Abstract
CD84 is a self-binding receptor from the CD150 family that is broadly expressed in hematopoietic cells. It has been described that the adaptors SAP and EAT-2 are critical for CD150 family members signaling and function. We observed that human mast cells express CD84 but lack SAP or EAT-2, that CD84 is tyrosine phosphorylated upon FcεRI engagement, and that the release of granule contents is reduced when FcεRI is co-engaged with CD84 in LAD2 and human CD34+-derived mast cells (huMCs). In addition, we observed that the release of IL-8 and GM-CSF was also reduced in FcεRI/CD84 costimulated cells as compared to FcεRI/Ig control. In order to understand how CD84 down-regulates FcεRI-mediated function, we analyzed signaling pathways affected by CD84 in human mast cells. Our results showed that CD84 dampens FcεRI-mediated calcium mobilization after its co-crosslinking with the receptor. Furthermore, FcεRI-mediated Syk-LAT-PLCγ1 axis activity is down-regulated after CD84 stimulation, compared to FcεRI/Ig control. The inhibitory kinase Fes phosphorylates mainly the inhibitory motif for CD84. Moreover Fes, which has been described to become phosphorylated after substrate binding, also gets phosphorylated when co-expressed with CD84. Consistently, Fes was observed to be more phosphorylated after CD84 and FcεRI co-crosslinking. The phosphorylation of the protein phosphatase SHP-1 also increases after CD84 and FcεRI coengagement. Taken together, our results show that CD84 is highly expressed in mast cells and that it contributes to the regulation of FcεRI signaling in a SAP and EAT-2 independent and Fes and SHP-1 dependent mechanisms.
INTRODUCTION
Mast cells (MCs) have been long recognized as the key effectors cells of allergic inflammation and disease (1). More recently, MCs have been described to play a diverse role within the immune system including the regulation of innate immune responses, autoimmunity, tolerance and transplant, among others (2). In order to manifest these biological functions, mast cells are equipped with a myriad of surface receptors that allow them to respond to environmental signals. The most widely studied receptor in MCs is the high affinity receptor for IgE (FcεRI) that belongs to the Fc receptor family. In human MCs, the FcεRI is a tetrameric receptor that comprises an IgE-binding α-chain, a signal amplification β-chain and a γ-chain homodimer which regulates signal transduction. After FcεRI engagement by cognate Ag, two independent but complementary pathways are triggered by the Src family kinases Lyn and Fyn, leading to optimal MC responses (3). The consequential phosphorylation of the β- and γ–chain ITAMs (Immunoreceptor tyrosine-based activation motifs) results in the recruitment and activation of Syk kinase, a key step in FcεRI signal transduction. The activation of Syk is essential for all known FcεRI-mediated responses including degranulation, secretion of allergic mediators, and induction of gene transcription (4). These early phosphorylation events lead to the recruitment of other molecules, e.g. linker for activation of T cells (LAT), SH2-containing leukocyte protein of 76 kDa (SLP-76), and the linker for activation of B cells (LAB, also called NTAL) (5), and to the activation of enzymes such as phospholipase Cγ (PLCγ), which regulates intracellular calcium release and protein kinase C activation. Downstream events regulate the activation of mitogen-activated protein kinase (MAPK) family members, extracellular signal-regulated kinase (ERK), c-Jun N terminal kinase and p38, which control the activity of numerous transcription factors important for the synthesis and secretion of lipid mediators and cytokines (6).
Mast cell responses are tightly regulated and a number of receptors that dampen FcεRI signals have been described. These include CD300a (7), CD300f (8), MAFA (9), gp49B1 (10) and inhibitory Fc receptors such as FcγRIIb (11) among others. Most of these receptors act by recruiting either protein tyrosine phosphatases such as SHP-1 or SHP-2, or inositol phosphatases such as SHIP-1 upon receptor co-ligation and phosphorylation of their ITIMs (immunoglobulin tyrosine-based inhibitor motifs) in their cytosolic domains.
The CD150 (SLAM) family of immune receptors is a sub-group of the CD2 family that is characterized by their capacity to recruit the cytoplasmic adaptor SAP by means of at least one SAP-binding motif known as immunoreceptor tyrosine-based switch motifs or ITSM (consensus sequence is TV/IYxxV/I where X denotes any amino acid) (12). SAP/SH2D1A consists almost entirely of a single SH2 domain protein and it was first identified as the product of the gene mutated in X-linked lymphoproliferative disease (XLP), a rare immune disorder commonly triggered by Epstein-Barr virus (13).
CD84 is a self-binding receptor (14) from the CD150 family that has 2 Ig-domains in the extracellular portion and 4 tyrosine residues in the cytoplasmic tail (15). CD84 is broadly expressed in the immune system and is present in T cells, B cells as well as several myeloid cells including macrophages and mast cells (16). Our group has shown that CD84 expression in transfected RBL 2H3 cells can down-regulate mast cell activation through homophilic interaction (17). However, these experiments were conducted in a heterologous system that required transfection of human CD84 into rat cells. Thus, the role of endogenous CD84 in the regulation of primary non-transfected MCs remained unclear. In the present work we therefore analyzed the contribution of CD84 to FcεRI mediated signaling in human mast cells showing that CD84 can inhibit FcεRI signaling through a SAP-EAT-2 independent and Fes, SHP-1 dependent mechanisms.
MATERIALS AND METHODS
Cells and antibodies
The LAD2 human mast cell line, kindly provided by Drs. A. Kirshenbaum and D.D Metcalfe (NIH, Bethesda, MD, USA), was grown in StemPro-34 Serum Free Medium (Invitrogen Life Technologies, Carlsbad, CA, USA), supplemented with StemPro-34 Nutrient, L-glutamine (2 mM), penicillin (100 U/ml), streptomycin (100 µg/ml), and 100 ng/ml recombinant stem cell factor (SCF) (Amgen, CA, USA). Primary human MCs derived from CD34-positive peripheral blood cells were obtained from healthy donors following informed consent on a protocol (98-I-0027; PI: Dr. A. Kirshenbaum) approved by the NIAID IRB and differentiated in vitro for 8 weeks in the presence of 100 ng/ml IL-6 and 100 ng/ml SCF as described (18). After 8 weeks, the purity of the cultures was assessed by surface FcεRI and KIT expression. COS-7 (107) cells were transiently transfected using Nucleofector (Amaxa inc, Gaithersburg, MD, USA) following the manufacturer’s instructions, and then lysed for immunoprecipitation assays, as previously described (19).
Anti-CD84 (clone CD84 1.7) (IgG1, k) and anti-human CD229 mAb (clone Ly9 1.25) (IgG1, k) used as isotypic control were described elsewhere (14, 19). Polyclonal rabbit anti-CD84 and anti-SHP-1 antibodies were from Santa Cruz (Santa Cruz Biotechnology, Inc. Santa Cruz, CA, USA). Anti-phosphotyrosine monoclonal cocktail and anti-total ERK antibodies were from Zymed Laboratories (Invitrogen). Anti-phospho (p)-LATY191, anti-p-SykY352, anti-p-PLC-γ1Y783, anti-Fes, anti-p-ERK and anti p-Akt were purchased from Cell Signaling (Cell Signaling Technology, Inc. Danvers, MA, USA). Biotinylated human IgE was from Abbiotec (San Diego, CA, USA). PE-conjugated FcεRI antibody was purchased from e-Bioscience (San Diego, CA, USA) and PE-conjugated c-kit antibody and PE-conjugated streptavidin were from BD-Biosciences, San José, CA, USA.
DNA reagents and transfections
Full-length human CD84 cloned in pCINeo, SAP cloned in pCMV2-Flag (20), and Fyn cloned in pSRα (21) were used. The murine lyn construct pcDNA1-mLyn and Lyn K275M plasmid coding for kinase dead mutant were kindly provided by Dr. Margaret Hibbs. Human Fes (Fes-pCMV6-XL4) was purchased from Trueclone (Origene, Rockville, MD, USA). Csk, SHP-1 and ILT2/CD85j cytoplasmic tail constructs for three hybrid system were previously described (22).
To analyze the contribution of CD84 cytoplasmic tail tyrosine residues, single mutant Y279F and deletion mutant ΔY324 were employed as described (17). Single Y324F mutant was generated using CD84 WT pCINeo as a template and primers for annealing were (F) 5´CCCAAGCTTTTCCACAGAAGGTTA GAC 3´, (R) 5´ GGATCCCTAGATCACAATTTCAAAGCT 3´. The double mutant Y297F/Y324F was generated using CD84 Y279F in pCDNA3 TOPO as a template and the primers mentioned above. PCR products from both mutants were cloned in pCDNA3 TOPO vector (Invitrogen). The integrity of all constructs was confirmed by sequencing with an ABI PRISM Big Dyes Terminator Cycle Sequencing Ready Reaction Kit (Applied Biosystems, Foster City, CA, USA) and universal T7 primer.
RNA extraction and RT-PCR
Total RNA was extracted with an RNAeasy Mini Kit, Qiagen (Qiagen, Hilden, Germany) from 2×106 mast cells sensitized with biotinylated IgE and incubated with CD84 or control antibodies and challenged with streptavidin. cDNA was generated from mRNA High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems) according to the manufacturer’s instructions.
To amplify CD84 from LAD2 or primary huMCs, the following primer pairs: (F): 5´ CCCGAATTCCGTTTGTTCAAGAG 3´, (R): 5´AAAGGATCCGCCCAGCAGCCT AG 3´ were used. The primers used to analyze SAP expression were: (F) 5´AGTGGCTGTGTATCATGGCAA 3´ and 5´ (R) CTTGATCTGGCTTCTGAAATG 3´.
Real Time PCR
Real-time PCR for SAP, EAT-2 and CD84 was performed using TaqMan Gene Expression Assay (Applied Biosystems) on an ABI-Prism 7300 Sequence Detector (Applied Biosystems). 18S RNA amplification control was used for cycle normalization. Data were analyzed with 7500 SDS Software (Applied Biosystems). All PCR reactions were set up in triplicates.
Yeast three-hybrid screen
Yeast three-hybrid system was performed as described elsewhere (20). The cytoplasmic tail of human CD84, cloned in pBridge, was co-transformed with SHP-1 or Csk (cloned in pGAD 424 and pACT2 respectively) in the yeast strain CG1945. ILT2 cloned in pBridge was used as a positive control. Yeast clones were then tested by the β-galactosidase assay. The β-galactosidase liquid culture assay using ONPG as a substrate was carried out as described in the Clontech (Palo Alto, CA, USA) yeast protocols handbook.
FACS staining
CD84 expression was analized using biotinylated anti-CD84 for 30 min at 4°C and the staining was developed by streptavidin-PE. FcεRI and c-kit expression were analyzed by direct staining with the indicated antibodies for 30 min at 4°C. Cells were then analyzed using a FACScalibur flow cytometer (FACScan Becton Dickinson & Co, Mountain View, CA, USA).
Lentiviral shRNA gene silencing
Lentiviral particles carrying human Fes shRNA or non-target control shRNA sequences were purchased from Santa Cruz. Infection of LAD2 cells was done according to manufacturer´s instructions. Targeted cells were selected with 0.4µg/ml puromycin for a week. Fes expression was analyzed by Western blot and silencing % was related to actin.
β-hexosaminidase release assay
Cells were sensitized overnight with 100 ng/ml biotinylated IgE, in StemPro-34 media with SCF and IL-6 (only SCF for LAD2 cells). Cells were then incubated with biotinylated anti-CD84 or anti-CD229 mAbs as isotype control in Tyrode´s buffer (10 mM Hepes, 137 mM NaCl, 2.7 mM, KCl, 0.4 mM NaH2PO4, 1.8 mM CaCl2, 1.3 mM MgSO4, 5.6 mM glucose and 0.025% BSA) for 30 min at 37 °C. After washing, cells were stimulated with 100 ng/ml streptavidin (Sigma, St. Louis, MO, USA) at 37 °C for 30 min. Several streptavidin concentrations were tested in order to verify excess of streptavidin in the assay and lack of competition between biotinylated IgE and either biotinylated anti-CD84 or isotype control. No significant differences were observed at all streptavidin concentrations studied (Supp Fig 2). IgE-dependent mast cell degranulation was monitored by β-hexosaminidase release as described (23). The resulting β-hexosaminidase activity was expressed as the percentage of maximum response (samples treated with triton X-100). % β-hexosaminidase release = (sample release minus spontaneous release/ maximum release minus spontaneous release) * 100.
Calcium mobilization
Calcium mobilization in LAD2 cells was followed by fluorimetric analysis of cytoplasmic free calcium with Fluo-4 AM fluorescent dye (Molecular Probes ®, Invitrogen). 0.2×106 cells/ point were loaded with 5µM Fluo-4 AM for 30 min at 4 °C in the dark, washed twice with Tyrode´s buffer and resuspended. Fluorimetric measurements were done in a Modulus ™ II Microplate Multimode Reader, Turner Biosystems (Promega, CA, USA) according to the manufacturer’s instructions.
Cell activation, immunoprecipitation and immunoblotting
Mast cells were sensitized overnight with biotinylated-IgE (100ng/ml) in culture medium. The following day, cells were incubated for 30 min at 37 °C with biotinylated anti-CD84 or anti-CD229 mAbs, washed twice with Tyrode´s buffer and then stimulated with 100 ng/ml of streptavidin in the same buffer for the indicated times at 37 °C. For immunoprecipitation studies, cells were washed twice with ice-cold PBS and solubilized in lysis buffer (0.5% Triton X-100, 0.5% sodium deoxycholate, 0.05% SDS, 50 mM Tris pH 7.4, 150 mM NaCl, 100 mM NaF, 1 mM Na3VO4, 1 mM phenylmethylsulfonyl fluoride (PMSF)). Cell lysates were pre-cleared by centrifugation; the whole cell lysate (WCL) fraction was collected, incubated first with the indicated primary antibodies and, subsequently with protein A-sepharose beads (Amersham Pharmacia Biotech, Sweden). After rotation for 2 h at 4 °C, the beads were washed three times with lysis buffer. Immunoprecipitated proteins were eluted by heat treatment at 100 °C for 5 min with 3 × sampling buffer. Immunoprecipitates and total cell lysates were separated by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and electrotransferred to polyvinylidene difluoride (PVDF) membranes (Millipore, Bedford, MA, USA). Blots were probed with the indicated antibodies. In all blots, proteins were visualized by enhanced chemiluminescence (Pierce, Rockford IL, USA).
GM-CSF and IL-8 production
IgE-dependent GM-CSF and IL-8 production and release was measured by ELISA with the DuoSet® ELISA Development System as described by the manufacturer (R&D Systems, Inc. MN, USA). Briefly, after overnight IgE sensitization, cells were incubated with either biotinylated anti-CD84 or anti-CD229 as an isotype control for 30 min at 37 °C, and then stimulated with streptavidin (1 µg/ml) for 6 h. Supernatants were then collected and analyzed by ELISA.
Biotinylation titration
Capture ELISA was performed by plating 1µg/ml goat anti-mouse Ig (Sigma, St. Louis, MO, USA) in PBS at 4°C overnight. Blocking was done for 1 hour at RT with 1% BSA in PBS. Biotinylated anti-CD84 and anti-CD229 were incubated for 2 h at RT at various concentrations. Streptavidin-HRP (R&D Systems, Inc. MN) was used to detect biotinylation and was incubated for 30 minutes. After several washes with PBS plus 0,01% Tween the assay was developed with TMB, 3,3,5,5-tetramethylbenzidine HRP substrate solution (R&D Systems, Inc. MN). The enzyme reaction was stopped after 10 minutes by adding diluted sulfuric acid. Quantitative measurement was carried out using microplate reader. The absorbance was measured at 450nm.
Protein concentration was previously determined by BCA protein assay (Pierce, Rockford IL) according to manufacturer´s instructions. Biotinylation titration showed that anti-CD84 and isotype control (anti-CD229) Abs have comparable levels of biotinylation. At equal protein concentration, no differences in streptavidin binding were observed for both Ab preparations (Supp Fig 1).
Statistical data analysis
All results are expressed as mean ± standard deviation (SD). Student´s t-test was used to determine significant differences (p value) between two experimental groups after determination of normal distribution of the sample and variance analysis.
RESULTS
CD84, but not SAP or EAT-2 is expressed in human mast cells
To explore the role of the CD150 family member CD84 in human mast cells in the context of FcεRI signaling, we initially examined the presence of CD84, both at the mRNA and protein level, in LAD2 cells and primary huMCs and indeed confirmed that CD84 was expressed in both cell types (Fig. 1 A, B, C). It has been reported that the effects of CD150 family members depend on the presence or absence of the adaptors SAP and/or EAT-2 (24). Despite observing the expression of CD84 in the human mast cells, we did not detect the expression of SAP and EAT-2 mRNA (Fig. 1 A) or SAP protein levels in these cells (Fig 1 D). The lack of efficient anti-EAT-2 antibodies available precluded us from examining the levels of this adaptor at the protein level. SAP and EAT-2 mRNA levels were also undetectable following FcεRI activation (data not shown). These observations led us to suggest that, in human mast cells, CD84 plays a role independently of SAP or EAT-2 signaling.
Figure 1. CD84 and SAP expression in mast cells.
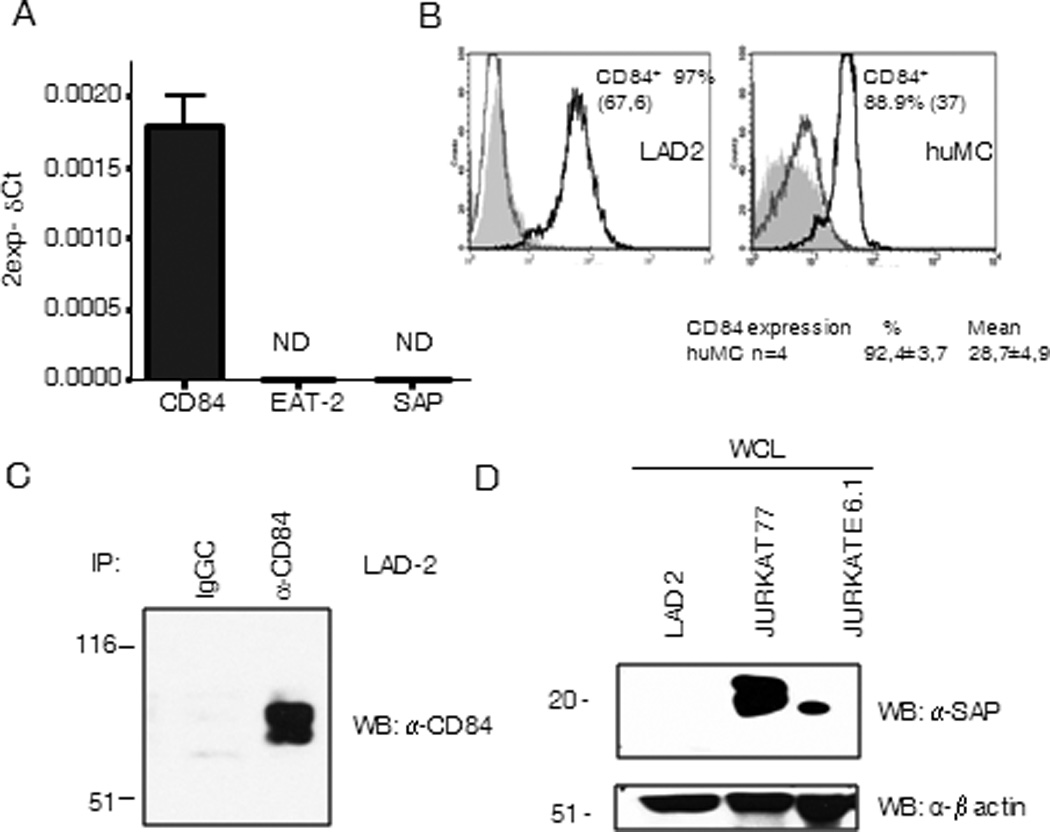
CD84, SAP and EAT-2 expression was analyzed in CD34+ peripheral blood-derived huMCs cells by real time PCR. Data are representative of three independent experiments (A). CD84 expression on LAD2 cells (left panel) and huMCs cells (right panel) surface was analyzed by FACS. Isotype CD229 antibody control (grey line) and streptavidin-PE (grey filled) are represented on the left histograms and CD84 expression on the right. Percentage of positive cells and mean of fluorescence (in brackets) are indicated in the figure. Number of donors analyzed is also stated below right panel (B). Western blot against CD84 from anti-CD84 immunoprecipitates from LAD2 cells lysates was performed (C), and western blot against SAP was done in whole cell lysates from indicated cells (D).
IgE-mediated huMC degranulation is inhibited by CD84
Following Ag-mediated FcεRI aggregation, mast cells rapidly release preformed pro-inflammatory molecules stored in cytoplasmic granules. We thus examined whether CD84 could enhance degranulation or modify FcεRI-mediated degranulation in LAD2 cells and huMCs cells after ligation or co-ligation of these two receptors. In both cell types, we observed no effect after CD84 triggering but a consistent reduction in β-hexosaminidase release following streptavidin mediated co-crosslinking of biotinylated IgE and anti-CD84 but not with an irrelevant isotype control mAb (Fig 2A and 2B respectively). We analyzed whether CD84 ligation had any effect on FcεRI expression on LAD2 cells surface. No changes on FcεRI were observed after 10 min and 30 min CD84 stimulation either at 37°C or 4°C. Thus, we can rule out that CD84 inhibitory role is due to effects on FcεRI expression (Supp Fig 3). Thus, CD84 when co-ligated with FCεRI inhibits antigen/IgE-mediated mast cell degranulation.
Figure 2. Inhibition of FcεRI-mediated degranulation by CD84.
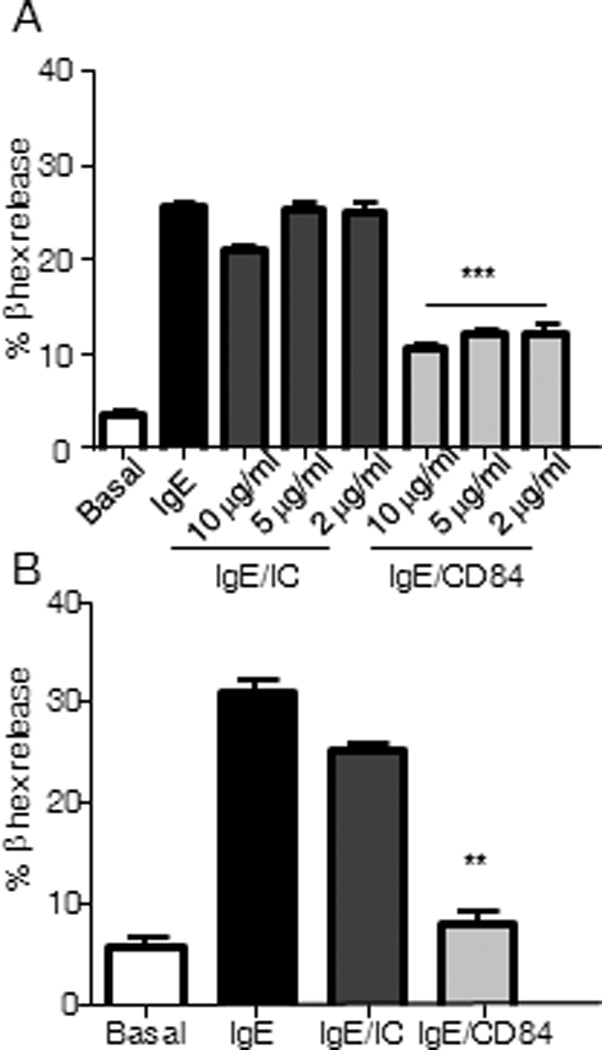
β-hexosaminidase contents were measured in the supernatants from activated LAD2 cells and huMCs. Biotinylated-IgE-sensitized LAD2 mast cells were incubated with the indicated concentrations of either biotinylated anti-CD84 mAb (IgE/CD84), isotype control (IgE/IC) or media (IgE). Co-engagement with 100 ng/ml streptavidin (SA) was allowed for 30 min (A). Degranulation of huMC’s was performed as described above in the presence of 5 µg/ml of either anti-CD84 or isotype control (IC) (B). Results, expressed as mean +/− SD, are representative of three independent experiments, p value (**p<0.001, ***p<0.0001) refers to IgE/CD84 compared against IgE/IC condition. Basal condition represents media with no stimuli.
FcεRI-mediated cytokine production is negatively regulated by CD84 in huMCs
Later events triggered after FcεRI aggregation include the de novo synthesis and secretion of cytokines such as IL-8 and GM-CSF. These mediators are the most abundant cytokines produced by huMCs, thus their release upon co-engagement of CD84 and FcεRI was explored. We observed that CD84 reduces FcεRI-produced IL-8 and GM-CSF detected in cultured supernatants of primary huMCs, when compared to cross-linking of FcεRI and an isotypic control (Fig.3A and 3B).
Figure 3. CD84 inhibits FcεRI-mediated cytokine secretion.
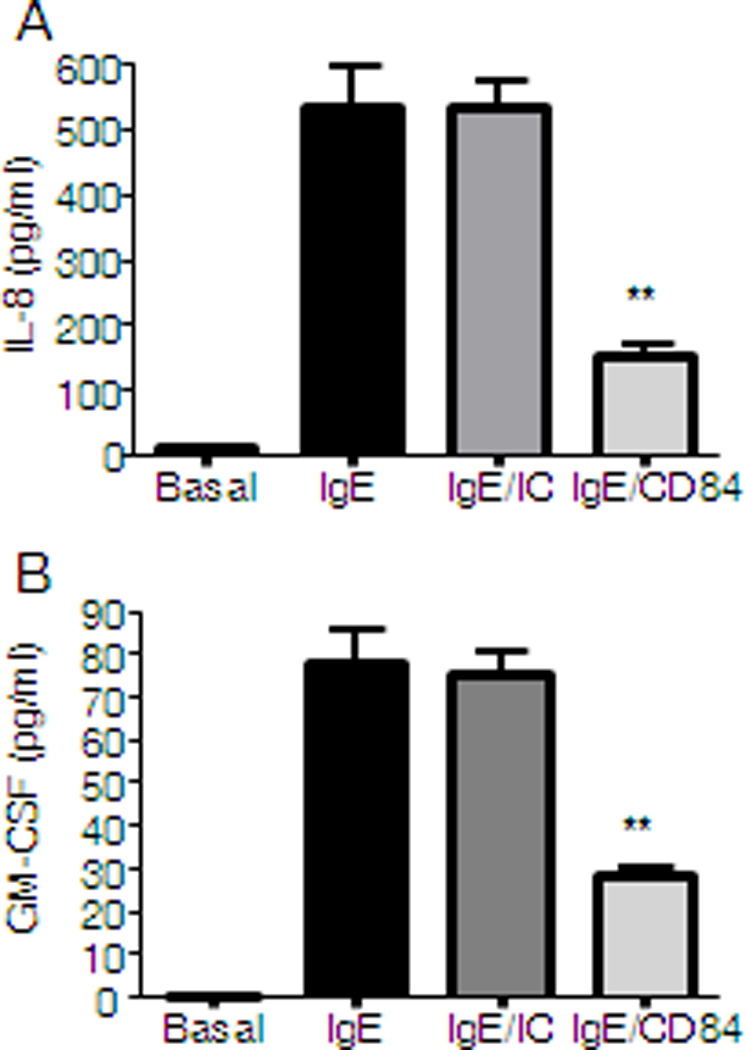
CD34+ peripheral blood-derived huMCs were sensitized overnight with biotinylated IgE and incubated with 5 µg/ml of biotinylated anti-CD84 (IgE/CD84) or isotype control (IgE/IC) or media (IgE) and then stimulated for 6 h with 1 µg/ml streptavidin (SA). IL-8 (A) and GM-CSF (B) production is represented. Results are representative of three independent experiments and are expressed as the mean +/− SD. Statistical significance (*p< 0.05, **p< 0.001) is relative to IgE/IC.
IgE-mediated calcium flux is reduced after CD84 engagement
Calcium mobilization is a key step required for mast cell degranulation and cytokine production (25). FcεRI aggregation leads to an increase in IP3 production, as a consequence of the activation of PLCγ, and the subsequent release of calcium from intracellular stores. This in turn promotes extracellular calcium influx to the cell. We thus evaluated if CD84 down-regulates FcεRI-mediated degranulation and cytokine production by attenuating the ability of aggregated FcεRI to generate the necessary calcium signal. As shown in Figure 4, CD84 was observed to dampen the FcεRI-dependent calcium response, however CD84 treated and control cells were able to respond equally to ionomycin suggesting that cells are viable and CD84 inhibits FcεRI-dependent calcium influx, thus providing support for our conclusion that an attenuated calcium signal accounts for the downregulated degranulation and cytokine production observed after CD84 engagement in human mast cells (Fig 4).
Figure 4. Calcium mobilization is down-regulated by FcεRI and CD84 co-ligation.
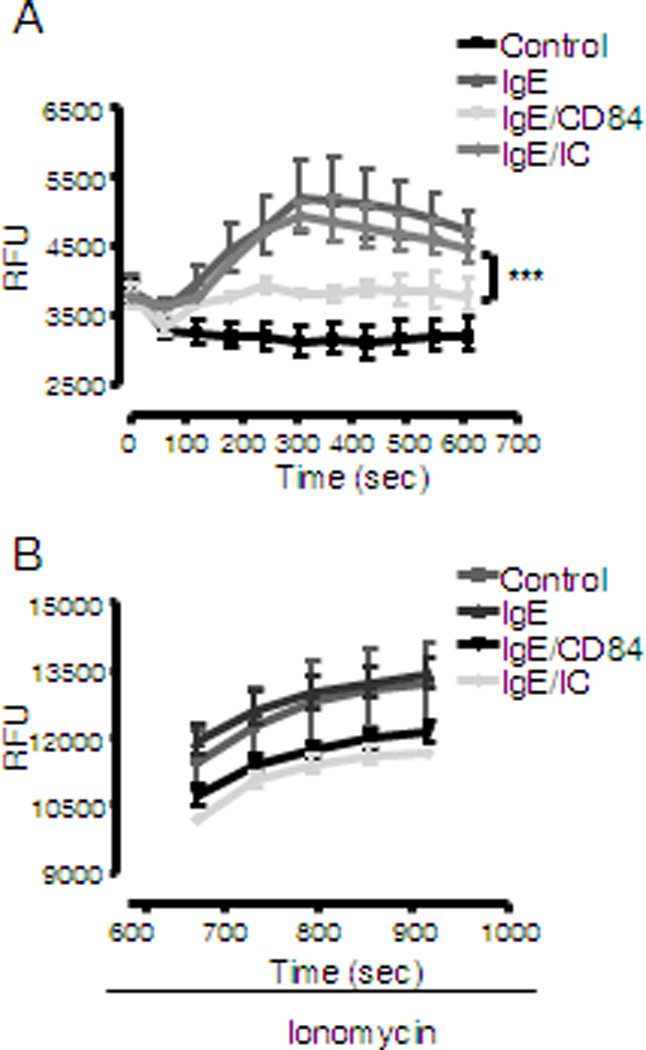
Determination of calcium flux following FcεRI stimulation was performed with biotinylated IgE sensitized LAD2 cells loaded with Fluo4 dye after incubation with 5 µg/ml biotinylated anti-CD84 mAb or isotype control (IC). Cells were stimulated with 100 ng/ml streptavidin (SA) and analyzed by fluorimetric measure of free calcium. Results are expressed as mean +/− SD and is representative of 3 independent experiments. Significance (***p<0.0001) represents IgE/CD84 compared to IgE/IC conditions. (A). Cells subsequently treated with different stimuli respond to a similar extent to ionomycin (B).
The Syk-LAT- PLCγ1 axis is down-regulated by CD84
The calcium signal elicited by FcεRI aggregation responsible for mediator release requires Syk kinase activation leading to the phosphorylation of the transmembrane adaptor LAT and subsequently PLC-γ1 activation. We thus analyzed whether the primary signaling pathway leading to calcium flux was altered after CD84 cross-linking. We performed a time course of co-stimulation with antigen/IgE together with anti-CD84 or isotype control mAb, and evaluated total phosphorylation status of whole cell lysates. As shown in Figure 5A, the level of tyrosine phosphorylation of several proteins induced by antigen was reduced after IgE/CD84 cross-linking. The molecular weight of proteins as indicated by the arrows, 160kDa, 70–72kDa and 36–38kDa, that appeared less phosphorylated were compatible with those of PLCγ1, Syk and LAT. Accordingly, when compared to the co-crosslinking with IgE and an isotype Ig control, co-engagement of CD84 and FcεRI showed a marked reduction in PLCγ1Y783, SykY352, and LATY191 phosphorylation as early as 10 seconds after stimulation (Fig 5B). These differences were maintained up to 3 min after stimulation and signals disappeared after 10 min.
Figure 5. (A) FcεRI and CD84 co-engagement reduce the phosphorylation of specific proteins.
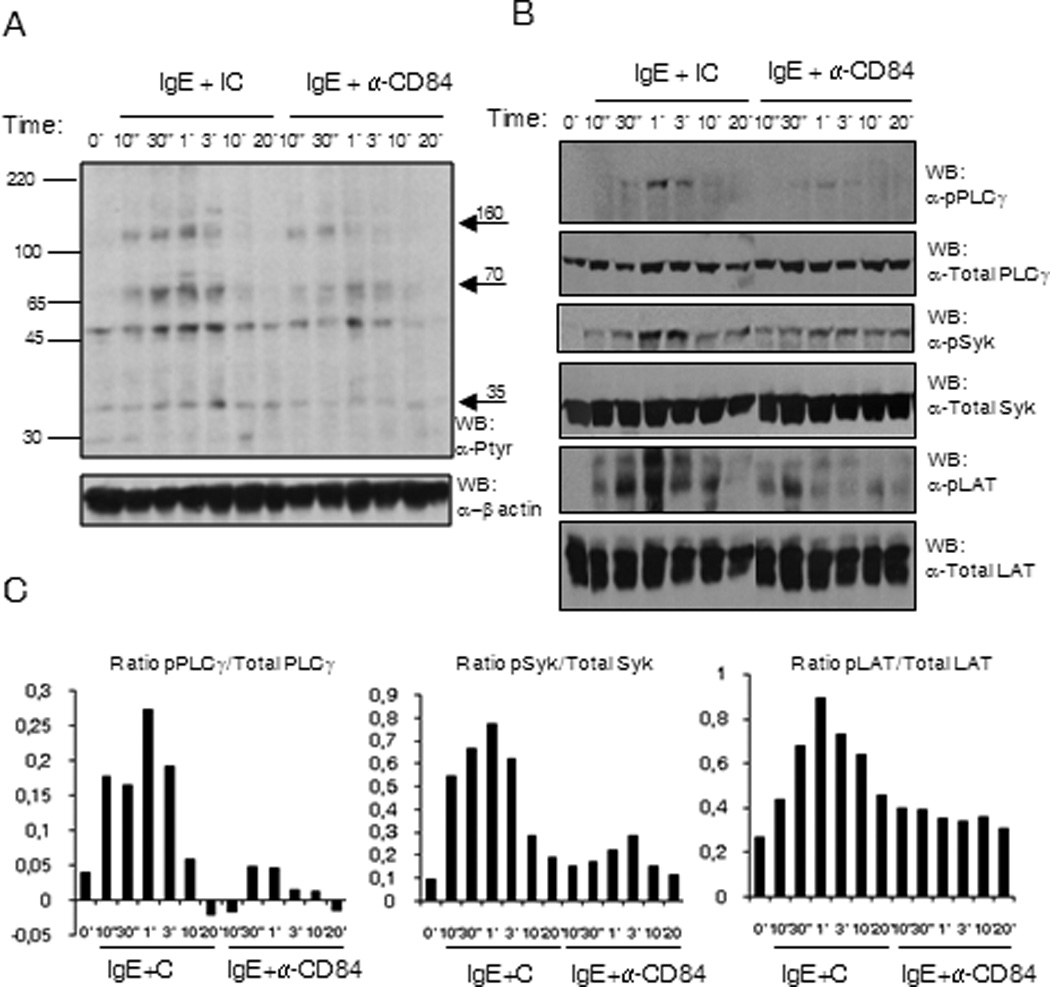
Phosphotyrosine western blot of LAD2 cell lysates upon biotinylated IgE and anti-CD84 or isotype control crosslinking with 250 ng/ml SA. The time of stimulation is indicated in the figure. Approximate molecular weight of differentially phosphorylated proteins are indicated by arrows on the right (B) SykY352, LATY191 and PLCγ1Y783 are less phosphorylated upon receptors co-ligation. Specific anti-phosphoresidue antibodies for pSykY352, pLATY191 and pPLCγ1Y783 were used to blot membrane with LAD2 cell lysates upon biotinylated IgE and anti-CD84 or isotype control crosslinking with 250 ng/ml SA. The time of stimulation is indicated in the figure. Membranes were reprobed with anti-total PLCγ1, anti-total Syk and anti-total LAT Abs to calculate phosphorylated/total protein ratios and to check loading levels per lane. Densitometric analysis and phosphorylated /total ratio for each molecule from 3 independent experiments is represented in the bar charts on the bottom (C).
PI3-kinase and ERK pathways are negatively regulated by CD84
The role of PI3K-mediated pathways in the control of cytokine production (26), have been well documented. To investigate whether the inhibitory action of CD84 on cytokine production could be due to the down-regulation of these signals, we examined the phosphorylation status of Akt, as a surrogate marker for PI3 kinase activation, at 5, 10 and 20 min following co-ligation of FcεRI and CD84 or FcεRI aggregation in the presence of an isotypic control. As shown in Figure 6A, FcεRI-dependent Akt phosphorylation was reduced when co-ligated with CD84 when compared with the isotype control (Fig 6A).
Figure 6. Akt and ERK phosphorylation are reduced by CD84 engagement.
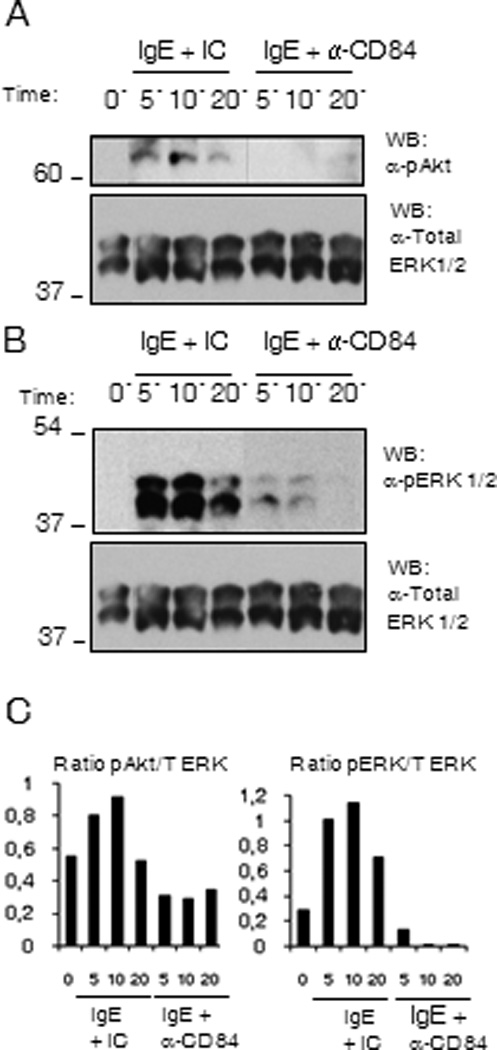
Biotinylated IgE sensitized (Bio-IgE) LAD2 cells were incubated with anti-CD84 mAb (CD84) or Ig isotype control (IC) and co-engaged with 100 ng/ml SA for 5, 10 and 20 min. Cells were lysed and total cell lysates were analyzed for (A) Akt phosphorylation as a surrogate marker for PI3K activation, and (B) ERK1/2 phosphorylation status. The blot was reprobed with total ERK. Membranes were reprobed with anti-total ERK 1/2 Abs to calculate phosphorylated/total protein ratios and to check loading levels per lane. Densitometric analysis and phosphorylated /total ratio for each molecule from 3 independent experiments is represented in the bar charts on the bottom (C).
Early events including LAT phosphorylation can activate the MAP kinases ERK1/2 through its interaction with guanine nucleotide exchange factors like Vav and SOS, allowing transcriptional regulation. As MAPK signaling is involved in the phosphorylation of transcription factors that control cytokine expression (27) defective ERK1/2 activation may also account for the reduced cytokine production observed in response to co-ligation with CD84. Therefore, we additionally investigated the effect of CD84 crosslinking on the ERK cascade, and observed a weaker activation of ERK 1/2 following FcεRI and CD84 co-engagement than the one observed when FcεRI was crosslinked alone or with an isotypic control (Fig 6B). These data support the conclusion that CD84 may down-regulate FcεRI-mediated cytokine production through the reversal of PI3K and ERK1/2 activation.
FcεRI aggregation induces CD84 phosphorylation
The ability of inhibitory receptors to down-regulate downstream signals vital for cell function requires the recruitment of inhibitory signaling molecules following phosphorylation of specific tyrosine residues contained within the ITIM, or in the case of CD150 family members, ITSM, consensus sequences. We thus examined the phosphorylation status of CD84 following FcεRI aggregation in LAD2 cells. We observed a very rapid and weak, but consistent increase in CD84 tyrosine phosphorylation after FcεRI stimulation that peaks at 30 s after IgE crosslinking (Fig. 7) which decreased by 3 min.
Figure 7. CD84 gets tyrosine phosphorylated upon FcεRI engagement.
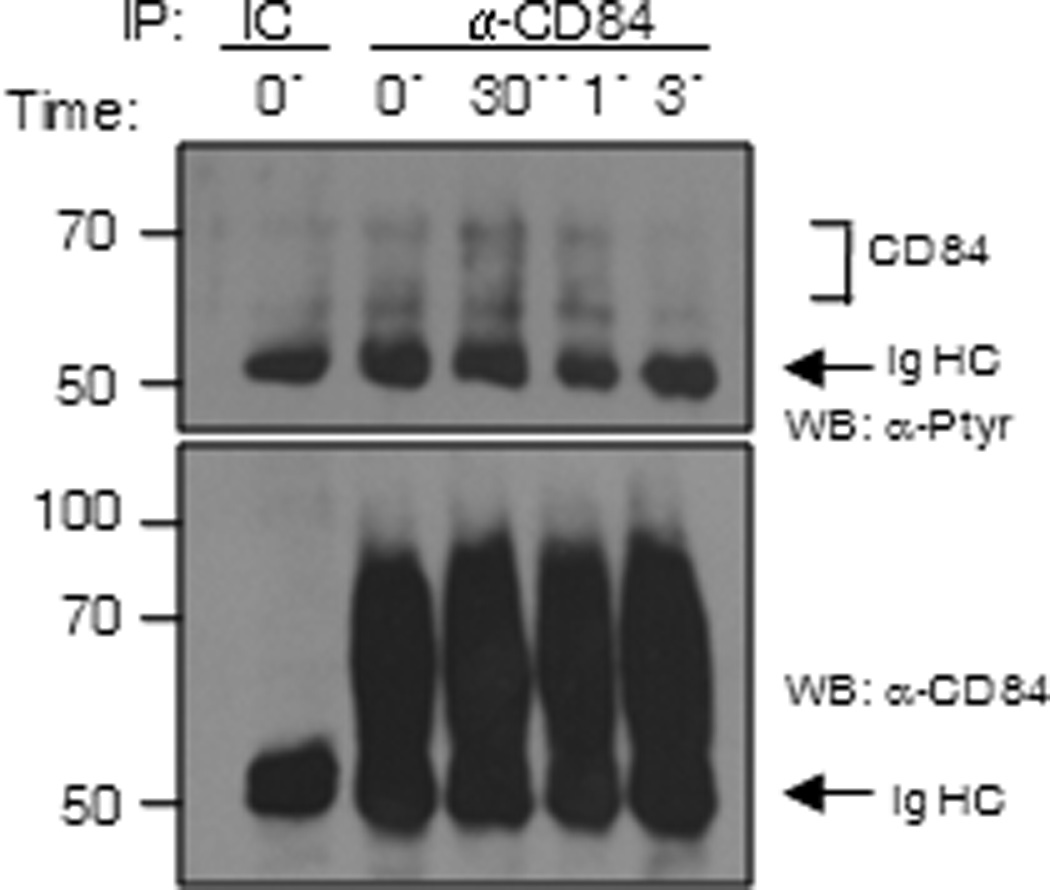
LAD2 cells were stimulated through the FcεRI for 0, 30 sec, 1 and 3 min, lysed and immunoprecipitated with anti-CD84 mAb. Membranes were blotted with anti-phosphotyrosine Ab and total CD84 levels were analyzed by western blot. Immunoprecipitation with an isotype control (IC) was also loaded.
Lyn and Fes kinases phosphorylate CD84 in COS-7 cells
Most inhibitory receptors on immune cells contain a consensus amino-acid sequence, termed the ITIM cytoplasmic domain (28). The prototype ITIM consists of the sequence (I/V/L/S)-x-Y-x-x-(L/V), where × denotes any amino acid. The two tyrosines in the CD84 cytoplasmic tail, Y279 and Y324, have proven equally crucial for CD84-mediated degranulation inhibition but none of them are a SAP-EAT2 consensus motif (17). Tyrosine 324 (S-S-Y-E-I-V-I) on CD84 features the ITIM consensus, whereas tyrosine 279 (R-I-Y-D-E-I) does not. Ligand-induced clustering of these ITIM-containing receptors results in tyrosine phosphorylation, often by a Src-family kinases, which provides a docking site for the recruitment of the tyrosine phosphatases SHP-1 and SHP-2, the inhibitory kinase, Csk or the inositol phosphatase, SHIP. Nevertheless, studies involving yeast in a phospho-dependent condition failed to show any direct interaction between CD84 and SHP-1 or Csk (Table 1). We used as a positive control for these experiments the previously described interaction between ILT2 receptor and SHP-1 and Csk (22). We also were unable to detect any interaction between CD84 and SHP-1, CSK or SHIP (data not shown) in COS-7, transfected RBL-2H3 cells or LAD2 cells. As shown previously, the CD84 Y279 or Y324 are not responsible for SHP-2 binding (17). Thus, the role of SHP-2 does not account for CD84-mediated inhibition.
Table I.
Interaction measured by three hybrid system between negative regulators SHP-1 or Csk and CD84 cytoplasmic tail. ILT2 receptor was used as a positive control. The activity range goes from − undetectable, + from 1 to 2 β-galactosidase units, ++ more than 2 units.
| ILT2 (positive control) | CD84 | |
|---|---|---|
| SHP-1 | ++ | − |
| Csk | + | − |
Lyn and Fes kinases have also been implicated in the negative regulation of FcεRI mediated signaling in mast cells (29). Therefore we analyzed the ability of both Lyn and Fes kinases to phosphorylate the CD84 residues Y279 and Y324. To do this, we co-transfected COS-7 cells with WT and mutant CD84 constructs with deletions of these residues together with either Lyn or Fes, then analyzed phosphorylation status. As shown in Figure 8A the major phosphorylation targets for Lyn in CD84 cytoplasmic tail are the residues corresponding to Y279 and Y324, and that disruption of these residues completely abolishes CD84 phosphorylation (Fig 8A). Lyn specificity was demonstrated by the lack of CD84 phosphorylation when co-transfected together with Lyn K275M mutant that has no kinase activity (lane 1 Fig 8A).
Figure 8. (A) CD84 Y279 is preferentially phosphorylated by Lyn.
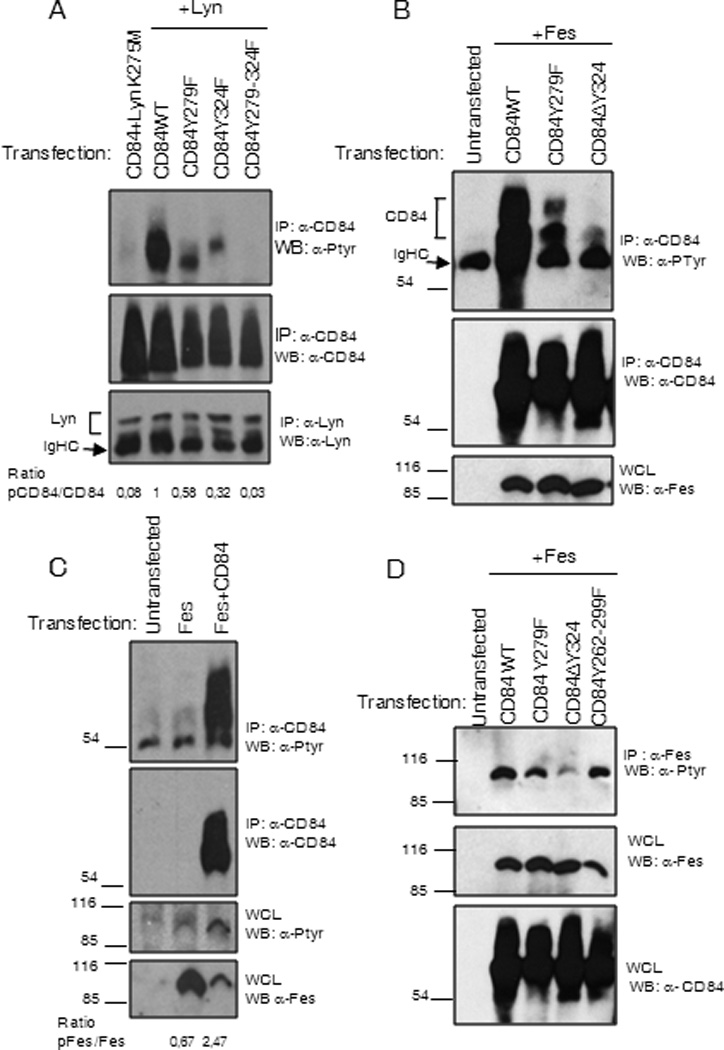
Anti-CD84 immunoprecipitation was performed from COS-7 cells co-transfected with WT and CD84 mutants and Lyn kinase. Lyn K275M represents Lyn mutant with ablated kinase activity. Membranes were blotted with anti-phosphotyrosine, anti–CD84 Abs. (B) CD84 Y324 is preferentially phosphorylated by Fes. Anti-CD84 immunoprecipitation was performed from COS-7 cells co-transfected with WT and CD84 mutants and Fes kinase. Membranes were blotted with anti-phosphotyrosine, anti–CD84 and anti-Fes Abs. (C) Fes phosphorylation is enhanced upon co-expression with CD84. Fes transfection in the presence or absence of CD84 was performed. Anti-Fes immunoprecipitation from COS-7 cells cotransfected with WT and CD84 mutants and Fes kinase was done (D). Membranes were blotted with anti-phosphotyrosine, anti-CD84 and anti-Fes Abs.
In a similar set of experiments, we found that CD84 Y324 fits with the consensus sequence for binding to Fes kinase SH2 domain. Thus, this residue that is part of a putative Fes binding motif (30), is the major residue within CD84 cytoplasmic tail that gets phosphorylated by Fes (Fig 8B). Interestingly, we detected a greater increase in Fes phosphorylation when co-transfected with CD84 (Fig.8C). Mutation of residue Y324 in CD84 almost completely abolished Fes tyrosine phosphorylation. As it has been described that Fes is activated following binding to its subtrates (31), these data strongly suggested that Fes could bind to CD84. However, we have been unable to consistently demonstrate this interaction.
Negative regulators Fes kinase and SHP-1 phosphatase are phosphorylated after FcεRI and CD84 crosslinking in human mast cells
In order to further delineate CD84 inhibitory mechanism in mast cells, particularly the contribution of the inhibitory kinase Fes, we examined the phosphorylation of Fes after FcεRI and CD84 crosslinking and compared it with that obtained upon FcεRI aggregation in the presence of an isotypic control. By blotting immunoprecipitated Fes for tyrosine phosphorylation, we observed a rapid phosphorylation of Fes after co-engagement of FcεRI and CD84, but not when FcεRI in the presence of an isotypic control was activated. As early as 30 seconds after FcεRI and CD84 co-ligation phosphorylated Fes was already detected and the kinase stayed phosphorylated 1 min after stimulation, concomitantly with CD84 phosphorylation after IgE stimulation. These data further support our conclusion that Fes participates in the inhibitory mechanism exerted by FcεRI in human mast cells (Fig 9A).
Figure 9. (A) Fes kinase is phosphorylated upon FcεRI and CD84 co-ligation.
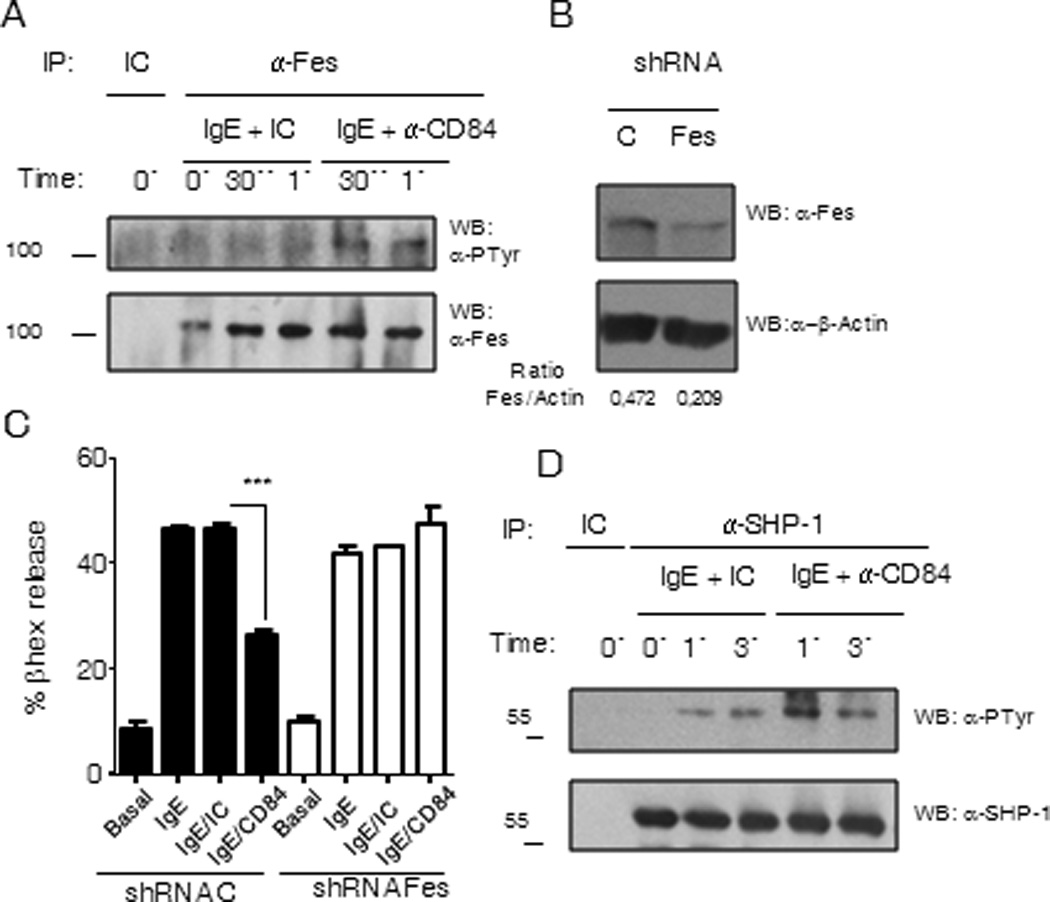
IgE-sensitized LAD2 cells were further incubated with biotinylated anti-CD84 or isotype control for 30 min. Cells were stimulated at 0, 30 sec or 1 min with 100 ng/ml SA, washed and lysed. Anti-Fes immunoprecipitation was performed and precipitates were analyzed by western blot against phosphotyrosine. Total Fes was evaluated by anti-Fes western blot. (B) shRNA Fes kinase silencing. Fes in LAD2 cells was targeted with Fes shRNA or a unspecific control shRNA (C) by lentiviral infection approach. Fes reduction was analyzed by anti-Fes western blot. Fes to actin ratio was measured to calculate Fes silencing %. (C) Fes knock down reverses CD84 mediated β-hexosaminidase release inhibition. Biotinylated-IgE-sensitized LAD2 cells with normal (black bars) versus reduced (white bars) levels of Fes kinase were incubated with 5µg/ml biotinylated anti-CD84 mAb (IgE/CD84), 5µg/ml biotinylated isotype control (IgE/IC) or media (IgE). Co-engagement with 100 ng/ml streptavidin (SA) was allowed for 30 min and β-hexosaminidase release was measured. Significance (***p<0.0001) represents IgE/CD84 compared to IgE/IC conditions. (D) SHP-1 phosphorylation increases after FcεRI and CD84 co-ligation. Sensitized IgE LAD2 cells, incubated with anti-CD84 antibody or isotype antibody control were stimulated as described above for 0, 1 or 3 min. Cells were lysed and SHP-1 was immunoprecipitated. SHP-1 phosphorylation was evaluated by anti-phosphotyrosine western blot of immunoprecipitates. Total SHP-1 was assessed by anti-SHP-1 western blot.
After CD84–FcεRI co-ligation there is a reduction in the phosphorylation of several proteins as indicated above. SHP-1 tyrosine phosphatase is a well known negative regulator of immune receptors signaling as it catalyzes dephosphorylation of tyrosine residues within signaling mediators (32). SHP-1 gets activated upon recruitment to phosphorylated ITIMs, and in several cases correlation between phosphorylation and increase in phosphatase activity has been described (33). Although we could not observed direct interaction between SHP-1 and CD84 we analyzed SHP-1 phosphorylation status after FcεRI and CD84 crosslinking in order to verify whether SHP-1 is activated under these conditions. We found an increase in SHP-1 phosphorylation after FcεRI and CD84 co-ligation as compared to FcεRI stimulation in the presence of an isotypic control. (Fig. 9 D)
Fes knock down reverses CD84 mediated inhibition
To further evaluate Fes contribution to CD84 negative regulatory function, we specifically targeted Fes with shRNA (Fig 9B). We obtained around 50% reduction of normal Fes expression in LAD2, and analyzed these cells for degranulation after FcεRI/IC or FcεRI/CD84 stimulation. We observed that CD84 inhibition on IgE mediated granule release observed in control shRNA cells was reverted in Fes targeted cells (Fig 9C). These results suggest that at least part of CD84 negative signals are mediated by the inhibitory kinase Fes.
DISCUSSION
Inhibitory receptors that reverse signals mediated by activating molecules have been described to play a crucial role in regulating the immune system (28). In the case of mast cells these receptors may be considered as potential targets for future therapeutics in allergic inflammation and mast cell dependent disease. Here, we describe that CD84, a member of the CD150 family of receptors, is a novel negative regulator of FcεRI-initiated signals in human mast cells. The CD150 family of receptors are key regulators that can mediate opposite functions in immune cells such as NK, NK-T, macrophages, T and B cells (34). A “switch of function” dual role model for CD150 receptor family members has been proposed by Veillette, whereby alternative activating/costimulatory and inhibitory functions are dictated by the presence or absence of the adaptors SAP and/or EAT-2 (35). It has been observed in NK cells that under conditions where SAP and EAT-2 are absent, CD150 family members can interact with inhibitory molecules including phosphatases or inhibitory kinases such as Csk, thereby dampening activating receptor responses (36). The role of the CD150 family of receptors and SAP adaptors have not been elucidated in human mast cells to date.
CD84 is highly expressed in mast cells (16) and is a self ligand receptor acting as a homophilic adhesion molecule (14). CD84 interacts with itself with very high affinity through its Ig-like domain 1 in a “head to head” interaction thus suggesting that CD84 interacts with the CD84 from another cell rather than in the same cell (14, 37). Human CD84 function has been described in T and B lymphocytes. In peripheral blood, CD84-CD84 interactions lead to INFγ production in response to sub optimal doses of anti CD3 (14). In T cells, CD84 appears to also signal in a SAP-independent manner, at least for certain responses. In this respect, cells from XLP patients are able to proliferate in a comparable way to WT cells after CD3-CD84 co-engagement, suggesting an additional level of complexity to the “switch of function” paradigm (38). In the case of B cells, CD84 is expressed and interacts both with SAP and EAT-2 (20),(39). The relevance of CD84 as a player in adaptive immunity and humoral responses has become evident in CD84-deficient mice which have a defect in the normal formation of germinal centers in the lymph nodes. CD84 mediates the adhesion necessary for a correct T cell-B cell interaction and for optimal orchestration of humoral responses (40). There is no data about mast cells function in CD84 knock out mice to date. It is of note, that murine CD84 cytoplasmic tail does not possess the inhibitory Y324 motif.
By transfecting CD84 into RBL 2H3 cells, our group previously determined that CD84 can dampen FcεRI-initiated signals in this cell line and that residues Y279 and Y324 (not involved in SAP and EAT-2 recruitment) in CD84 cytoplasmic tail are critical for such inhibition (17). Given the limitations of previous study due to the necessary heterologous transfection of human CD84 into a rat cell line, it was however unclear whether these findings were indicative of a potential biological role of endogenous CD84 in human mast cell function. In the present work we provide evidence using the more physiologically appropriate primary cultured CD34+-peripheral blood derived human mast cells as well as LAD2 cell line to further demonstrate a negative role for CD84 in FcεRI-mediated responses in the absence of SAP and EAT-2 expression. Our current data suggest that CD84 signals independently of SAP or EAT-2 and our previous results (17) pointed at the tyrosine motifs, that are not involved in SAP and EAT-2 direct binding, as crucial for CD84 inhibitory role giving altogether the information that this receptor can play an important role as a modulator beyond SAP and EAT-2 recruitment or dependent signaling of these adaptors. Additionally, as reported (41), we also found that CD84 is expressed in mast cells isolated from human lung tissue, and have evidence that it also reduces degranulation in such cells (data not shown).
Consistent with these findings, is that CD84 gets rapidly phosphorylated upon FcεRI engagement, which most likely allows it to regulate its inhibitory function, as previous results with RBL cells suggested. Although the Src family tyrosine kinase Lck phosphorylates CD84 in T cells (38), and Fyn phosphorylates all tyrosine residues in CD84 transfected COS-7 cells (20),(17) selective CD84 phosphorylation in its inhibitory residues has not been investigated to date. We found that in co-transfected COS-7 cells, Lyn is able to phosphorylate CD84 primarily at Y279 and also Y324. Indeed, double deletion of these residues completely abolishes Lyn-mediated phosphorylation of CD84. These data indicates that Lyn is not responsible for phosphorylation of SAP-EAT-2 dependent motifs present in CD84 cytoplasmic tail.
The Lyn-regulated negative pathway of mast cell modulation involves Csk-dependent activation of Fyn, mediated by Lyn dependent Cbp phosphorylation (42). However we were unable to detect differences in Cbp phosphorylation upon FcεRI-CD84 versus FcεRI crosslinking. Given that signals downstream of Fyn activation lead to PI3K activation via Gab2 phosphorylation (43) and that we show that Akt phosphorylation (a surrogate marker for PI3K activation) is reduced upon FcεRI/CD84 co-ligation, a direct activation of Csk leading to a Fyn activity reduction could not be ruled out. Csk has been shown to interact with specific members of CD150 family such as CD244 (44), however, we were unable to detect such an interaction with CD84 in a three hybrid system assay using 2B4 as a positive control.
PI3K signaling can be also downregulated by SHIP-1 in mast cells (45). SHIP-1 antagonizes the action of the PI3K pathway by converting the PI3K-produced signaling intermediate PI (3,4,5)P3 to PI(3,4)P2 thereby preventing recruitment of PH domain-containing proteins such as Akt to signaling complexes at the membrane. Thus we analyzed SHIP-1 phosphorylation as indicative of SHIP-1 activity. However, again we observed no differences between CD84 and control co-ligation in LAD 2 cells (data not shown).
The more distal tyrosine Y324 conforms to a Fes kinase binding docking site (YExV/I) (30), and we show that Fes is able to strongly phosphorylate CD84 at this residue. Although we have consistently observed Fes activation in the presence of WT CD84, we have failed to detect a direct interaction between these two molecules. It cannot be ruled out the possibility that CD84 could also indirectly affect Fes activity. Given that CD84 is a self-binding adhesion molecule that could modify cell adhesive properties one could speculate that cytoskeleton modification under such conditions could in turn affect Fes activation status. Fps/Fes and Fer are a group of non-receptor cytoplasmic protein-tyrosine kinases, that have been implicated in the regulation of innate immunity through generating signals downstream of a number of cytokines, growth factor and immunoglobulin receptors (46). In mast cells, Fes negatively regulates FcεRI responses, thus, Fes-deficient BMMCs are hyper responsive at low doses of Ag (29). In addition, Fes kinase is tyrosine phosphorylated in a Lyn-dependent manner upon Ag engagement of FcεRI, therefore a negative regulatory axis involving Lyn-Fes kinases has been proposed. We observed that Fes phosphorylation in LAD2 cells is higher after co-crosslinking of FcεRI with CD84, than with a negative control. Furthermore, Fes expression reduction leads to abrogation of CD84 inhibitory effect on IgE mediated responses such as degranulation. We failed to observe IgE mediated hyperdegranulation in LAD2 cells wit low levels of Fes. One possibility is that the remaining levels of Fes in these cells still can control granule release in the context of FcεRI signaling alone. Another possibility could be that Fes negative action on FcεRI has been describe for low Ag doses, which would not necessarily be comparable to our streptavidin crosslinking system. Considering that Fes has been shown to phosphorylate inhibitory receptors such as Pecam in BMMCs (29), a scenario where Fes gets activated via CD84 and phosphorylates another inhibitory receptor that then recruits phosphatases is conceivable. Low but detectable phosphorylation of CD84 could reflect its role in the initiation of the negative signal that would be then amplified by other inhibitory receptors able to recruit SHP-1. This would suggest that CD84 could be a player in the negative regulatory axis upstream of Lyn and Fes. Higher concentration of Lyn kinase brought by activated FcεRI could increase Fes activation and trigger CD84 phosphorylation at inhibitory tyrosines.
It is recognized that phosphorylation of Syk at Y352 residue is associated with the conformational change in Syk required for activation. Accordingly, we observed that Syk is less phosphorylated at this residue upon FcεRI-CD84 co-ligation when compared to that induced by FcεRI aggregation in the absence of CD84. Consistent with a lower activation of Syk, we also observed that the FcεRI-dependent phosphorylation of LAT (Y191) is reduced when CD84 is engaged. LAT is critical for recruitment of PLCγ1 and calcium signaling (6). Calcium signals are essential for degranulation and optimal production of cytokines in mast cells in response to antigen and other stimulants (47). Consistent with these reports is our observation that CD84 triggering decreases calcium signals. In addition to inhibition of the calcium signal, we found that CD84 co-engagement with the FcεRI strongly dampens both Akt and ERK phosphorylation. Consistent with weaker upstream signals observed upon co-stimulation we observed lower production of the IL-8/CXCL8 and GM-CSF cytokines.
In conclusion, in the present work we demonstrate that CD84 a member of the CD150 family of receptors can negatively regulate FcεRI mediated signals, degranulation and cytokine secretion in human mast cells in a SAP-EAT-2 independent and a Fes and SHP-1 dependent mechanisms. Considering that CD84 is a self ligand we hypothesize that this receptor could set a threshold for FcεRI activation that would be overcome when IgE receptor is aggregated by an encounter with Ag. Defining the precise pathway that regulates CD84 signaling in mast cells as well as the mechanisms that modulate its expression would be crucial in order to establish the potential therapeutic value of this dual receptor.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Dr. Margaret Hibbs (Ludwig Institute for Cancer Research, Melbourne) for providing the Lyn constructs and Feilah Mohamed for technical assistance.
This work was supported by grant SAF2009-07548 from the Plan Nacional Ministerio de Ciencia e Innovación. Spain. D. A–E and E. A–E are supported by a Juan de la Cierva contract and a FPI fellowship respectively, both from Ministerio de Ciencia e Innovación, Spain. I. O–V is funded by a contract from CIBERES. J.S. is supported by a contract Miguel Servet from Fondo de Investigaciones Sanitarias from Instituto de Salud Carlos III (CP06/00058). Work conducted in the laboratory of A.M.G. is supported by the Intramural Research Program within the National Institute of Allergy and Infectious Disease of the National Insitutes of Health, USA.
REFERENCES
- 1.Galli SJ, Grimbaldeston M, Tsai M. Immunomodulatory mast cells: negative, as well as positive, regulators of immunity. Nat Rev Immunol. 2008;8:478–486. doi: 10.1038/nri2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hershko AY, Rivera J. Mast cell and T cell communication; amplification and control of adaptive immunity. Immunol Lett. 128:98–104. doi: 10.1016/j.imlet.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rivera J, Olivera A. Src family kinases and lipid mediators in control of allergic inflammation. Immunol Rev. 2007;217:255–268. doi: 10.1111/j.1600-065X.2007.00505.x. [DOI] [PubMed] [Google Scholar]
- 4.Zhang J, Berenstein EH, Evans RL, Siraganian RP. Transfection of Syk protein tyrosine kinase reconstitutes high affinity IgE receptor-mediated degranulation in a Syk-negative variant of rat basophilic leukemia RBL-2H3 cells. J Exp Med. 1996;184:71–79. doi: 10.1084/jem.184.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gilfillan AM, Rivera J. The tyrosine kinase network regulating mast cell activation. Immunol Rev. 2009;228:149–169. doi: 10.1111/j.1600-065X.2008.00742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gilfillan AM, Tkaczyk C. Integrated signalling pathways for mast-cell activation. Nat Rev Immunol. 2006;6:218–230. doi: 10.1038/nri1782. [DOI] [PubMed] [Google Scholar]
- 7.Bachelet I, Munitz A, Moretta A, Moretta L, Levi-Schaffer F. The inhibitory receptor IRp60 (CD300a) is expressed and functional on human mast cells. J Immunol. 2005;175:7989–7995. doi: 10.4049/jimmunol.175.12.7989. [DOI] [PubMed] [Google Scholar]
- 8.Alvarez-Errico D, Aguilar H, Kitzig F, Brckalo T, Sayos J, Lopez-Botet M. IREM-1 is a novel inhibitory receptor expressed by myeloid cells. Eur J Immunol. 2004;34:3690–3701. doi: 10.1002/eji.200425433. [DOI] [PubMed] [Google Scholar]
- 9.Abramson J, Licht A, Pecht I. Selective inhibition of the Fc epsilon RI-induced de novo synthesis of mediators by an inhibitory receptor. Embo J. 2006;25:323–334. doi: 10.1038/sj.emboj.7600932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katz HR, Vivier E, Castells MC, McCormick MJ, Chambers JM, Austen KF. Mouse mast cell gp49B1 contains two immunoreceptor tyrosine-based inhibition motifs and suppresses mast cell activation when coligated with the high-affinity Fc receptor for IgE. Proc Natl Acad Sci U S A. 1996;93:10809–10814. doi: 10.1073/pnas.93.20.10809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ujike A, Ishikawa Y, Ono M, Yuasa T, Yoshino T, Fukumoto M, Ravetch JV, Takai T. Modulation of immunoglobulin (Ig)E-mediated systemic anaphylaxis by low-affinity Fc receptors for IgG. J Exp Med. 1999;189:1573–1579. doi: 10.1084/jem.189.10.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwartzberg PL, Mueller KL, Qi H, Cannons JL. SLAM receptors and SAP influence lymphocyte interactions, development and function. Nat Rev Immunol. 2009;9:39–46. doi: 10.1038/nri2456. [DOI] [PubMed] [Google Scholar]
- 13.Sayos J, Wu C, Morra M, Wang N, Zhang X, Allen D, van Schaik S, Notarangelo L, Geha R, Roncarolo MG, Oettgen H, De Vries JE, Aversa G, Terhorst C. The X-linked lymphoproliferative-disease gene product SAP regulates signals induced through the co-receptor SLAM. Nature. 1998;395:462–469. doi: 10.1038/26683. [DOI] [PubMed] [Google Scholar]
- 14.Martin M, Romero X, de la Fuente MA, Tovar V, Zapater N, Esplugues E, Pizcueta P, Bosch J, Engel P. CD84 functions as a homophilic adhesion molecule and enhances IFN-gamma secretion: adhesion is mediated by Ig-like domain 1. J Immunol. 2001;167:3668–3676. doi: 10.4049/jimmunol.167.7.3668. [DOI] [PubMed] [Google Scholar]
- 15.de la Fuente MA, Pizcueta P, Nadal M, Bosch J, Engel P. CD84 leukocyte antigen is a new member of the Ig superfamily. Blood. 1997;90:2398–2405. [PubMed] [Google Scholar]
- 16.Romero X, Benitez D, March S, Vilella R, Miralpeix M, Engel P. Differential expression of SAP and EAT-2-binding leukocyte cell-surface molecules CD84, CD150 (SLAM), CD229 (Ly9) and CD244 (2B4) Tissue Antigens. 2004;64:132–144. doi: 10.1111/j.1399-0039.2004.00247.x. [DOI] [PubMed] [Google Scholar]
- 17.Oliver-Vila I, Saborit-Villarroya I, Engel P, Martin M. The leukocyte receptor CD84 inhibits Fc epsilon RI-mediated signaling through homophilic interaction in transfected RBL-2H3 cells. Mol Immunol. 2008;45:2138–2149. doi: 10.1016/j.molimm.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 18.Radinger M, Jensen BM, Kuehn HS, Kirshenbaum A, Gilfillan AM. Generation, isolation, and maintenance of human mast cells and mast cell lines derived from peripheral blood or cord blood. Curr Protoc Immunol Chapter. 7 doi: 10.1002/0471142735.im0737s90. Unit 7 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin M, Del Valle JM, Saborit I, Engel P. Identification of Grb2 as a novel binding partner of the signaling lymphocytic activation molecule-associated protein binding receptor CD229. J Immunol. 2005;174:5977–5986. doi: 10.4049/jimmunol.174.10.5977. [DOI] [PubMed] [Google Scholar]
- 20.Sayos J, Martin M, Chen A, Simarro M, Howie D, Morra M, Engel P, Terhorst C. Cell surface receptors Ly-9 and CD84 recruit the X-linked lymphoproliferative disease gene product SAP. Blood. 2001;97:3867–3874. doi: 10.1182/blood.v97.12.3867. [DOI] [PubMed] [Google Scholar]
- 21.Del Valle JM, Engel P, Martin M. The cell surface expression of SAP-binding receptor CD229 is regulated via its interaction with clathrin-associated adaptor complex 2 (AP-2) J Biol Chem. 2003;278:17430–17437. doi: 10.1074/jbc.M301569200. [DOI] [PubMed] [Google Scholar]
- 22.Sayos J, Martinez-Barriocanal A, Kitzig F, Bellon T, Lopez-Botet M. Recruitment of C-terminal Src kinase by the leukocyte inhibitory receptor CD85j. Biochem Biophys Res Commun. 2004;324:640–647. doi: 10.1016/j.bbrc.2004.09.097. [DOI] [PubMed] [Google Scholar]
- 23.Furumoto Y, Brooks S, Olivera A, Takagi Y, Miyagishi M, Taira K, Casellas R, Beaven MA, Gilfillan AM, Rivera J. Cutting Edge: Lentiviral short hairpin RNA silencing of PTEN in human mast cells reveals constitutive signals that promote cytokine secretion and cell survival. J Immunol. 2006;176:5167–5171. doi: 10.4049/jimmunol.176.9.5167. [DOI] [PubMed] [Google Scholar]
- 24.Veillette A. SLAM-family receptors: immune regulators with or without SAP-family adaptors. Cold Spring Harb Perspect Biol. 2 doi: 10.1101/cshperspect.a002469. a002469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vig M, Kinet JP. Calcium signaling in immune cells. Nat Immunol. 2009;10:21–27. doi: 10.1038/ni.f.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gu H, Saito K, Klaman LD, Shen J, Fleming T, Wang Y, Pratt JC, Lin G, Lim B, Kinet JP, Neel BG. Essential role for Gab2 in the allergic response. Nature. 2001;412:186–190. doi: 10.1038/35084076. [DOI] [PubMed] [Google Scholar]
- 27.Karin M. Mitogen-activated protein kinase cascades as regulators of stress responses. Ann N Y Acad Sci. 1998;851:139–146. doi: 10.1111/j.1749-6632.1998.tb08987.x. [DOI] [PubMed] [Google Scholar]
- 28.Ravetch JV, Lanier LL. Immune inhibitory receptors. Science. 2000;290:84–89. doi: 10.1126/science.290.5489.84. [DOI] [PubMed] [Google Scholar]
- 29.Udell CM, Samayawardhena LA, Kawakami Y, Kawakami T, Craig AW. Fer and Fps/Fes participate in a Lyn-dependent pathway from FcepsilonRI to platelet-endothelial cell adhesion molecule 1 to limit mast cell activation. J Biol Chem. 2006;281:20949–20957. doi: 10.1074/jbc.M604252200. [DOI] [PubMed] [Google Scholar]
- 30.Songyang Z, Shoelson SE, McGlade J, Olivier P, Pawson T, Bustelo XR, Barbacid M, Sabe H, Hanafusa H, Yi T, et al. Specific motifs recognized by the SH2 domains of Csk, 3BP2, fps/fes, GRB-2, HCP, SHC, Syk, and Vav. Mol Cell Biol. 1994;14:2777–2785. doi: 10.1128/mcb.14.4.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Filippakopoulos P, Kofler M, Hantschel O, Gish GD, Grebien F, Salah E, Neudecker P, Kay LE, Turk BE, Superti-Furga G, Pawson T, Knapp S. Structural coupling of SH2-kinase domains links Fes and Abl substrate recognition and kinase activation. Cell. 2008;134:793–803. doi: 10.1016/j.cell.2008.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bolland S, Ravetch JV. Inhibitory pathways triggered by ITIM-containing receptors. Adv Immunol. 1999;72:149–177. doi: 10.1016/s0065-2776(08)60019-x. [DOI] [PubMed] [Google Scholar]
- 33.Lorenz U. SHP-1 and SHP-2 in T cells: two phosphatases functioning at many levels. Immunol Rev. 2009;228:342–359. doi: 10.1111/j.1600-065X.2008.00760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cannons JL, Tangye SG, Schwartzberg PL. SLAM family receptors and SAP adaptors in immunity. Annu Rev Immunol. 29:665–705. doi: 10.1146/annurev-immunol-030409-101302. [DOI] [PubMed] [Google Scholar]
- 35.Veillette A, Dong Z, Perez-Quintero LA, Zhong MC, Cruz-Munoz ME. Importance and mechanism of 'switch' function of SAP family adapters. Immunol Rev. 2009;232:229–239. doi: 10.1111/j.1600-065X.2009.00824.x. [DOI] [PubMed] [Google Scholar]
- 36.Dong Z, Cruz-Munoz ME, Zhong MC, Chen R, Latour S, Veillette A. Essential function for SAP family adaptors in the surveillance of hematopoietic cells by natural killer cells. Nat Immunol. 2009;10:973–980. doi: 10.1038/ni.1763. [DOI] [PubMed] [Google Scholar]
- 37.Yan Q, Malashkevich VN, Fedorov A, Fedorov E, Cao E, Lary JW, Cole JL, Nathenson SG, Almo SC. Structure of CD84 provides insight into SLAM family function. Proc Natl Acad Sci U S A. 2007;104:10583–10588. doi: 10.1073/pnas.0703893104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tangye SG, Nichols KE, Hare NJ, van de Weerdt BC. Functional requirements for interactions between CD84 and Src homology 2 domain-containing proteins and their contribution to human T cell activation. J Immunol. 2003;171:2485–2495. doi: 10.4049/jimmunol.171.5.2485. [DOI] [PubMed] [Google Scholar]
- 39.Tangye SG, van de Weerdt BC, Avery DT, Hodgkin PD. CD84 is up-regulated on a major population of human memory B cells and recruits the SH2 domain containing proteins SAP and EAT-2. Eur J Immunol. 2002;32:1640–1649. doi: 10.1002/1521-4141(200206)32:6<1640::AID-IMMU1640>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 40.Cannons JL, Qi H, Lu KT, Dutta M, Gomez-Rodriguez J, Cheng J, Wakeland EK, Germain RN, Schwartzberg PL. Optimal germinal center responses require a multistage T cell:B cell adhesion process involving integrins, SLAM-associated protein, and CD84. Immunity. 32:253–265. doi: 10.1016/j.immuni.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ghannadan M, Baghestanian M, Wimazal F, Eisenmenger M, Latal D, Kargul G, Walchshofer S, Sillaber C, Lechner K, Valent P. Phenotypic characterization of human skin mast cells by combined staining with toluidine blue and CD antibodies. J Invest Dermatol. 1998;111:689–695. doi: 10.1046/j.1523-1747.1998.00359.x. [DOI] [PubMed] [Google Scholar]
- 42.Ohtake H, Ichikawa N, Okada M, Yamashita T. Cutting Edge: Transmembrane phosphoprotein Csk-binding protein/phosphoprotein associated with glycosphingolipid-enriched microdomains as a negative feedback regulator of mast cell signaling through the FcepsilonRI. J Immunol. 2002;168:2087–2090. doi: 10.4049/jimmunol.168.5.2087. [DOI] [PubMed] [Google Scholar]
- 43.Parravicini V, Gadina M, Kovarova M, Odom S, Gonzalez-Espinosa C, Furumoto Y, Saitoh S, Samelson LE, O'Shea JJ, Rivera J. Fyn kinase initiates complementary signals required for IgE-dependent mast cell degranulation. Nat Immunol. 2002;3:741–748. doi: 10.1038/ni817. [DOI] [PubMed] [Google Scholar]
- 44.Eissmann P, Beauchamp L, Wooters J, Tilton JC, Long EO, Watzl C. Molecular basis for positive and negative signaling by the natural killer cell receptor 2B4 (CD244) Blood. 2005;105:4722–4729. doi: 10.1182/blood-2004-09-3796. [DOI] [PubMed] [Google Scholar]
- 45.Rauh MJ, Kalesnikoff J, Hughes M, Sly L, Lam V, Krystal G. Role of Src homology 2-containing-inositol 5'-phosphatase (SHIP) in mast cells and macrophages. Biochem Soc Trans. 2003;31:286–291. doi: 10.1042/bst0310286. [DOI] [PubMed] [Google Scholar]
- 46.Greer P. Closing in on the biological functions of Fps/Fes and Fer. Nat Rev Mol Cell Biol. 2002;3:278–289. doi: 10.1038/nrm783. [DOI] [PubMed] [Google Scholar]
- 47.Ma HT, Beaven MA. Regulation of Ca2+ signaling with particular focus on mast cells. Crit Rev Immunol. 2009;29:155–186. doi: 10.1615/critrevimmunol.v29.i2.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


