Abstract
A novel reaction of indole with aryldiazonium salts leading to the formation of 2-aryl-3-(arylazo)indoles was discovered. The products were found to possess potent anti-MRSA and anti-LLVRE activities. The SAR studies indicate that the potentially metabolically labile azo functionality can be replaced with ether oxygen and thioether sulphur atoms without any loss of activity.
Keywords: MRSA, low-level VRE, indole, arylation
Nosocomial infections caused by multi-drug resistant bacteria, such as methicillin-resistant Staphylococcus aureus (MRSA) or vancomycin-resistant Enterococcus faecalis (VRE) present a considerable challenge in the clinic.1,2 Therefore, the search for new antibacterial agents capable of combating these microorganisms continues unabated.3–5 Because a number of azo-containing compounds have been reported to exhibit promising antimicrobial properties,6–10 we initiated a project aimed at the synthesis of diverse azo-containing heterocycles and their evaluation for antibacterial and antifungal activities with the emphasis on hospital-acquired infections. These efforts led to the identification of a compound active against MRSA, whose structure was originally assigned as 2,3-di(p-chlorophenylazo)indole (Compound A, Figure 1).
Figure 1.
The originally assigned erroneous and corrected structures for compound A.
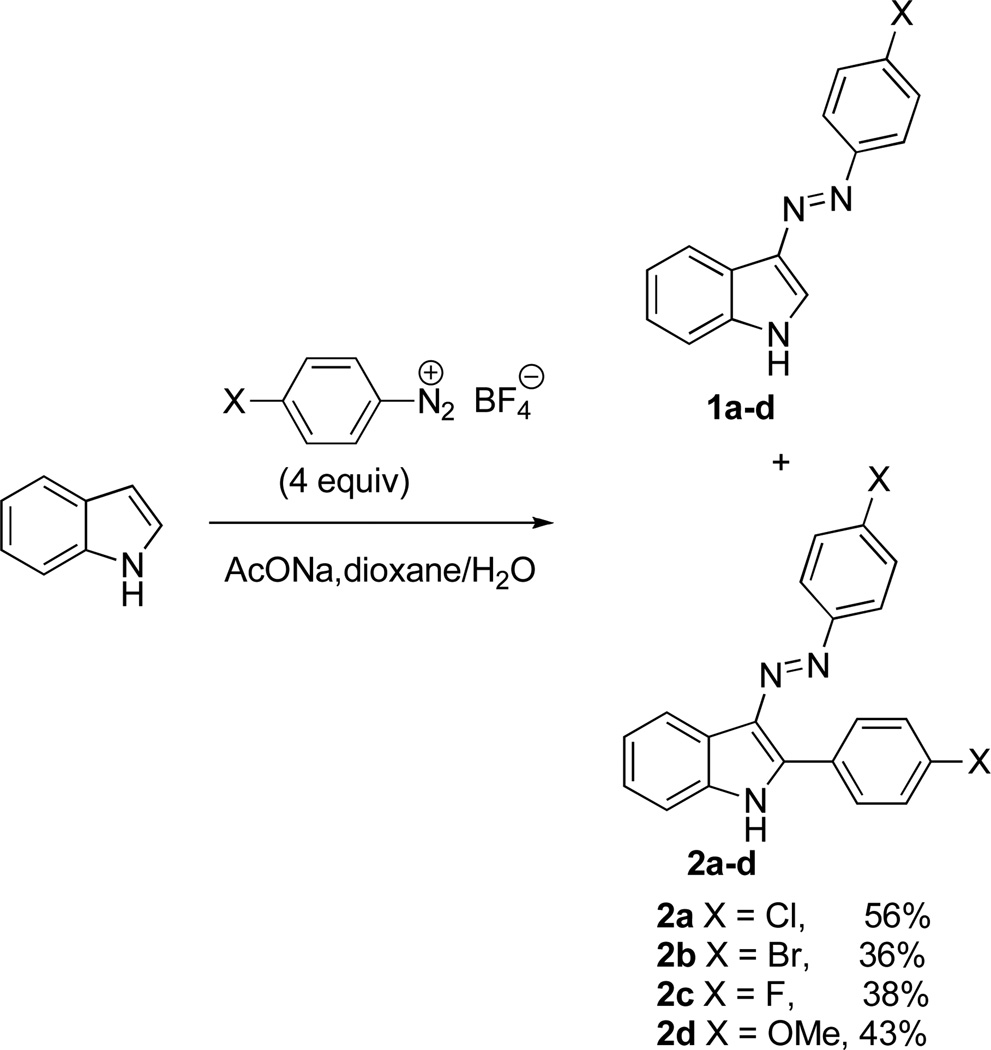
This compound was originally synthesized by the reaction of indole with 2.5 equiv of p-ClPhN2+Cl−, which gave 87% of the C-3 monoazo product (1a in Scheme 1) and only 3% of compound A. The subsequent optimization of this procedure resulted in the use of 4 equiv of p-ClPhN2+BF4− and afforded an improved 56% yield of this desired product. Although the NMR spectra of compound A were consistent with its originally assigned structure as 2,3-di(p-chlorophenylazo)indole, the mass spectral analysis suggested that only one azo group was present. While either C-2 or C-3 positioning of the azo functionality was theoretically possible, the mechanistic considerations led us to propose structure 2a (Figure 1, Scheme 1) for this indole derivative.
Scheme 1.
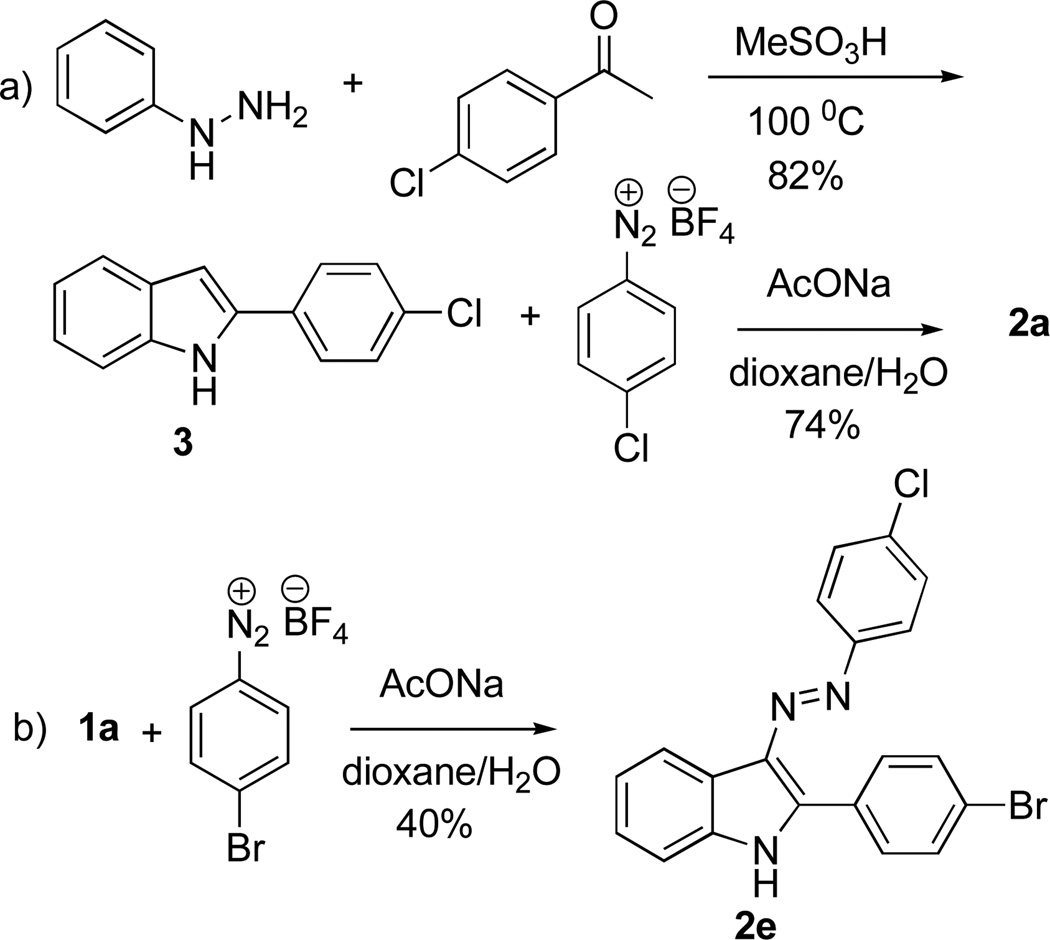
To confirm the structure of 2a unequivocally, this compound was synthesized by an independent route, involving the Fisher synthesis of indole 3 and its subsequent reaction with p-ClPhN2+BF4− (Scheme 2a). The spectral data for the obtained compound were indistinguishable with those for 2a. The reaction in Scheme 1 also allowed for the synthesis of analogues 2b–d, while the unsymmetrical derivative 2e was obtained via a stepwise introduction of the substituents (Scheme 2b).
Scheme 2.
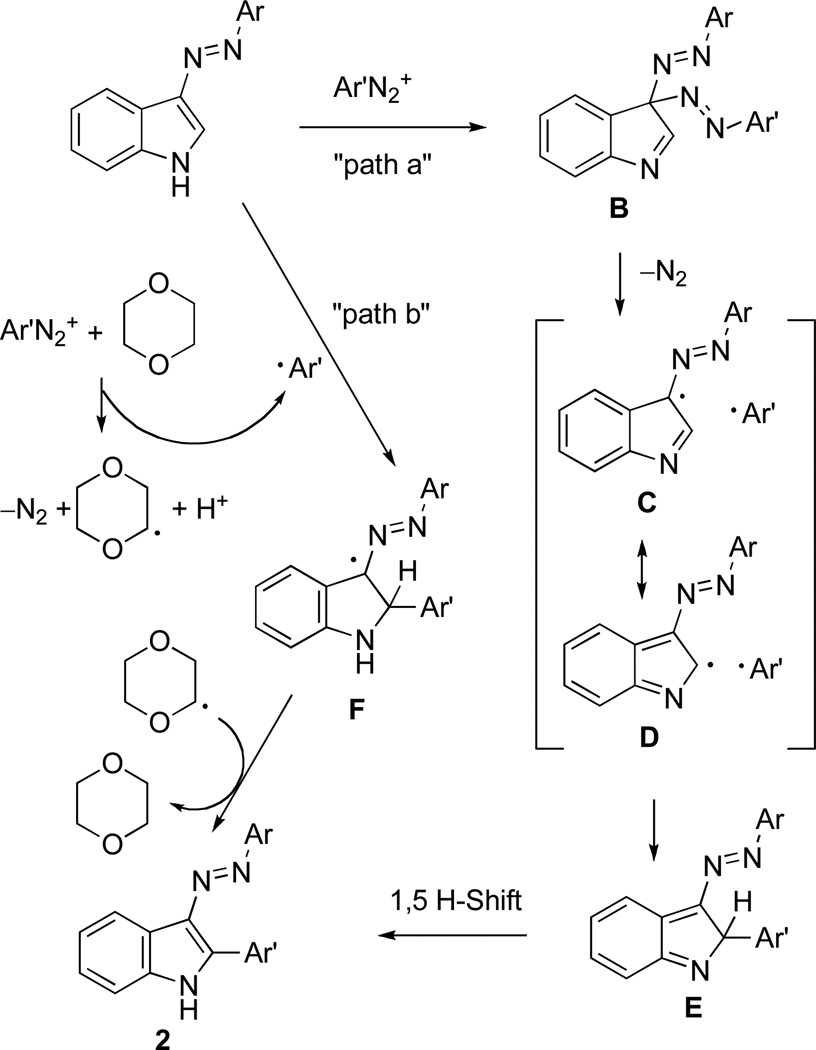
It could be surmised that mechanistically this new process involves an ipso-attack at C-3 of the indole ring11 with the formation of the geminal diazo compound B (“path a” in Scheme 3). The homolytic bond breakage and elimination of nitrogen can then lead to radical recombination at C-3 (C) or more sterically accessible C-2 (D). The 1,5 hydrogen shift results in the observed product 2. However, this mechanism is inconsistent with the formation of 2e (in Scheme 2b), which proceeds without scrambling of the halogens. Therefore, a more likely mechanism is based on the Meerwein-type arylation shown as “path b.” It involves an aromatic radical formation from the diazonium salt and addition of this radical at C-2 position of the indole to form azo-stabilized radical F, which results in product 2. The aryl radical formation from the diazonium salt can be promoted by either acetate or dioxane (shown is Scheme 3) as has been reported previously for the metal-free Meerwein arylations.12,13 Although the classical Meerwein arylations of olefins are promoted with copper or palladium salts,14 the use of Cu(OAc)2 or Pd(OAc)2 did not result in higher yields in our case.
Scheme 3.
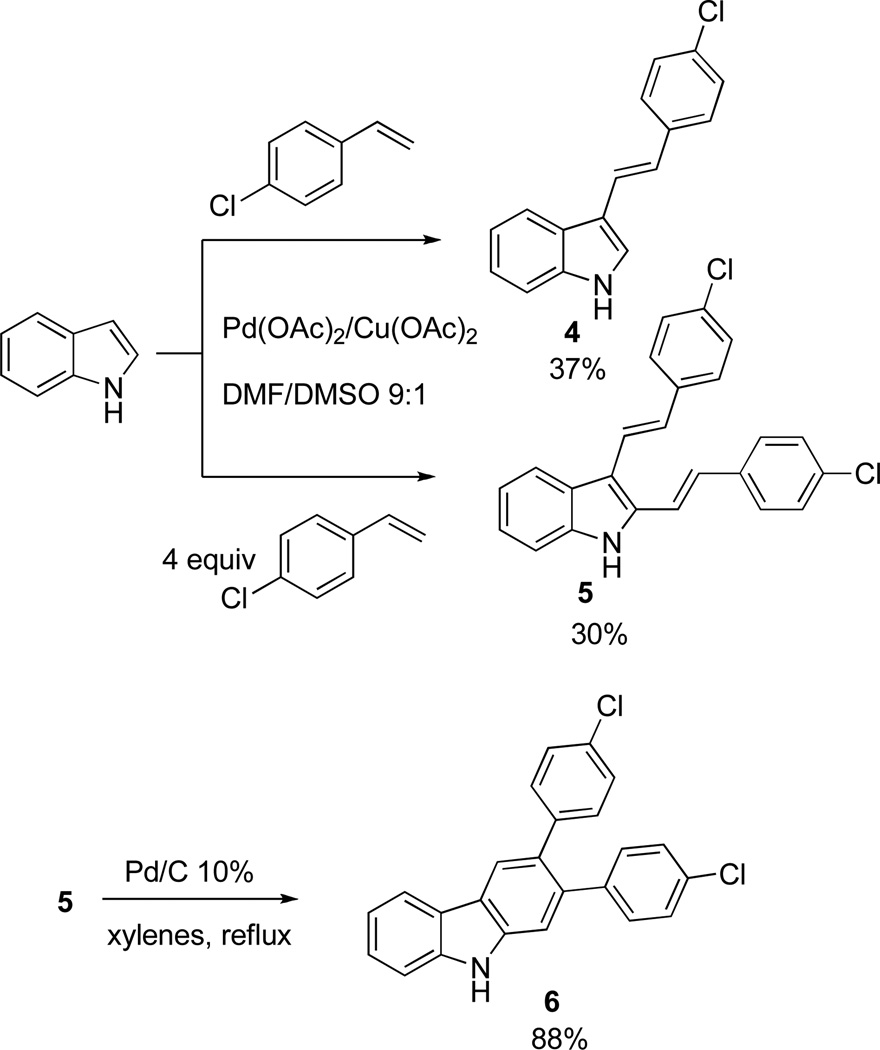
The evaluation of compounds 2a–e for antibacterial activity showed promising results, specifically against gram-positive organisms (see Table 1). However, we had concerns about the stability of the azo functionality under physiological conditions. Therefore, to explore the SAR within this series of compounds we first addressed the issue of whether this moiety is absolutely required for activity. Thus, we synthesized the “carba” analogues 4 and 5, utilizing Fujiwara/Heck chemistry and varying the number of equivalents of the olefin (Scheme 4).15 Distyrylindole 5 was then converted to carbazole 6 upon reflux in xylenes in the presence of Pd/C. We believe this approach provides a useful synthetic access to the carbazole skeleton.16
Table 1.
Antimicrobial (MICs, µM) and Cancer Cell Growth Inhibitory (GI50, µM) Activities of the Synthesized Indole Derivativesa
| Compound | Gram-(+) bacterial strains | Gram-(−) bacterial strains | Fungus | Cancer cell line |
|||
|---|---|---|---|---|---|---|---|
|
S. aureusb BAA-44 |
S. aureusc 29213 |
E. faecalisd 51299 |
P. aeruginosa 27853 |
A. baumannii 15151 |
C. albicans 26555 |
HeLa | |
| 1a | 12.5 | 12.5 | 25 | >100 | >100 | 12.5 | 23 ± 3e |
| 1b | 6.3 | 6.3 | 12.5 | >100 | >100 | 25 | 49 ± 5 |
| 2a | 1.6 | 0.6 | 8 | >100 | >100 | >100 | 26 ± 0 |
| 2b | 12.5 | 6.3 | 6.3 | >100 | >100 | >100 | 9 ± 1 |
| 2c | 1.6 | 1.3 | 3.1 | >100 | >100 | >100 | 12 ± 1 |
| 2d | 50 | 3.1 | > 100 | >100 | >100 | >100 | 23 ± 1 |
| 2e | 6.3 | 6.3 | 6.3 | >100 | >100 | >100 | 13 ± 0 |
| 3 | >100 | >100 | >100 | >100 | >100 | >100 | >100 |
| 4 | >100 | 25 | >100 | >100 | >100 | >100 | 64 ± 5 |
| 5 | >100 | >100 | > 100 | > 100 | > 100 | > 100 | 69 ± 5 |
| 6 | >100 | >100 | >100 | > 100 | > 100 | > 100 | 16 ± 1 |
| 7a | 3.1 | 1.6 | 3.1 | >100 | >100 | >100 | 6 ± 1 |
| 7b | 3.1 | 1.6 | 3.1 | >100 | >100 | >100 | 12 ± 2 |
| 7c | 1.3 | 1.6 | 6.3 | >100 | >100 | >100 | 8 ± 1 |
| 8a | 3.1 | 1.6 | 12.5 | >100 | >100 | >100 | 9 ± 0 |
| 8b | 0.6 | 1.6 | 12.5 | >100 | >100 | >100 | 8 ± 2 |
| 8c | 3.1 | 3.1 | 12.5 | >100 | >100 | >100 | 3 ± 0 |
| 8d | 1.6 | 1.6 | 6.3 | >100 | >100 | >100 | 13 ± 3 |
| 8e | 6.3 | 12.5 | 25 | >100 | >100 | >100 | 24 ± 2 |
| 8f | 1.3 | 1.6 | 3.1 | >100 | >100 | >100 | 13 ± 4 |
| 8g | 6.3 | 12.5 | 50 | >100 | >100 | >100 | 19 ± 1 |
| 9 | >100 | >100 | >100 | >100 | >100 | >100 | 14 ± 1 |
| 10 | >100 | >100 | >100 | >100 | >100 | >100 | 19 ± 1 |
| Vancomycin | 2.8 | 2.8 | 86.3 | NDf | ND | ND | ND |
| Penicillin G | >100 | 23.9 | 11.9 | ND | ND | ND | ND |
Bacterial suspensions were adjusted to ~5×105 CFU/mL.20,21 The suspensions were treated with compounds, serially diluted 2-fold and were incubated according to ATCC recommendations for 18 hours. Fungal testing was accomplished according to a previously published method.22 Bacterial and fungal viability was determined using the MTT method due to coloring of compounds.23 HeLa testing was also accomplished according to a previously published method.24
Methicillin-resistant S. aureus
Susceptible S. aureus strain
Low-level vancomycin-resistant Enterococcus
The data are presented as GI50 ± SD from four experiments.
ND = not determined
Scheme 4.
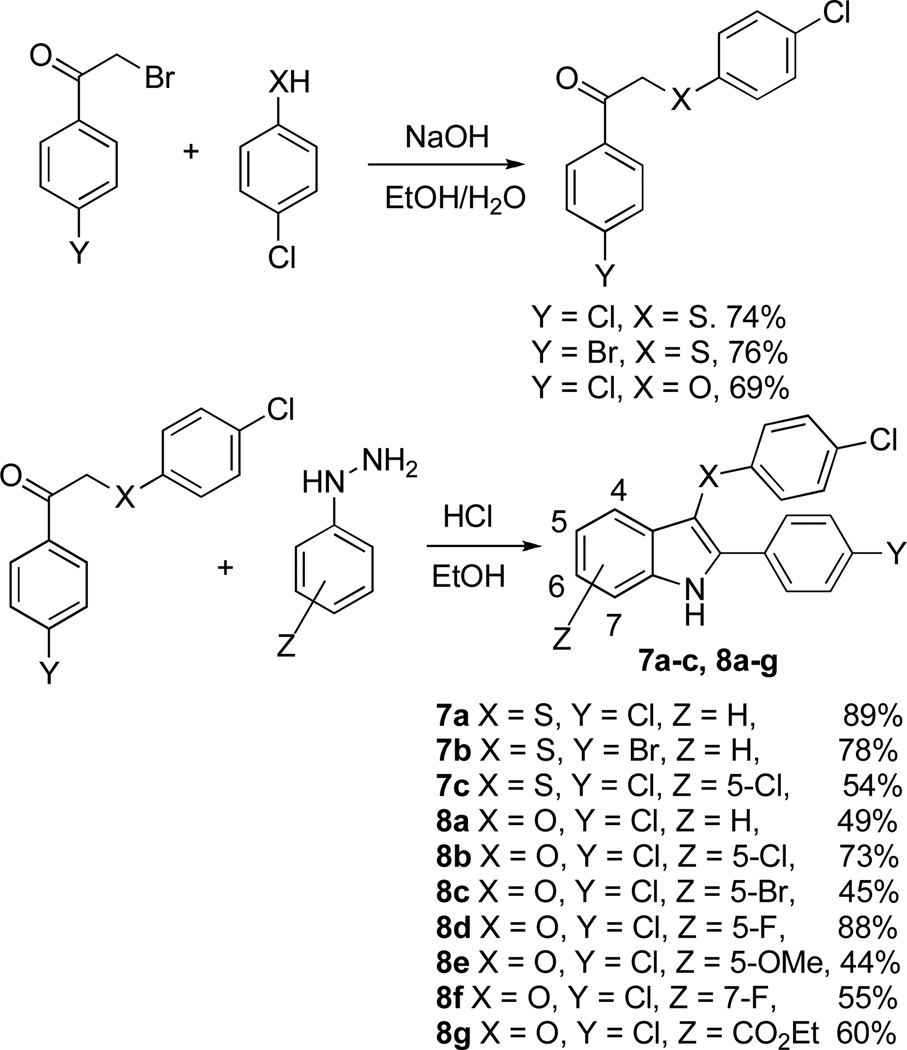
We also explored a bioisosteric substitution of the azo group with sulfur and oxygen atoms. Such compounds were prepared using the Fisher indole synthesis starting with the requisite arylthia- and aryloxa-acetophenones (Scheme 5). To our knowledge, these syntheses constitute the first examples of the preparation of 2-aryl-3-aryloxa- and 2-aryl-3-arylthia-indoles (7a–c and 8a–g) using the Fisher reaction.
Scheme 5.
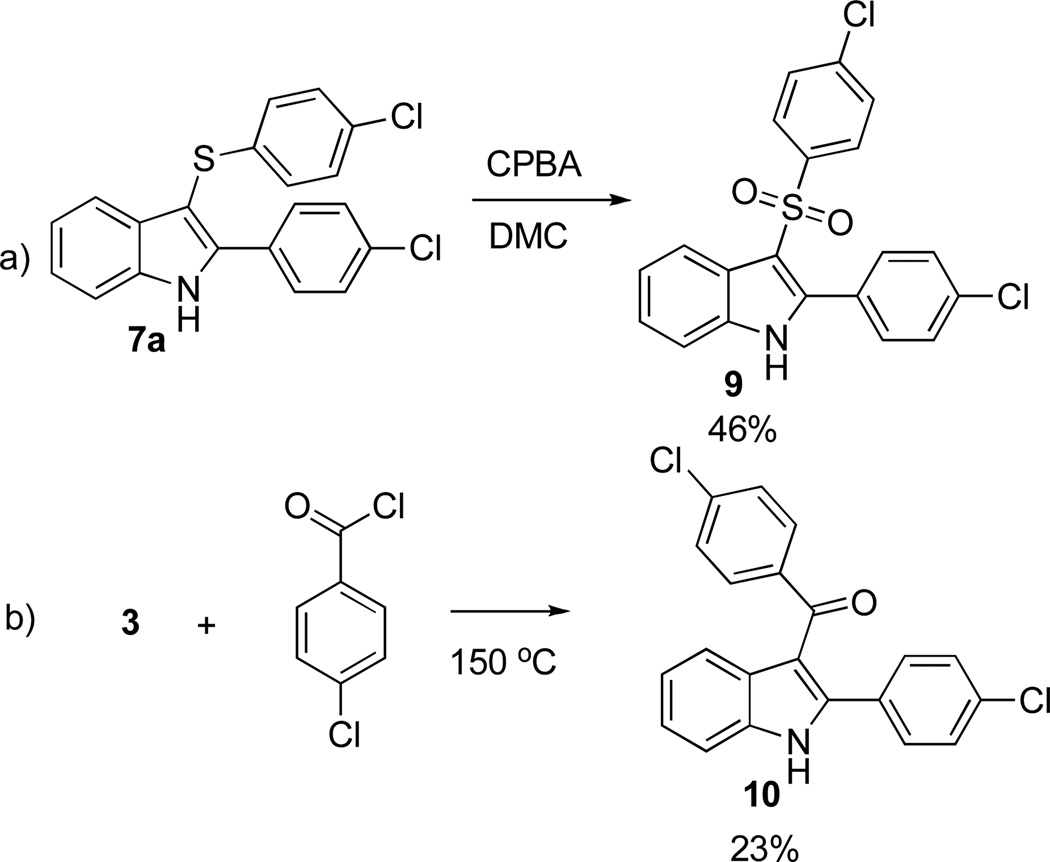
Finally, compound 9, containing the sulfone isostere of the azo moiety, was prepared by oxidation of 7a with MCPBA (Scheme 6a),17 while compound 10, the carbono analogue, was obtained by acylation of 3 with p-Cl-PhCOCl (Scheme 6b).18,19
Scheme 6.
The data in Table 1 indicate that all our compounds that show antibacterial activity inhibit the growth of gram-positive microorganisms only. Furthermore, there is no discrimination between the bacterial strains on the basis of their resistance status. Thus, all 3-azo-indole derivatives synthesized (1a,b and 2a–e) are equally potent toward the susceptible S. aureus strain (S. aureus 29213), its resistant counterpart MRSA (S. aureus BAA-44) or low-level vancomycin-resistant Enterococcus (LLVRE, E. faecalis 51299). Although the activity is lost when the 3-azo functionality is replaced by “carba” bridges (4–6), or sulfono (9) and carbono (10) groups, even more potent compounds result from substituting the 3-azo moiety with the thioether sulphur (7a–c) and ether oxygen (8a–g) atoms. For example, aryloxyindole 8b is submicromolar toward MRSA. These results compare favorably with the clinically used antibiotics vancomycin and penicillin G, which are less potent in this minipanel of gram-positive bacteria and show organism-dependent growth inhibitory properties.
The synthesized indole derivatives were also tested against a nosocomial fungus C. albicans (Table 1). Only the 3-monoazo compounds 1a and 1b exhibited modest levels of activity, which is consistent with an earlier report of antifungal activities associated with 3-phenylazoindole.25 Importantly, we found that the antibacterial properties of our promising analogues 7a–c and 8a–g are paralleled with an antiproliferative effect against cultured human cancer cells, such as HeLa (Table 1). These findings warrant future rodent studies, in which the toxicity of these compounds can be properly evaluated.
In conclusion, an unprecedented reaction of indole with aryldiazonium salts affords 2-aryl-3-(arylazo)indoles, which display promising anti-MRSA and anti-LLVRE activities. The successful bioisosteric substitution of the labile azo group with ether oxygen and thioether sulphur atoms indicates that the azo functionality is not required for activity and, thus, the potential metabolic instability of these indole-based antibacterial agents is not of concern. Experiments aimed at elucidating the mode of action of these compounds in gram-positive bacteria are underway and will be reported in due course.
Acknowledgments
US National Institutes of Health (grants RR-16480 and CA-99957) are gratefully acknowledged for financial support of this work. We are grateful to the reviewer of this paper for pointing out the similarity of our proposed mechanism to the metal-free Meerwein arylation processes.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Pichereau S, Rose WE. Expert Opin. Pharmacother. 2010;11:3009. doi: 10.1517/14656566.2010.511614. [DOI] [PubMed] [Google Scholar]
- 2.Tacconelli E, Cataldo MA. Int. J. Antimicrob. Agents. 2008;31:99. doi: 10.1016/j.ijantimicag.2007.08.026. [DOI] [PubMed] [Google Scholar]
- 3.Kobayashi Y, Ichioka M, Hirose T, Nagai K, Matsumoto A, Matsui H, Hanaki H, Masuma R, Takahashi Y, Omura S, Sunazuka T. Bioorg. Med. Chem. Lett. 2010;20:6116. doi: 10.1016/j.bmcl.2010.08.037. [DOI] [PubMed] [Google Scholar]
- 4.Hossion AML, Otsuka N, Kandahary RK, Tsuchiya T, Ogawa W, Iwado A, Zamami Y, Sasaki K. Bioorg. Med. Chem. Lett. 2010;20:5349. doi: 10.1016/j.bmcl.2010.02.060. [DOI] [PubMed] [Google Scholar]
- 5.Hu L, Kully ML, Boykin DW, Abood N. Bioorg. Med. Chem. Lett. 2009;19:4626. doi: 10.1016/j.bmcl.2009.06.077. [DOI] [PubMed] [Google Scholar]
- 6.Dixit BC, Patel HM, Desai DJ. J. Serb. Chem. Soc. 2007;72:119. [Google Scholar]
- 7.Jarrahpour AA, Motamedifar M, Pakshir K, Hadi N, Zarei M. Molecules. 2004;9:815. doi: 10.3390/91000815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ozturk A, Abdullah MI. Sci. Total. Environ. 2006;358:137. doi: 10.1016/j.scitotenv.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 9.Joshi KC, Pathak VN, Arya P, Chand P. Agric. Biol. Chem. 1979;43:171. [Google Scholar]
- 10.Srivastava N, Sengupta AK. Indian J. Chem. 1983;22B:707. [Google Scholar]
- 11.Jackson AH, Prasitpan N, Shannon PVR, Tinker AC. J. C. S. Perkin Trans. I. 1987:2543. [Google Scholar]
- 12.Doyle MP, Dellaria JF, Siegfried B, Bishop SW. J. Org. Chem. 1977;42:3494. [Google Scholar]
- 13.Molinaro C, Mowat J, Gosselin F, O’Shea PD, Marcoux J-F, Angelaud R, Davies IW. J. Org. Chem. 2007;72:1856. doi: 10.1021/jo062483g. [DOI] [PubMed] [Google Scholar]
- 14.Raucher S, Koolpe GA. J. Org. Chem. 1983;48:2066. [Google Scholar]
- 15.Grimster NP, Gauntlett C, Godfrey CRA, Gaunt MJ. Angew. Chem. Int. Ed. 2005;44:3125. doi: 10.1002/anie.200500468. [DOI] [PubMed] [Google Scholar]
- 16.As our work was in progress, a similar synthesis of carbazoles, utilizing the sequential Heck/electrocyclization process, appeared in the literature: Hussain M, Tung DT, Langer P. Synlett. 2009:1822.
- 17.De Martino G, Edler MC, La Regina G, Coluccia A, Barbera MC, Barrow D, Nicholson RI, Chiosis G, Brancale A, Hamel E, Artico M, Silvestri R. J. Med. Chem. 2006;49:947. doi: 10.1021/jm050809s. [DOI] [PubMed] [Google Scholar]
- 18.Inion H, De Vogelaer H, Descamps M, Bauthier J, Colot M, Richard J, Charlier R. Eur. J. Med. Chem. 1977;12:483. [Google Scholar]
- 19.Selected synthetic procedure (2a–d): To a solution of indole (0.001 mol) in dioxane (2 mL) were added a solution of sodium acetate (0.004 mol) in water (0.5 mL) and an appropriate diazonium tetrafluoroborate (0.004 mol) at room temperature. The reaction mixture was stirred at room temperature for six hours and then water was added (10 mL). After the extraction with AcOEt, the organic layer was dried (Na2SO4) and the solvent was removed under reduced pressure. The product was purified by flash chromatography on silica gel (hexane-ethyl acetate, 15:1). Selected characterization data (2a): 56%; 1H NMR (CDCl3) δ 8.60–8.59 (m, 1H), 7.90-7.87 (d, J=8.52 Hz, 2H), 7.80-7.77 (d, J=8.52 Hz, 2H), 7.50-7.47 (d, J=8.52 Hz, 2H), 7.46-7.43 (d, J=8.79 Hz, 2H), 7.36-7.31 (m, 3H); 13C NMR (CDCl3) δ 135.7, 134.8, 132.7, 130.5, 129.3, 129.2, 125.1, 123.8, 123.3, 119.8, 111.2; HRMS m/z (ESI) calcd for C20H14Cl2N3 (M+H+) 366.0565, found 366.0557.
- 20.Forbes BA, Sahm DF, Weissfeld AS. Bailey & Scott’s diagnostic microbiology. 10th ed. St. Louis: Mosby, Inc.; 1998. [Google Scholar]
- 21.Jett BD, Hatter KL, Huycke MM, Gilmore MS. Biotechniques. 1997;23:648. doi: 10.2144/97234bm22. [DOI] [PubMed] [Google Scholar]
- 22.Subcommittee on Antifungal Susceptibility Testing (AFST) of the ESCMID European Committee for Antimicrobial Susceptibility Testing (EUCAST). Clin. Microbiol. Infect. 2008;14:398.
- 23.Mohtar M, Johari SA, Li AR, Isa MM, Mustafa S, Ali AM, Basri DF. Curr. Microbiol. 2009;59:181. doi: 10.1007/s00284-009-9416-9. [DOI] [PubMed] [Google Scholar]
- 24.Evdokimov NM, Van slambrouck S, Heffeter P, Tu L, Le Calve B, Lamoral-Theys D, Hooten CJ, Uglinskii PY, Rogelj S, Kiss R, Steelant WFA, Berger W, Yang JJ, Bologa CJ, Kornienko A, Magedov IV. J. Med. Chem. 2011;54:2012. doi: 10.1021/jm1009428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dekker WH, Selling HA, Overeem JC. J. Agric. Food Chem. 1975;23:785. doi: 10.1021/jf60200a041. [DOI] [PubMed] [Google Scholar]