Abstract
Background
Drinking alcohol during pregnancy has a range of negative consequences for the developing fetus. Screening and brief intervention approaches have significant promise, but their population impact may be limited by a range of challenges to implementation. We, therefore, conducted preliminary acceptability and feasibility evaluation of a computer-delivered brief intervention for alcohol use during pregnancy.
Methods
Participants were 50 pregnant women who screened positive for risky drinking during a routine prenatal clinic visit and were randomly assigned to computer-delivered brief intervention or assessment-only conditions.
Results
Ratings of intervention ease of use, helpfulness, and other factors were high (4.7–5.0 on a 1–5 scale). Participants in both conditions significantly decreased alcohol use at follow-up, with no group differences; however, birth weights for infants born to women in the intervention group were significantly higher (p<0.05, d = 0.62).
Conclusions
Further development and study of computer-delivered screening and intervention for alcohol use during pregnancy are warranted.
Introduction
Alcohol use during pregnancy is associated with clear negative consequences for the developing fetus, known collectively as fetal alcohol spectrum disorders (FASD).1–6 Although many women stop or reduce alcohol use during pregnancy,7,8 many others continue to drink. African American women, in particular, may be less likely to reduce binge drinking (four or more drinks on one occasion) during pregnancy.9 Data from the 2007–2008 National Survey on Drug Use and Health7 suggest that 10% of African American pregnant women report binge drinking in the past month; this is up from 6% in 2005–2006 and is higher than for pregnant women overall, regardless of race or ethnicity (4.5% in 2007–2008).
Screening, brief intervention, and referral for treatment (SBIRT) efforts for problem alcohol use in primary care settings have received substantial empirical support.10–12 Efforts to apply this approach to the prenatal care setting have also been successful,13,14 although effects may be restricted to women with heavier alcohol use.14–16 In its report on preventing alcohol-exposed pregnancies, the Centers for Disease Control and Prevention (CDC) lists screening and brief intervention as 1 of 10 key recommendations.17
Despite the promise of SBIRT approaches to reach pregnant women who otherwise may go unidentified, it has been noted that the “implementation of brief interventions in ‘real world’ settings is slow.”17 The potential obstacles are many and varied. For example, one study suggested that doing all recommended screening and prevention tasks would take a primary care provider 4.4 hours per working day,18 and another showed that few obstetricians engage in all recommended screening and brief intervention elements, even after training.19 Similar findings have been reported by multiple surveys of physicians providing care for pregnant women, suggesting that few of them fully implement recommended brief intervention strategies.20–22
Advantages of computer delivery
Computer-delivered screening and brief intervention may address some of these obstacles and has a number of potential advantages over current approaches. First, some evidence has suggested that only a third of women are assessed for alcohol use during prenatal care visits.23 If technology-based screening becomes a routine part of prenatal care, it could dramatically increase the proportion of at-risk pregnant women who are screened. Second, because women may disclose stigmatized behaviors more readily in computer-based interviewing,24 computer-delivered screening could also identify a higher proportion of at-risk women. Third, computer-delivered brief intervention can potentially take place in waiting or examination rooms without limitation by provider time, willingness, or ability.25 Of course, whether or not these potential advantages translate into improved implementation is an empirical question. However, these potential advantages are strong enough to support the initial step of evaluating the feasibility, acceptability, and efficacy of computer-delivered approaches.
Study aims
As a first step in evaluating a computer-delivered intervention for alcohol use during pregnancy, we assessed (1) the feasibility of the computer-delivered approach through evaluation of the rate of identification of at-risk drinking and of the proportion of participants able to complete the computer-delivered session and (2) acceptability of computer-delivered SBIRT via participant report of ease of use, helpfulness, and overall satisfaction. Secondary aims of this study were to conduct preliminary effect size estimation of intervention-related changes in (1) alcohol consumption (frequency, quantity, and binge use) 30 days after the single-session intervention and (2) birth outcome variables (i.e., gestational age, birth weight, and head circumference).
Materials and Methods
Participants were 50 pregnant women attending an inner-city prenatal care clinic. Inclusion criteria included being pregnant, between ages 18 and 45 (with at least 1 month expected gestation remaining), able to understand spoken English, and either (1) meeting T-ACE criteria for problem alcohol use, (2) exceeding the National Institute on Alcohol Abuse and Alcoholism (NIAAA) “normal” sensible drinking limits before pregnancy (more than seven standard drinks a week or more than two drinks at a time), or (3) reporting drinking at least one time per month during pregnancy These inclusion criteria are based on previous research and are in keeping with the recommendations of the American College of Obstetricians and Gynecologists (ACOG).26,27 Exclusion criteria included inability to provide informed consent (e.g., due to psychosis, intoxication, or other clear cognitive impairment), inability to communicate in English, and not having access to a phone (for follow-up). All women who completed the screening portion and were not eligible for the study received a small gift for their baby (equivalent to $1 value). All eligible participants received gift cards (equivalent to $30) for their participation at the baseline visit. Women who completed the follow-up session received an additional gift card (equivalent to $5) by mail. This study was approved by the Wayne State University Institutional Review Board as well as the Detroit Medical Center Research Review Committee.
Women were approached while in the prenatal clinic waiting room and briefly told of the study. This initial anonymous portion of the study (screener) was described as questions that ask about the participants' feelings, social contacts, health, and possible substance use. After obtaining verbal informed consent, participants completed a self-administered brief screener via computer. Participants who met alcohol risk criteria on the screener (an automated procedure, using predetermined decision rules) were invited to participate in the full study, which was described as a study of alcohol use during pregnancy, and told that the computer would randomly assign them (like a coin toss) to either participate in the Healthy Pregnancy Check-Up, where they would receive feedback from the computer about past and present alcohol use, or to only answer questions. Those wishing to continue and willing to provide signed informed consent were included in the clinical trial.
Measures
The T-ACE (a widely used screening tool for problem alcohol use) was embedded within a larger screening measure consisting of general health-related items. Previous research has demonstrated that this tool can improve the identification of women with alcohol use problems in clinical settings better than can clinical questioning alone.28,29 The screen takes <1 minute to administer and consists of four items: (1) How many drinks does it take to make you feel high? (Tolerance); (1) Have people Annoyed you by criticizing your drinking? (3) Have you ever felt you should Cut down on your drinking? (4) Have you ever had a drink first thing in the morning to steady your nerves or get rid of a hangover (i.e., an Eye-opener)? The general health-related items tapped diet, exercise, smoking, and other factors.
Participants in the clinical trial completed an approximately 40-minute assessment session on the Tablet PC. This battery included the following measures:
Timeline follow-back (TLFB)-modified computer version
This method 30 involves the use of a calendar and multiple procedures to aid recall of drinking, drug use, or smoking. Computer administrations, as well as phone follow-up assessments, have yielded comparable results to face-to-face interview administrations.31–33 Participants were asked to recall their alcohol use over the past month.
Readiness to change questionnaire
This measure is based on Prochaska and DiClemente's stages-of-change model, which assigns nontreatment-seeking people to the precontemplation, contemplation, or action stage of change based on how they feel about their drinking at present (during pregnancy).34
Acceptability of software
Acceptability of the computer-delivered system was assessed by examining the satisfaction questions embedded in the assessment. All participants rated their satisfaction with the software after completing the intervention section (or assessment section for the control group); ratings were based on a 1–5 Likert scale, where 1=low and 5=high. These items were developed and used in previous studies to assess the acceptability of the software in a similar population of women in the perinatal period.35
Center for Epidemiologic Studies Short Depression Scale (CES-D10)
This is a well-validated and reliable self-report scale designed to measure depressive symptomatology.36,37
Birth outcome variables
Birth outcome data collected from the medical record included gestational age, birth weight, and head circumference.
Computer-delivered brief intervention
The computer randomly assigned half of participants to the computer-delivered intervention group. This brief intervention was self-administered and solely computer-delivered, with assistance available from the investigator as needed, and took approximately 15–20 minutes to complete. The software used has been shown previously to be highly acceptable and easy to use, even for participants with no prior computer experience.35 To ensure privacy, participants listened to the narrator by using headphones; all questions were read out loud by the narrator, and response options could be read if tapped by the participant. The Tablet PC used in this study included a touch screen, such that participants simply tapped the screen with a stylus to choose their responses. Moreover, the automated software allows participants the option to go back and revisit questions as needed.
The computer-based brief motivational intervention was specifically tailored to pregnant women in a number of ways. For example, the intervention itself included a brief educational component that delivered current information about FASD. All images and examples in the software were specifically tailored to pregnant women.
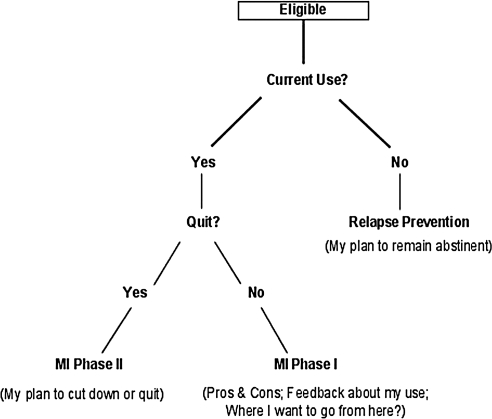
The software also tailored content based on the current drinking status of each participant (Fig. 1 shows intervention flow). For women who reported they had already quit, the narrator presented a section that focused on relapse prevention (“My plan to remain abstinent”) while asking the participant to provide the reasons/benefits to them of having made this change. The remaining participants were asked about their current interest in quitting (Are you willing/ready to quit?), leading to a bifurcated treatment response such that those participants reporting a goal of immediate abstinence moved more quickly to a section consistent with phase 2 of MI (primarily goal setting), whereas those who did not wish to quit received elements consistent with phase 1 of MI (e.g., pros and cons, normed feedback). This intervention process is consistent with evidence, albeit inconsistent, that motivational approaches may work best with less motivated individuals.38,39
FIG. 1.
Intervention flow chart.
Control group
Participants randomly assigned to the control group were administered a series of questions about television show preferences and viewed a brief series of videos of popular entertainers/shows, with subsequent requests for ratings of subjective preference. The duration and level of interactivity for this control condition were equivalent to that of the intervention condition, thus controlling for time effects and facilitating blinding of the investigator to experimental condition. On completion of study, all women in the control condition received a brochure specifically designed to facilitate reductions in drinking during pregnancy.
Follow-up evaluation
Follow-up was conducted 1 month after the intervention (average follow-up duration of 33 days, standard deviation [SD] 7.9, range 25–72). This session took place over the phone, took approximately 10–15 minutes, and included the TLFB assessment of drinking in the past month (TLFB by phone has been shown to be a valid and reliable means of assessing alcohol use outcomes).31
Results
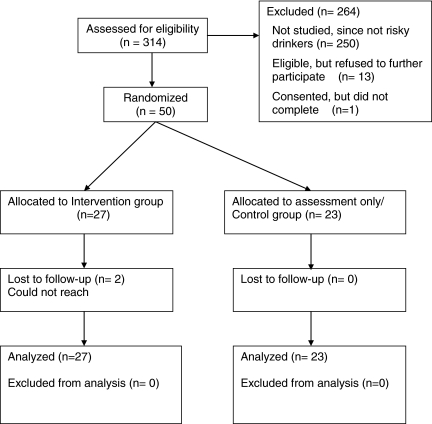
Study flow is summarized in Figure 2. A total of 490 consecutive women were approached about the study; 314 (64%) were interested and agreed to be screened. Of those screened, 64 (20%) met inclusion criteria for participation. Of those who met the inclusion criteria, 13 women (20%) chose not to participate after being informed of the nature of the study. Additionally, 1 woman who consented was interrupted and did not complete the study. Overall, 64 women (20%) were identified as meeting eligibility criteria, with 50 (16%) completing the study. All 50 women were randomized, with 27 being allocated to the intervention group and 23 to the control group.
FIG. 2.
Study flow chart.
Data were analyzed using SPSS version 17 (Chicago, IL). All data were assessed for normality and appropriateness of the proposed statistical tests. Any covariate related to an outcome or group membership (intervention or control) at p≤0.20 was included in the analyses for that outcome. Outcome variables were dichotomized if they did not meet criteria for normality.
To assess randomization success at baseline, comparisons of demographic characteristics between intervention and control groups were conducted with t tests for normally distributed data or chi-square tests for dichotomous data; nonparametric analyses (e.g., Mann-Whitney U test) were conducted for nonnormal data that were not dichotomized but instead were treated as ordinal (e.g., skew of ≥2). On an a priori basis, statistically or clinically significant differences between the groups were covaried in later analyses of the relationship between treatment status and outcome. Analyses revealed significant differences between the two groups in family history status and stage of change (Table 1); these variables were covaried in subsequent analyses. No other significant between-group differences were found in reported alcohol quantity or frequency, T-ACE screen status, previous risky drinking status, depression status, or smoking status.
Table 1.
Demographic Characteristics of Participants (n=50)
| Variable | Control, n=23 | Intervention, n=27 | p valuea |
|---|---|---|---|
| Age (mean±SD) | 26.4 (5.52) | 25.0 (4.93) | 0.38 |
| Pregnancy week (mean±SD) | 25.5 (7.63) | 25.0 (8.45) | 0.83 |
| Race (%) | 0.38 | ||
| African American | 20 (87%) | 21 (78%) | |
| Caucasian | 3 (13%) | 5 (19%) | |
| Hispanic | - | 1 (3%) | |
| Education (%) | 0.29 | ||
| 0–8 grades | 3 (13%) | 2 (7%) | |
| 9–11 grades | 12 (52%) | 12 (44%) | |
| High school graduate or GED | 6 (26%) | 9 (33%) | |
| Some college | 2 (9%) | 4 (15%) | |
| Marital status (%) | 0.78 | ||
| Single | 6 (26%) | 7 (26%) | |
| Family history of alcohol abuse (%) | 4 (27%) | 11 (55%) | 0.09b |
| Alcohol, quantity (SD) (g of ethanol per week)c | 83.4 (147) | 89.5 (91.3) | 0.62 |
| Received assistance in past year (%) | |||
| WIC food assistance | 16 (70%) | 20 (74%) | 0.73 |
| FIA assistance | 10 (44%) | 12 (44%) | 0.95 |
| CES-D, depressed status (%) | |||
| Yes | 14 (61%) | 13 (48%) | 0.41 |
| Stage of change (%) | 0.14b | ||
| Precontemplation | 3 (13%) | 8 (30%) | |
| Action | 20 (87%) | 19 (70%) | |
p values for differences between conditions were calculated using chi-square analyses for dichotomous data; independent t tests for continuous data, and nonparametric analyses (Mann-Whitney U) for nonnormally distributed data.
Covaried in subsequent analyses.
One standard drink is equivalent to 14 g of alcohol.
CES-D, Center for Epidemiologic Studies Depression Scale; FIA, Family Independent Agency; GED, general equivalency diploma; SD, standard deviation; WIC, Women, Infants, and Children.
The follow-up rate was very good overall, with 48 of 50 participants (96%) assessed at 1 month. Data from the 2 dropouts were examined by comparing those who completed the 1-month follow-up (n=48) with those who dropped out (n=2) by treatment status and by baseline characteristics. No significant differences were found, and, therefore, these missing data were seen as not threatening the validity of the study. Eight participants gave birth before the 1-month follow-up session; evaluation context (before or after birth) was thus entered as a covariate in subsequent follow-up analyses. Chi-square and independent samples t test analyses revealed no significant differences (and none <p=0.2) in sociodemographic characteristics between the intervention and control groups at baseline (Table 1).
Participant characteristics
Participants had a mean age of 26 years (SD 5.2), and a mean gestational age of 25 weeks (SD 8). They were predominantly African American (82%), with 72% reporting receiving some form of public assistance, 50% reporting having graduated from high school or completed a general equivalency diploma (GED), and 54% meeting CES-D screening criteria for depression risk. A total of 74% of participants reported quitting their alcohol use before participating in the study.
Feasibility of screening and brief intervention
Of the 314 participants screened for eligibility using the computer, all were able to complete screening without any reported difficulty; similarly, of the 50 participants participating in the clinical trial, all were able to complete the session without assistance. Of the 314 women assessed for eligibility, 64 (20%) were identified as either currently drinking or at risk for drinking during pregnancy.
Acceptability and satisfaction
Participant ratings of software acceptability were consistently high (Table 2). Mean ratings ranged from a low of 4.7 (out of 5) (in response to: How much did you like working with the computer?) to a high of 5.0 (in response to: How easy was it to use?). Ratings of acceptability were equally high among intervention and control group participants and for participants who reported very low levels of drinking and no drinking at all during pregnancy.
Table 2.
Mean Ratings on Software Satisfaction Questions
| Question | Intervention condition, n=27 Mean (SD) |
|---|---|
| How much did you like working with the computer? | 4.78 (0.70) |
| How interesting was it? | 4.70 (0.61) |
| How easy was it to use? | 4.96 (0.19) |
| How respectful of you was it? | 4.85 (0.60) |
| How much did it annoy you? | 2.00 (1.27) |
All ratings were based on a 1–5 Likert scale, where 1=not at all, and 5=very much.
Assessing changes in alcohol use
Because typical drinking patterns as seen in the TLFB results were nonnormal, these data were treated as ordinal at baseline and dichotomous (no/any drinking) at follow-up.
Change in alcohol use in the entire study sample
Both treatment and control conditions demonstrated significant decreases in reported quantity of alcohol use at 1-month follow-up (Wilcoxon signed-rank test, W=25, p<0.01, r=−0.73). Overall, 72% of all participants reported any drinking at baseline assessment, and 10% reported any drinking at follow-up.
Intervention effects on alcohol use and birth outcome variables
Bivariate logistic regression analysis was conducted to examine the effect of treatment on alcohol use at follow-up, controlling for baseline alcohol use as well as variables that were significantly different between the two groups as revealed in previous analyses (stage of change and family history). This analysis showed no effect of treatment on alcohol use at follow-up (p=0.71).
Treatment group differences were examined for the following birth outcomes: gestational age, birth weight, and head circumference. Controlling for baseline alcohol use, smoking status, and maternal weight, treatment and control groups did not differ on gestational age, F(1,44)=01.97, p=0.17, or head circumference, F(1, 44)=0.13, p=0.72. However, there was a significant difference in birth weight in favor of the intervention condition, F(1,44)=4.80, p=0.03, ηp2=0.11. The mean birth weight of infants born to women in the intervention condition (mean=3189.6, SD=328.0) was significantly higher than that for infants of women in the control condition (mean=2965.3, SD=387.7), d=0.62.
Discussion
The results of this study suggest that computer-delivered screening can identify at-risk drinking among pregnant women and that working with the computer did not pose a challenge for this group of primarily low-income women. Our rate of identification of at-risk drinking of 20% is higher than that seen in similar studies.14,40 Although this finding, of course, could be due to our somewhat more liberal inclusion criteria and differences in sample, it argues for the overall ability of the computer-delivered approach to identify at-risk drinking during pregnancy. This finding is consistent with other evidence that self-report of stigmatized behaviors is greater in computer-interview formats.24
Results were encouraging and consistent in overall high ratings of acceptability of the software across all domains of assessment (e.g., likability, interest). Similar results have been found in previous studies with this software, many of which also included qualitative feedback that was consistent with quantitative ratings.35 Concerns that computer literacy could be a barrier to widespread use of technology in healthcare settings are not supported by these data.
Importantly, ratings of acceptability in this study were high even among participants who were included in this study based only on reports of elevated drinking before pregnancy (most of whom denied any drinking, or any intention of drinking, during pregnancy). This suggests that broad inclusion criteria designed to capture a higher proportion of at-risk drinkers, even those who disclose only minimal risk, may be practical if the intervention for this group is sensitively targeted. For example, in the present study, relapse prevention techniques were used as a platform for addressing the risks of drinking and how to avoid it during pregnancy, without implying substantial risk on the part of the participant herself. The ability to present acceptable brief interventions to women who are thought to be at some added risk, despite denying any current drinking, is encouraging. The development of acceptable brief interventions for women who are potentially at risk of drinking during pregnancy, despite the absence of disclosure of actual drinking, may be an important focus of future research.
Our results indicating that both groups reduced their alcohol use at follow-up, with no difference between conditions, were similar to findings from previous studies.13,40,41 There are several possible explanations for this finding. First, and most parsimoniously, this lack of treatment effect may simply reflect a general lack of efficacy for this computer-delivered brief intervention. However, this lack of effect could also be potentially explained by a number of other factors. It may be that beneficial effects could become evident at a later date. A recent study assessing a brief intervention in problem drinkers found no effects of treatment at the 6-month follow-up but demonstrated significant effects of treatment at the 12-month follow-up.42 Evidence for the delayed effects of brief interventions for alcohol use within a primary care setting was also noted in a meta-analysis.11 It must be noted, however, that other meta-analyses have suggested that the effect of motivational interventions may peak early and dissipate quickly over time.43
Second, previous work has identified the active role of assessment alone in contributing toward behavior change.41,42;44–46 In the current study, the assessment was nearly 40 minutes long and included several measures regarding alcohol use and motivation to change. Thus, it is possible that asking a participant to consider her alcohol use in such a thoughtful manner may have facilitated behavior change. Two studies that included the Alcohol Use Disorders Identification Test (AUDIT) in their brief assessment reported decreased levels of hazardous drinking at follow-up; this includes one study that used random assignment to assessment vs. screening only.42,45 The substantial reductions in alcohol use among both treatment and control participants in this study are consistent with the suggestion that both arms may have had an active effect on drinking behavior.
Finally, the use of a phone-based follow-up with participants who have not had sufficient time to build up a trusting relationship with the investigators, particularly in the perinatal period, may have suppressed disclosure of alcohol use. This method of evaluation may have been more vulnerable to social desirability bias than other possible approaches. Notably, this suggestion (that the intervention may have led to real decreases in drinking that were not detectable because of severe underreporting in both conditions) is incompatible with the previous suggestion that assessment effects may have overwhelmed intervention effects. Given that outcome analyses were secondary in this phase 1 study, we offer this and the previous suggestions only as possibilities that might inform future, fully powered trials.
The finding with respect to birth weight is encouraging and may support the suggestion that underreporting masked intervention effects in this study. A similar study14 found intervention-related effects on birth weight, a finding that is consistent with the known effects of alcohol use during pregnancy.6 Given social desirability, recall, and other biases in self-report of alcohol use, infant birth weight and other birth outcomes may be important supplemental outcomes in evaluating the success of alcohol interventions during pregnancy. This finding must also be interpreted with caution, however; given the number of comparisons in this preliminary study, this group difference could simply reflect type I error. Further, even if the computer-delivered motivational intervention caused this effect, it may have done so through a mechanism other than reduced drinking (e.g., it may have increased concern about fetal health in general, leading to changes in diet, decreased smoking, and other behaviors).
Limitations
A number of limitations must be noted. First, the current sample consisted predominantly of African American (82%) women of lower socioeconomic status (SES) who received care in an urban hospital setting; these findings may not be apply to other groups of pregnant women. An additional limitation of this study is the reliance on self-report data. Although this study used (1) the TLFB approach, which has been shown to be a reliable and valid method for retrospective evaluation of all forms of substance use;47,48 (2) ACASI (Audio Computer Assisted Self-Interview) technology, and (3) a confidential approach outside of any legal or treatment setting, it is possible that social desirability bias played a role in the study, particularly with respect to the telephone-based follow-up. Long-term outcome measures are recommended with a longer follow-up assessment. Although reduction of alcohol use at any point during pregnancy could lead to improved outcomes for the fetus,49,50 future studies may benefit from targeting intervention earlier in pregnancy. Finally, the small number in this preliminary study also limited power and, therefore, ability to detect intervention effects.
Conclusions
The demonstrated acceptability and feasibility of computer-delivered brief intervention for alcohol use during pregnancy is encouraging. Although there was no effect of treatment on reported alcohol use at follow-up, both groups significantly reduced their self-reported alcohol use at follow-up, and infants born to women in the intervention group had significantly higher birth weights. Future research in this area is clearly merited and should take such factors as underreporting and assessment effects into consideration. Verification of the potential implementation advantages of computer-delivered brief intervention should follow evidence that this approach can indeed lead to behavior change.
The high acceptability of the intervention evaluated in this study, even among participants who denied active drinking, raises an intriguing possibility. Rather than relying on accurate disclosure, the identification of specific risk factors associated with prenatal alcohol use could lead to more efficient screening and identification, as well as augmenting intervention strategies to prevent or minimize FASDs.51,52 For example, several studies have already identified factors that increase the risk of alcohol use during pregnancy, including low (SES), having an early age of drinking onset, being unmarried, and reporting heavy drinking by male partner.53–57 It may be that screeners using indirect risk factors, such as these, in combination with replicable and low-cost computer-delivered interventions, could facilitate efforts to have a meaningful population impact on FASD even among women who choose not to disclose their drinking.
Acknowledgments
This research was supported under NIAAA training grant AA16256 (to G. K.T; mentor, S.J.O.). The authors also thank Nancy Lockhart for her technical assistance with adapting the software for this study and the staff at University Health Center for their support in recruitment. These results were presented, in part, at the annual meeting of the Research Society on Alcoholism (RSA) in June 2009 in San Diego, CA.
Disclosure Statement
No competing financial interests exist.
References
- 1.Burden M. Jacobson S. Sokol R. Jacobson J. Effects of prenatal alcohol exposure on attention and working memory at 7.5 years of age. Alcohol Clin Exp Res. 2005;29:443–452. doi: 10.1097/01.alc.0000156125.50577.ec. [DOI] [PubMed] [Google Scholar]
- 2.Burden M. Jacobson S. Jacobson J. Relation of prenatal alcohol exposure to cognitive processing speed and efficiency in childhood. Alcohol Clin Exp Res. 2005;29:1473–1483. doi: 10.1097/01.alc.0000175036.34076.a0. [DOI] [PubMed] [Google Scholar]
- 3.Jacobson J. Jacobson S. Sokol R. Effects of prenatal exposure to alcohol, smoking, and illicit drugs on postpartum somatic growth. Alcohol Clin Exp Res. 1994;18:317–323. doi: 10.1111/j.1530-0277.1994.tb00020.x. [DOI] [PubMed] [Google Scholar]
- 4.Jacobson J. Jacobson S. Sokol R. Martier S. Ager J. Prenatal alcohol exposure and infant information processing abilities. Child Dev. 1993;64:1706–1721. [PubMed] [Google Scholar]
- 5.Jacobson S. Jacobson J. Sokol RJ. Chiodo LM. Corobana R. Maternal age, alcohol abuse history, and quality of parenting as moderators of the effects of prenatal alcohol exposure on 7.5-year intellectual function. Alcohol Clin Exp Res. 2004;28:1732–1745. doi: 10.1097/01.alc.0000145691.81233.fa. [DOI] [PubMed] [Google Scholar]
- 6.Sokol RJ. Delaney-Black V. Nordstrom B. Fetal alcohol spectrum disorder. JAMA. 2003;290:2996–2999. doi: 10.1001/jama.290.22.2996. [DOI] [PubMed] [Google Scholar]
- 7.Office of Applied Studies. Substance use among women during pregnancy and following childbirth. 2009. www.oas.samhsa.gov/2k3/pregnancy/pregnancy.htm. [Mar 20;2010 ]. www.oas.samhsa.gov/2k3/pregnancy/pregnancy.htm
- 8.Lucas ET. Goldschmidt L. Day NL. Alcohol use among pregnant African American women: Ecological considerations. Health Soc Work. 2003;28:273–283. doi: 10.1093/hsw/28.4.273. [DOI] [PubMed] [Google Scholar]
- 9.Tenkku LE. Morris DS. Salas J. Xaverius PK. Racial disparities in pregnancy-related drinking reduction. Matern Child Health J. 2009;13:604–613. doi: 10.1007/s10995-008-0409-2. [DOI] [PubMed] [Google Scholar]
- 10.Kaner E. Dickinson H. Beyer F, et al. The effectiveness of brief alcohol interventions in primary care settings: A systematic review. Drug Alcohol Rev. 2009;28:301–323. doi: 10.1111/j.1465-3362.2009.00071.x. [DOI] [PubMed] [Google Scholar]
- 11.Bertholet N. Daeppen JB. Wietlisback V. Fleming M. Burnand B. Reduction of alcohol consumption by brief alcohol interventionin primary care: Systematic review and meta-analysis. Arch Intern Med. 2005;165:986–995. doi: 10.1001/archinte.165.9.986. [DOI] [PubMed] [Google Scholar]
- 12.Moyer A. Finney JW. Swearingen CE. Vergun P. Brief interventions for alcohol problems: A meta-analytic review of controlled investigations in treatment-seeking and non-treatment-seeking populations. Addict. 2002;97:279–292. doi: 10.1046/j.1360-0443.2002.00018.x. [DOI] [PubMed] [Google Scholar]
- 13.Hankin J. Sokol R. Identification and care of problems associated with alcohol ingestion in pregnancy. Semin Perinatol. 1995;19:286–292. doi: 10.1016/s0146-0005(05)80043-6. [DOI] [PubMed] [Google Scholar]
- 14.O'Connor MJ. Whaley SE. Brief intervention for alcohol use by pregnant women. Am J Public Health. 2007;97:252–258. doi: 10.2105/AJPH.2005.077222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang G. McNamara T. Orav E, et al. Brief interventions for prenatal alcohol use: A randomized trial. Obstet Gynecol. 2005;105:991–998. doi: 10.1097/01.AOG.0000157109.05453.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Handmaker NS. Miller WR. Manicke M. Finding of a pilot study of motivational interviewing with pregnant drinkers. J Stud Alcohol. 1999;60:285–287. doi: 10.15288/jsa.1999.60.285. [DOI] [PubMed] [Google Scholar]
- 17.Barry K. Caetano R. Chang G, et al. Reducing alcohol-exposed pregnancies: A report of the National Task Force on Fetal Alcohol Syndrome and Fetal Alcohol Effect. Atlanta, GA: Centers for Disease Control and Prevention; 2009. [Google Scholar]
- 18.Yarnall KS. Pollak KI. Ostbye T. Krause KM. Michener J. Primary care: Is there enough time for prevention? Am J Public Health. 2003;93:635–641. doi: 10.2105/ajph.93.4.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DePue JD. Goldstein MG. Schilling A, et al. Dissemination of the AHCPR clinical practice guideline in community health centres. Tobacco Control. 2002;11:329–335. doi: 10.1136/tc.11.4.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chapin J. Root W. Improving obstetrician-gynecologist implementation of smoking cessation guidelines for pregnant women: An interim report of the American College of Obstetricians and Gynecologists. Nicotine Tobacco Res. 2004;6:S253–257. doi: 10.1080/14622200410001669123. [DOI] [PubMed] [Google Scholar]
- 21.Grimley DM. Bellis JM. Raczynski JM. Henning K. Smoking cessation counseling practices: A survey of Alabama obstetrician-gynecologists. South Med J. 2001;94:297–303. [PubMed] [Google Scholar]
- 22.Anderson BL. Parra Dang E. Floyd RL. Sokol RJ. Mahoney J. Schulkin J. Knowledge, opinions, and practice patterns of obstetrician-gynecologists regarding their patients' use of alcohol. J Addict Med. 2010;4:114–121. doi: 10.1097/ADM.0b013e3181b95015. [DOI] [PubMed] [Google Scholar]
- 23.Lemola S. Grob A. Drinking and smoking in pregnancy: Which questions do Swiss physicians ask? Swiss Med Weekly. 2007;137:66–69. doi: 10.4414/smw.2007.11648. [DOI] [PubMed] [Google Scholar]
- 24.Newman JC. Des Jarlais DC. Turner CF. Gribble J. Cooley P. Paone D. The differential effects of face-to-face and computer interview modes. Am J Public Health. 2002;92:294–297. doi: 10.2105/ajph.92.2.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morse B. Hutchins E. Reducing complications from alcohol use during pregnancy through screening. J Am Med Womens Assoc. 2000;55:225–227. [PubMed] [Google Scholar]
- 26.Chang G. Alcohol screening instruments for pregnant women. Alcohol Res Health. 2001;25:204–209. [PMC free article] [PubMed] [Google Scholar]
- 27.American College of Obstetricians and Gynecologists. Drinking and reproductive health: A fetal alcohol spectrum disorders prevention took kit. 2006.
- 28.Sokol RJ. Martier SS. Ager JW. The T-ACE questions: Practical prenatal detection of risk-drinking. Am J Obstet Gynecol. 1989;160:863–870. doi: 10.1016/0002-9378(89)90302-5. [DOI] [PubMed] [Google Scholar]
- 29.Bertrand J. Floyd RL. Weber MK, et al. National Task Force on FAS/FAE. Fetal alcohol syndrome: Guidelines for referral and diagnosis. Atlanta: Centers for Disease Control and Prevention; 2004. [Google Scholar]
- 30.Sobell LC. Sobell MB. Timeline follow-back: A calendar method for assessing alcohol and drug use. Toronto, Ontario, Canada: Addiction Research Foundation; 1996. [Google Scholar]
- 31.Sobell LC. Sobell MB. Leo GI. Cancilla A. The reliability of the alcohol timeline followback when administered by telepohone and by computer. Drug Alcohol Depend. 1996;42:49–54. doi: 10.1016/0376-8716(96)01263-x. [DOI] [PubMed] [Google Scholar]
- 32.Brown RA. Burgess ES. Sales SD. Whitely JA. Evans DM. Miller IW. Reliability and validity of a smoking timeline follow-back interview. Psychol Addict Behav. 1998;12:101–112. [Google Scholar]
- 33.Carney MA. Tennen H. Affleck G. Del Boca FK. Kranzler HR. Levels and patterns of alcohol consumption using timeline follow-back, daily diaries and real-time “electronic interviews.”. J Stud Alcohol. 1998;59:447–454. doi: 10.15288/jsa.1998.59.447. [DOI] [PubMed] [Google Scholar]
- 34.Rollnick S. Heather N. Gold R. Hall W. Development of a short “readiness to change” questiónnaire for use in brief, opportunistic interventions among excessive drinkers. Br J Addict. 1992;87:743–754. doi: 10.1111/j.1360-0443.1992.tb02720.x. [DOI] [PubMed] [Google Scholar]
- 35.Ondersma SJ. Chase SK. Svikis DS. Schuster CR. Computer-based brief motivational intervention for perinatal drug use. J Subst Abuse Treat. 2005;28:305–312. doi: 10.1016/j.jsat.2005.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Andresen EM. Malmgren JA. Carter WB. Patrick DL. Screening for depression in well older adults: Evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale) Am J Prev Med. 1994;10:77–84. [PubMed] [Google Scholar]
- 37.Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 38.Ondersma S. Winhusen T. Erickson S. Stine S. Wang Y. Motivational enhancement therapy with pregnant substance-abusing women: Does baseline motivation moderate efficacy? Drug Alcohol Depend. 2009;101:74–79. doi: 10.1016/j.drugalcdep.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rohsenow DJ. Monti P. Martin RA, et al. Motivational enhancement and coping skills training for cocaine abusers: Effects on substance use outcomes. Addiction. 2004;99:862–874. doi: 10.1111/j.1360-0443.2004.00743.x. [DOI] [PubMed] [Google Scholar]
- 40.Chang G. Wilkins-Haug L. Berman S. Goetz M. A brief intervention for prenatal alcohol use: An in-depth look. J Subst Abuse Treat. 2000;18:365–369. doi: 10.1016/s0740-5472(99)00105-1. [DOI] [PubMed] [Google Scholar]
- 41.Chang G. Goetz MA. Wilkins-Haug Berman S. Brief intervention for alcohol use in pregnancy: A randomized trial. Addiction. 1999;94:1499–1508. doi: 10.1046/j.1360-0443.1999.941014996.x. [DOI] [PubMed] [Google Scholar]
- 42.Tsai YF. Tsai MC. Lin YP. Chen CY. Brief intervention for problem drinkers in a Chinese population: A randomized controlled trial in a hospital setting. Alcohol Clin Exp Res. 2009;33:95–101. doi: 10.1111/j.1530-0277.2008.00815.x. [DOI] [PubMed] [Google Scholar]
- 43.Hettema J. Steele J. Miller WR. Motivational interviewing. Annu Rev Clin Psychol. 2005;1:91–111. doi: 10.1146/annurev.clinpsy.1.102803.143833. [DOI] [PubMed] [Google Scholar]
- 44.Bernstein J. Bernstein E. Heeren TC. Mechanisms of change in control group drinking in clinical trials of brief alcohol intervention: Implications for bias toward the null. Drug Alcohol Rev. 2010;29:498–507. doi: 10.1111/j.1465-3362.2010.00174.x. [DOI] [PubMed] [Google Scholar]
- 45.Kypri K. Langley L. Saunders JB. Cashell-Smith ML. Assessment may conceal therapeutic benefit: Findings from a randomized controlled trial for hazardous drinking. Addiction. 2007;102:62–70. doi: 10.1111/j.1360-0443.2006.01632.x. [DOI] [PubMed] [Google Scholar]
- 46.Epstein EE. Drapkin ML. Yusko DA. Cook SM. McCrady BS. Jensen NK. Is alcohol assessment therapeutic? Pretreatment change in drinking among alcohol-dependent women. J Stud Alcohol. 2005;66:369–378. doi: 10.15288/jsa.2005.66.369. [DOI] [PubMed] [Google Scholar]
- 47.Carey KB. Clinically useful assessments: Substance use and comorbid psychiatric disorders. Behav Res Ther. 2002;40:1345–1361. doi: 10.1016/s0005-7967(02)00039-6. [DOI] [PubMed] [Google Scholar]
- 48.Sobell LC. Sobell MB. Leo GI. Cancilla A. Reliability of a timeline method: Assessing normal drinkers' reports of recent drinking and a comparative evaluation across several populations. Br J Addict. 1998;83:393–402. doi: 10.1111/j.1360-0443.1988.tb00485.x. [DOI] [PubMed] [Google Scholar]
- 49.Coles CD. Brown RT. Smith IE. Plazman KA. Erickson S. Falek A. Effects of prenatal alcohol exposure at school age. I. Physical and cognitive development. Neurotoxicol Teratol. 1991;13:357–367. doi: 10.1016/0892-0362(91)90084-a. [DOI] [PubMed] [Google Scholar]
- 50.Rosett HL. Weiner L. Zuckerman B. McKinlay S. Edelin KC. Reduction of alcohol consumption during pregnancy with benefits to the newborn. Alcohol Clin Exp Res. 1980;4:178–184. doi: 10.1111/j.1530-0277.1980.tb05632.x. [DOI] [PubMed] [Google Scholar]
- 51.Flynn H. Chermack S. Prenatal alcohol use: The role of lifetime problems with alcohol, drugs, depression, and violence. J Stud Alcohol Drug. 2008;69:500–509. doi: 10.15288/jsad.2008.69.500. [DOI] [PubMed] [Google Scholar]
- 52.Chiodo L. Janisse J. Delaney-Black V. Sokol R. Hannigan J. A metric of maternal prenatal risk drinking predicts neurobehavioral outcomes in preschool children. Alcohol Clin Exp Res. 2009;33:634–644. doi: 10.1111/j.1530-0277.2008.00878.x. [DOI] [PubMed] [Google Scholar]
- 53.Mengel MB. Searight R. Cook K. Preventing alcohol-exposed pregnancies. J Am Board Fam Med. 2006;19:494–505. doi: 10.3122/jabfm.19.5.494. [DOI] [PubMed] [Google Scholar]
- 54.Sidhu JS. Floyd R. Alcohol use among women of childbearing age—United States 1991–1999. MMWR. 2002;51:273–276. [PubMed] [Google Scholar]
- 55.Haynes G. Dunnagan T. Christopher S. Determinants of alcohol use in pregnant women at risk for alcohol consumption. Neurotoxicol Teratol. 2003;25:659–666. doi: 10.1016/j.ntt.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 56.Abel EL. An update on incidence of FAS: FAS is not an equal opportunity birth defect. Neurotoxicol Teratol. 1995;17:437–443. doi: 10.1016/0892-0362(95)00005-c. [DOI] [PubMed] [Google Scholar]
- 57.Smith IE. Lancaster JS. Moss-Wells S. Coles CD. Falek A. Identifying high risk pregnant drinkers: Biological and behavioral correlates of continuous heavy drinking during pregnancy. J Stud Alcohol. 1987;48:304–309. doi: 10.15288/jsa.1987.48.304. [DOI] [PubMed] [Google Scholar]