Abstract
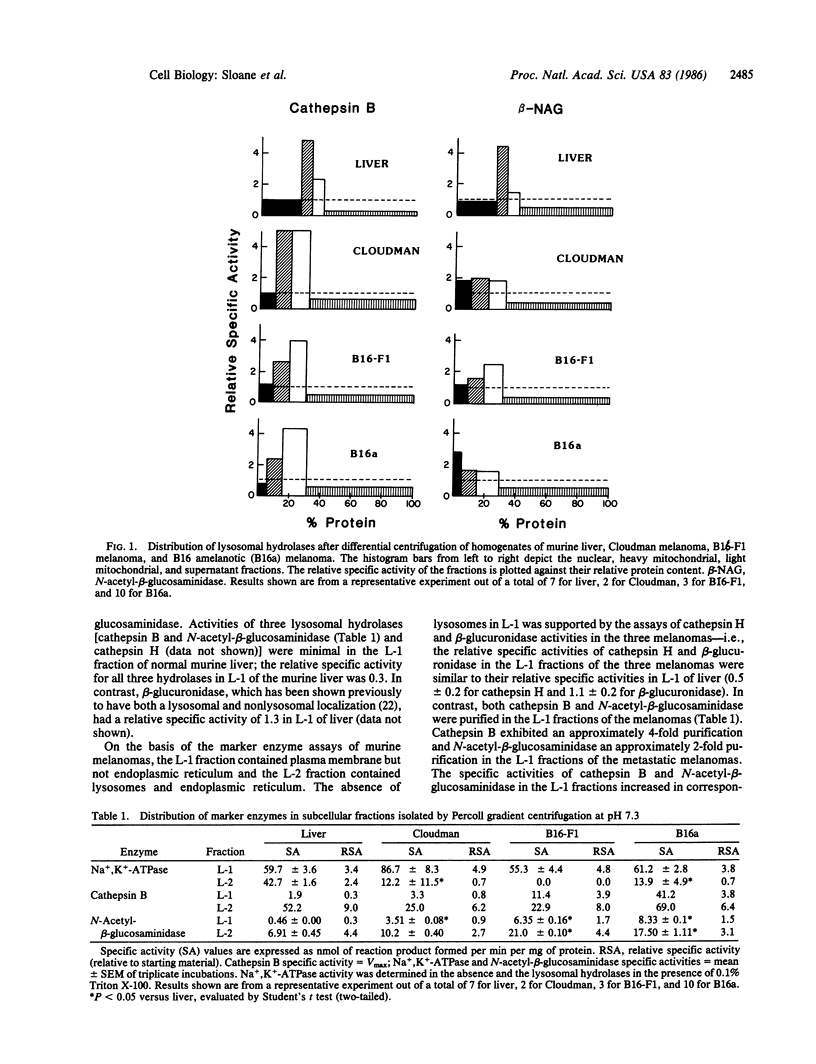
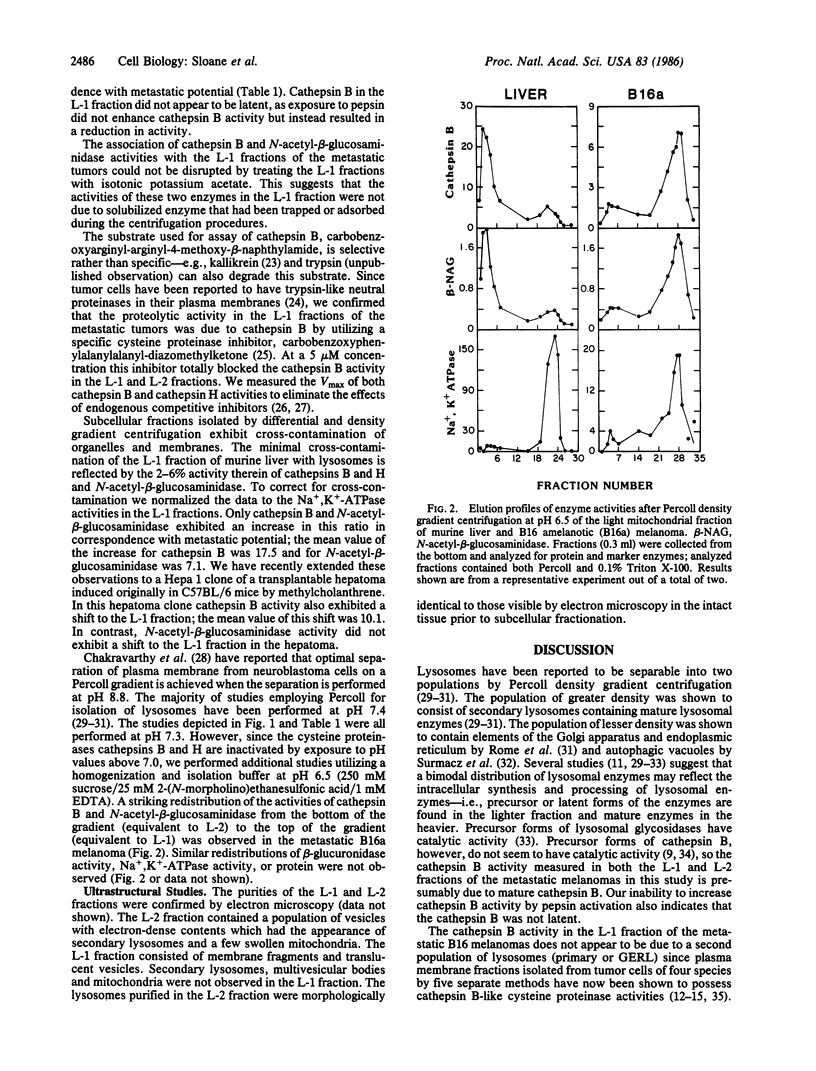
The subcellular localization of cathepsin B activity (EC 3.4.22.1) in three murine melanomas of increasing metastatic potential (Cloudman less than B16-F1 less than B16 amelanotic) was determined. Cathepsin B activity was localized in the heavy mitochondrial fraction of normal murine liver but in the light mitochondrial fraction of the metastatic melanomas; the localization of three other lysosomal hydrolases did not shift. Further purification of the light mitochondrial fraction into L-1 (density = 1.045 g/ml) and L-2 (density = 1.07 g/ml) fractions was achieved on a 30% iso-osmotic Percoll gradient. The L-1 fraction of liver and melanomas contained Na+, K+-ATPase activity; the L-2 fraction of liver contained four lysosomal hydrolase (cathepsins B and H, N-acetyl-beta-glucosaminidase, and beta-glucuronidase) and glucose-6-phosphatase activities. Ultrastructural examination revealed that the L-1 fraction consisted of membrane vesicles and the L-2 fraction of secondary lysosomes. In the B16 melanomas cathepsin B and N-acetyl-beta-glucosaminidase activities were found in both L-1 and L-2 fractions. Specific activities of the two enzymes in the plasma membrane (L-1) fractions increased in correspondence with metastatic potential. Cathepsin H and beta-glucuronidase activities were not localized in the plasma membrane fractions of the B16 melanomas. Localization of hydrolytic enzymes in the plasma membrane of metastatic tumor cells could result in focal dissolution of the extracellular matrix and thereby invasion and metastasis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronson N. N., Jr, Touster O. Isolation of rat liver plasma membrane fragments in isotonic sucrose. Methods Enzymol. 1974;31:90–102. doi: 10.1016/0076-6879(74)31009-9. [DOI] [PubMed] [Google Scholar]
- Barrett A. J., Kirschke H. Cathepsin B, Cathepsin H, and cathepsin L. Methods Enzymol. 1981;80(Pt 100):535–561. doi: 10.1016/s0076-6879(81)80043-2. [DOI] [PubMed] [Google Scholar]
- Brown J. A., Swank R. T. Subcellular redistribution of newly synthesized macrophage lysosomal enzymes. Correlation between delivery to the lysosomes and maturation. J Biol Chem. 1983 Dec 25;258(24):15323–15328. [PubMed] [Google Scholar]
- Böhmer F. D., Schmidt H. E., Schön R. Proteolytic activities associated with plasma membrane preparations from tumour cells in enzootic bovine leukosis and from normal bovine lymphoid cells. Acta Biol Med Ger. 1982;41(10):883–890. [PubMed] [Google Scholar]
- Cavanaugh P. G., Sloane B. F., Bajkowski A. S., Gasic G. J., Gasic T. B., Honn K. V. Involvement of a cathepsin B-like cysteine proteinase in platelet aggregation induced by tumor cells and their shed membrane vesicles. Clin Exp Metastasis. 1983 Oct-Dec;1(4):297–307. doi: 10.1007/BF00121192. [DOI] [PubMed] [Google Scholar]
- Chakravarthy B. R., Spence M. W., Clarke J. T., Cook H. W. Rapid isolation of neuroblastoma plasma membranes on Percoll gradients. Characterization and lipid composition. Biochim Biophys Acta. 1985 Jan 10;812(1):223–233. doi: 10.1016/0005-2736(85)90542-5. [DOI] [PubMed] [Google Scholar]
- Docherty K., Hutton J. C., Steiner D. F. Cathepsin B-related proteases in the insulin secretory granule. J Biol Chem. 1984 May 25;259(10):6041–6044. [PubMed] [Google Scholar]
- Gieselmann V., Pohlmann R., Hasilik A., Von Figura K. Biosynthesis and transport of cathepsin D in cultured human fibroblasts. J Cell Biol. 1983 Jul;97(1):1–5. doi: 10.1083/jcb.97.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honn K. V., Onoda J. M., Pampalona K., Battaglia M., Neagos G., Taylor J. D., Diglio C. A., Sloane B. F. Inhibition by dihydropyridine class calcium channel blockers of tumor cell-platelet-endothelial cell interactions in vitro and metastasis in vivo. Biochem Pharmacol. 1985 Jan 15;34(2):235–241. doi: 10.1016/0006-2952(85)90130-3. [DOI] [PubMed] [Google Scholar]
- Jorgensen P. L. Isolation of (Na+ plus K+)-ATPase. Methods Enzymol. 1974;32:277–290. [PubMed] [Google Scholar]
- Kirschke H., Shaw E. Rapid interaction of cathepsin L by Z-Phe-PheCHN12 and Z-Phe-AlaCHN2. Biochem Biophys Res Commun. 1981 Jul 30;101(2):454–458. doi: 10.1016/0006-291x(81)91281-x. [DOI] [PubMed] [Google Scholar]
- Köppel P., Baici A., Keist R., Matzku S., Keller R. Cathepsin B-like proteinase as a marker for metastatic tumor cell variants. Exp Cell Biol. 1984;52(5):293–299. doi: 10.1159/000163273. [DOI] [PubMed] [Google Scholar]
- Liotta L. A., Thorgeirsson U. P., Garbisa S. Role of collagenases in tumor cell invasion. Cancer Metastasis Rev. 1982;1(4):277–288. doi: 10.1007/BF00124213. [DOI] [PubMed] [Google Scholar]
- Mierendorf R. C., Jr, Cardelli J. A., Dimond R. L. Pathways involved in targeting and secretion of a lysosomal enzyme in Dictyostelium discoideum. J Cell Biol. 1985 May;100(5):1777–1787. doi: 10.1083/jcb.100.5.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mort J. S., Leduc M. S. The combined action of two enzymes in human serum can mimic the activity of cathepsin B. Clin Chim Acta. 1984 Jul 16;140(2):173–182. doi: 10.1016/0009-8981(84)90342-5. [DOI] [PubMed] [Google Scholar]
- Mort J. S., Leduc M., Recklies A. D. A latent thiol proteinase from ascitic fluid of patients with neoplasia. Biochim Biophys Acta. 1981 Dec 15;662(2):173–180. doi: 10.1016/0005-2744(81)90027-9. [DOI] [PubMed] [Google Scholar]
- Mullins D. E., Rohrlich S. T. The role of proteinases in cellular invasiveness. Biochim Biophys Acta. 1983 Dec 29;695(3-4):177–214. doi: 10.1016/0304-419x(83)90011-2. [DOI] [PubMed] [Google Scholar]
- Pietras R. J., Roberts J. A. Cathepsin B-like enzymes. Subcellular distribution and properties in neoplastic and control cells from human ectocervix. J Biol Chem. 1981 Aug 25;256(16):8536–8544. [PubMed] [Google Scholar]
- Read S. M., Northcote D. H. Minimization of variation in the response to different proteins of the Coomassie blue G dye-binding assay for protein. Anal Biochem. 1981 Sep 1;116(1):53–64. doi: 10.1016/0003-2697(81)90321-3. [DOI] [PubMed] [Google Scholar]
- Recklies A. D., Tiltman K. J., Stoker T. A., Poole A. R. Secretion of proteinases from malignant and nonmalignant human breast tissue. Cancer Res. 1980 Mar;40(3):550–556. [PubMed] [Google Scholar]
- Rome L. H., Garvin A. J., Allietta M. M., Neufeld E. F. Two species of lysosomal organelles in cultured human fibroblasts. Cell. 1979 May;17(1):143–153. doi: 10.1016/0092-8674(79)90302-7. [DOI] [PubMed] [Google Scholar]
- Rosenfeld M. G., Kreibich G., Popov D., Kato K., Sabatini D. D. Biosynthesis of lysosomal hydrolases: their synthesis in bound polysomes and the role of co- and post-translational processing in determining their subcellular distribution. J Cell Biol. 1982 Apr;93(1):135–143. doi: 10.1083/jcb.93.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan R. E., Crissman J. D., Honn K. V., Sloane B. F. Cathepsin B-like activity in viable tumor cells isolated from rodent tumors. Cancer Res. 1985 Aug;45(8):3636–3641. [PubMed] [Google Scholar]
- Skudlarek M. D., Swank R. T. Turnover of two lysosomal enzymes in macrophages. J Biol Chem. 1981 Oct 10;256(19):10137–10144. [PubMed] [Google Scholar]
- Sloane B. F., Bird J. W. Effect of ovarian hormones on lysosomal acid hydrolase activities in rat myometrium. Am J Physiol. 1977 Apr;232(4):E423–E431. doi: 10.1152/ajpendo.1977.232.4.E423. [DOI] [PubMed] [Google Scholar]
- Sloane B. F., Honn K. V. Cysteine proteinases and metastasis. Cancer Metastasis Rev. 1984;3(3):249–263. doi: 10.1007/BF00048388. [DOI] [PubMed] [Google Scholar]
- Sloane B. F., Honn K. V., Sadler J. G., Turner W. A., Kimpson J. J., Taylor J. D. Cathepsin B activity in B16 melanoma cells: a possible marker for metastatic potential. Cancer Res. 1982 Mar;42(3):980–986. [PubMed] [Google Scholar]
- Steven F. S., Griffin M. M., Itzhaki S., Al-Habib A. A trypsin-like neutral protease on Ehrlich ascites cell surfaces: its role in the activation of tumour-cell zymogen of collagenase. Br J Cancer. 1980 Nov;42(5):712–721. doi: 10.1038/bjc.1980.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmacz C. A., Wert J. J., Jr, Mortimore G. E. Role of particle interaction on distribution of liver lysosomes in colloidal silica. Am J Physiol. 1983 Jul;245(1):C52–C60. doi: 10.1152/ajpcell.1983.245.1.C52. [DOI] [PubMed] [Google Scholar]
- Tulsiani D. R., Six H., Touster O. Rat liver microsomal and lysosomal beta-glucuronidases differ in both carbohydrate and amino acid compositions. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3080–3084. doi: 10.1073/pnas.75.7.3080. [DOI] [PMC free article] [PubMed] [Google Scholar]



