Abstract
Sensory inputs triggered by external stimuli are projected into discrete arrays of neuronal modules in the primary sensory cortex. This whisker-to-barrel pathway has gained in popularity as a model system to study the development of cortical circuits and sensory processing because its clear patterns facilitate the identification of genetically modified mice with whisker map deficits and make possible coordinated in vitro and in vivo electrophysiological studies. Numerous whisker map determinants have been identified in the past two decades. In this review, we summarize what have we learned from the detailed studies conducted in various mutant mice with cortical whisker map deficits. We will specifically focus on the anatomical and functional establishment of the somatosensory thalamocortical circuits.
Keywords: barrel, cortical map, neural circuit, the primary somatosensory cortex, thalamocortical synapses
Introduction
In cortical sensory maps, thalamocortical afferents (TCAs) transmit peripheral sensations in organized arrays into distinct cortical neuronal modules to provide a topographic representation of the external sensory world (Buonomano & Merzenich, 1998). Mis-wiring of neuronal circuits during early life is likely to be a major cause of neurological disorders, including autism and schizophrenia, which may arise from defects in cortical development (Calhoun et al., 2009; Luscher & Huber, 2010; Rubenstein, 2011). The rodent whisker map in the primary somatosensory (S1) cortex (Fig. 1) has emerged as a popular model system to elucidate the molecular mechanism underlying the formation of cortical neural circuits as well as to explore how sensory experience affects the development of neural networks (Erzurumlu & Kind, 2001; Feldman & Brecht, 2005; Fox & Wong, 2005; Inan & Crair, 2007; Petersen, 2007; Fox, 2008).
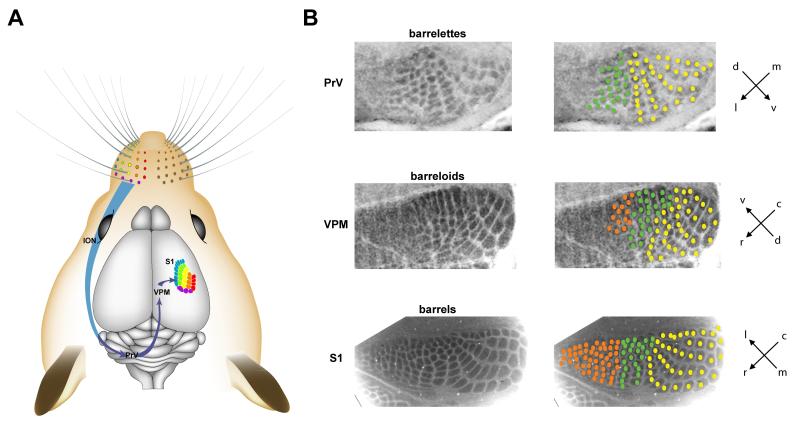
Figure 1.
The lemniscal whisker-to-barrel pathway in the mouse brain. (A) The whiskers on the snout are innervated by the infraorbital branch of the maxillary nerve (ION), which transmit sensory information to the rostral principal nucleus (PrV) in the brainstem. Trigeminothalamic axons from the PrV project to the ventral posteromedial nucleus (VPM) in the thalamus in the contralateral hemisphere. Thalamocortical axons from the VPM project to the primary somatosensory cortex (S1). The five rows of whiskers and the straddle whiskers are color-coded. (B) Representative images of CO-stained coronal sections through PrV of brain stem (barrelettes), VPM of thalamus (barreloids), and tangential sections through cortical layer IV (barrels). CO patches corresponding to rostroventral whiskers are marked with orange and green circles, and caudodorsal whiskers with yellow circles. Drawing in A is modified from an Adobe Illustrator file generously provided by Dr. Knott (Knott et al., 2002).
In this review, we focus on the molecular determinants of mouse cortical whisker map formation and their roles in neuronal morphogenesis and synaptic function/plasticity. First, the developmental processes occurring during embryonic and early postnatal ages that lead to the formation of “barrels”, neuronal modules in the S1 cortex representing individual whiskers, will be described as has been informed by studies conducted with mice. Next, the distinctive characteristics of the whisker map deficits found in a variety of genetically modified mice (transgenic mice) will be summarized. This section will focus on how specific mutations affect the arborization pattern of thalamocortical axons and the dendritic morphogenesis of cortical layer IV glutamatergic neurons within the S1 cortex. Finally, the functional development of thalamocortical synapses will be discussed in wildtype and mutant mice with cortical whisker map deficits.
The rodent whisker-to-barrel pathway
Rodents have poor vision and depend on tactile information derived from whiskers on their snout to navigate through their environment. Facial whiskers are innervated by the infraorbital branch of the trigeminal nerve (ION). The central afferents of these primary sensory neurons innervate brain stem trigeminal nuclear complex (BSTC), including the rostral principal nucleus (PrV) and the caudal spinal nucleus (SpV) (Fox, 2008; Erzurumlu et al., 2010) (Fig 1). The spatial arrangements of neuronal modules for individual whiskers in the BSTC, named “barrelettes”, are arranged into a row-and arc pattern recapitulating arrangement of the facial whiskers. Trigeminothalamic axons from the PrV project to the contralateral thalamic ventral postero-medial (VPM) nucleus, via the lemniscal pathway. In VPM they form the whisker-related pattern called “barreloids” with an inverted orientation. The TCAs of VPM innervate the S1 cortex and form whisker-related clusters in cortical layer IV. Each TCA cluster relaying sensory information from an individual whisker is encircled by a distinctive ring of cortical layer IV neurons (barrels) (Woolsey & Van der Loos, 1970; Killackey & Leshin, 1975). Barrelettes in the BSTC begin to appear at late P0 (Li et al., 1994) or P1 (Ma, 1993) depending on the mouse strain. Around P3, barreloids and barrels begin to develop in the thalamus (Woolsey, 1990) and the S1 cortex (Rebsam et al., 2002), respectively. Sensory information from individual whiskers is relayed to the corresponding barrelette, barreloid, and barrel in a clear one-to-one relationship.
The developing axons of trigeminal ganglion neurons are guided by a combination of molecular cues to target their peripheral and central targets and sculpt their synaptic connections to form the whisker map (reviewed in Erzurumlu et al., 2010). The identified positional cues that lay out the rough topography include various neurotrophins, axon guidance molecules, transcription factors, and glutamate receptors. Loss-of-function mutation in the gene encoding Drg11, a paired-domain transcription factor expressed in the principal nucleus of BSTC but not in the thalamus or cortex, results in the absence of whisker map throughout the whisker-to-barrel pathway (Ding et al., 2003). Interestingly, mutant mice with extra whiskers have extra barrels in the corresponding locations (van der Loos & Dorfl, 1978; Van der Loos et al., 1984). These findings suggest that the spatial layout in the principal brainstem nucleus, the first sensory relay station, forms the basis for the whisker map in higher sensory stations. The refinement of sensory maps and the establishment of precise stimulus-response features, however, may depend on other cellular and synaptic processes that rely, at least in part, on neural activity-dependent mechanisms (Katz & Shatz, 1996; Crair, 1999).
In addition to the lemniscal pathway, sensory information derived from whiskers can also be relayed to the cortex through the paralemniscal pathway (Petersen, 2007; Fox, 2008). Here, sensory afferents from SpV in the BSTC project to the posteriomedial nucleus of thalamus (POm), and then POm TCAs innervate the inter-barrel septal area of the S1 layer IV. In this review we have focused on the lemniscal pathway because it is the major whisker-to-barrel pathway.
The development of reciprocal connections between the thalamus and the cortex
Thalamic axons projecting into the cortex relay sensory inputs, while reciprocal innervations from the cortex to the thalamus provide critical feed-back to modulate the thalamic responses required for the complex information processing and integration that underlie cognitive behaviors (Jones, 2002; Alitto & Usrey, 2003; Temereanca & Simons, 2004; Theyel et al., 2010). Around embryonic day 13 (E13) (Fig. 2), TCAs derived from the dorsal thalamus begin to project ventrally and then turn dorso-laterally at the boundary between the diencephalon and the telencephalon (Lopez-Bendito & Molnar, 2003; Price et al., 2006). At E13.5, TCAs pass through a restricted but permissive corridor between the lateral and medial ganglionic eminences (LGE and MGE) as tight bundles (Molnar et al., 1998). This corridor is formed by a special population of GABAergic neurons, called guidepost cells, originating from the lateral ganglion eminence and migrating to the mantle region of the MGE (Lopez-Bendito et al., 2006). After entering striatum proper, TCAs separate into multiple fascicles and reach the pallial-subpallial boundary (PSPB). In the mean time, corticothalamic axons (CTAs), originating from preplate principal neurons, leave the cortical plate and extend to the PSPB (Jacobs et al., 2007). The PSPB has been proposed to be a critical region for the reciprocal interactions between the TCAs and CTAs (Molnar & Butler, 2002). Deleting the transcription factor Pax6 in this area causes aberrant routing of many TCAs and CTAs (Simpson et al., 2009). After a period of potential interaction between TCAs and CTAs in the PSPB, the respective axons resume their progression toward their specific targets.
Figure 2.
Timeline for development of the mouse cortical whisker map.
The long distance navigation of TCAs and CTAs towards their targets involves an elaborate coordination of multiple factors that initiate and guide axon outgrowth, fasciculation, navigation, target recognition, and refinement (Katz & Constantine-Paton, 1988; Molnar et al., 2003; Garel & Rubenstein, 2004; Price et al., 2006; Inan & Crair, 2007). Several ligand/receptor families involved in axon guidance have been identified (reviewed in O’Donnell et al., 2009). These include semaphorins/plexin and neuropilin, ephrins/Eph, netrins/DCC and UNC5, as well as Slits/Robo. Slit2 is expressed in the hypothalamus and its activity repel the Robo-expressing guidepost cells that form the corridor for TCAs (Bielle et al., 2011). Thus they prevent TCAs from erroneously entering the hypothalamus and from crossing the midline (Bagri et al., 2002; Braisted et al., 2009). A “handshake hypothesis” for TCA and CTA interactions has been postulated based on the close association of these tracts (Molnar & Blakemore, 1995), and the observation that deleting a particular transcription factor expressed only in the cortex or only in the thalamus leads to abnormalities of both tracts (Hevner et al., 2002). This hypothesis posits that corticothalamic and thalamocortical axons serve as scaffolds for each other and axon-axon interactions are important for the correct patterning and targeting of these axons. Recently, we found that removing cannabinoid receptor type 1 (CB1R) from cortical glutamatergic neurons leads to morphological changes in both CTAs and TCAs (Wu et al., 2010). These observations suggest that endocannabinoid signaling may be a modulator of the handshake interactions between CTAs and TCAs, especially for the interactions mediating the fasciculation process.
The subplate (SP) neurons, which are among the first born cortical neurons, send “pioneer” axons to meet TCAs in the PSPB, and defects in SP neurons lead to failure of TCA innervation of the cortex (McConnell et al., 1989; Zhou et al., 1999; Kanold & Luhmann, 2010). Once TCAs reach the cortex at ~E15 (Molnar et al., 1998), they pause in the SP layer and then begin innervating the cortex at ~ postnatal day (P) 0. Whether early neural activity is required for correct TCA targeting is still controversial. Genetic ablation of SNAP25, a protein that is involved in evoked neurotransmitter release, did not affect the growth, targeting or innervation of TCAs into the S1 cortex during embryonic development (Molnar et al., 2002). This suggests that TCA growth, early topographic sorting of these fibers and innervation into cortex does not rely on activity-dependent mechanisms requiring evoked neurotransmitter release.
The development of whisker map in the S1 cortex
Barrels, the neuronal modules representing whiskers, can be easily identified as periodic, cell dense walls separated by a cell sparse area in cortical layer IV of the S1 cortex. During postnatal development, TCAs reach layer IV and VI neurons immediately after birth (Rebsam et al., 2002). At P2, TCAs in the cortex separate into two loosely distributed bands, one in layer VI and one in layer IV (Rebsam et al., 2002). At P3, when TCAs begin to segregate into whisker-related clusters in layer IV, the distributions of dendrites projecting out from individual spiny stellate neurons are minimally polarized (Espinosa et al., 2009). At P4, the whisker map can also be revealed by the distribution of post-synaptic molecules like PKARIIβ (protein kinase A regulatory subunit IIß) (Watson et al., 2006), mGluR5 (metabotropic glutamate receptor 5) (Wijetunge et al., 2008), and FMRP (Fragile X mental retardation protein) (Harlow et al., 2010). At P5, glial processes revealed by either glutamate transporter GLAST or GLT-1 immunoreactivity also distribute in a whisker-related manner (Voutsinos-Porche et al., 2003). At P6, the time when the barrel ring is just beginning to form, spiny stellate neurons develop a polarized dendritic pattern (Espinosa et al., 2009). After P6, the dendritic segment number of layer IV neurons and the complexity of TCA arbors continue to rise until the end of the second postnatal week.
Molecular determinants of whisker map formation
The prominent anatomical features of “barrels” have facilitated the identification of several mutant mice with defective whisker maps in the S1 cortex (Erzurumlu & Kind, 2001). In this review, we will focus on the mutant mice that have normal cortical laminations and normal axonal targeting (Table). There are two types of whisker map deficits found in these mutant mice that can be distinguished based on the characteristics of their pattern deficits. The first type is the complete absence of whisker map. This group includes, but is not limited to, the null mutant mice of the following genes: NMDAR (N-methyl-D-aspartate receptor) subunit 1 or 2B (NR1 and NR2B) (Li et al., 1994; Kutsuwada et al., 1996; Iwasato et al., 1997), AC1 (adenylyl cyclase I; Adcy1brl) (Welker et al., 1996), MAOA (monoamine oxidase A) (Cases et al., 1996), 5-HTT (serotonin transporter) (Persico et al., 2001; Salichon et al., 2001). In other mutant mice, the organization of layer IV neurons and whisker-related TCA clusters are “mismatched” with complete absence of the architectonic organization of layer IV neurons while the whisker-related TCA clusters are visible. This group contains the null mutant mice of mGluR5 (Wijetunge et al., 2008; She et al., 2009), PLC-ß1 (phospholipase C-ß1) (Hannan et al., 2001), PKARIIß (Inan et al., 2006; Watson et al., 2006), SynGAP (a synaptic Ras GTPase activating protein) (Barnett et al., 2006), NeuroD2 (Neurogenic Differentiation 2) (Ince-Dunn et al., 2006), and LMO4 (LIM domain-only 4) (Kashani et al., 2006). It also includes cortex-specific knockouts (KOs) of NR1 (Cx-NR1), mGluR5 (Iwasato et al., 2000; Ballester-Rosado et al., 2010), and NF1 (neurofibromin) (Lush et al., 2008). The nature of mutations leading to whisker map deficits suggest that glutamatergic and serotonergic neurotransmission, cAMP/ protein kinase A (PKA) signaling, PLC/inositol 1,4,5-trisphosphate (IP3) signaling, and activity-dependent transcription are all involved in barrel development.
Table.
A list of mutant mice with whisker map deficits
| protein | gene name / global or cortex-specific KO |
barreletts in BSTC |
barreloids in thalamus |
whisker-related TCA clusters in the S1 cortex |
barrels in the S1 cortex | synaptic function/ plasticity | references |
|---|---|---|---|---|---|---|---|
| adenylyl cyclase 1 | Adcy1 / global KO |
Normal | Small barreloids not well delineated |
No whisker related pattern; single TCA analysis found normal number of axonal length, branch and bouton numbers while axonal spans are larger |
No whisker related pattern | Defective glutamatergic release, AMPAR trafficking, and long-term plasticity in thalamocortical synapses. In vivo single unit recording revealed normal topography for whisker map despite a decrease in the response latency for surrounding whiskers |
(Welker et al., 1996; Abdel-Majid et al., 1998; Lu et al., 2003; Gheorghita et al., 2006; Lu et al., 2006) |
| adenylyl cyclase 1 | Adcy1 / cortex- specific KO by EMX-Cre |
Normal | Normal | Normal | Barrels are evident but layer IV spiny stellate neurons have reduced dendritic asymmetry and increased dendritic span |
AMPA/NMDA current ratio is reduced at P11; reduced amplitude of evoked AMPA mediated currents |
(Iwasato et al., 2008) |
|
cAMP-dependent
protein kinase type II regulatory subunit |
Pka-R2 / global KO |
Normal | Normal | Normal | No whisker related pattern | Impaired thalamocortical LTP formation and AMPAR trafficking |
(Inan et al., 2006; Watson et al., 2006) |
|
NMDA receptor
subunit 1 |
Grin1 / global KO |
No whisker related pattern |
No whisker related pattern |
No whisker related pattern |
No whisker related pattern | Evoked NMDAR-responses are reduced in layer IV neurons of S1 |
(Li et al., 1994; Iwasato et al., 1997; Rudhard et al., 2003) |
|
NMDA receptor
subunit 1 |
Grin1 / cortex- specific KO by EMX-Cre |
Normal | Normal | TCAs form rudimentary pattern for caudo-dorsal whiskers; analysis of single TCA shows reduced branching and reduced total axon arbor length but wider distributions |
No whisker related pattern; layer IV spiny stellate neurons have symmetric dendritic morphology and their dendritic spans, total dendritic lengths, and spine numbers are increased |
N. A. | (Datwani et al., 2002; Lee et al., 2005; Iwasato et al., 2000) |
|
NMDA receptor
subunit 2B |
Grin2b / genetic mosaic* |
N. A. | N. A. | N. A. | KO neurons have symmetric dendritic morphology |
N. A. | (Espinosa et al., 2009) |
|
metabotropic
glutamate receptor 5 |
Grm5 or Mglur5 / global KO |
Small barreletts not well delineated |
Small barreloids not well delineated |
TCAs form rudimentary pattern for caudo-dorsal whiskers; analysis of single TCA shows reduced complexity and reduced total axon arbor length while the axonal span is increased |
No whisker related pattern; layer IV spiny stellate neurons have symmetric dendritic morphology and increased dendritic span. |
Abnormal thalamocortical LTP /LTD formation; faster NMDA current decay kinetics; normal presynaptic function; mEPSC’s frequency is increased while mIPSC’s frequency is reduced |
(Hannan et al., 2001; Wijetunge et al., 2008; She et al., 2009), (Ballester-Rosado et al., 2010) |
|
metabotropic
glutamate receptor 5 |
Grm5 or Mglur5 /cortex-specific KO with NEX- Cre |
Normal | Normal | TCAs form rudimentary pattern for caudo-dorsal whiskers; analysis of single TCA shows reduced complexity and reduced total axon arbor length while the axonal span is increased |
No whisker related pattern; layer IV spiny stellate neurons have symmetric dendritic morphology and increased dendritic span and segment numbers |
mIPSC’s frequency is reduced while mEPSC’s frequency is normal |
(Ballester-Rosado et al., 2010) |
|
Phospholipase C,
beta1 |
Plcb1 / global KO |
N. A. | N. A. | Normal | No whisker related pattern | N. A. | (Hannan et al., 2001) |
|
synaptic Ras
GTPase activating protein 1 |
Syngap1 / global KO |
Normal | Small barreloids are not well delineated |
Rudimentary pattern for caudo-dorsal whiskers |
KO: No whisker related pattern; het: reduced barrel segregation |
N. A. | (Barnett et al., 2006) |
|
monoamine
oxidase type A |
MAOA / global KO |
Normal | Small barreloids are not well delineated |
No whisker related pattern or rudimentary pattern for caudo- dorsal whiskers |
No whisker related pattern | N. A. | (Cases et al., 1996) |
|
sodium-
dependent serotonin transporter |
SERT; 5-HTT / global KO |
KO: barreletts are poorly defined; HET: small barreletts that are not well delineated |
KO: barreloids are poorly defined, VPM is smaller; het: barreloids are not well delineated |
KO: no whisker related pattern except a rudimentary pattern for a few large caudal whiskers; het: blurry boundaries for TCA clusters |
KO: no whisker related pattern; het: septa between barrels are larger |
N. A. | (Persico et al., 2001) |
|
monoamine
oxidase A; serotonin transporter |
MAOA; 5-HTT DKO |
N. A. | Small medioventral barreloids are poorly delineated |
No whisker related pattern |
No whisker related pattern | N. A. | (Salichon et al., 2001) |
|
monoamine
oxidase A; serotonin receptor 1B |
MAOA;5-HT1B DKO |
N. A. | Normal | Normal | Fuzzy whisker-related pattern with reduced barrel segregation. |
N. A. | (Salichon et al., 2001) |
|
MAOA; 5-HTT; 5-
HT1B TKO |
MAOA; 5-HTT; 5-HT1B TKO |
N. A. | N. A. | Normal | Fuzzy whisker-related pattern with reduced barrel segregation. |
N. A. | (Salichon et al., 2001) |
|
LIM domain
transcription factor LMO4 |
Lmo4 / cortex-specific KO with NEX- Cre |
N. A. | N. A. | TCAs form rudimentary pattern for caudo-dorsal whiskers |
No whisker related pattern | N. A. | (Kashani et al., 2006) |
|
neurogenic
differentiation factor 2 |
Neurod2 / global KO |
Normal | Normal | Rudimentary pattern for caudo-dorsal whiskers |
No whisker related pattern | Reduced AMPA/NMDA current ratios and reduced spontaneous EPSC amplitude |
(Ince-Dunn et al., 2006) |
|
brain derived
neurotrophic factor |
BDNF / global KO |
N. A. | N. A. | Delayed appearance of whisker pattern |
N. A. | Reduced AMPA/NMDA current ratios; defective LTP formation, and increased the number of silent synapses in TC synapses; altered development of fast-spiking GABAergic neurons |
(Itami et al., 2003; Lush et al., 2005; Itami et al., 2007) |
|
tropomyosin
receptor kinase B receptor |
NTRK2; TrkB/ global KO |
N. A. | N. A. | Delayed appearance of whisker pattern |
N. A. | N. A. | (Lush et al., 2005) |
|
tropomyosin
receptor kinase B receptor |
NTRK2; TrkB / cortex-specific KO with hGFAP-Cre |
N. A. | N. A. | Normal | N. A. | N. A. | (Lush et al., 2005) |
| EphrinA5 | EFNAS / global KO |
N. A. | N. A. | Normal whisker-related pattern but the number and length of TCA arbors are reduced |
N. A. | N. A. | (Uziel et al., 2008) |
| neurofibromin | Nf1 / cortex- specific with hGFAP-Cre |
N. A. | N. A. | Smaller TCA clusters and bigger septa area |
No whisker related pattern | N. A. | (Lush et al., 2008) |
Protein names and gene names based on www.uniprot.org
The serotonin (5-HT) afferents arising from the raphe nuclei of brain stem innervate the S1 cortex early in development, coinciding with the clustered ingrowth of thalamocortical fibers (Bennett-Clarke et al., 1993). 5-HT released into the thalamocortical synaptic cleft is taken up by TCAs via the 5-HTT and packed into synaptic vesicles via the vesicular monoamine transporter 2 (VMAT2) (Lebrand et al., 1996; Cases et al., 1998; Lebrand et al., 1998). 5-HT not packed into vesicles is degraded by MAOA, an enzyme that catalyzes the oxidative deamination of monoamines. 5-HT can activate the G-protein coupled 5-HT1B receptor on TCAs, and has been reported to inhibit the release of glutamate (Rhoades et al., 1994; Laurent et al., 2002). Deleting either MAOA or 5-HTT genes in mice results in excess 5-HT in S1 cortex and barrel map deficits (Cases et al., 1996; Persico et al., 2001). Deleting 5-HT1B receptors in either MAOA or 5-HTT KO mice rescues the barrel map deficit in these mutants (Salichon et al., 2001), suggesting that 5-HT1B receptor hyper-activity disrupts barrel map formation. However, the incomplete rescue of barrel cytoarchitecture in MAOA or 5-HTT KO mice by 5-HT1B removal suggests that additional mediators of 5-HT signaling in S1 cortex define barrel pattern. Conversely, the normal whisker map in 5-HT1B knockout mice suggests that 5-HT signaling through 5-HT1B receptors is not required for barrel formation under physiological condition (Salichon et al., 2001).
Ectopic expression of Fibroblast Growth Factor (FGF) in cortical neurons leads to duplication of barrel fields (Fukuchi-Shimogori & Grove, 2001) and TCA patterns are defective in both Cx-NR1 and Cx-mGluR5 KO mice (Iwasato et al., 2000; Ballester-Rosado et al., 2010). These latter findings argue that factors intrinsic to the cortex also instruct TCA patterning. Nevertheless, the rudimentary whisker-related TCA patterns in Cx-NR1 or Cx-mGluR5 knockouts argue against an absolute requirement for postsynaptic NMDAR and mGluR5 activity in guiding the somatotopy of TCAs. It is likely that TCAs themselves play an instructive role in segregating TCAs into whisker-related clusters.
In the adult S1 cortex, neurons located in a particular barrel receive sensory input predominantly from their principal whisker (Welker, 1976; Simons & Woolsey, 1979; Armstrong-James & Fox, 1987; Welker et al., 1993). In ‘barrelless’ (Adcy1brl) mice (Van der Loos et al., 1986), the cytoarchitectonic features characteristic of barrels are missing in the cortex, and the distribution of thalamocortical afferents is continuous over an area of up to ten presumptive barrels (Welker et al., 1996). In vivo single cell recordings from the presumptive barrel field of barrelless mice show that layer IV neurons have somatotopically appropriate receptive fields for their principal whiskers but also respond to neighboring whiskers with short latency. In mGluR5 KO mice, barrel rings are missing, but the representations for large whiskers are identifiable as clusters of TCAs. Recordings of whisker-evoked activity also found preserved topographical organization of facial vibrissae in mGluR5 KO mice, but a significantly diminished temporal discrimination of center to surround whiskers in the responses of individual neurons (She et al., 2009). These results suggest that the cortical representations for individual whiskers are grossly preserved in the S1 cortex of these mutant mice despite the absence of barrel cytoarchitecture.
Whisker-related arborizations of single TCAs
In the adult, TCAs arborize in cortical layers IV and VI with densely segregated clusters of arbors in layer IV and a loosely diffuse pattern in layer VI (Agmon et al., 1995; Rebsam et al., 2002; Lee et al., 2005). Anatomical studies of individual TCAs at different developmental stages (Rebsam et al., 2002; Lee et al., 2005) found that at P2-3 TCAs innervating the barrel field have relatively diffuse projection patterns. After P6-7, individual TCAs form highly branched and densely clustered arbors corresponding to the mapped facial whisker. These observations suggest that a progressive addition of branches at the appropriate cortical location with the commensurate elimination of inappropriate branches leads to the formation of whisker-related TCA arborizations (Senft & Woolsey, 1991; Rebsam et al., 2002).
In both global- and Cx-mGluR5 KO mice, TCAs formed significantly fewer branches but spanned a wider area (Fig. 3) (Ballester-Rosado et al., 2010). The total lengths of their axonal arbors were also significantly shorter. These data suggest that mGluR5 signaling in cortical glutamatergic neurons instructs TCAs to develop a compact and highly branched axonal patterning in layer IV. Similar to Cx-mGluR5 KO mice, cortex-specific removal of NR1 also leads to rudimentary TCA patterns (Iwasato et al., 2000; Datwani et al., 2002). However, in contrast to the reduced TCA complexity in mGluR5 KO mice, single TCAs in Cx-NR1 KO mice have exuberant branches (Lee et al., 2005). Thus, mGluR5 and NMDAR exert different effects on TCA arborization.
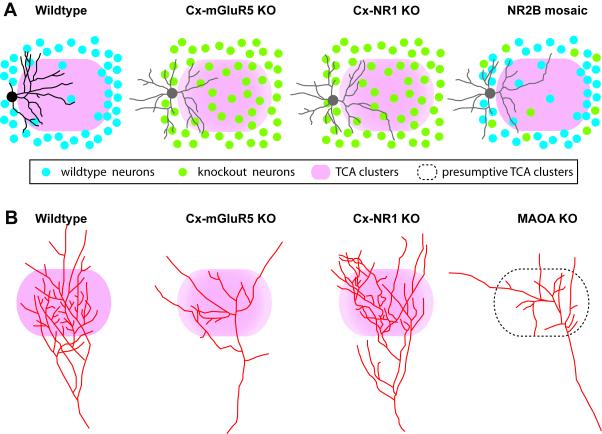
Figure 3.
Schematic diagram showing examples of reconstructed layer IV spiny stellate neurons, and single thalamocortical axons in wildtype, Cx-mGluR5 KO, Cx-NR1 KO and NR2B mosaic mutant mice. (A) In wildtype mice, layer IV spiny stellate neurons show polarized dendritic morphology with the majority of their dendrites projecting toward the barrel hollow (pink). For simplicity, only spiny stellate neurons are depicted here. In both Cx-mGluR5 and Cx-NR1 KO mice, layer IV neurons are evenly distributed in the barrel field, and have symmetric dendritic morphology (non-polarized pattern, depicted in grey). In NR2B mosaic KO mice, mutant layer IV neurons located in the barrel walls show non-polarized dendritic pattern. (B) Examples of reconstructed single TCAs. In wildtype mice, the axon arbor in layer IV develops from a single axon, forming numerous axon collaterals, with a dominant orientation of the branches toward the barrel center, individual TCAs form highly branched and densely clustered arbors corresponding to the mapped facial whisker. In Cx-mGluR5 KO mice and MAOA KO mice, the complexity of TCA arbors is much reduced, and collaterals grow in divergent directions instead of forming a narrow cluster. In Cx-NR1 KO mice, TCAs have exuberant branches. Drawings were based on data shown in Datwani et al. (2002), Rebsam et al. (2002), Lee et al. (2005), Espinosa et al. (2009), and Ballester-Rosado et al. (2010).
The TCA patterning deficits observed in Cx-mGluR5 and Cx-NR1 KO mice suggest that retrograde signaling induced by glutamate receptors plays an important role in guiding the growth of TCAs into a compact and highly branched pattern in their corresponding barrels. Several activity-dependent retrograde messengers made postsynaptically and acting presynaptically have been identified (for review see Regehr et al., 2009), including endocannabinoids, nitric oxide, neuropeptides, neurotransmitters (e.g., glutamate), trophic factors (e.g. Brain Derived Neurotrophic factor (BDNF)), ephrin/Eph, etc. Many studies have found that mGluR5 signaling regulates the postsynaptic synthesis of endocannabinoids and BDNF to modulate neurotransmission presynaptically. BDNF and several ephrin/Ephs are expressed in the developing S1 cortex (Itami et al., 2000; Vanderhaeghen et al., 2000; Bolz et al., 2004) and in vitro studies found that BDNF and ephrin/Eph can promote TCA branching (Gao et al., 1998; Mann et al., 2002; Hanamura et al., 2004). Despite a grossly normal whisker map in S1 cortex, the complexity of TCA arbors is reduced in Ephrin-A5 KO mice (Vanderhaeghen et al., 2000; Uziel et al., 2008). In BDNF and TrkB (Tyrosine Kinase B Receptor) KO mice, a delay TCA clustering was reported (Lush et al., 2005). Therefore, BDNF signaling through TrkB receptors is likely to be involved in TCA patterning. The factors mediating the retrograde influence of NMDAR and mGluR5 signaling on TCA patterning remain to be elucidated.
Layer IV neurons form barrel rings and develop a polarized dendritic morphology
The barrel cytoarchitecture consists of distinctive cell-dense rings of layer IV neurons that project their dendrites towards the center of the cell spare barrel hollow where TCA arbors are situated (Woolsey et al., 1975; Steffen & Van der Loos, 1980; Simons & Woolsey, 1984; Rice, 1985; Lubke et al., 2000). The absence of barrel cytoarchitecture in Cx-NR1 and Cx-mGluR5 KO mice (Fig. 3) indicates a requirement for NMDAR and mGluR5 signaling in cortical excitatory neurons in orchestrating the lateral placement of layer IV neurons as they form barrel rings (Iwasato et al., 2000; Ballester-Rosado et al., 2010). In both mutant mouse lines, the dendritic polarity of layer IV spiny stellate neurons is also reduced (Datwani et al., 2002; Ballester-Rosado et al., 2010). In addition, dendritic outgrowths are also perturbed in these mutant mice with a higher segment number and increased total length. Taken together these results suggest that both NR1 and mGluR5 are required to guide layer IV neurons to form barrel rings and develop a polarized dendritic pattern.
The cell-autonomous role of NMDAR in dendritic patterning of layer IV cortical neurons is most elegantly demonstrated by Espinosa et al. (2009) using the MADM (mosaic analysis with double markers) method to generate NR2B mosaic mutant mice. Analyzing the dendritic morphology of NR2B KO neurons surrounded by wildtype neurons, they found that even at the barrel wall NR2B KO neurons failed to develop a polarized pattern (Espinosa et al., 2009). Interestingly, Wijetunge et al. (2008) found that NR2B is reduced in mGluR5 KO S1 cortex. mGluR5 can regulate NR2B expression through the FMRP-mediated local dendritic translational machinery (Westmark & Malter, 2007; Edbauer et al., 2010). NMDAR and mGluR5 interact through adaptor proteins (Fagni et al., 2004) and reciprocally modulate each other’s function (Alagarsamy et al., 2002; Heidinger et al., 2002; Guo et al., 2004; Alagarsamy et al., 2005). In addition, through a protein kinase C (PKC)-src pathway, mGluR5 activation leads to NR2B tyrosine phosphorylation (Guo et al., 2004). Taken together, NR2B is likely to be regulated by mGluR5 activity in modulating dendritic patterning.
Postnatal apoptosis doesn’t seem to have a major impact on barrel wall formation (Sanno et al., 2010). In transgenic mice expressing a dominant-negative RhoA mutant in developing neurons, barrel cytoarchitecture develops normally despite a reduction in apoptosis and a significant increase in the number and density of cortical neurons.
Lesion-induced anatomical rearrangement in cortical whisker maps
The anatomical organization of both TCAs and cortical neurons will be dramatically rearranged if sensory input is disrupted by nerve damage within a few days of birth (Van der Loos & Woolsey, 1973; Killackey & Leshin, 1975; Woolsey & Wann, 1976; Simons & Land, 1987; Woolsey, 1990; Kossut, 1992). The developmental time window to trigger anatomical map plasticity ends around the time when the whisker-related pattern becomes obvious. However, delaying the appearance of the cortical whisker map by reducing the uptake of 5-HT with a 5-HTT blocker doesn’t lead to alterations in the critical period of anatomical whisker map plasticity (Rebsam et al., 2005). In addition, normal anatomical whisker map plasticity has been found in several mutant mice with whisker map deficits, including PKARIIß (Inan et al., 2006), Cx-NR1 (Iwasato et al., 2000), and Cx-AC1 (Iwasato et al., 2008). Taken together, these results suggest that the formation and the rearrangement of whisker-related pattern in the S1 cortex use different mechanisms. Recently, Takasaki et al (2008) found that, at young ages, the glutamate transporters GLT-1 and GLAST-1 are expressed mainly in astrocytes and are required for anatomical whisker map plasticity. Reduced anatomical rearrangements of TCAs triggered by electrocauterization of C-row hair follicles were found in both GLT-1 and GLAST-1 KO mice. This finding suggests that the regulation of ambient glutamate level in the extracellular space is critical for the anatomical rearrangements of TCAs and layer IV neurons. It remains an open question as to how the critical period of anatomical whisker map plasticity in the cortex is terminated prior to P4 and whether it is possible to extend the critical period for the anatomical rearrangement of thalamocortical connections.
The functional development of thalamocortical synapses
The preparation of the acute mouse thalamocortical brain slice described by Agmon and Connors (1991) allows one to preserve the neural circuits connecting the barreloids in the ventrobasal (VB) thalamus with the barrels in the S1 cortex. This preparation has proven to be extremely valuable. A wealth of knowledge in the synaptic function and plasticity of thalamocortical (TC) synapses has been gained using various electrophysiological paradigms in conjunction with pharmacological reagents and transgenic mice in this slice preparation. TC synapses are glutamatergic (White, 1978; Agmon & O’Dowd, 1992) and this neurotransmission is mediated through ionotropic glutamate receptors, including AMPAR (amino-3-hydroxyl-5-methyl-4-isoxazolepropicreaseonate receptor), NMDAR, and kainate receptors (Agmon & O’Dowd, 1992; Crair & Malenka, 1995; Kidd & Isaac, 1999). Below, we discuss the functional changes occurred in TC synapses during the first two postnatal weeks when barrels are forming.
Maturation of thalamocortical synapses
Excitatory cortical layer IV principal neurons receive glutamatergic inputs from TCAs and neighboring glutamatergic neurons, while receiving inhibitory inputs from cortical GABAergic interneurons (Agmon & Connors, 1991; Crair & Malenka, 1995; Petersen & Sakmann, 2000). The majority of interneurons connected to layer IV principal neurons receive direct inputs from TCAs (Porter et al., 2001; Bruno & Simons, 2002; Inoue & Imoto, 2006; Sun et al., 2006; Cruikshank et al., 2007; Daw et al., 2007a; Tan et al., 2008). Thus, thalamic axons drive both excitatory and inhibitory cortical neurons, the latter triggering a fast feed-forward inhibition (Agmon & Connors, 1991; Beierlein et al., 2002; Beierlein et al., 2003; Swadlow, 2003; Gabernet et al., 2005) to limit the temporal window for integration and to sharpen the temporal resolution of thalamic inputs (Pinto et al., 2000; Pouille & Scanziani, 2001; Pinto et al., 2003; Wilent & Contreras, 2004).
Serial developmental changes in synaptic functions during the first two postnatal weeks, when barrels are forming, have been characterized (Fig. 2). First, AMPAR-mediated currents in TC synapses increase (Crair & Malenka, 1995; Barth & Malenka, 2001; Lu et al., 2001). Second, the number of silent synapses (synapses containing only NMDARs) decreases with age (Isaac et al., 1997). Third, the NMDAR subunit composition changes from NR2B-dominant to a mixed NR2A/2B NMDAR composition accompanied by the shortening of NMDAR decay kinetics (Barth & Malenka, 2001; Lu et al., 2001). The developmental increase in NR2A is likely to account for the acceleration of NMDA current kinetics in TC synapses (Lu et al., 2001). Fourth, the feed-forward connections from TCAs to inhibitory neurons become strengthened toward the end of the first postnatal week (Daw et al., 2007a). After the first postnatal week, thalamic stimulation often triggers a monosynaptic excitatory postsynaptic current immediately followed by an inhibitory postsynaptic current in both excitatory and inhibitory neurons (e.g. Fig. 3 in Porter et al., 2001).
Thalamocortical synaptic plasticity
Synaptic plasticity in TC synapses can be induced with Hebbian-based pairing protocols (Daw et al., 2007b; Inan & Crair, 2007; Fox, 2008). However, long-term potentiation (LTP) (Crair & Malenka, 1995; Barth & Malenka, 2001; Lu et al., 2001) or long-term depression (LTD) (Feldman et al., 1998; Lu et al., 2003) can only be induced during the first postnatal week when barrels are forming. TC-LTP formation requires NR2B-containing NMDAR, calcium, and protein kinase A (PKA) signaling (Crair & Malenka, 1995; Lu et al., 2001; Lu et al., 2003). NMDAR is also required for LTD induction in rat thalamocortical synapses (Feldman et al., 1998) but not in mice (unpublished observations by Lu, H.C.). The temporal coincidence between the critical period for the synaptic plasticity of thalamocortical connections and the period of anatomical development of barrels is intriguing. This led to the hypothesis that Hebbian-based synaptic learning rules that adjust synaptic strength are the cellular mechanisms underlying cortical somatosensory map formation (Crair & Malenka, 1995).
‘barrelless’ (Adcy1brl) mice were the first mutant mice identified to lack barrel cytoarchitecture (Van der Loos et al., 1986; Welker et al., 1996). The mutation in barrelless mice is a result of a loss-of-function in the calcium/calmodulin stimulated AC1 (Abdel-Majid et al., 1998). The robust cAMP synthesis triggered by calcium/calmodulin in wildtype cortices is greatly reduced in barrelless cortices (Abdel-Majid et al., 1998). These findings imply that calcium influx triggered by neuronal activity may activate AC1 to increase cAMP concentration to instruct cortical map development. Detailed analysis of the functional properties of barrelless thalamocortical synapses found that barrelless synapses are stuck in an immature state that contains few functional AMPARs, but they are rarely silent (NMDAR-only) (Lu et al., 2003). Both LTP and LTD formation in TC synapses are difficult to induce in barrelless mice, probably due to an inability to properly regulate synaptic AMPAR trafficking. Despite these synaptic deficits, the developmental switch in NMDAR subunit composition occurs normally in barrelless mice.
PKA, the main target of cAMP, has been repeatedly implicated in synaptic plasticity (e.g. Gutlerner et al., 2002; Lee et al., 2003). PKARII ß is expressed in the dendritic spines of cortical layer IV neurons when barrels are forming. In both PKARII ß and Cx-AC1 KO mice (Inan et al., 2006; Iwasato et al., 2008), thalamocortical-LTP formation is defective and AMPAR mediated current is reduced. These data support the role of the cAMP/PKA signaling in cortical layer IV neurons in regulating AMPAR trafficking upon LTP-like processes occurred during normal development. The reduced relative contribution of AMPA current to NMDA current in the TC synapses of NeuroD2 KO mice suggest that calcium-dependent transcription activity is likely to be involved as well (Ince-Dunn et al., 2006). Taken together, the developmental strengthening of AMPAR-mediated currents in TC synapses are regulated through calcium and cAMP/PKA signaling.
mGluR5, a group I mGluR, primarily activates Gαq, which stimulates PLC to generate the second messengers IP3 and diacylglycerol (DAG) by hydrolyzing phosphatidyl inositol bisphosphate (PIP2). These cumulative actions modulate various kinases, ion channels, and IP3-gated intracellular calcium stores (reviewed in Luscher & Huber, 2010; Niswender & Conn, 2010). mGluR5 is predominantly present in postsynaptic dendrites and spines (Romano et al., 1995; Takasaki et al., 2008) and has a prominent role in synaptic plasticity at many synapses. Activation of mGluR5 triggers rapid protein synthesis through local dendritic translational machinery (Merlin et al., 1998; Huber et al., 2000; Raymond et al., 2000; Karachot et al., 2001; Vanderklish & Edelman, 2002). At thalamocortical synapses of mGluR5 KO mouse, LTD formation is enhanced while LTP is absent (She et al., 2009). At these synapses NMDAR-mediated currents also decay faster than at wildtype TC synapses. Faster decay kinetics may lead to altered calcium influx, which may be insufficient for LTP but adequate for LTD induction. Interestingly, the developmental increase in AMPAR current occurs normally in mGluR5 KO TC synapses. mGluR5 signaling maybe dispensable for the developmental increase in AMPAR-mediated current.
Prior to the formation of barrels, a significant proportion of thalamocortical synapses are functionally silent (NMDAR-only) and they can be converted to functional synapses (AMPAR-containing synapses) by an LTP pairing paradigm (Isaac et al., 1997). FMRP is an RNA-binding protein and acts as a negative regulator of protein translation (Penagarikano et al., 2007; Garber et al., 2008). In Fmr1 KO (null mutant for FMRP) mice, there is an increase in the number of silent synapses towards the end of the first postnatal week and the temporal window for thalamocortical-LTP formation is shifted to later ages (Harlow et al., 2010). Interestingly, Fmr1 KO mice don’t have barrel map deficits or alterations in the critical period for anatomical barrel map plasticity. An increase in the number of silent synapses after the first postnatal week has also been described in the BDNF null mutant mice (Itami et al., 2003). Despite a delay in the appearance of whisker map, both the whisker-related TCA pattern and the critical period of barrel map plasticity are normal in BDNF KO mice (Itami et al., 2000). Taken together, the presence of silent synapses is associated with the time window of LTP formation. The normal critical period for lesion-induced anatomical barrel map plasticity in both Frm1 and BDNF KO mice suggests that the activity-dependent conversion of silent synapses to functional synapses may not account for the cellular mechanism underlying anatomical rearrangements of thalamocortical synapses.
The functional development of the glutamatergic release machinery in TCAs
Mature thalamocortical synapses have a very high probability of release (Pr) and often contain many release sites per axon (Gil et al., 1999). As a result, the transmission of an individual TCA is very reliable and efficient. In vivo recordings in adult rat somatosensory cortex revealed that synaptic responses in barrel neurons adapt rapidly to repetitive facial whisker deflections. The robust short-term depression at thalamocortical synapses is the main contributor to this adaptation (Chung et al., 2002). These specialized features of the TCA release machinery are thus critical for sensory function. Nicotine and 5-HT can modulate TCA release machinery. Exogenous application of nicotine enhances TCA glutamate release as observed by an increase in the amplitude of evoked postsynaptic current and in the degree of short-term depression triggered by paired stimuli (Gil et al., 1999). In contrast, 5-HT treatment leads to a reduction of evoked excitatory response (Rhoades et al., 1994) and reduced short-term depression (Laurent et al., 2002). It is plausible that the disrupted neurotransmission caused by excess 5-HT in the MAOA and 5-HTT KO mice prevents whisker map formation by acting on 5-HT1B expressed on TCAs (Bennett-Clarke et al., 1993).
In barrelless and RIM1α (Rab3-interacting molecule 1α) KO thalamocortical synapses, the release efficiency from TCAs is reduced as indicated by the decrease in short-term depression and Pr compared to control TCAs (Lu et al., 2006). Despite dramatic alterations in the presynaptic function of RIM1α KO TCAs, barrel map deficits found in these mutant mice are subtle and mainly localized to the post-synaptic part of thalamocortical synapses: the barrel cytoarchitecture formed by layer IV neurons. Normal short-term plasticity and release probabilities were found in the thalamocortical synapses of Cx-AC1, PKARIIß, and mGluR5 KO mice, all of which have defective LTP formation (Inan et al., 2006; Iwasato et al., 2008; She et al., 2009). These findings suggest that alterations of synaptic plasticity do not result in deficiencies in the release machinery.
In summary, all the barrel mutant mice found with dendritic patterning deficits are also defective in synaptic plasticity (including Cx-AC1, mGluR5, and PKARIIß KO mice). This strongly supports the hypothesis that Hebbian-based synaptic learning rules are the cellular mechanism underlying the establishment and refinement of thalamocortical connections. In contrast, TCAs in Cx-AC1, PKARII ß, and BDNF KO mice form whisker-related clusters in the S1 cortex despite the absence of thalamocortical-LTP formation (Itami et al., 2000; Inan et al., 2006; Iwasato et al., 2008). These data argue strongly against the role of LTP-like mechanisms in instructing TCA segregation into whisker-related clusters.
Conclusion
The participants involved in cortical whisker map formation often play additional and important roles in the functional maturation of thalamocortical synapses and in synaptic plasticity in mature synapses from diverse brain areas. It remains to be determined whether they activate similar or different signaling cascades for anatomically precise development of thalamocortical circuits and for synaptic function / plasticity. In many developmental processes, the anatomical organization is determined soon after the appearance of a primordium. Indeed, the expression of many molecules required for barrel formation (e.g. AC1, NMDAR and mGluR5) can be detected as soon as embryonic neurons become post-mitotic. Are these molecules needed from the beginning of their expression in the presumptive area for barrels? Do glutamate receptors in developing neurons respond to regulated neurotransmitter release or spontaneous release? What is the nature of the activity-dependent processes required for fine-tuning thalamocortical connections? Answering these questions will undoubtedly advance our knowledge in the development cortical circuits in sensory processing.
Acknowledgements
We would like to thank Dr. Melis Inan for her helpful comments on the manuscript. This work is supported by the NIH NS048884 (HCL), DA029381 (HCL), HD065561 (HCL).
Abbreviations
- 5-HT
serotonin
- 5-HTT
serotonin transporter
- AC1
adenylyl cyclase I
- AMPAR
amino-3-hydroxyl-5-methyl-4-isoxazolepropicreaseonate receptor
- BDNF
Brain Derived Neurotrophic factor
- BSTC
brain stem trigeminal nuclear complex
- CB1R
cannabinoid receptor type 1
- CTA(s)
corticothalamic axon(s)
- Cx-
cortex-specific knockouts of -
- DAG
diacylglycerol
- E
embryonic day
- FGF
fibroblast growth factor
- FMRP
fragile X mental retardation protein
- IP3
inositol 1,4,5-trisphosphate
- ION
infraorbital branch of the trigeminal nerve
- KO
knockout
- LGE
lateral ganglionic eminence
- LMO4
LIM domain-only 4
- LTD
long-term depression
- LTP
long-term potentiation
- MADM
mosaic analysis with double markers)
- MAOA
monoamine oxidase A
- MGE
medial ganglionic eminence
- mGluR5
metabotropic glutamate receptor 5
- NeuroD2
Neurogenic Differentiation 2
- NF1
neurofibromin
- NMDAR
N-methyl-D-aspartate receptor
- NR1
NMDAR subunit 1
- NR2B
NMDAR subunit 2B
- OD
ocular dominance
- P
postnatal day
- PIP2
phosphatidyl inositol bisphosphate
- PKA
protein kinase A
- PKARIIß
PKA regulatory subunit IIß
- PKC
protein kinase C
- PLC
phospholipase C
- PLC-ß1
phospholipase C-ß1
- POm
posteriomedial nucleus of thalamus
- Pr
probability of release
- PrV
rostral principal nucleus
- PSPB
pallial-subpallial boundary
- RIM1α
Rab3-interacting molecule 1α
- S1
primary somatosensory
- SP
subplate
- SpV
caudal spinal nucleus
- SynGAP
synaptic Ras GTPase activating protein
- TC
thalamocortical
- TCA(s)
thalamocortical afferent(s)
- TrkB
Tyrosine Kinase B Receptor
- VB
ventrobasal thalamus
- VMAT2
vesicular monoamine transporter 2
- VPM
ventral postero-medial nucleus of thalamus
References
- Abdel-Majid RM, Leong WL, Schalkwyk LC, Smallman DS, Wong ST, Storm DR, Fine A, Dobson MJ, Guernsey DL, Neumann PE. Loss of adenylyl cyclase I activity disrupts patterning of mouse somatosensory cortex. Nat Genet. 1998;19:289–291. doi: 10.1038/980. [DOI] [PubMed] [Google Scholar]
- Agmon A, Connors BW. Thalamocortical responses of mouse somatosensory (barrel) cortex in vitro. Neuroscience. 1991;41:365–379. doi: 10.1016/0306-4522(91)90333-j. [DOI] [PubMed] [Google Scholar]
- Agmon A, O’Dowd DK. NMDA receptor-mediated currents are prominent in the thalamocortical synaptic response before maturation of inhibition. J Neurophysiol. 1992;68:345–349. doi: 10.1152/jn.1992.68.1.345. [DOI] [PubMed] [Google Scholar]
- Agmon A, Yang LT, Jones EG, O’Dowd DK. Topological precision in the thalamic projection to neonatal mouse barrel cortex. J Neurosci. 1995;15:549–561. doi: 10.1523/JNEUROSCI.15-01-00549.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alagarsamy S, Rouse ST, Junge C, Hubert GW, Gutman D, Smith Y, Conn PJ. NMDA-induced phosphorylation and regulation of mGluR5. Pharmacol Biochem Behav. 2002;73:299–306. doi: 10.1016/s0091-3057(02)00826-2. [DOI] [PubMed] [Google Scholar]
- Alagarsamy S, Saugstad J, Warren L, Mansuy IM, Gereau R.W.t., Conn PJ. NMDA-induced potentiation of mGluR5 is mediated by activation of protein phosphatase 2B/calcineurin. Neuropharmacology. 2005;49(Suppl 1):135–145. doi: 10.1016/j.neuropharm.2005.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alitto HJ, Usrey WM. Corticothalamic feedback and sensory processing. Curr Opin Neurobiol. 2003;13:440–445. doi: 10.1016/s0959-4388(03)00096-5. [DOI] [PubMed] [Google Scholar]
- Armstrong-James M, Fox K. Spatiotemporal convergence and divergence in the rat S1 “barrel” cortex. J Comp Neurol. 1987;263:265–281. doi: 10.1002/cne.902630209. [DOI] [PubMed] [Google Scholar]
- Bagri A, Marin O, Plump AS, Mak J, Pleasure SJ, Rubenstein JL, Tessier-Lavigne M. Slit proteins prevent midline crossing and determine the dorsoventral position of major axonal pathways in the mammalian forebrain. Neuron. 2002;33:233–248. doi: 10.1016/s0896-6273(02)00561-5. [DOI] [PubMed] [Google Scholar]
- Ballester-Rosado CJ, Albright MJ, Wu CS, Liao CC, Zhu J, Xu J, Lee LJ, Lu HC. mGluR5 in cortical excitatory neurons exerts both cell-autonomous and -nonautonomous influences on cortical somatosensory circuit formation. J Neurosci. 2010;30:16896–16909. doi: 10.1523/JNEUROSCI.2462-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett MW, Watson RF, Vitalis T, Porter K, Komiyama NH, Stoney PN, Gillingwater TH, Grant SG, Kind PC. Synaptic Ras GTPase activating protein regulates pattern formation in the trigeminal system of mice. J Neurosci. 2006;26:1355–1365. doi: 10.1523/JNEUROSCI.3164-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth AL, Malenka RC. NMDAR EPSC kinetics do not regulate the critical period for LTP at thalamocortical synapses. Nat Neurosci. 2001;4:235–236. doi: 10.1038/85070. [DOI] [PubMed] [Google Scholar]
- Beierlein M, Fall CP, Rinzel J, Yuste R. Thalamocortical bursts trigger recurrent activity in neocortical networks: layer 4 as a frequency-dependent gate. J Neurosci. 2002;22:9885–9894. doi: 10.1523/JNEUROSCI.22-22-09885.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beierlein M, Gibson JR, Connors BW. Two dynamically distinct inhibitory networks in layer 4 of the neocortex. J Neurophysiol. 2003;90:2987–3000. doi: 10.1152/jn.00283.2003. [DOI] [PubMed] [Google Scholar]
- Bennett-Clarke CA, Leslie MJ, Chiaia NL, Rhoades RW. Serotonin 1B receptors in the developing somatosensory and visual cortices are located on thalamocortical axons. Proc Natl Acad Sci U S A. 1993;90:153–157. doi: 10.1073/pnas.90.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielle F, Marcos-Mondejar P, Keita M, Mailhes C, Verney C, Ba-Charvet K. Nguyen, Tessier-Lavigne M, Lopez-Bendito G, Garel S. Slit2 activity in the migration of guidepost neurons shapes thalamic projections during development and evolution. Neuron. 2011;69:1085–1098. doi: 10.1016/j.neuron.2011.02.026. [DOI] [PubMed] [Google Scholar]
- Bolz J, Uziel D, Muhlfriedel S, Gullmar A, Peuckert C, Zarbalis K, Wurst W, Torii M, Levitt P. Multiple roles of ephrins during the formation of thalamocortical projections: maps and more. J Neurobiol. 2004;59:82–94. doi: 10.1002/neu.10346. [DOI] [PubMed] [Google Scholar]
- Braisted JE, Ringstedt T, O’Leary DD. Slits are chemorepellents endogenous to hypothalamus and steer thalamocortical axons into ventral telencephalon. Cereb Cortex. 2009;19(Suppl 1):i144–151. doi: 10.1093/cercor/bhp035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno RM, Simons DJ. Feedforward mechanisms of excitatory and inhibitory cortical receptive fields. J Neurosci. 2002;22:10966–10975. doi: 10.1523/JNEUROSCI.22-24-10966.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonomano DV, Merzenich MM. Cortical plasticity: from synapses to maps. Annu Rev Neurosci. 1998;21:149–186. doi: 10.1146/annurev.neuro.21.1.149. [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Eichele T, Pearlson G. Functional brain networks in schizophrenia: a review. Front Hum Neurosci. 2009;3:17. doi: 10.3389/neuro.09.017.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cases O, Lebrand C, Giros B, Vitalis T, De Maeyer E, Caron MG, Price DJ, Gaspar P, Seif I. Plasma membrane transporters of serotonin, dopamine, and norepinephrine mediate serotonin accumulation in atypical locations in the developing brain of monoamine oxidase A knock-outs. J Neurosci. 1998;18:6914–6927. doi: 10.1523/JNEUROSCI.18-17-06914.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cases O, Vitalis T, Seif I, De Maeyer E, Sotelo C, Gaspar P. Lack of barrels in the somatosensory cortex of monoamine oxidase A-deficient mice: role of a serotonin excess during the critical period. Neuron. 1996;16:297–307. doi: 10.1016/s0896-6273(00)80048-3. [DOI] [PubMed] [Google Scholar]
- Chung S, Li X, Nelson SB. Short-term depression at thalamocortical synapses contributes to rapid adaptation of cortical sensory responses in vivo. Neuron. 2002;34:437–446. doi: 10.1016/s0896-6273(02)00659-1. [DOI] [PubMed] [Google Scholar]
- Crair MC. Neuronal activity during development: permissive or instructive? Curr Opin Neurobiol. 1999;9:88–93. doi: 10.1016/s0959-4388(99)80011-7. [DOI] [PubMed] [Google Scholar]
- Crair MC, Malenka RC. A critical period for long-term potentiation at thalamocortical synapses. Nature. 1995;375:325–328. doi: 10.1038/375325a0. [DOI] [PubMed] [Google Scholar]
- Cruikshank SJ, Lewis TJ, Connors BW. Synaptic basis for intense thalamocortical activation of feedforward inhibitory cells in neocortex. Nat Neurosci. 2007;10:462–468. doi: 10.1038/nn1861. [DOI] [PubMed] [Google Scholar]
- Datwani A, Iwasato T, Itohara S, Erzurumlu RS. NMDA receptor-dependent pattern transfer from afferents to postsynaptic cells and dendritic differentiation in the barrel cortex. Mol Cell Neurosci. 2002;21:477–492. doi: 10.1006/mcne.2002.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daw MI, Ashby MC, Isaac JT. Coordinated developmental recruitment of latent fast spiking interneurons in layer IV barrel cortex. Nat Neurosci. 2007a;10:453–461. doi: 10.1038/nn1866. [DOI] [PubMed] [Google Scholar]
- Daw MI, Scott HL, Isaac JT. Developmental synaptic plasticity at the thalamocortical input to barrel cortex: Mechanisms and roles. Mol Cell Neurosci. 2007b doi: 10.1016/j.mcn.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding YQ, Yin J, Xu HM, Jacquin MF, Chen ZF. Formation of whisker-related principal sensory nucleus-based lemniscal pathway requires a paired homeodomain transcription factor, Drg11. J Neurosci. 2003;23:7246–7254. doi: 10.1523/JNEUROSCI.23-19-07246.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edbauer D, Neilson JR, Foster KA, Wang CF, Seeburg DP, Batterton MN, Tada T, Dolan BM, Sharp PA, Sheng M. Regulation of synaptic structure and function by FMRP-associated microRNAs miR-125b and miR-132. Neuron. 2010;65:373–384. doi: 10.1016/j.neuron.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erzurumlu RS, Kind PC. Neural activity: sculptor of ‘barrels’ in the neocortex. Trends Neurosci. 2001;24:589–595. doi: 10.1016/s0166-2236(00)01958-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erzurumlu RS, Murakami Y, Rijli FM. Mapping the face in the somatosensory brainstem. Nat Rev Neurosci. 2010;11:252–263. doi: 10.1038/nrn2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinosa JS, Wheeler DG, Tsien RW, Luo L. Uncoupling dendrite growth and patterning: single-cell knockout analysis of NMDA receptor 2B. Neuron. 2009;62:205–217. doi: 10.1016/j.neuron.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagni L, Ango F, Perroy J, Bockaert J. Identification and functional roles of metabotropic glutamate receptor-interacting proteins. Semin Cell Dev Biol. 2004;15:289–298. doi: 10.1016/j.semcdb.2003.12.018. [DOI] [PubMed] [Google Scholar]
- Feldman DE, Brecht M. Map plasticity in somatosensory cortex. Science. 2005;310:810–815. doi: 10.1126/science.1115807. [DOI] [PubMed] [Google Scholar]
- Feldman DE, Nicoll RA, Malenka RC, Isaac JT. Long-term depression at thalamocortical synapses in developing rat somatosensory cortex. Neuron. 1998;21:347–357. doi: 10.1016/s0896-6273(00)80544-9. [DOI] [PubMed] [Google Scholar]
- Fox K. Barrel cortex. Cambridge University Press; Cambridge: 2008. [Google Scholar]
- Fox K, Wong RO. A comparison of experience-dependent plasticity in the visual and somatosensory systems. Neuron. 2005;48:465–477. doi: 10.1016/j.neuron.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Fukuchi-Shimogori T, Grove EA. Neocortex patterning by the secreted signaling molecule FGF8. Science. 2001;294:1071–1074. doi: 10.1126/science.1064252. [DOI] [PubMed] [Google Scholar]
- Gabernet L, Jadhav SP, Feldman DE, Carandini M, Scanziani M. Somatosensory integration controlled by dynamic thalamocortical feed-forward inhibition. Neuron. 2005;48:315–327. doi: 10.1016/j.neuron.2005.09.022. [DOI] [PubMed] [Google Scholar]
- Gao PP, Yue Y, Zhang JH, Cerretti DP, Levitt P, Zhou R. Regulation of thalamic neurite outgrowth by the Eph ligand ephrin-A5: implications in the development of thalamocortical projections. Proc Natl Acad Sci U S A. 1998;95:5329–5334. doi: 10.1073/pnas.95.9.5329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber KB, Visootsak J, Warren ST. Fragile X syndrome. Eur J Hum Genet. 2008;16:666–672. doi: 10.1038/ejhg.2008.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garel S, Rubenstein JL. Intermediate targets in formation of topographic projections: inputs from the thalamocortical system. Trends Neurosci. 2004;27:533–539. doi: 10.1016/j.tins.2004.06.014. [DOI] [PubMed] [Google Scholar]
- Gheorghita F, Kraftsik R, Dubois R, Welker E. Structural basis for map formation in the thalamocortical pathway of the barrelless mouse. J Neurosci. 2006;26:10057–10067. doi: 10.1523/JNEUROSCI.1263-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil Z, Connors BW, Amitai Y. Efficacy of thalamocortical and intracortical synaptic connections: quanta, innervation, and reliability. Neuron. 1999;23:385–397. doi: 10.1016/s0896-6273(00)80788-6. [DOI] [PubMed] [Google Scholar]
- Guo W, Wei F, Zou S, Robbins MT, Sugiyo S, Ikeda T, Tu JC, Worley PF, Dubner R, Ren K. Group I metabotropic glutamate receptor NMDA receptor coupling and signaling cascade mediate spinal dorsal horn NMDA receptor 2B tyrosine phosphorylation associated with inflammatory hyperalgesia. J Neurosci. 2004;24:9161–9173. doi: 10.1523/JNEUROSCI.3422-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutlerner JL, Penick EC, Snyder EM, Kauer JA. Novel protein kinase A-dependent long-term depression of excitatory synapses. Neuron. 2002;36:921–931. doi: 10.1016/s0896-6273(02)01051-6. [DOI] [PubMed] [Google Scholar]
- Hanamura K, Harada A, Katoh-Semba R, Murakami F, Yamamoto N. BDNF and NT-3 promote thalamocortical axon growth with distinct substrate and temporal dependency. Eur J Neurosci. 2004;19:1485–1493. doi: 10.1111/j.1460-9568.2004.03228.x. [DOI] [PubMed] [Google Scholar]
- Hannan AJ, Blakemore C, Katsnelson A, Vitalis T, Huber KM, Bear M, Roder J, Kim D, Shin HS, Kind PC. PLC-beta1, activated via mGluRs, mediates activity-dependent differentiation in cerebral cortex. Nat Neurosci. 2001;4:282–288. doi: 10.1038/85132. [DOI] [PubMed] [Google Scholar]
- Harlow EG, Till SM, Russell TA, Wijetunge LS, Kind P, Contractor A. Critical period plasticity is disrupted in the barrel cortex of FMR1 knockout mice. Neuron. 2010;65:385–398. doi: 10.1016/j.neuron.2010.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidinger V, Manzerra P, Wang XQ, Strasser U, Yu SP, Choi DW, Behrens MM. Metabotropic glutamate receptor 1-induced upregulation of NMDA receptor current: mediation through the Pyk2/Src-family kinase pathway in cortical neurons. J Neurosci. 2002;22:5452–5461. doi: 10.1523/JNEUROSCI.22-13-05452.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hevner RF, Miyashita-Lin E, Rubenstein JL. Cortical and thalamic axon pathfinding defects in Tbr1, Gbx2, and Pax6 mutant mice: evidence that cortical and thalamic axons interact and guide each other. J Comp Neurol. 2002;447:8–17. doi: 10.1002/cne.10219. [DOI] [PubMed] [Google Scholar]
- Huber KM, Kayser MS, Bear MF. Role for rapid dendritic protein synthesis in hippocampal mGluR-dependent long-term depression. Science. 2000;288:1254–1257. doi: 10.1126/science.288.5469.1254. [DOI] [PubMed] [Google Scholar]
- Inan M, Crair MC. Development of cortical maps: perspectives from the barrel cortex. Neuroscientist. 2007;13:49–61. doi: 10.1177/1073858406296257. [DOI] [PubMed] [Google Scholar]
- Inan M, Lu HC, Albright MJ, She WC, Crair MC. Barrel map development relies on protein kinase A regulatory subunit II beta-mediated cAMP signaling. J Neurosci. 2006;26:4338–4349. doi: 10.1523/JNEUROSCI.3745-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ince-Dunn G, Hall BJ, Hu SC, Ripley B, Huganir RL, Olson JM, Tapscott SJ, Ghosh A. Regulation of thalamocortical patterning and synaptic maturation by NeuroD2. Neuron. 2006;49:683–695. doi: 10.1016/j.neuron.2006.01.031. [DOI] [PubMed] [Google Scholar]
- Inoue T, Imoto K. Feedforward inhibitory connections from multiple thalamic cells to multiple regular-spiking cells in layer 4 of the somatosensory cortex. J Neurophysiol. 2006;96:1746–1754. doi: 10.1152/jn.00301.2006. [DOI] [PubMed] [Google Scholar]
- Isaac JT, Crair MC, Nicoll RA, Malenka RC. Silent synapses during development of thalamocortical inputs. Neuron. 1997;18:269–280. doi: 10.1016/s0896-6273(00)80267-6. [DOI] [PubMed] [Google Scholar]
- Itami C, Kimura F, Kohno T, Matsuoka M, Ichikawa M, Tsumoto T, Nakamura S. Brain-derived neurotrophic factor-dependent unmasking of “silent” synapses in the developing mouse barrel cortex. Proc Natl Acad Sci U S A. 2003;100:13069–13074. doi: 10.1073/pnas.2131948100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itami C, Kimura F, Nakamura S. Brain-derived neurotrophic factor regulates the maturation of layer 4 fast-spiking cells after the second postnatal week in the developing barrel cortex. J Neurosci. 2007;27:2241–2252. doi: 10.1523/JNEUROSCI.3345-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itami C, Mizuno K, Kohno T, Nakamura S. Brain-derived neurotrophic factor requirement for activity-dependent maturation of glutamatergic synapse in developing mouse somatosensory cortex. Brain Res. 2000;857:141–150. doi: 10.1016/s0006-8993(99)02352-5. [DOI] [PubMed] [Google Scholar]
- Iwasato T, Datwani A, Wolf AM, Nishiyama H, Taguchi Y, Tonegawa S, Knopfel T, Erzurumlu RS, Itohara S. Cortex-restricted disruption of NMDAR1 impairs neuronal patterns in the barrel cortex. Nature. 2000;406:726–731. doi: 10.1038/35021059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasato T, Erzurumlu RS, Huerta PT, Chen DF, Sasaoka T, Ulupinar E, Tonegawa S. NMDA receptor-dependent refinement of somatotopic maps. Neuron. 1997;19:1201–1210. doi: 10.1016/s0896-6273(00)80412-2. [DOI] [PubMed] [Google Scholar]
- Iwasato T, Inan M, Kanki H, Erzurumlu RS, Itohara S, Crair MC. Cortical adenylyl cyclase 1 is required for thalamocortical synapse maturation and aspects of layer IV barrel development. J Neurosci. 2008;28:5931–5943. doi: 10.1523/JNEUROSCI.0815-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs EC, Campagnoni C, Kampf K, Reyes SD, Kalra V, Handley V, Xie YY, Hong-Hu Y, Spreur V, Fisher RS, Campagnoni AT. Visualization of corticofugal projections during early cortical development in a tau-GFP-transgenic mouse. Eur J Neurosci. 2007;25:17–30. doi: 10.1111/j.1460-9568.2006.05258.x. [DOI] [PubMed] [Google Scholar]
- Jones EG. Thalamic circuitry and thalamocortical synchrony. Philos Trans R Soc Lond B Biol Sci. 2002;357:1659–1673. doi: 10.1098/rstb.2002.1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanold PO, Luhmann HJ. The subplate and early cortical circuits. Annu Rev Neurosci. 2010;33:23–48. doi: 10.1146/annurev-neuro-060909-153244. [DOI] [PubMed] [Google Scholar]
- Karachot L, Shirai Y, Vigot R, Yamamori T, Ito M. Induction of long-term depression in cerebellar Purkinje cells requires a rapidly turned over protein. J Neurophysiol. 2001;86:280–289. doi: 10.1152/jn.2001.86.1.280. [DOI] [PubMed] [Google Scholar]
- Kashani AH, Qiu Z, Jurata L, Lee SK, Pfaff S, Goebbels S, Nave KA, Ghosh A. Calcium activation of the LMO4 transcription complex and its role in the patterning of thalamocortical connections. J Neurosci. 2006;26:8398–8408. doi: 10.1523/JNEUROSCI.0618-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz LC, Constantine-Paton M. Relationships between segregated afferents and postsynaptic neurones in the optic tectum of three-eyed frogs. J Neurosci. 1988;8:3160–3180. doi: 10.1523/JNEUROSCI.08-09-03160.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- Kidd FL, Isaac JT. Developmental and activity-dependent regulation of kainate receptors at thalamocortical synapses. Nature. 1999;400:569–573. doi: 10.1038/23040. [DOI] [PubMed] [Google Scholar]
- Killackey HP, Leshin S. The organization of specific thalamocortical projections to the posteromedial barrel subfield of the rat somatic sensory cortex. Brain Res. 1975;86:469–472. doi: 10.1016/0006-8993(75)90897-5. [DOI] [PubMed] [Google Scholar]
- Knott GW, Quairiaux C, Genoud C, Welker E. Formation of dendritic spines with GABAergic synapses induced by whisker stimulation in adult mice. Neuron. 2002;34:265–273. doi: 10.1016/s0896-6273(02)00663-3. [DOI] [PubMed] [Google Scholar]
- Kossut M. Effects of sensory deprivation upon a single cortical vibrissal column: a 2DG study. Exp Brain Res. 1992;90:639–642. doi: 10.1007/BF00230950. [DOI] [PubMed] [Google Scholar]
- Kutsuwada T, Sakimura K, Manabe T, Takayama C, Katakura N, Kushiya E, Natsume R, Watanabe M, Inoue Y, Yagi T, Aizawa S, Arakawa M, Takahashi T, Nakamura Y, Mori H, Mishina M. Impairment of suckling response, trigeminal neuronal pattern formation, and hippocampal LTD in NMDA receptor epsilon 2 subunit mutant mice. Neuron. 1996;16:333–344. doi: 10.1016/s0896-6273(00)80051-3. [DOI] [PubMed] [Google Scholar]
- Laurent A, Goaillard JM, Cases O, Lebrand C, Gaspar P, Ropert N. Activity-dependent presynaptic effect of serotonin 1B receptors on the somatosensory thalamocortical transmission in neonatal mice. J Neurosci. 2002;22:886–900. doi: 10.1523/JNEUROSCI.22-03-00886.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebrand C, Cases O, Adelbrecht C, Doye A, Alvarez C, El Mestikawy S, Seif I, Gaspar P. Transient uptake and storage of serotonin in developing thalamic neurons. Neuron. 1996;17:823–835. doi: 10.1016/s0896-6273(00)80215-9. [DOI] [PubMed] [Google Scholar]
- Lebrand C, Cases O, Wehrle R, Blakely RD, Edwards RH, Gaspar P. Transient developmental expression of monoamine transporters in the rodent forebrain. J Comp Neurol. 1998;401:506–524. [PubMed] [Google Scholar]
- Lee HK, Takamiya K, Han JS, Man H, Kim CH, Rumbaugh G, Yu S, Ding L, He C, Petralia RS, Wenthold RJ, Gallagher M, Huganir RL. Phosphorylation of the AMPA receptor GluR1 subunit is required for synaptic plasticity and retention of spatial memory. Cell. 2003;112:631–643. doi: 10.1016/s0092-8674(03)00122-3. [DOI] [PubMed] [Google Scholar]
- Lee LJ, Iwasato T, Itohara S, Erzurumlu RS. Exuberant thalamocortical axon arborization in cortex-specific NMDAR1 knockout mice. J Comp Neurol. 2005;485:280–292. doi: 10.1002/cne.20481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Erzurumlu RS, Chen C, Jhaveri S, Tonegawa S. Whisker-related neuronal patterns fail to develop in the trigeminal brainstem nuclei of NMDAR1 knockout mice. Cell. 1994;76:427–437. doi: 10.1016/0092-8674(94)90108-2. [DOI] [PubMed] [Google Scholar]
- Lopez-Bendito G, Cautinat A, Sanchez JA, Bielle F, Flames N, Garratt AN, Talmage DA, Role LW, Charnay P, Marin O, Garel S. Tangential neuronal migration controls axon guidance: a role for neuregulin-1 in thalamocortical axon navigation. Cell. 2006;125:127–142. doi: 10.1016/j.cell.2006.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Bendito G, Molnar Z. Thalamocortical development: how are we going to get there? Nat Rev Neurosci. 2003;4:276–289. doi: 10.1038/nrn1075. [DOI] [PubMed] [Google Scholar]
- Lu HC, Butts DA, Kaeser PS, She WC, Janz R, Crair MC. Role of efficient neurotransmitter release in barrel map development. J Neurosci. 2006;26:2692–2703. doi: 10.1523/JNEUROSCI.3956-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu HC, Gonzalez E, Crair MC. Barrel cortex critical period plasticity is independent of changes in NMDA receptor subunit composition. Neuron. 2001;32:619–634. doi: 10.1016/s0896-6273(01)00501-3. [DOI] [PubMed] [Google Scholar]
- Lu HC, She WC, Plas DT, Neumann PE, Janz R, Crair MC. Adenylyl cyclase I regulates AMPA receptor trafficking during mouse cortical ‘barrel’ map development. Nat Neurosci. 2003;6:939–947. doi: 10.1038/nn1106. [DOI] [PubMed] [Google Scholar]
- Lubke J, Egger V, Sakmann B, Feldmeyer D. Columnar organization of dendrites and axons of single and synaptically coupled excitatory spiny neurons in layer 4 of the rat barrel cortex. J Neurosci. 2000;20:5300–5311. doi: 10.1523/JNEUROSCI.20-14-05300.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher C, Huber KM. Group 1 mGluR-dependent synaptic long-term depression: mechanisms and implications for circuitry and disease. Neuron. 2010;65:445–459. doi: 10.1016/j.neuron.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lush ME, Li Y, Kwon CH, Chen J, Parada LF. Neurofibromin is required for barrel formation in the mouse somatosensory cortex. J Neurosci. 2008;28:1580–1587. doi: 10.1523/JNEUROSCI.5236-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lush ME, Ma L, Parada LF. TrkB signaling regulates the developmental maturation of the somatosensory cortex. Int J Dev Neurosci. 2005;23:523–536. doi: 10.1016/j.ijdevneu.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Ma PM. Barrelettes--architectonic vibrissal representations in the brainstem trigeminal complex of the mouse. II. Normal post-natal development. J Comp Neurol. 1993;327:376–397. doi: 10.1002/cne.903270306. [DOI] [PubMed] [Google Scholar]
- Mann F, Peuckert C, Dehner F, Zhou R, Bolz J. Ephrins regulate the formation of terminal axonal arbors during the development of thalamocortical projections. Development. 2002;129:3945–3955. doi: 10.1242/dev.129.16.3945. [DOI] [PubMed] [Google Scholar]
- McConnell SK, Ghosh A, Shatz CJ. Subplate neurons pioneer the first axon pathway from the cerebral cortex. Science. 1989;245:978–982. doi: 10.1126/science.2475909. [DOI] [PubMed] [Google Scholar]
- Merlin LR, Bergold PJ, Wong RK. Requirement of protein synthesis for group I mGluR-mediated induction of epileptiform discharges. J Neurophysiol. 1998;80:989–993. doi: 10.1152/jn.1998.80.2.989. [DOI] [PubMed] [Google Scholar]
- Molnar Z, Adams R, Goffinet AM, Blakemore C. The role of the first postmitotic cortical cells in the development of thalamocortical innervation in the reeler mouse. J Neurosci. 1998;18:5746–5765. doi: 10.1523/JNEUROSCI.18-15-05746.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar Z, Blakemore C. How do thalamic axons find their way to the cortex? Trends Neurosci. 1995;18:389–397. doi: 10.1016/0166-2236(95)93935-q. [DOI] [PubMed] [Google Scholar]
- Molnar Z, Butler AB. The corticostriatal junction: a crucial region for forebrain development and evolution. Bioessays. 2002;24:530–541. doi: 10.1002/bies.10100. [DOI] [PubMed] [Google Scholar]
- Molnar Z, Higashi S, Lopez-Bendito G. Choreography of early thalamocortical development. Cereb Cortex. 2003;13:661–669. doi: 10.1093/cercor/13.6.661. [DOI] [PubMed] [Google Scholar]
- Molnar Z, Lopez-Bendito G, Small J, Partridge LD, Blakemore C, Wilson MC. Normal development of embryonic thalamocortical connectivity in the absence of evoked synaptic activity. J Neurosci. 2002;22:10313–10323. doi: 10.1523/JNEUROSCI.22-23-10313.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niswender CM, Conn PJ. Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu Rev Pharmacol Toxicol. 2010;50:295–322. doi: 10.1146/annurev.pharmtox.011008.145533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell M, Chance RK, Bashaw GJ. Axon growth and guidance: receptor regulation and signal transduction. Annu Rev Neurosci. 2009;32:383–412. doi: 10.1146/annurev.neuro.051508.135614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penagarikano O, Mulle JG, Warren ST. The pathophysiology of fragile x syndrome. Annu Rev Genomics Hum Genet. 2007;8:109–129. doi: 10.1146/annurev.genom.8.080706.092249. [DOI] [PubMed] [Google Scholar]
- Persico AM, Mengual E, Moessner R, Hall FS, Revay RS, Sora I, Arellano J, DeFelipe J, Gimenez-Amaya JM, Conciatori M, Marino R, Baldi A, Cabib S, Pascucci T, Uhl GR, Murphy DL, Lesch KP, Keller F. Barrel pattern formation requires serotonin uptake by thalamocortical afferents, and not vesicular monoamine release. J Neurosci. 2001;21:6862–6873. doi: 10.1523/JNEUROSCI.21-17-06862.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen CC. The functional organization of the barrel cortex. Neuron. 2007;56:339–355. doi: 10.1016/j.neuron.2007.09.017. [DOI] [PubMed] [Google Scholar]
- Petersen CC, Sakmann B. The excitatory neuronal network of rat layer 4 barrel cortex. J Neurosci. 2000;20:7579–7586. doi: 10.1523/JNEUROSCI.20-20-07579.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto DJ, Brumberg JC, Simons DJ. Circuit dynamics and coding strategies in rodent somatosensory cortex. J Neurophysiol. 2000;83:1158–1166. doi: 10.1152/jn.2000.83.3.1158. [DOI] [PubMed] [Google Scholar]
- Pinto DJ, Hartings JA, Brumberg JC, Simons DJ. Cortical damping: analysis of thalamocortical response transformations in rodent barrel cortex. Cereb Cortex. 2003;13:33–44. doi: 10.1093/cercor/13.1.33. [DOI] [PubMed] [Google Scholar]
- Porter JT, Johnson CK, Agmon A. Diverse types of interneurons generate thalamus-evoked feedforward inhibition in the mouse barrel cortex. J Neurosci. 2001;21:2699–2710. doi: 10.1523/JNEUROSCI.21-08-02699.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouille F, Scanziani M. Enforcement of temporal fidelity in pyramidal cells by somatic feed-forward inhibition. Science. 2001;293:1159–1163. doi: 10.1126/science.1060342. [DOI] [PubMed] [Google Scholar]
- Price DJ, Kennedy H, Dehay C, Zhou L, Mercier M, Jossin Y, Goffinet AM, Tissir F, Blakey D, Molnar Z. The development of cortical connections. Eur J Neurosci. 2006;23:910–920. doi: 10.1111/j.1460-9568.2006.04620.x. [DOI] [PubMed] [Google Scholar]
- Raymond CR, Thompson VL, Tate WP, Abraham WC. Metabotropic glutamate receptors trigger homosynaptic protein synthesis to prolong long-term potentiation. J Neurosci. 2000;20:969–976. doi: 10.1523/JNEUROSCI.20-03-00969.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebsam A, Seif I, Gaspar P. Refinement of thalamocortical arbors and emergence of barrel domains in the primary somatosensory cortex: a study of normal and monoamine oxidase a knock-out mice. J Neurosci. 2002;22:8541–8552. doi: 10.1523/JNEUROSCI.22-19-08541.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebsam A, Seif I, Gaspar P. Dissociating barrel development and lesion-induced plasticity in the mouse somatosensory cortex. J Neurosci. 2005;25:706–710. doi: 10.1523/JNEUROSCI.4191-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regehr WG, Carey MR, Best AR. Activity-dependent regulation of synapses by retrograde messengers. Neuron. 2009;63:154–170. doi: 10.1016/j.neuron.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoades RW, Bennett-Clarke CA, Shi MY, Mooney RD. Effects of 5-HT on thalamocortical synaptic transmission in the developing rat. J Neurophysiol. 1994;72:2438–2450. doi: 10.1152/jn.1994.72.5.2438. [DOI] [PubMed] [Google Scholar]
- Rice FL. Gradual changes in the structure of the barrels during maturation of the primary somatosensory cortex in the rat. J Comp Neurol. 1985;236:496–503. doi: 10.1002/cne.902360406. [DOI] [PubMed] [Google Scholar]
- Romano C, Sesma MA, McDonald CT, O’Malley K, Van den Pol AN, Olney JW. Distribution of metabotropic glutamate receptor mGluR5 immunoreactivity in rat brain. J Comp Neurol. 1995;355:455–469. doi: 10.1002/cne.903550310. [DOI] [PubMed] [Google Scholar]
- Rubenstein JL. Annual Research Review: Development of the cerebral cortex: implications for neurodevelopmental disorders. J Child Psychol Psychiatry. 2011;52:339–355. doi: 10.1111/j.1469-7610.2010.02307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudhard Y, Kneussel M, Nassar MA, Rast GF, Annala AJ, Chen PE, Tigaret CM, Dean I, Roes J, Gibb AJ, Hunt SP, Schoepfer R. Absence of Whisker-related pattern formation in mice with NMDA receptors lacking coincidence detection properties and calcium signaling. J Neurosci. 2003;23:2323–2332. doi: 10.1523/JNEUROSCI.23-06-02323.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salichon N, Gaspar P, Upton AL, Picaud S, Hanoun N, Hamon M, De Maeyer E, Murphy DL, Mossner R, Lesch KP, Hen R, Seif I. Excessive activation of serotonin (5-HT) 1B receptors disrupts the formation of sensory maps in monoamine oxidase a and 5-ht transporter knock-out mice. J Neurosci. 2001;21:884–896. doi: 10.1523/JNEUROSCI.21-03-00884.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanno H, Shen X, Kuru N, Bormuth I, Bobsin K, Gardner HA, Komljenovic D, Tarabykin V, Erzurumlu RS, Tucker KL. Control of postnatal apoptosis in the neocortex by RhoA-subfamily GTPases determines neuronal density. J Neurosci. 2010;30:4221–4231. doi: 10.1523/JNEUROSCI.3318-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senft SL, Woolsey TA. Growth of thalamic afferents into mouse barrel cortex. Cereb Cortex. 1991;1:308–335. doi: 10.1093/cercor/1.4.308. [DOI] [PubMed] [Google Scholar]
- She WC, Quairiaux C, Albright MJ, Wang YC, Sanchez DE, Chang PS, Welker E, Lu HC. Roles of mGluR5 in synaptic function and plasticity of the mouse thalamocortical pathway. Eur J Neurosci. 2009;29:1379–1396. doi: 10.1111/j.1460-9568.2009.06696.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons DJ, Land PW. Early experience of tactile stimulation influences organization of somatic sensory cortex. Nature. 1987;326:694–697. doi: 10.1038/326694a0. [DOI] [PubMed] [Google Scholar]
- Simons DJ, Woolsey TA. Functional organization in mouse barrel cortex. Brain Res. 1979;165:327–332. doi: 10.1016/0006-8993(79)90564-x. [DOI] [PubMed] [Google Scholar]
- Simons DJ, Woolsey TA. Morphology of Golgi-Cox-impregnated barrel neurons in rat SmI cortex. J Comp Neurol. 1984;230:119–132. doi: 10.1002/cne.902300111. [DOI] [PubMed] [Google Scholar]
- Simpson TI, Pratt T, Mason JO, Price DJ. Normal ventral telencephalic expression of Pax6 is required for normal development of thalamocortical axons in embryonic mice. Neural Dev. 2009;4:19. doi: 10.1186/1749-8104-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffen H, Van der Loos H. Early lesions of mouse vibrissal follicles:: their influence on dendrite orientation in the cortical barrelfield. Exp Brain Res. 1980;40:419–431. doi: 10.1007/BF00236150. [DOI] [PubMed] [Google Scholar]
- Sun QQ, Huguenard JR, Prince DA. Barrel cortex microcircuits: thalamocortical feedforward inhibition in spiny stellate cells is mediated by a small number of fast-spiking interneurons. J Neurosci. 2006;26:1219–1230. doi: 10.1523/JNEUROSCI.4727-04.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swadlow HA. Fast-spike interneurons and feedforward inhibition in awake sensory neocortex. Cereb Cortex. 2003;13:25–32. doi: 10.1093/cercor/13.1.25. [DOI] [PubMed] [Google Scholar]
- Takasaki C, Okada R, Mitani A, Fukaya M, Yamasaki M, Fujihara Y, Shirakawa T, Tanaka K, Watanabe M. Glutamate transporters regulate lesion-induced plasticity in the developing somatosensory cortex. J Neurosci. 2008;28:4995–5006. doi: 10.1523/JNEUROSCI.0861-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Z, Hu H, Huang ZJ, Agmon A. Robust but delayed thalamocortical activation of dendritic-targeting inhibitory interneurons. Proc Natl Acad Sci U S A. 2008;105:2187–2192. doi: 10.1073/pnas.0710628105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temereanca S, Simons DJ. Functional topography of corticothalamic feedback enhances thalamic spatial response tuning in the somatosensory whisker/barrel system. Neuron. 2004;41:639–651. doi: 10.1016/s0896-6273(04)00046-7. [DOI] [PubMed] [Google Scholar]
- Theyel BB, Llano DA, Sherman SM. The corticothalamocortical circuit drives higher-order cortex in the mouse. Nat Neurosci. 2010;13:84–88. doi: 10.1038/nn.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uziel D, Muhlfriedel S, Bolz J. Ephrin-A5 promotes the formation of terminal thalamocortical arbors. Neuroreport. 2008;19:877–881. doi: 10.1097/WNR.0b013e3282ffdeec. [DOI] [PubMed] [Google Scholar]
- van der Loos H, Dorfl J. Does the skin tell the somatosensory cortex how to construct a map of the periphery? Neurosci Lett. 1978;7:23–30. doi: 10.1016/0304-3940(78)90107-6. [DOI] [PubMed] [Google Scholar]
- Van der Loos H, Dorfl J, Welker E. Variation in pattern of mystacial vibrissae in mice. A quantitative study of ICR stock and several inbred strains. J Hered. 1984;75:326–336. [PubMed] [Google Scholar]
- Van der Loos H, Welker E, Dorfl J, Rumo G. Selective breeding for variations in patterns of mystacial vibrissae of mice. Bilaterally symmetrical strains derived from ICR stock. J. Hered. 1986;77:66–82. doi: 10.1093/oxfordjournals.jhered.a110201. [DOI] [PubMed] [Google Scholar]
- Van der Loos H, Woolsey TA. Somatosensory cortex: structural alterations following early injury to sense organs. Science. 1973;179:395–398. doi: 10.1126/science.179.4071.395. [DOI] [PubMed] [Google Scholar]
- Vanderhaeghen P, Lu Q, Prakash N, Frisen J, Walsh CA, Frostig RD, Flanagan JG. A mapping label required for normal scale of body representation in the cortex. Nat Neurosci. 2000;3:358–365. doi: 10.1038/73929. [DOI] [PubMed] [Google Scholar]
- Vanderklish PW, Edelman GM. Dendritic spines elongate after stimulation of group 1 metabotropic glutamate receptors in cultured hippocampal neurons. Proc Natl Acad Sci U S A. 2002;99:1639–1644. doi: 10.1073/pnas.032681099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voutsinos-Porche B, Bonvento G, Tanaka K, Steiner P, Welker E, Chatton JY, Magistretti PJ, Pellerin L. Glial glutamate transporters mediate a functional metabolic crosstalk between neurons and astrocytes in the mouse developing cortex. Neuron. 2003;37:275–286. doi: 10.1016/s0896-6273(02)01170-4. [DOI] [PubMed] [Google Scholar]
- Watson RF, Abdel-Majid RM, Barnett MW, Willis BS, Katsnelson A, Gillingwater TH, McKnight GS, Kind PC, Neumann PE. Involvement of protein kinase A in patterning of the mouse somatosensory cortex. J Neurosci. 2006;26:5393–5401. doi: 10.1523/JNEUROSCI.0750-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welker C. Receptive fields of barrels in the somatosensory neocortex of the rat. J Comp Neurol. 1976;166:173–189. doi: 10.1002/cne.901660205. [DOI] [PubMed] [Google Scholar]
- Welker E, Armstrong-James M, Bronchti G, Qurednik W, Gheorghita-Baechler F, Dubois R, Guernsey DL, Van der Loos H, Neumann PE. Altered sensory processing in the somatosensory cortex of the mouse mutant barrelless. Science. 1996;271:1864–1867. doi: 10.1126/science.271.5257.1864. [DOI] [PubMed] [Google Scholar]
- Welker E, Armstrong-James M, Van der Loos H, Kraftsik R. The mode of activation of a barrel column: response properties of single units in the somatosensory cortex of the mouse upon whisker deflection. Eur J Neurosci. 1993;5:691–712. doi: 10.1111/j.1460-9568.1993.tb00534.x. [DOI] [PubMed] [Google Scholar]
- Westmark CJ, Malter JS. FMRP mediates mGluR5-dependent translation of amyloid precursor protein. PLoS Biol. 2007;5:e52. doi: 10.1371/journal.pbio.0050052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White EL. Identified neurons in mouse Sml cortex which are postsynaptic to thalamocortical axon terminals: a combined Golgi-electron microscopic and degeneration study. J Comp Neurol. 1978;181:627–661. doi: 10.1002/cne.901810310. [DOI] [PubMed] [Google Scholar]
- Wijetunge LS, Till SM, Gillingwater TH, Ingham CA, Kind PC. mGluR5 regulates glutamate-dependent development of the mouse somatosensory cortex. J Neurosci. 2008;28:13028–13037. doi: 10.1523/JNEUROSCI.2600-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilent WB, Contreras D. Synaptic responses to whisker deflections in rat barrel cortex as a function of cortical layer and stimulus intensity. J Neurosci. 2004;24:3985–3998. doi: 10.1523/JNEUROSCI.5782-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolsey TA. Peripheral alteration and somatosensory development. In: Coleman EJ, editor. Development of sensory systems in mammals. Wiley; New York: 1990. pp. 461–516. [Google Scholar]
- Woolsey TA, Dierker ML, Wann DF. Mouse SmI cortex: qualitative and quantitative classification of golgi-impregnated barrel neurons. Proc Natl Acad Sci U S A. 1975;72:2165–2169. doi: 10.1073/pnas.72.6.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolsey TA, Van der Loos H. The structural organization of layer IV in the somatosensory region (SI) of the mouse cerebral cortex. Brain Res. 1970;17:205–242. doi: 10.1016/0006-8993(70)90079-x. [DOI] [PubMed] [Google Scholar]
- Woolsey TA, Wann JR. Areal changes in mouse cortical barrels following vibrissal damage at different postnatal ages. J Comp Neurol. 1976;170:53–66. doi: 10.1002/cne.901700105. [DOI] [PubMed] [Google Scholar]
- Wu CS, Zhu J, Wager-Miller J, Wang S, O’Leary D, Monory K, Lutz B, Mackie K, Lu HC. Requirement of cannabinoid CB(1) receptors in cortical pyramidal neurons for appropriate development of corticothalamic and thalamocortical projections. Eur J Neurosci. 2010;32:693–706. doi: 10.1111/j.1460-9568.2010.07337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C, Qiu Y, Pereira FA, Crair MC, Tsai SY, Tsai MJ. The nuclear orphan receptor COUP-TFI is required for differentiation of subplate neurons and guidance of thalamocortical axons. Neuron. 1999;24:847–859. doi: 10.1016/s0896-6273(00)81032-6. [DOI] [PubMed] [Google Scholar]