Abstract
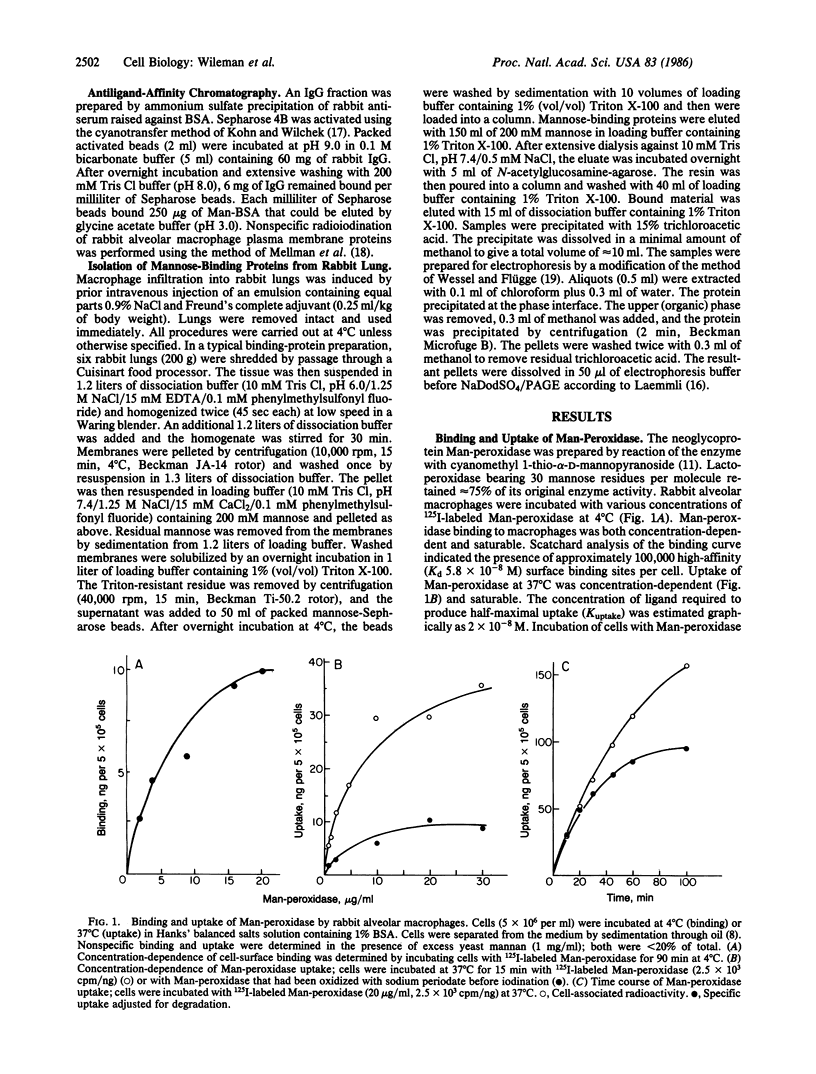
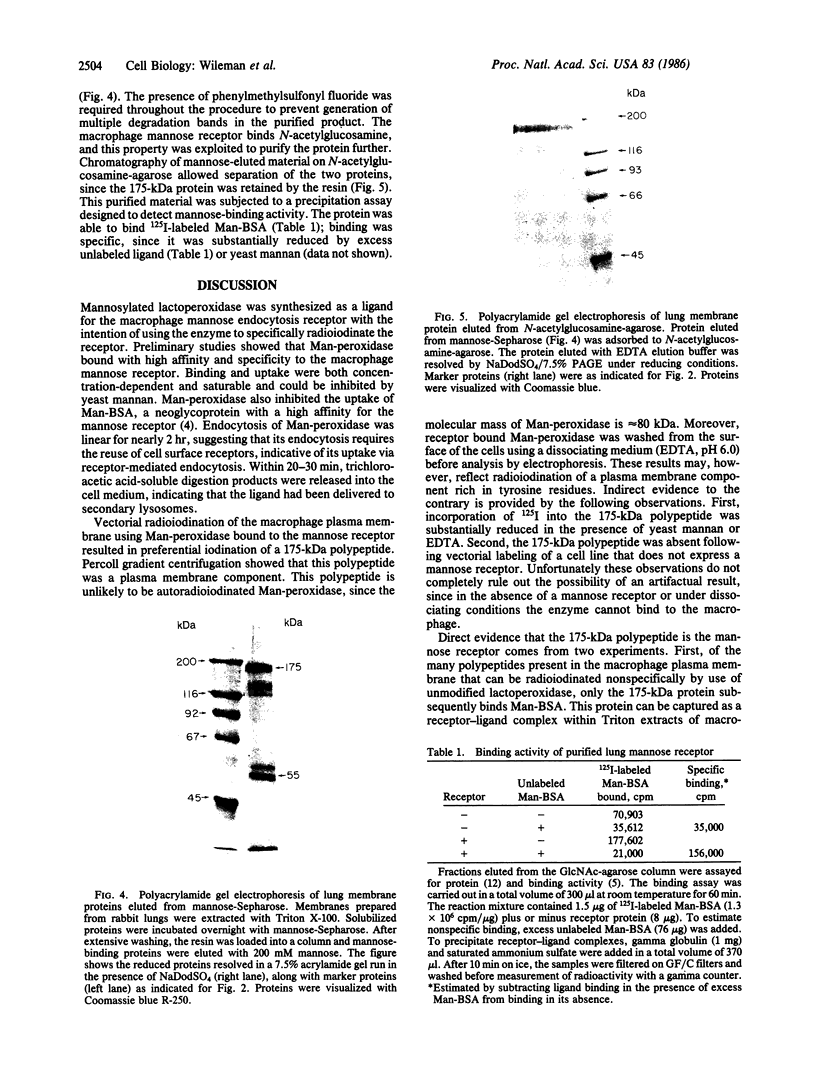
Mannose-lactoperoxidase, a neoglycoprotein prepared by reaction of lactoperoxidase with cyanomethyl 1-thiomannoside, bound to alveolar macrophages at 4 degrees C (Kd = 5.8 X 10(-8) M) and was rapidly internalized at 37 degrees C (K uptake = 2 X 10(-8) M). Mannose-lactoperoxidase binding and uptake were blocked by yeast mannan, and mannose-lactoperoxidase inhibited uptake of 125I-labeled mannose-BSA (bovine serum albumin). Radioiodination of cells with surface-bound mannose-lactoperoxidase was carried out in the presence of glucose and glucose oxidase. A major polypeptide (175 kDa) was radioiodinated by this procedure. Iodination of the 175-kDa polypeptide appeared to be receptor-mediated, since it was blocked by the presence of yeast mannan. Specific iodination was absent from receptor-negative cells. To demonstrate that the 175-kDa species is a ligand-binding protein, cells were iodinated by the standard lactoperoxidase method. Washed cells were then allowed to bind mannose-BSA. Receptor-ligand complexes, prepared by detergent extraction, were passed over anti-BSA IgG affinity columns. Mannose, but not mannose 6-phosphate or galactose, eluted a radioactive protein from the column that migrated with an apparent molecular mass of 175 kDa on NaDodSO4/PAGE. Detergent extracts of crude membranes prepared from macrophage-enriched whole rabbit lung were adsorbed to mannose-Sepharose; the fraction obtained by elution with mannose contained two protein components of 175 and 55 kDa. Subsequent chromatography on N-acetylglucosamine-agarose yielded a single protein of 175 kDa. The 175-kDa polypeptide was shown to bind 125I-labeled mannose-BSA in a precipitation assay. This binding could be blocked with mannan or mannose-BSA. The results indicate that the cell-surface mannose receptor is a 175-kDa protein.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brownell M. D., Colley K. J., Baenziger J. U. Synthesis, processing, and secretion of the core-specific lectin by rat hepatocytes and hepatoma cells. J Biol Chem. 1984 Mar 25;259(6):3925–3932. [PubMed] [Google Scholar]
- Harding C., Levy M. A., Stahl P. Morphological analysis of ligand uptake and processing: the role of multivesicular endosomes and CURL in receptor-ligand processing. Eur J Cell Biol. 1985 Mar;36(2):230–238. [PubMed] [Google Scholar]
- Kawasaki T., Etoh R., Yamashina I. Isolation and characterization of a mannan-binding protein from rabbit liver. Biochem Biophys Res Commun. 1978 Apr 14;81(3):1018–1024. doi: 10.1016/0006-291x(78)91452-3. [DOI] [PubMed] [Google Scholar]
- Keller R. K., Touster O. Physical and chemical properties of beta-glucuronidase from the preputial gland of the female rat. J Biol Chem. 1975 Jun 25;250(12):4765–4769. [PubMed] [Google Scholar]
- Kohn J., Wilchek M. A new approach (cyano-transfer) for cyanogen bromide activation of Sepharose at neutral pH, which yields activated resins, free of interfering nitrogen derivatives. Biochem Biophys Res Commun. 1982 Aug;107(3):878–884. doi: 10.1016/0006-291x(82)90604-0. [DOI] [PubMed] [Google Scholar]
- Kozutsumi Y., Kawasaki T., Yamashina I. Isolation and characterization of a mannan-binding protein from rabbit serum. Biochem Biophys Res Commun. 1980 Jul 31;95(2):658–664. doi: 10.1016/0006-291x(80)90836-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leake E. S., Myrvik Q. N. Changes in morphology and in lysozyme content of free alveolar cells after the intravenous injection of killed BCG in oil. J Reticuloendothel Soc. 1968 Feb;5(1):33–53. [PubMed] [Google Scholar]
- Lee Y. C., Stowell C. P., Krantz M. J. 2-Imino-2-methoxyethyl 1-thioglycosides: new reagents for attaching sugars to proteins. Biochemistry. 1976 Sep 7;15(18):3956–3963. doi: 10.1021/bi00663a008. [DOI] [PubMed] [Google Scholar]
- Lin H. S., Gordon S. Secretion of plasminogen activator by bone marrow-derived mononuclear phagocytes and its enhancement by colony-stimulating factor. J Exp Med. 1979 Aug 1;150(2):231–245. doi: 10.1084/jem.150.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard Y., Baenziger J. U. Characterization of a mannose and N-acetylglucosamine-specific lectin present in rat hepatocytes. J Biol Chem. 1982 Apr 10;257(7):3788–3794. [PubMed] [Google Scholar]
- Mellman I. S., Steinman R. M., Unkeless J. C., Cohn Z. A. Selective iodination and polypeptide composition of pinocytic vesicles. J Cell Biol. 1980 Sep;86(3):712–722. doi: 10.1083/jcb.86.3.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd V. L., Lee Y. C., Schlesinger P. H., Stahl P. D. L-Fucose-terminated glycoconjugates are recognized by pinocytosis receptors on macrophages. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1019–1022. doi: 10.1073/pnas.78.2.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl P. D., Rodman J. S., Miller M. J., Schlesinger P. H. Evidence for receptor-mediated binding of glycoproteins, glycoconjugates, and lysosomal glycosidases by alveolar macrophages. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1399–1403. doi: 10.1073/pnas.75.3.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl P., Schlesinger P. H., Sigardson E., Rodman J. S., Lee Y. C. Receptor-mediated pinocytosis of mannose glycoconjugates by macrophages: characterization and evidence for receptor recycling. Cell. 1980 Jan;19(1):207–215. doi: 10.1016/0092-8674(80)90402-x. [DOI] [PubMed] [Google Scholar]
- Steinman R. M., Mellman I. S., Muller W. A., Cohn Z. A. Endocytosis and the recycling of plasma membrane. J Cell Biol. 1983 Jan;96(1):1–27. doi: 10.1083/jcb.96.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tietze C., Schlesinger P., Stahl P. Mannose-specific endocytosis receptor of alveolar macrophages: demonstration of two functionally distinct intracellular pools of receptor and their roles in receptor recycling. J Cell Biol. 1982 Feb;92(2):417–424. doi: 10.1083/jcb.92.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend R., Stahl P. Isolation and characterization of a mannose/N-acetylglucosamine/fucose-binding protein from rat liver. Biochem J. 1981 Jan 15;194(1):209–214. doi: 10.1042/bj1940209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel D., Flügge U. I. A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal Biochem. 1984 Apr;138(1):141–143. doi: 10.1016/0003-2697(84)90782-6. [DOI] [PubMed] [Google Scholar]
- Wileman T., Boshans R. L., Schlesinger P., Stahl P. Monensin inhibits recycling of macrophage mannose-glycoprotein receptors and ligand delivery to lysosomes. Biochem J. 1984 Jun 15;220(3):665–675. doi: 10.1042/bj2200665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wileman T., Boshans R., Stahl P. Uptake and transport of mannosylated ligands by alveolar macrophages. Studies on ATP-dependent receptor-ligand dissociation. J Biol Chem. 1985 Jun 25;260(12):7387–7393. [PubMed] [Google Scholar]