Abstract
Over the last decade, the search for a method able to reliably predict seizures hours in advance has been largely replaced by a more realistic goal of very early detection of seizure onset which would allow therapeutic or warning devices to be triggered prior to the onset of disabling clinical symptoms. We explore in this article the steps along the pathway from data acquisition to closed loop applications that can and should be considered to design the most efficient early seizure detection. Microelectrodes, high-frequency oscillations, high sampling rate, high-density arrays, and modern analysis techniques are all elements of the recording and detection process that in combination with modeling studies can provide new insights into the dynamics of seizure onsets. Each of these step needs to be considered if one wants to implement improved detection devices that will favorably impact the quality of life of patients.
Keywords: seizure onset, early detection, EEG acquisition, warning devices, therapeutic devices
Introduction
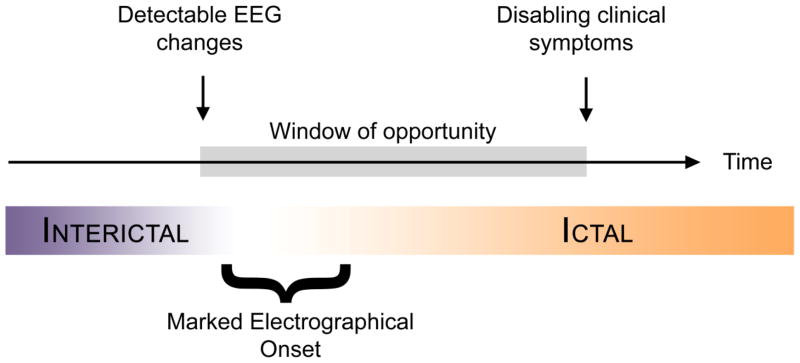
The initial hope to be able to predict seizures hours in advance has more recently shifted toward a more realistic goal of short term prediction or early detection. In the latest workshop on seizure prediction in 2009 in Kansas City, a patient representative stated clearly that it would be most useful for patients to get a reliable warning just before the onset of disabling clinical symptoms. Warnings hours before a seizure will just produce a long period of anxiety, diminishing quality of life. Therefore there is renewed interest in methods able to detect seizures prior to the onset of disabling clinical symptoms. This early detection paradigm is distinct from seizure prediction in that it does not require or assume the presence of a preictal period. Instead, early detection targets the potential window of time existing between the start of measurable changes in brain activity that are part of the ictal evolution and the onset of disabling symptoms for the patient as illustrated in Figure 1.
Figure 1.
The window of opportunity is the time of the earliest detectable changes in the EEG and the onset of disabling clinical symptoms. Closed-loop therapy must be delivered within that timeframe to provide optimum benefit to the patient.
Early seizure detection poses a number of challenges. In most patients, intracranial electrodes allow for earlier seizure detection than do scalp recordings, particularly for partial seizures. If an entire seizure can be utilized, particularly with intracranial recordings, then it is relatively straightforward to detect seizures with reasonable sensitivity and specificity since one is dealing with events often lasting over a minute [1]. Detection in these instances is used for seizure identification and retrospective analysis of seizures, often in the context of presurgical evaluations. If, however, one wishes to detect a seizure within seconds of onset for the purpose of warning or closed-loop therapy (e.g. neurostimulation), then the challenges are much greater. Even though early seizure dynamics (e.g. frequency, complexity) are similar for multiple seizures from a given focus in a given patient [2], the requirements for early detection may compromise specificity if high sensitivity is desired. Often these are not false positive detections since epileptiform activity is detected, but events that do not evolve into clinical seizures may be included.
The length of this window of opportunity (Figure 1) in which to intervene and potentially alter seizure evolution is, however, dependent on the ability of the detection method to perceive the changes in EEG features as early as possible and also on the region of the seizure onset (e.g. orbital frontal, mesial temporal) which can influence clinical features and rate of seizure propagation. Despite decades of progress made in the design of an automatic seizure detection method, there is still to this day no algorithm or device approved by the Federal and Drug Administration (FDA) for seizure detection in epilepsy monitoring unit in the United States. We look for areas for potential improvement along the path of the recording process. The following discussion will address potential ways to optimize early seizure detection in the context of improved and developing recording techniques.
High density microelectrodes arrays; what they will show? Do micro-electrodes capture more or less?
Although seizure detection based on other physiological data have been proposed [3], the most appropriate source of information to rapidly assess the onset of a seizure remains, for the moment, electroencephalograms, either recorded from scalp electrodes or from electrodes implanted intracranially using subdural strips and grids or depth electrode arrays. Over the last few years, there has been increased interest in developing high-density arrays of electrodes or microelectrodes incorporating microwires, placed separately or in conjunction with regular macroelectrode grids or depths. In addition to providing denser sampling of cortical activity, these arrays are better suited to recording high frequency activity. These new recordings electrodes have been using mostly for research purposes without established clinical applications, but reports raise the possibility that such high density arrays with smaller electrodes can record information important for identifying the ictal onset zone and planning surgical resections. Their development was linked to the push for exploring the content of the EEG in a higher frequency domain than what is permitted by commonly used macroelectrodes. Worell et al. reported that the recording of highly localized high-frequency oscillations (HFO) [4] was most effectively done by microwires. Using microelectrodes, Schevon reports the recordings of highly focal microdischarges [5] are likely to be from a single cortical macrocolumn. Stead recorded microseizures and microdischarges, more frequently in the seizure onset zone but also occurring less frequently in control patients [6]. These recordings do not assess single unit activity but multineuronal activity. Still these studies report the ability of these new electrodes to assess highly localized activity, and in the case of the microseizures reported by Stead, activity that may correlate with region of onset of partial seizures. If that would be the case, those microseizures would be ideal candidates for targeted therapies. A recent report [7] suggests that removal of brain regions generating interictal fast ripples correlates with seizure freedom. The fact that this activity can be highly localized raises the issue that planning such recordings requires a good idea of the ictal onset zone prior to implantation or the utilization of large electrode arrays. While in a long term closed-loop therapy paradigm, one would hope that the focus of the partial seizures is correctly identified prior to implantation so electrodes can be placed appropriately, this may not be the case in the standard settings (e.g. epilepsy monitoring unit) where seizure localization is still often part of the evaluation. In any case, if high frequency activity (e.g. 250–500 Hz) accompanies the period of earliest ictal onset, then incorporation of these specialized arrays has the potential to improve early seizure detection.
What about extending coverage?
The extent of coverage by recording arrays is in part dependent upon the knowledge of the localization of the ictal onset zone, that region that generates the epileptic seizure. The ictal onset zone may be much more localized than the epileptogenic zone (the region targeted for resection for optimal surgical outcome) or the irritative zone (corresponding to interictal discharges), but even experts acknowledge that these zones are often overlapping and often not clearly defined [8]. For the purposes of early seizure detection, one would want to be as close as possible to the ictal onset zone. In some patients this is a discrete region; in other patients (e.g. non-lesional neocortical partial epilepsy) the ictal onset zone may be broad or regional. While it is not uncommon to incorporate 100–200 intracranial contacts into macroelectrode recording arrays, there are some limits on the size and extent of the arrays posed by the extent of the craniotomy and inherent risks of larger arrays.
The use of high-density EEG using larger arrays of electrodes for scalp recordings is oriented towards dipole source localization. These larger arrays have been shown to provide improvements in effective spatial resolution up to 512 electrodes if the noise level remains low [9]. In intracranial recording the incorporation of microelectrodes arrays placed within the space of traditional grid arrays offers a increased density of points of measure which can provide a superior understanding of the local network dynamic involved in the early stages of the seizure [6]. Still the limitations on the number of channels that can be recorded simultaneously limits the number of microelectrodes that can be considered. The use of macroelectrodes remains a valid compromise between size of the electrodes and area of the brain covered, especially in the context of the presurgical evaluation where the location of the seizure focus is not yet clearly identified.
Sampling rate of acquisition
Over the years the sampling frequency of data has increased as the electronics and computational power allow. Ever since the advent of digital EEG recordings, and particularly over the last 10 years since the first report of pathological HFO (pHFO), research and clinical equipment have been regularly upgraded with ones able to perform higher sampling rates in an attempt to better characterize the higher end of the spectrum of the EEG and determine its role in epileptogenesis and its potential for the delineation of the ictal onset and epileptogenic zones [10–12]. Rates of acquisition range from 200Hz for routine clinical scalp recordings to 10–32kHz for the latest experimental intracranial recordings. However at such high rates, the amount of data generated and processing this data remain important issues. Stead reports in a study of 14 patients, that 2256h of EEG recordings at 32kHz required the storage and analysis of 12.5TB of data [6]. The need for microelectrode recordings and the accompanying high sampling rates is still evolving and will probably be individualized.
Digitization: bits and noise
Quantization of the EEG signals has been sometimes overlooked as part of the problem of assessing onset of the ictal period. Even with current 16 bit converters, it is not uncommon to see virtually flat signals during post-ictal periods, where the amplitude of the EEG is so low, it cannot be distinguished from background noise levels. Sixteen bits A/D converters often offer only a 12 bit effective range due to the signal to noise ratio of the amplifier. Even 24 bit converters realistically often only offer 16 to 19 bits of effective range. The current interest for HFO and the high frequency low voltage components of the EEG raise the issue of maintaining a dynamic range of over 110dB (the ratio of the higher bit to the lower effective bit) when recording with microelectrodes. As more and more 24bit A/D converters appear on the market and propose theoretical sensitivity for the least significant bit of 3nV, the remaining issue will be to improve the signal-to-noise ratio to be able to take advantage of this decrease in quantization error of the EEG signals. The increasing use of microelectrodes and microwires aimed at measuring increasingly smaller electric potentials highlights the need for improved electrical integrity of the measuring circuits.
What to measure? Is there an ever elusive quantity which will work better?
The choice of which quantity should be used to assess the early changes of the EEG during ictal onset has been the focus of most studies dealing with early seizure detection. In the last decade, as the center of attention shifted towards the prediction of seizures, most of the same measures were used to attempt to detect a “pre-ictal state,” a state that has, however, remained elusive. The initial enthusiasm for seizure prediction was muted when none of the initial reports could be reproduced and the claim of superiority of non-linear measures was being reconsidered (see summary in [13]). At the same time, a combination of linear and non-linear features was suggested to be a potentially better approach for seizure detection [14]. While the hope of finding a new quantifying measure which will provide reliable earlier changes predictive of an impending seizure remains, two other paths of investigation have also been explored. One is the multichannel approach which we will address below, the other one is the decision component of the process. Deciding whether or not the change in the measured value indicates the onset of a seizure is often treated as a binary classification problem and can involve advanced classification making procedures such as support vector machines [15, 16], nearest neighbors [17, 18], Gaussian process modeling [19] or neural networks [20]. The classification procedure itself will be the step where the definition of what is a seizure or what we want the detector to classify as such will be inserted in the detection process.
Definition of a seizure
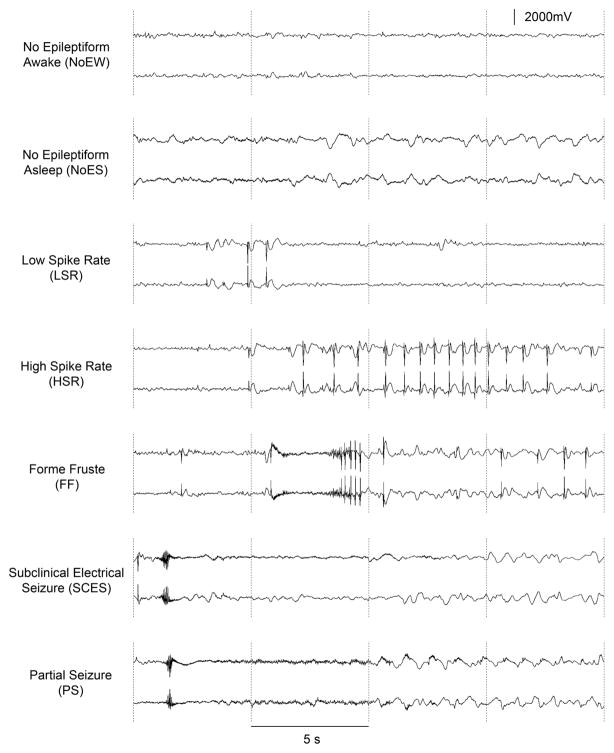
The definition of a seizure and what constitutes a seizure remain difficult to unequivocally establish, and this lack of a definition often impairs the meta-analysis of seizure detection algorithms because not all studies define a seizure in the same way. From a recent meeting between clinicians, scientists, regulators, and industry, the notion that a working standard is needed to make progress was raised. The most common issue deals with interictal activity which often looks fairly identical to the onset of a clinical seizure but will often not evolve much further. These “form fruste” are often not marked as seizures because they do not progress sufficiently and often terminate within seconds of onset. Despite their short duration, the dynamics of these events often possess identical characteristics to the partial seizures of the patient. Closed-loop systems that require early seizure detection (e.g. within 2 seconds) may have therapy triggered by this interictal epileptiform activity.
One suggested path is to at least detect the seizures that are commonly agreed upon by the expert clinicians with a threshold at the level of certainty based on the number of experts who agree (e.g. can the algorithm detect the seizures that 85% of clinicians agree upon?). Of course obtaining such a gold standard of seizure markings by experts is a difficult task in itself. The best tool that could be used to produce the most consistent meta-analysis of seizure detectors would be the use of a common database of seizures. While such an initiative has been undertaken, the vast diversity of ictal patterns that partial onset seizures comprise requires that such a database would be extremely vast. It also raises the question of whether or not a database can ever include all ictal patterns. Despite the stereotypical nature of partial seizures in one patient, the spectrum of partial seizures from different patients onset remains quite diverse.
Analysis of single channel versus multichannel?
Recordings of EEG and ECoG include multiple channels. Signal features derived from single channels analyzes can be used in multistage, multivariate, multichannel detection procedures to enhance reliability [21, 22]. Analysis of single channel activity, however, does not provide information about interactions between different brain structures. To analyze the network interactions before and during seizures multichannel methods are required. Study of the predictive performance of a number of univariate and bivariate measures [23] demonstrate advantages of bivariate measures which could capture synchronization between channels. Some studies find that channels remote from the seizure onset zone carry relevant information about epileptiform activity [23, 24]. Expanding these analyzes to multiple channels may provide more inside into interactions within the network generating and propagating the seizure activity. Methods based on multivariate autoregressive methods (MVAR) provide information about connectivity patterns. To date these methods have been used primarily for localization of the seizure onset region [25,26]. However, the MVAR model can be also used to measure dynamic changes in synchronization between multiple channels [27]. Further development of these and other methods of investigating connectivity and synchronization patterns from multichannel recordings may facilitate the reliable early detection of seizures.
Modeling
Increases in computational power allow for more complex and detailed models of brain structures which may provide new insights into the dynamics of transitions to the ictal state. Models including relatively detailed simulations of neuronal networks provide some insight into relations between changes in ion concentrations and dynamics of epileptiform activity [28]. This may suggest a new approach to early detection possibly including measurements of variables other than electric or magnetic activity of neurons, measurements that could facilitate earlier seizure detection. Some modeling studies suggest that some types of epilepsy (i.e. primary generalized absence seizures) may be described using bistable models [29]. In these situations there is an abrupt transition from the interictal to ictal state. Another sophisticated modeling study (personal communication, Anderson et al.) reveals that partial seizures arising from abnormal focal neural networks can be evoked by normal random fluctuations of background activity, fluctuations that would not result in seizures in normal neural networks. While this model illustrates a situation where seizure prediction would be difficult, early seizure detection would still be possible. Future development of early detection methods needs to include a diversity of seizure types. Modeling studies of simulated electrical stimulation provide some information as what features are important to optimize the effectiveness of stimulation in termination of seizures [30]. The timing of the stimulation in relation to the phase of ongoing activity was important. This suggests that future detection algorithms may need to provide not only time of detection but some additional parameters of the signal for optimal application of closed-loop therapeutic devices. Modeling studies of newborn EEG provide encouraging results of model based seizure detection [31, 32].
Embedding
The prospect of embedded detectors raises the issue of low-power computing and poses limit to the amount of processing that can be done by those devices in the assessment of ictal onset [33, 34]. Engineering of these implantable devices is forcing a compromise of detection power versus consumption. Although increasing processing power of embedded CPUs opens the door to new possibilities as time progresses, current embedded devices are often limited to the very basic detection procedures, while others propose limited channel selection to reduce the computational burden [35]. With the potential application of closed-loop therapies there is a need for improved early seizure detection. A closed-loop device designed to detect seizures after only two seconds has been demonstrated to significantly reduce disabling seizures in controlled trials in 191 patients with drug-resistant partial seizures and is currently under review by the FDA [36]. This small responsive neurostimulator is implanted in a recess in the skull and is connected to two intracranial electrodes (strips or depth arrays) placed near the presumed ictal onset zone. The device is programmed to detect early seizure activity within two seconds of onset (i.e. during the window of opportunity) and to deliver stimulation designed to disrupt the seizure and prevent evolution to a disabling seizure. Seizure detection can be tuned to the parameters of the seizures in the specific patient. During the blinded evaluation period seizures were reduced by 37.9% compared to 17.3 for the sham group (p = 0.012). Because of the device limitations, the detection algorithms in the responsive neurostimulator are based on simple parameters of line length, area under the curve, and half wave. Tuning of the detection algorithm results in highly sensitive detection but with detection of many epileptiform events that are not destined to evolve into seizures (see Figure 2 above and related discussion). Nevertheless we are at the point where implementation of such closed-loop therapy based on early seizure detection is a reality. The potential for using similar devices for delivery of other types of therapy (e.g. drugs, focal cooling) also exists [37–39].
Figure 2.
Spectrum of ICEEG activities recorded in the same patient. From awake without epileptiform activity to a partial seizure, the ICEEG can present numerous patterns, including form fruste which are the most likely to trigger false positive response from early detection algorithms.
Timing of detection
The specific issue facing early seizure detection is of course the time frame in which the seizure can be detected. As discussed above seizure detection in the context of clinical review during an epilepsy monitoring evaluation is done a posteriori and does not require the detection to be done prior to the end of the seizure. Nevertheless, it would be beneficial to have capable warning systems in EMU settings to alert personnel and allow assessment of the patient during the seizure. The feasibility of patient warning systems has already been demonstrated [40]. The assessment of the delay between electrographical onset of ictal event and the clinical impairment of the patient was shown to be of up to 30 seconds even with the more stringent definitions. While this does not apply to all seizures, since the onset of clinical symptoms and impairment is related to the cerebral region of onset, this gives an estimate of the length of the window of opportunity mentioned in the introduction.
Conclusion
Potential improvements in seizure detection devices and methods are several. Each step of the detection paradigm can be a target of future experiments to bring to fruition a seizure warning mechanism that can significantly improve the quality of life of patient or to optimize closed-loop therapy devices. From the choice of electrodes to the decision making algorithm, it is important to take a comprehensive look at the various elements which are part of this complicated and evolving process.
Highlights.
Therapeutic or warning devices rely on the very early detection of seizure onset.
We explore the pathways from data acquisition to applications that can be considered.
Improved detection devices would favorably impact the quality of life of patients.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Afra P, Jouny CC, Bergey GK. Duration of complex partial seizures: an intracranial EEG study. Epilepsia. 2008;49:677–684. doi: 10.1111/j.1528-1167.2007.01420.x. [DOI] [PubMed] [Google Scholar]
- 2.Jouny CC, Adamolekun B, Franaszczuk PJ, Bergey GK. Intrinsic ictal dynamics at the seizure focus: effects of secondary generalization revealed by complexity measures. Epilepsia. 2007;48:297–304. doi: 10.1111/j.1528-1167.2006.00963.x. [DOI] [PubMed] [Google Scholar]
- 3.Jeppesen J, Beniczky S, Fuglsang-Frederiksen A, Sidenius P, Jasemian Y. Detection of epileptic-seizures by means of power spectrum analysis of heart rate variability: a pilot study. Technol Health Care. 2010;18:417–426. doi: 10.3233/THC-2010-0606. [DOI] [PubMed] [Google Scholar]
- 4.Worrell GA, Gardner AB, Stead SM, Hu S, Goerss S, Cascino GJ, et al. High-frequency oscillations in human temporal lobe: simultaneous microwire and clinical macroelectrode recordings. Brain. 2008;131:928–937. doi: 10.1093/brain/awn006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schevon CA, Goodman RR, McKhann G, Jr, Emerson RG. Propagation of Epileptiform Activity on a Submillimeter Scale. J Clin Neurophysiol. 2010:406–411. doi: 10.1097/WNP.0b013e3181fdf8a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stead M, Bower M, Brinkmann BH, Lee K, Marsh WR, Meyer FB, et al. Microseizures and the spatiotemporal scales of human partial epilepsy. Brain. 2010;133:2789–2797. doi: 10.1093/brain/awq190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu JY, Sankar R, Lerner JT, Matsumoto JH, Vinters HV, Mathern GW. Removing interictal fast ripples on electrocorticography linked with seizure freedom in children. Neurology. 2010;75:1686–1694. doi: 10.1212/WNL.0b013e3181fc27d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Worrell GA. Sensing the body electric: biomarkers of epileptic brain. Epilepsy Curr. 2011;11(4):118–119. doi: 10.5698/1535-7511-11.4.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ryynanen OR, Hyttinen JA, Malmivuo JA. Effect of measurement noise and electrode density on the spatial resolution of cortical potential distribution with different resistivity values for the skull. IEEE Trans Biomed Eng. 2006;53:1851–1858. doi: 10.1109/TBME.2006.873744. [DOI] [PubMed] [Google Scholar]
- 10.Jacobs J, Levan P, Chatillon CE, Olivier A, Dubeau F, Gotman J. High frequency oscillations in intracranial EEGs mark epileptogenicity rather than lesion type. Brain. 2009;132:1022–1037. doi: 10.1093/brain/awn351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bragin A, Engel J, Jr, Staba RJ. High-frequency oscillations in epileptic brain. Curr Opin Neurol. 2010;23:151–156. doi: 10.1097/WCO.0b013e3283373ac8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andrade-Valenca LP, Dubeau F, Mari F, Zelmann R, Gotman J. Interictal scalp fast oscillations as a marker of the seizure onset zone. Neurology. 2011 doi: 10.1212/WNL.0b013e318228bee2. epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stam CJ. Nonlinear dynamical analysis of EEG and MEG: review of an emerging field. Clin Neurophysiol. 2005;116:2266–2301. doi: 10.1016/j.clinph.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 14.Päivinen N, Lammi S, Pitkänen A, Nissinen J, Penttonen M, Grönfors T. Epileptic seizure detection: A nonlinear viewpoint. Comput Methods Programs Biomed. 2005;79:151–159. doi: 10.1016/j.cmpb.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 15.Gardner AB, Krieger AM, Vachtsevanos G, Litt B. One-Class Novelty Detection for Seizure Analysis from Intracranial EEG. J Mach Learn Res. 2006;7:1025–1044. [Google Scholar]
- 16.Sorensen TL, Olsen UL, Conradsen I, Henriksen J, Kjaer TW, Thomsen CE, et al. Automatic epileptic seizure onset detection using Matching Pursuit: A case study. Engineering in Medicine and Biology Society (EMBC), 2010 Annual International Conference of the IEEE; 2010. pp. 3277–3280. [DOI] [PubMed] [Google Scholar]
- 17.Qu H, Gotman J. A patient-specific algorithm for the detection of seizure onset in long- term EEG monitoring: possible use as a warning device. IEEE Trans Biomed Eng. 1997;44:115–22. doi: 10.1109/10.552241. [DOI] [PubMed] [Google Scholar]
- 18.Polychronaki GE, Ktonas PY, Gatzonis S, Siatouni A, Asvestas PA, Tsekou H, et al. Comparison of fractal dimension estimation algorithms for epileptic seizure onset detection. J Neural Eng. 2010;7:046007. doi: 10.1088/1741-2560/7/4/046007. [DOI] [PubMed] [Google Scholar]
- 19.Faul S, Gregorcic G, Boylan G, Marnane W, Lightbody G, Connolly S. Gaussian process modeling of EEG for the detection of neonatal seizures. IEEE Trans Biomed Eng. 2007;54(12):2151–62. doi: 10.1109/tbme.2007.895745. [DOI] [PubMed] [Google Scholar]
- 20.Tzallas A, Tsipouras M, Fotiadis D. Epileptic Seizure Detection in Electroencephalograms using Time-Frequency Analysis. IEEE Trans Inf Technol Biomed. 2009;13(5):703–710. doi: 10.1109/TITB.2009.2017939. [DOI] [PubMed] [Google Scholar]
- 21.Aarabi A, Grebe R, Wallois F. A multistage knowledge-based system for EEG seizure detection in newborn infants. Clin Neurophysiol. 2007;118(12):2781–2797. doi: 10.1016/j.clinph.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 22.Mitra J, Glover JR, Ktonas PY, Thitai Kumar A, Mukherjee A. Epilepsia. 2010;51(4):564–72. [Google Scholar]; Karayiannis NB, Frost JD, Jr, Hrachovy RA, Mizrahi EM. A multistage system for the automated detection of epileptic seizures in neonatalelectroencephalography. J Clin Neurophysiol. 2009;26(4):218–226. doi: 10.1097/WNP.0b013e3181b2f29d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mormann F, Kreuz T, Rieke C, Andrzejak RG, Kraskov A, David P, et al. On predictability of epileptic seizures. Clin Neurophysiol. 2005;116:569–87. doi: 10.1016/j.clinph.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 24.D’Allesandro M, Esteller R, Vachtsevanos G, Hinson A, Echauz J, Litt B. Epileptic seizure prediction using hybrid feature selection over multiple intracranial EEG electrode contacts:a report of four patients. IEEE Trans Biomed Eng. 2003;50:571–83. doi: 10.1109/tbme.2003.810706. [DOI] [PubMed] [Google Scholar]
- 25.Franaszczuk PJ, Bergey GK, Kaminski MJ. Analysis of mesial temporal seizure onset and propagation using directed transfer function method. Electroencephalogr Clin Neurophysiol. 1994;91:413–427. doi: 10.1016/0013-4694(94)90163-5. [DOI] [PubMed] [Google Scholar]
- 26.Wilke C, van Drongelen W, Kohrman M, He B. Neocortical seizure foci localization by means of a directed transfer function method. Epilepsia. 2010;51(4):564–572. doi: 10.1111/j.1528-1167.2009.02329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Franaszczuk PJ, Bergey GK. An autoregressive method for the measurement of synchronization of interictal and ictal EEG signals. Biol Cybern. 1999;81:3–9. doi: 10.1007/s004220050540. [DOI] [PubMed] [Google Scholar]
- 28.Kudela P, Franaszczuk P, Bergey GK. Reduction of intracellular calcium removal rate can explain changes in seizure dynamics: studies in neuronal network models. Epilepsy Res. 2003;57:95–109. doi: 10.1016/j.eplepsyres.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 29.Suffczynski P, Lopes da Silva FH, Parra J, Velis DN, Bouwman BM, van Rijn CM, van Hese P, Boon P, Khosravani H, Derchansky M, Carlen P, Kalitzin S. Dynamics of epileptic phenomena determined from statistics of ictal transitions. IEEE Trans Biomed Eng. 2006;53(3):524–32. doi: 10.1109/TBME.2005.869800. [DOI] [PubMed] [Google Scholar]
- 30.Anderson WS, Weinberg S, Kudela P, Bergey GK, Franaszczuk P. Phase dependent stimulation effects on bursting activity in a neural network cortical simulation. Epilepsy Res. 2009;84:42–55. doi: 10.1016/j.eplepsyres.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roessgen M, Zoubir AM, Boashash B. Seizure detection of newborn EEG using a model-based approach. IEEE Trans Biomed Eng. 1998;45(6):673–85. doi: 10.1109/10.678601. [DOI] [PubMed] [Google Scholar]
- 32.Stevenson NJ, Mesbah M, Boylan GB, Colditz PB, Boashash B. A nonlinear model of newborn EEG with nonstationary inputs. Ann Biomed Eng. 2010;38(9):3010–21. doi: 10.1007/s10439-010-0041-3. [DOI] [PubMed] [Google Scholar]
- 33.Shoeb A, Pang T, Guttag J, Schachter S. Non-invasive computerized system for automatically initiating vagus nerve stimulation following patient-specific detection of seizures or epileptiform discharges. Int J Neural Syst. 2009;19:157–172. doi: 10.1142/S0129065709001938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salam MT, Sawan M, Nguyen DK. Epileptic seizure onset detection prior to clinical manifestation. Engineering in Medicine and Biology Society (EMBC), 2010 Annual International Conference of the IEEE; 2010. pp. 6210–6213. [DOI] [PubMed] [Google Scholar]
- 35.Faul SD. Dynamic channel selection to reduce computational burden in seizure detection. Engineering in Medicine and Biology Society (EMBC), 2010 Annual International Conference of the IEEE; 2010. pp. 6365–6368. [DOI] [PubMed] [Google Scholar]
- 36.Morrell MJ RNS System in Epilepsy Study Group. Responsive cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology. 2011 doi: 10.1212/WNL.0b013e3182302056. in press. [DOI] [PubMed] [Google Scholar]
- 37.Stein AG, Eder HG, Blum DE, Drachev A, Fisher RS. An automated drug delivery system for focal epilepsy. Epilepsy Res. 2000;2000(39):103–114. doi: 10.1016/s0920-1211(99)00107-2. [DOI] [PubMed] [Google Scholar]
- 38.Burton JM, Peebles GA, Binder DK, Rothman SM, Smyth MD. Transcortical cooling inhibits hippocampal-kindled seizures in the rat. Epilepsia. 2005;2005(46):1881–1887. doi: 10.1111/j.1528-1167.2005.00299.x. [DOI] [PubMed] [Google Scholar]
- 39.Fujii M, Fujioka H, Oku T, Tanaka N, Imoto H, Maruta Y, Nomura S, Kajiwara K, Saito T, Yamakawa T, Yamakawa T, Suzuki M. Application of focal cerebral cooling for the treatment of intractable epilepsy. Neurol Med Chir (Tokyo) 2010;50:839–844. doi: 10.2176/nmc.50.839. [DOI] [PubMed] [Google Scholar]
- 40.Osorio I, Frei M. Feasibility of automated warning in subjects with localization-related epilepsies. Epilepsy & Behavior. 2010;19(4):602–7. doi: 10.1016/j.yebeh.2010.09.021. [DOI] [PubMed] [Google Scholar]