whether there is a relationship between vitamin D deficiency and these racial differences in immune response is unclear and requires further mechanistic study.
Limitations
This is a cross-sectional study; confirmation of these findings will require larger longitudinal epidemiologic studies. BV in our study was defined by the Amsel criteria, which is not the gold standard diagnostic test. When compared against the gold standard Nugent gram stain, Amsel criteria are found to be 36%–70% sensitive and 94%–98% specific, with the lower sensitivity reported in a previous study in the WIHS.36,37 Because of the specificity of the Amsel criteria, we are confident that the vast majority of cases are true cases. However, the lack of sensitivity implies that cases of BV were missed and could have biased our analysis; thus, our findings should be considered preliminary. For the women participating in the metabolic substudy, vitamin D measurements were performed in the context of a substudy looking at bone health. The inclusion criteria for this study (lack of diabetes, corticosteroids, or bisphosphonates medications) would not be expected to alter the relationship between BV and vitamin D deficiency. Because the population was not chosen randomly, however, there may be unanticipated effects for which we have not controlled.
Conclusions
In this cross-sectional study of nonpregnant HIV-infected and uninfected women, BV and vitamin D deficiency were both common. Vitamin D deficiency was significantly correlated with BV among HIV-infected women, with a trend toward correlation among all women. The relationship between vitamin D and BV, if it is confirmed by larger longitudinal or mechanistic studies, may partially explain the increased incidence of BV among black women and may be a modifiable risk factor for the disorder. Further study is needed to determine if nutritional or behavioral interventions aimed at increasing vitamin D levels will decrease the occurrence of BV.
Acknowledgments
This report was presented at the 17th Conference on Retroviruses and Opportunistic Infections, San Francisco, 2010.
Data in this article were collected by the Women's Interagency HIV Study (WIHS) Collaborative Study Group. The WIHS is funded by the National Institute of Allergy and Infectious Diseases (UO1-AI-35004, UO1-AI-31834, UO1-AI-34994, UO1-AI-34989, UO1-AI-34993, and UO1-AI-42590) and by the National Institute of Child Health and Human Development (UO1-HD-32632). The study is cofunded by the National Cancer Institute, the National Institute on Drug Abuse, and the National Institute on Deafness and Other Communication Disorders. Funding is also provided by the National Center for Research Resources (MO1-RR-00071, MO1-RR-00079, MO1-RR-00083). This study was also supported by the Chicago Developmental Center for AIDS Research (P30-A1-082151).
Disclosure Statement
The authors have no financial or other conflict of interest with regard to the content of this article.
References
- 1.Marrazzo JM. Martin DH. Watts DH, et al. Bacterial vaginosis: Identifying research gaps. Sex Transm Dis. 2010;37:732–744. doi: 10.1097/OLQ.0b013e3181fbbc95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leitich H. Bodner-Adler B. Brunbauer M. Kaider A. Egarter C. Husslein P. Bacterial vaginosis is a risk factor for preterm delivery: A meta-analysis. Am J Obstet Gynecol. 2003;189:139–147. doi: 10.1067/mob.2003.339. [DOI] [PubMed] [Google Scholar]
- 3.Martin HL. Richardon BA. Nyange PM, et al. Vaginal lactobacilli, microbial flora and risk of HIV-1 and sexually transmitted disease acquisition. J Infect Dis. 1999;180:1863–1868. doi: 10.1086/315127. [DOI] [PubMed] [Google Scholar]
- 4.Myer L. Denny L. Telerant R, et al. Bacterial vaginosis and susceptibility to HIV infection in South African women: A nested case-control study. J Infect Dis. 2005;192:1372–1380. doi: 10.1086/462427. [DOI] [PubMed] [Google Scholar]
- 5.Wiesenfeld HC. Hillier SL. Krohn MA, et al. Bacterial vagnosis is a strong predictor of Neisseria gonorrhoeae and Chlamydia trachomatis infection. Clin Infect Dis. 2003;36:663–668. doi: 10.1086/367658. [DOI] [PubMed] [Google Scholar]
- 6.Watts DH. Springer G. Minkoff H, et al. The occurrence of vaginal infections among HIV-infected and high risk HIV-uninfected women. J Acquir Immune Defic Syndr. 2006;43:161–168. doi: 10.1097/01.qai.0000242448.90026.13. [DOI] [PubMed] [Google Scholar]
- 7.Bodnar LM. Krohn MA. Simhan HN. Maternal vitamin D deficiency is associated with bacterial vaginosis in the first trimester of pregnancy. J Nutr Epidemiol. 2009;139:1157–1161. doi: 10.3945/jn.108.103168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis LM. Chang SC. Mancini J. Nathanson MS. Witter FR. O'Brien KO. Vitamin D insufficiency is prevalent among pregnant African American adolescents. J Pediatr Adolesc Gynecol. 2010;23:45–52. doi: 10.1016/j.jpag.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 9.Hensel KJ. Tandis TM. Gelber SE. Ratner AJ. Pregnancy-specific association of vitamin D deficiency and bacterial vaginosis. Am J Obstet Gynecol. 2011;204:41.e1–e9. doi: 10.1016/j.ajog.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 10.Sha BE. Zarriffard R. Wang QJ, et al. Female genital-tract HIV load correlates inversely with Lactobacillus species but positively with bacterial vaginosis and Mycoplasma hominis. J Infect Di. 2005;191:25–32. doi: 10.1086/426394. [DOI] [PubMed] [Google Scholar]
- 11.Jamieson DJ. Duerr A. Klein RS. Longitudinal analysis of bacterial vaginosis: Findings from the HIV Epidemiology Research Study. Obstet Gynecol. 2001;98:656–663. doi: 10.1016/s0029-7844(01)01525-3. [DOI] [PubMed] [Google Scholar]
- 12.Hessol NA. Schneider M. Greenblatt RM, et al. Retention of women enrolled in a prospective study of HIV infection: Impact of race, unstable housing and use of HIV therapy. Am J Epidemiol. 2001;154:563–573. doi: 10.1093/aje/154.6.563. [DOI] [PubMed] [Google Scholar]
- 13.Barkan SE. Melnick SL. Preston-Martin S, et al. The Women's Interagency HIV Study. Epidemiology. 1998;9:117–125. [PubMed] [Google Scholar]
- 14.Amsel R. Totten PA. Spiegal CA. Chen KC. Eshenbach D. Holmes KK. Nonspecific vaginitis. Diagnositic criteria and microbial and epidemiologic associations. Am J Med. 1983;74:14–22. doi: 10.1016/0002-9343(83)91112-9. [DOI] [PubMed] [Google Scholar]
- 15.Lensmeyer GL. Wiebe DA. Binkley N. Drezner MK. High-performing HPLC methods for 25-hydroxyvitamin D measurement: Comparison with contemporary assays. Clin Chem. 2006;52:1120–1126. doi: 10.1373/clinchem.2005.064956. [DOI] [PubMed] [Google Scholar]
- 16.Strickler HD. Burk RD. Fazzari M, et al. Natural history and possible reactivation of human papillomavirus in HIV-positive women. J Natl Cancer Inst. 2005;97:577–586. doi: 10.1093/jnci/dji073. [DOI] [PubMed] [Google Scholar]
- 17.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 18.Cole AM. Innate host defense of human vaginal and cervical mucosae. Curr Top Microbiol Immunol. 2006;306:199–230. [PubMed] [Google Scholar]
- 19.Valore EV. Park CH. Igreti SL. Ganz T. Antimicrobial components of vaginal fluid. Am J Obstet Gynecol. 2002;187:561–568. doi: 10.1067/mob.2002.125280. [DOI] [PubMed] [Google Scholar]
- 20.Chesney RW. Vitamin D and the Magic Mountain: The anti-infectious role of the vitamin. J Pediatr. 2010;156:698–703. doi: 10.1016/j.jpeds.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 21.Schleitohoff SS. Zitterman A. Tendric G. Berthold HK. Stehle P. Koerfer R. Vitamin D supplementation improves cytokine profiles in patients with congestive heart failue: A double-blind randomized placebo-controlled trial. Am J Clin Nutr. 2006;83:754–759. doi: 10.1093/ajcn/83.4.754. [DOI] [PubMed] [Google Scholar]
- 22.Overton ET. Yin MT. The rapidly evolving research on vitamin D among HIV-infected populations. Curr Infect Dis Rep. 2011;13:83–93. doi: 10.1007/s11908-010-0144-x. [DOI] [PubMed] [Google Scholar]
- 23.Stein EM. Yin MT. McMahon DJ, et al. Vitamin D deficiency in HIV-infected post-menopausal Hispanic and African-American women. Osteoporos Int. 2011;22:477–487. doi: 10.1007/s00198-010-1299-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Den Bout-Van Den Beukel CJ. Fievez L. Michels M, et al. Vitamin D deficiency among HIV-infected individuals in The Netherlands; Effects of antiretroviral therapy. AIDS Res Hum Retrovir. 2008;24:1375–1382. doi: 10.1089/aid.2008.0058. [DOI] [PubMed] [Google Scholar]
- 25.Arpadi SM. MeMahon D. Abransm EJ, et al. Effect of bimonthly supplementation with oral cholecalcifereol on serum 25-hyroxyvitamin concentrations in HIV-infected children and adolescents. Pediatrics. 2009;123:121–126. doi: 10.1542/peds.2008-0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilson TE. Massad LS. Riester KA, et al. Sexual, contraceptive and drug use behaviors of women with HIV and those at high risk for infection: Results from the Women's Interagency HIV Study. AIDS. 1999;13:591–598. doi: 10.1097/00002030-199904010-00008. [DOI] [PubMed] [Google Scholar]
- 27.Verstraelen H. Delanghe J. Roelens K. Blot S. Claeys G. Temmerman M. Subclinical iron deficiency is a strong predictor of bacterial vaginosis in early pregnancy. BMC Infect Dis. 2005;5:55–65. doi: 10.1186/1471-2334-5-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tohill BC. Heilig CM. Klein RS, et al. Nutritional biomarkers associated with gynecological conditions among US women with or at risk of HIV infection. Am J Clin Nutr. 2007;85:1327–1334. doi: 10.1093/ajcn/85.5.1327. [DOI] [PubMed] [Google Scholar]
- 29.Neggers YH. Nansel TR. Andrews WW, et al. Dietary intake of selected nutrients affects bacterial vaginosis in women. J Nutr. 2007;137:2128–2133. doi: 10.1093/jn/137.9.2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yasodhara P. Raghuath M. Sreeramulu D. Venu L. Hemalatha R. Krishna TP. Local immunity in Indian women with bacterial vaginosis. J Reprod Immunol. 2006;70:133–141. doi: 10.1016/j.jri.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 31.Ness RB. Hillier S. Richter HE, et al. Can known risk factors explain racial differences in the occurrence of bacterial vaginosis? J Natl Med Assoc. 2003;95:201–212. [PMC free article] [PubMed] [Google Scholar]
- 32.Ryckman KK. WIlliams SM. Krohn MA. Simhan HN. Racial difference in cervical cytokine concentrations between pregnant women with and without bacterial vaginosis. J Reprod Immunol. 2008;78:166–171. doi: 10.1016/j.jri.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ryckman KK. Simhan HN. Krohn MA. Williams SM. Cervical cytokine network patterns during pregnancy: The role of bacterial vaginosis and geographic ancestry. J Reprod Immunol. 2009;79:174–182. doi: 10.1016/j.jri.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nansel TR. Riggs MA. Yu K-F. Andrews WW. Schwebke JR. Klebanoff MA. The association of psychosocial stress and bacterial vaginosis in a longitudinal cohort. Am J Obstet Gynecol. 2006;194:381–386. doi: 10.1016/j.ajog.2005.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ryckman KK. Simhan HN. Krohn MA. Williams SM. Predicting risk of bacterial vaginosis: The role of race, smoking and corticotrophin-releasing hormone-related genes. Mol Hum Reprod. 2009;15:131–137. doi: 10.1093/molehr/gan081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nugent RP. Krohn MA. Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol. 1991;29:297–301. doi: 10.1128/jcm.29.2.297-301.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sha BE. Chen HY. Wang QJ. Zariffard MR. Cohen MH. Spear GT. Utility of Amsel criteria, Nugent score, quantitative PCR for G. vaginalis, M. hominis and Lactobacillus spp for diagnosis of bacterial vaginosis in human immunodeficiency virus infected women. J Clin Microbiol. 2005;43:4607–4612. doi: 10.1128/JCM.43.9.4607-4612.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]