Abstract
Acquired resistance to Herceptin is a major clinical problem in the treatment of HER2-overexpressing breast cancer. Understanding the molecular mechanisms leading to resistance will allow identification of novel therapeutic targets and predictors of therapeutic response. To this end, up-regulation of anti-apoptotic proteins has been associated with resistance to the HER2-targeted drug lapatinib, but has not yet been linked to Herceptin resistance. The aim of the current study was to determine if the Bcl-2 anti-apoptotic protein is a potential therapeutic target in cells with acquired Herceptin resistance. The BT474 HER2-overexpressing breast cancer cell line and BT474-derived acquired Herceptin-resistant clones were used as models in this study. Bcl-2 and Bax expression were assessed by Western blotting. Proliferation assays were performed on cells treated with the Bcl-2 inhibitor ABT-737 in the absence or presence of Herceptin. Finally, the effect of PI3K inhibition or IKK inhibition on Bcl-2 expression and Herceptin sensitivity was examined by Western blotting and established proliferation assays. We show that cells with acquired resistance to Herceptin have an increased Bcl-2:Bax ratio. Resistant cells have increased sensitivity to ABT-737. Further, pharmacologic inhibition of Bcl-2 improved sensitivity to Herceptin in acquired resistant cells. Finally, PI3K and IKK inhibition down-regulated Bcl-2 expression and increased sensitivity to Herceptin in resistant cells. Taken together, these new observations support further study of Bcl-2-targeted therapies in Herceptin-resistant breast cancers, and importantly, future investigation of Bcl-2 expression as a potential predictor of Herceptin response in patients with HER2-overexpressing breast cancer.
Keywords: Bcl2, erbB2, Herceptin, lapatinib, trastuzumab
1. INTRODUCTION
Overexpression of the HER2 receptor tyrosine kinase occurs in approximately 30% of metastatic breast cancers, and is associated with poor disease-free survival [1]. Herceptin (trastuzumab; Genentech, South San Francisco, CA) is a recombinant humanized anti-HER2 monoclonal antibody approved for treatment of HER2-overexpressing metastatic breast cancer. Clinical studies have shown that the response rates to Herceptin monotherapy in patients with metastatic breast cancer ranged from 12% to 34% for a median duration of 9 months [2-4]. Combination of Herceptin with chemotherapy [5-7] improved response rates, increased time to disease progression, and increased overall survival rates compared with chemotherapy alone. Unfortunately, as is the case for Herceptin monotherapy, many patients who were treated with Herceptin plus chemotherapy developed progressive disease within one year [5-7]. Thus, acquired resistance to Herceptin remains as an important issue in the clinical treatment of HER2-overexpressing breast cancer. Understanding the molecular mechanisms contributing to acquired resistance will ultimately allow identification of new molecular targets and predictors of response to therapy.
In the current study, we used the HER2-overexpressing BT474 breast cancer cell line and clones derived from BT474 that have acquired trastuzumab resistance to determine the role of the Bcl-2 anti-apoptotic protein in Herceptin resistance.
2. MATERIAL AND METHODS
Materials
Herceptin was purchased from the Winship Cancer Institute pharmacy and dissolved in sterile water at a stock concentration of 20 mg/mL. ABT-737 was purchased from SelleckChem (Houston, TX), and dissolved in DMSO at a final 10 mM stock solution. LY294002 (Cell Signaling; Danvers, MA) was dissolved in DMSO at 10 mM. IKK inhibitor II wedelolactone (EMD Biosciences; Gibbstown, NJ) was dissolved in DMSO at 15 mM stock concentration.
Cell culture
The BT474 HER2-overexpressing breast cancer cell line was purchased from American Type Culture Collection (ATCC; Manassas, VA). BT474-derived acquired Herceptin-resistant clones were developed by chronically growing the parental line in a clinically relevant dose of 4 μg/mL Herceptin, as previously described [8]. All cells were maintained in DMEM + 10% FBS and 1% penicillin/streptomycin; Herceptin-resistant cells were maintained in media containing 4 μg/mL Herceptin. Prior to experiments, Herceptin-resistant cells were maintained in Herceptin-free media for at least 24 hours. All cells were cultured in humidified incubators at 37°C with 5% CO2.
Cell proliferation assays
Cells were treated with vehicle control or drug in 6 replicates per group. After 6 d, MTS proliferation assay (Promega; Madison, WI) was performed according to manufacturer directions.
Western blotting
Cells were lysed in RIPA buffer (Cell Signaling) supplemented with protease and phosphatase inhibitors (Sigma-Aldrich; St. Louis, MO). Total protein extracts were run on SDS-PAGE and blotted onto nitrocellulose. Blots were probed overnight using the following antibodies: from Cell Signaling, p-S473 Akt-XP used at 1:200, polyclonal antibodies against total Akt (1:1000), and Bax; Bcl-2 monoclonal 83-8B from Assay Designs / Enzo Life Sciences (Ann Arbor, MI); β-actin monoclonal AC-15 (Sigma-Aldrich) at 1:10,000. Protein bands were detected using the Odyssey Imaging System (Li-Cor Biosciences; Lincoln, NE). Quantification of bands was done using NIH ImageJ software; each band was normalized to its actin loading control.
Data analysis
Statistical analyses were carried out using the Student’s t-test using Microsoft Excel 2007 for each experimental group versus control group. P-values were determined across doses using single-variable analysis of variance (ANOVA). A two sided P-value of less than 0.05 was considered as significant in all analysis.
3. RESULTS
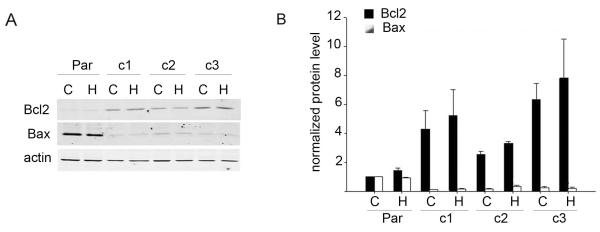
3.1. Bcl-2 expression is increased and Bax expression is decreased in Herceptin-resistant breast cancer cells
Herceptin has been shown to reduce expression of the bcl-2 gene in BT474 cells and in patient tumor samples [9]. We examined the expression of Bcl-2 and pro-apoptotic protein Bax in BT474 cells and BT474-derived Herceptin-resistant clones in the absence or presence of Herceptin (Figure 1A). While Herceptin treatment did not affect Bcl-2 or Bax protein levels in our cell lines, dramatic differences in baseline expression of Bcl-2 and Bax were seen in resistant versus parental cells (Figure 1B). BT-HRc1, c2, and c3 cells showed a 4.3-fold, 2.5-fold, and 6.3-fold higher Bcl-2 level versus parental cells, respectively. Conversely, Bax levels were significantly reduced in all three resistant clones, with BT-HRc1, c2, and c3 showing an 11-fold, 6-fold, and 4-fold down-regulation in Bax expression versus parental cells, respectively.
Figure 1.
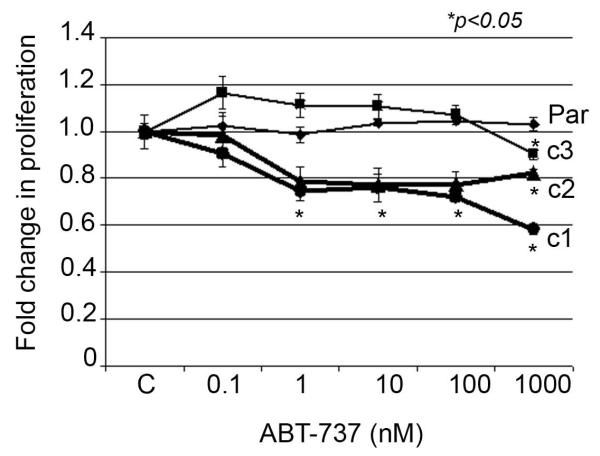
3.2. Herceptin-resistant cells show increased sensitivity to pharmacologic inhibition of Bcl-2
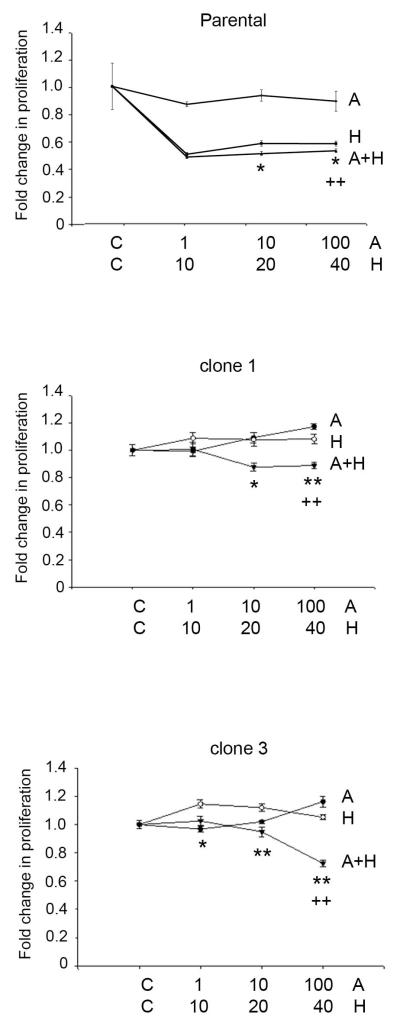
We examined the dose-response of parental and resistant cells to the BH3 mimetic pan-Bcl-2 inhibitor ABT-737. Resistant cells showed significantly higher sensitivity to inhibition of Bcl-2 versus parental cells (Figure 2). At the highest dose of 1000 nM ABT-737, the majority of parental cells remained viable, while BT-HRc1, c2, and c3 resistant cells showed viability of 58%, 82%, and 90%, respectively. Further, combined treatment of parental or resistant cells with ABT-737 plus Herceptin showed statistically significant reduction in proliferation versus Herceptin alone, with the combination achieving the most significant effect in resistant cells (Figure 3).
Figure 2.
Figure 3.
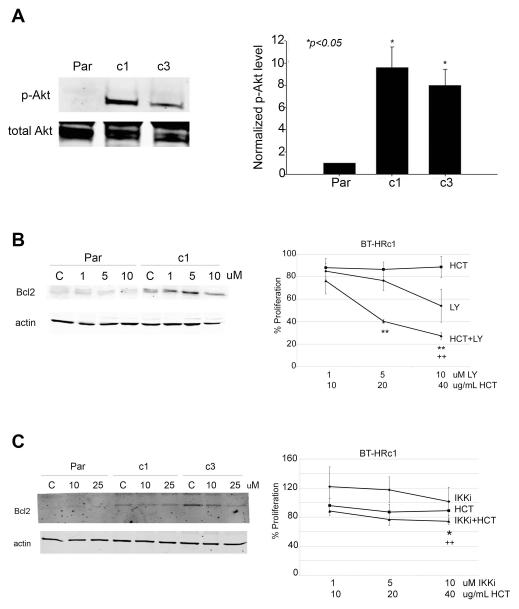
3.3. Analysis of the effects of PI3K inhibition and IKK inhibition on Bcl-2 expression and proliferation in Herceptin-resistant cells
We examined levels of phosphorylated Akt in parental cells and acquired Herceptin-resistant clones (Figure 4A). Both Herceptin-resistant clones showed significantly elevated p-Akt levels versus parental cells. BT474 parental and resistant clone 1 cells were treated with the pharmacologic inhibitor of PI3K, LY294002, at 1, 5, or 10 μM for 48 h, at which point Bcl-2 expression was examined (Figure 4B left panel). PI3K inhibition did not significantly alter Bcl-2 expression in parental cells, but reduced Bcl-2 expression in resistant cells. Consistent with this potential reduction in Bcl-2 expression, LY294002 inhibited proliferation of BT-HRc1 cells in a dose-dependent manner (Figure 4B right panel). Combination treatment with LY294002 and Herceptin resulted in dramatic inhibition of proliferation in resistant cells, supporting published reports that PI3K inhibition restores sensitivity to Herceptin.
Figure 4.
Next, we examined the effects of IKK inhibition on Bcl-2 expression since NF-kB is a known regulator of Bcl-2 transcription. Wedelolactone, a pharmacologic inhibitor of IKK, caused a dose-dependent decrease in Bcl-2 expression in Herceptin-resistant cells (Figure 4C left panel). Co-treatment of resistant cells with wedelolactone plus Herceptin resulted in statistically significant reductions in proliferation (Figure 4C right panel), although the effect was not as dramatic as that observed with PI3K inhibition.
4. DISCUSSION
Our results collectively indicate that cells with acquired resistance to Herceptin have an increased Bcl-2 to Bax ratio. Cells with acquired resistance showed increased sensitivity to pharmacologic Bcl-2 inhibitor ABT-737 versus parental cells. In addition, the combination of ABT-737 and Herceptin reduced proliferation of Herceptin-resistant cells more significantly than either drug alone. Thus, our results support further study of Bcl-2 inhibition as a strategy for improving response to Herceptin, and investigation of Bcl-2 expression level as a predictor of response to Herceptin.
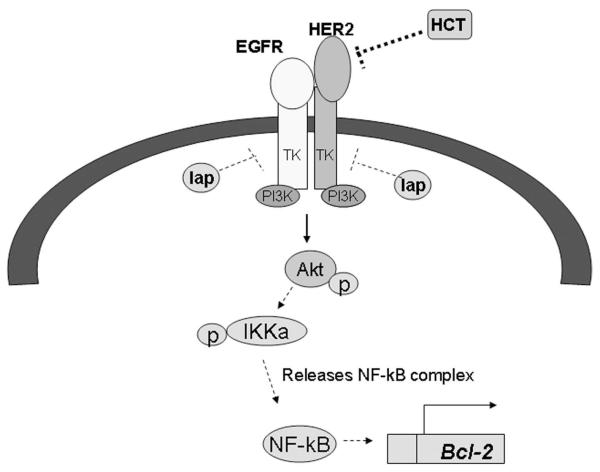
Our data also suggest that increased PI3K signaling in resistant cells may contribute to Bcl-2 overexpression, mediated in part by the NF-kB transcription factor. HER2 is known to signal downstream through PI3K causing phosphorylation of Akt. One of the downstream substrates of Akt is IKK alpha, whose phosphorylation results in release of the NF-kappaB transcription factor. Free NF-kB can translocate to the nucleus and activate transcription of a number of genes whose promoters contain NF-kB response elements, including Bcl-2 (Figure 5). Activation of IKKalpha/NF-kB downstream of PI3K/Akt has been shown to activate Bcl-2 transcription [10]. In addition, PI3K/Akt can directly activate Bcl-2 transcription [11]. We showed that PI3K inhibition and/or IKK inhibition down-regulates Bcl-2 expression and inhibits proliferation of Herceptin-resistant cells. Our data are consistent with the earlier findings of increased PI3K/Akt signaling in cells with Herceptin resistance [12-15]. Further, our current data support our previous study showing that PI3K inhibition blocks survival of Herceptin-resistant cells [16].
Figure 5.
Modulation of apoptotic regulators including Bcl-2 family members as a potential strategy for treating HER2-overexpressing breast cancer is supported by a number of published studies. Stable transfection of wild-type or mutant HER2 into breast cancer cells has been shown to increase Bcl-2 expression [17-20], supporting Bcl-2 as a potential target in HER2-dependent cancers. In addition, genetic knockdown of other anti-apoptotic proteins (Mcl-1 and survivin) has been shown to induce apoptosis in HER2-overexpressing breast cancer cell lines that were resistant to Herceptin and the dual EGFR/HER2 kinase inhibitor lapatinib [21]. Combining lapatinib or Herceptin with the cyclin-dependent kinase inhibitor flavopirodol, which suppresses Mcl-1 expression [22], also resulted in synergistic induction of cell death in HER2-overexpressing breast cancer [22-25]. Overexpression of Mcl-1 or knockdown of Bax or Bak abrogated the apoptotic effects of combination flavopiridol plus lapatinib [25], indicating that regulation of Bcl-2 family members is critical to achieving response to this novel drug combination.
Our data lend evidence for further study of the Bcl-2 inhibitor ABT-737 in Herceptin-resistant breast cancer. Other Bcl-2 antagonists have also been studied in the context of HER2-overexpressing breast cancer. Obatoclax (GX15-070), which inhibits Bcl-2, Bcl-xL, and MCL-1, increased lapatinib-mediated autophagic cell death independent of endogenous EGFR or HER2 level in breast cancer cells [26]. Similarly, synergistic cell death was induced by lapatinib combined with the Bcl-2 inhibitor HA14-1 or Obatoclax in MCF-7, MCF-7 HER2 stable transfectant, or MCF-7 tamoxifen-resistant cells [27].
Future Directions
Our data suggest that increased Bcl-2 expression contributes to Herceptin resistance. The implications of these new findings are several fold: (1) Bcl-2 expression levels may be used to predict response to Herceptin, and (2) Bcl-2 may serve as a novel molecular target for improving response to Herceptin. In addition, our observations suggest that NF-kB and PI3K may contribute to Bcl-2 up-regulation in resistant cancers. Thus, IKK and PI3K inhibitors may potentially be used therapeutically in Herceptin-resistant breast cancers that have increased expression of Bcl-2.
The major limitation of our study is that the results have not been confirmed in vivo using animal models or human tumor samples. Future studies should therefore examine pharmacologic inhibition of Bcl-2 in combination with Herceptin in xenografts of Herceptin-resistant breast cancer cell lines. In addition, the exact mechanism by which Bcl-2 is up-regulated warrants evaluation. In particular, it would be worthwhile to determine if up-regulation is transcriptional, and, further, if a PI3K/Akt/IKK/NF-kB pathway regulates Bcl-2 levels in resistant cells. Finally, IKK inhibition will be tested in combination with Herceptin in xenografts of Herceptin-resistant cancer cell lines to determine if this is a novel molecular target for improving Herceptin sensitivity. In this regard, our previous work showed that cells with acquired resistance to Herceptin have an increased sensitivity to curcumin, an inhibitor of NF-kB [28], supporting the concept that NF-KB may be hyper-activated and serve as a potential pharmacologic target in breast cancers that have progressed on Herceptin.
Implications for Pharmacogenomics and Personalized Medicine
Published work and our studies support the hypothesis that increased PI3K signaling and increased Bcl-2 expression may serve as predictors of resistance to Herceptin. Initial studies correlated increased baseline expression of Bcl-2 with ER-positive breast cancer, positive response to hormonal therapy, and favorable prognosis [29]. However, additional studies showed that increased expression of Bcl-2 in residual cancer cells following preoperative chemotherapy reduced apoptosis and increased resistance to chemotherapy [30]. Furthermore, reports have shown that the ratio between anti-apoptotic and pro-apoptotic Bcl-2 family members determines breast cancer cell response to chemotherapy [31], with chemo-sensitivity correlating with a high Bax:Bcl-2 ratio [32]. Several studies have also suggested that estrogen promotes resistance to chemotherapeutic drugs by increasing the Bcl-2:Bax ratio [31, 33]. These reports are consistent with our new findings that Herceptin resistance is associated with an increased level of Bcl-2:Bax.
Taken together, we propose that Bcl-2 inhibition may be an effective personalized therapeutic strategy for HER2-overexpressing breast cancers that have progressed on prior Herceptin treatment and that show elevated expression of Bcl-2. Indeed, clinical trials of several Bcl-2 family-targeted drugs are ongoing. The preclinical data shown here lend further support for the clinical investigation of Bcl-2 inhibitors in the specific context of HER2-positive metastatic breast cancer that shows poor response to HER2-targeted therapies and elevated expression of Bcl-2.
5. CONCLUSIONS
In summary, we showed that Bcl-2 expression is increased in cells that have acquired resistance to Herceptin. Cells with acquired resistance showed increased sensitivity to a Bcl-2 inhibitor, and the combination of Bcl-2 inhibitor plus Herceptin showed significantly greater inhibition of proliferation versus either agent alone. Ongoing studies will examine the mechanism of Bcl-2 up-regulation, and will determine if Bcl-2 inhibition restores Herceptin sensitivity in vivo. These studies are necessary to move toward establishing Bcl-2 as a new therapeutic target in Herceptin-resistant breast cancers. Once clinical correlations have been validated between high Bcl-2 level and poor response to Herceptin, Bcl-2 expression may be established as a novel predictor of Herceptin resistance.
Acknowledgements
Grant support for the research presented herein is gratefully acknowledged from the National Cancer Institute (K01CA118174) and the Georgia Cancer Coalition Distinguished Cancer Scholars Program to Dr. Rita Nahta. Anonymous peer review comments are also acknowledged.
List of Abbreviations
- EGFR
epidermal growth factor receptor
- ER
estrogen receptor
- FISH
fluorescent in situ hybridization
- HER2
human epidermal growth factor receptor 2
- IGF-IR
insulin-like growth factor-I receptor
- IHC
immunohistochemical
- IKK
inhibitor of kappa B kinase
- MMTV
mouse mammary tumor virus
- NF-kB
nuclear factor kappa-light-chain-enhancer of activated B cells
- PI3K
phosphatidylinositol 3 kinase
- PTEN
phosphatase and tensin homolog
Footnotes
Conflict of Interests
None declared/applicable.
References
- [1].Slamon DJ, Clark GM, Wong SG, et al. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235(4785):177–82. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- [2].Baselga J, Tripathy D, Mendelsohn J, et al. Phase II study of weekly intravenous recombinant humanized anti-p185HER2 monoclonal antibody in patients with HER2/neu-overexpressing metastatic breast cancer. J Clin Oncol. 1996;14(3):737–44. doi: 10.1200/JCO.1996.14.3.737. [DOI] [PubMed] [Google Scholar]
- [3].Cobleigh MA, Vogel CL, Tripathy D, et al. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER-2 overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol. 1999;17(9):2639–48. doi: 10.1200/JCO.1999.17.9.2639. [DOI] [PubMed] [Google Scholar]
- [4].Vogel CL, Cobleigh MA, Tripathy D, et al. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20(3):719–26. doi: 10.1200/JCO.2002.20.3.719. [DOI] [PubMed] [Google Scholar]
- [5].Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344(11):783–92. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- [6].Esteva FJ, Valero V, Booser D, et al. Phase II study of weekly docetaxel and trastuzumab for patients with HER-2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20(7):1800–8. doi: 10.1200/JCO.2002.07.058. [DOI] [PubMed] [Google Scholar]
- [7].Seidman AD, Fornier MN, Esteva FJ, et al. Weekly trastuzumab and paclitaxel therapy for metastatic breast cancer with analysis of efficacy by HER2 immunophenotype and gene amplification. J Clin Oncol. 2001;19(10):2587–95. doi: 10.1200/JCO.2001.19.10.2587. [DOI] [PubMed] [Google Scholar]
- [8].Rowe DL, Ozbay T, Bender LM, et al. Nordihydroguaiaretic acid, a cytotoxic insulin-like growth factor-I receptor/HER2 inhibitor in trastuzumab-resistant breast cancer. Mol Cancer Ther. 2008;7(7):1900–8. doi: 10.1158/1535-7163.MCT-08-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Milella M, Trisciuoglio D, Bruno T, et al. Trastuzumab down-regulates Bcl-2 expression and potentiates apoptosis induction by Bcl-2/Bcl-XL bispecific antisense oligonucleotides in HER-2 gene--amplified breast cancer cells. Clin Cancer Res. 2004;10(22):7747–56. doi: 10.1158/1078-0432.CCR-04-0908. [DOI] [PubMed] [Google Scholar]
- [10].Sun ZJ, Chen G, Hu X, et al. Activation of PI3K/Akt/IKK-alpha/NF-kappaB signaling pathway is required for the apoptosis-evasion in human salivary adenoid cystic carcinoma: its inhibition by quercetin. Apoptosis. 2010;15(7):850–63. doi: 10.1007/s10495-010-0497-5. [DOI] [PubMed] [Google Scholar]
- [11].Pugazhenthi S, Nesterova A, Sable C, et al. Akt/protein kinase B up-regulates Bcl-2 expression through cAMP-response element-binding protein. J Biol Chem. 2000;275(15):10761–6. doi: 10.1074/jbc.275.15.10761. [DOI] [PubMed] [Google Scholar]
- [12].Nagata Y, Lan KH, Zhou X, et al. PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell. 2004;6(2):117–27. doi: 10.1016/j.ccr.2004.06.022. [DOI] [PubMed] [Google Scholar]
- [13].Dave B, Migliaccio I, Gutierrez MC, et al. Loss of phosphatase and tensin homolog or phosphoinositol-3 kinase activation and response to trastuzumab or lapatinib in human epidermal growth factor receptor 2-overexpressing locally advanced breast cancers. J Clin Oncol. 2011;29(2):166–73. doi: 10.1200/JCO.2009.27.7814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Berns K, Horlings HM, Hennessy BT, et al. A functional genetic approach identifies the PI3K pathway as a major determinant of trastuzumab resistance in breast cancer. Cancer Cell. 2007;12(4):395–402. doi: 10.1016/j.ccr.2007.08.030. [DOI] [PubMed] [Google Scholar]
- [15].Esteva FJ, Guo H, Zhang S, et al. PTEN, PIK3CA, p-AKT, and p-p70S6K status: association with trastuzumab response and survival in patients with HER2-positive metastatic breast cancer. Am J Pathol. 2010;177(4):1647–56. doi: 10.2353/ajpath.2010.090885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ozbay T, Durden DL, Liu T, et al. In vitro evaluation of pan-PI3-kinase inhibitor SF1126 in trastuzumab-sensitive and trastuzumab-resistant HER2-over-expressing breast cancer cells. Cancer Chemother Pharmacol. 2010;65(4):697–706. doi: 10.1007/s00280-009-1075-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kumar R, Mandal M, Lipton A, et al. Overexpression of HER2 modulates bcl-2, bcl-XL, and tamoxifen-induced apoptosis in human MCF-7 breast cancer cells. Clin Cancer Res. 1996;2(7):1215–9. [PubMed] [Google Scholar]
- [18].Siddiqa A, Long LM, Li L, et al. Expression of HER-2 in MCF-7 breast cancer cells modulates anti-apoptotic proteins Survivin and Bcl-2 via the extracellular signal-related kinase (ERK) and phosphoinositide-3 kinase (PI3K) signalling pathways. BMC Cancer. 2008;8:129–137. doi: 10.1186/1471-2407-8-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Mitra D, Brumlik MJ, Okamgba SU, et al. An oncogenic isoform of HER2 associated with locally disseminated breast cancer and trastuzumab resistance. Mol Cancer Ther. 2009;8(8):2152–62. doi: 10.1158/1535-7163.MCT-09-0295. [DOI] [PubMed] [Google Scholar]
- [20].Cittelly DM, Das PM, Salvo VA, et al. Oncogenic HER2{Delta}16 suppresses miR-15a/16 and deregulates BCL-2 to promote endocrine resistance of breast tumors. Carcinogenesis. 2010;31(12):2049–57. doi: 10.1093/carcin/bgq192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Valabrega G, Capellero S, Cavalloni G, et al. HER2-positive breast cancer cells resistant to trastuzumab and lapatinib lose reliance upon HER2 and are sensitive to the multitargeted kinase inhibitor sorafenib. Breast Cancer Res Treat. 2010 doi: 10.1007/s10549-010-1281-5. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- [22].Gojo I, Zhang B, Fenton RG. The cyclin-dependent kinase inhibitor flavopiridol induces apoptosis in multiple myeloma cells through transcriptional repression and down-regulation of Mcl-1. Clin Cancer Res. 2002;8(11):3527–38. [PubMed] [Google Scholar]
- [23].Nahta R, Iglehart JD, Kempkes B, Schmidt EV. Rate-limiting effects of Cyclin D1 in transformation by ErbB2 predicts synergy between herceptin and flavopiridol. Cancer Res. 2002;62(8):2267–71. [PubMed] [Google Scholar]
- [24].Nahta R, Trent S, Yang C, et al. Epidermal growth factor receptor expression is a candidate target of the synergistic combination of trastuzumab and flavopiridol in breast cancer. Cancer Res. 2003;63(13):3626–31. [PubMed] [Google Scholar]
- [25].Mitchell C, Yacoub A, Hossein H, et al. Inhibition of MCL-1 in breast cancer cells promotes cell death in vitro and in vivo. Cancer Biol Ther. 2010;10(9):903–17. doi: 10.4161/cbt.10.9.13273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Martin AP, Mitchell C, Rahmani M, et al. Inhibition of MCL-1 enhances lapatinib toxicity and overcomes lapatinib resistance via BAK-dependent autophagy. Cancer Biol Ther. 2009;8(21):2084–96. doi: 10.4161/cbt.8.21.9895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Witters LM, Witkoski A, Planas-Silva MD, et al. Synergistic inhibition of breast cancer cell lines with a dual inhibitor of EGFR-HER-2/neu and a Bcl-2 inhibitor. Oncol Rep. 2007;17(2):465–9. [PubMed] [Google Scholar]
- [28].Nahta R, Shabaya S, Ozbay T, Rowe DL. Personalizing HER2-targeted therapy in metastatic breast cancer beyond HER2 status: what we have learned from clinical specimens. Curr Pharmacogenomics Person Med. 2009;7(4):263–74. doi: 10.2174/187569209790112337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Gee JM, Robertson JF, Ellis IO, et al. Immunocytochemical localization of BCL-2 protein in human breast cancers and its relationship to a series of prognostic markers and response to endocrine therapy. Int J Cancer. 1994;59(5):619–28. doi: 10.1002/ijc.2910590508. [DOI] [PubMed] [Google Scholar]
- [30].Ellis PA, Smith IE, Detre S, et al. Reduced apoptosis and proliferation and increased Bcl-2 in residual breast cancer following preoperative chemotherapy. Breast Cancer Res Treat. 1998;48(2):107–16. doi: 10.1023/a:1005933815809. [DOI] [PubMed] [Google Scholar]
- [31].Zhang GJ, Kimijima I, Onda M, et al. Tamoxifen-induced apoptosis in breast cancer cells relates to down-regulation of bcl-2, but not bax and bcl-X(L), without alteration of p53 protein levels. Clin Cancer Res. 1999;5(10):2971–7. [PubMed] [Google Scholar]
- [32].Chresta CM, Masters JR, Hickman JA. Hypersensitivity of human testicular tumors to etoposide-induced apoptosis is associated with functional p53 and a high Bax:Bcl-2 ratio. Cancer Res. 1996;56(8):1834–41. [PubMed] [Google Scholar]
- [33].Huang Y, Ray S, Reed JC, et al. Estrogen increases intracellular p26Bcl-2 to p21Bax ratios and inhibits taxol-induced apoptosis of human breast cancer MCF-7 cells. Breast Cancer Res Treat. 1997;42(1):73–81. doi: 10.1023/a:1005777219997. [DOI] [PubMed] [Google Scholar]