Abstract
Rationale
Diphenhydramine (DPH) is an over-the-counter medication used in the treatment of allergic symptoms. While DPH abuse is infrequent, recent preclinical evidence suggests that DPH and cocaine combinations may have enhanced reinforcing properties.
Objective
The aims were to assess the reinforcing effectiveness of cocaine and DPH alone or in combination under a second-order schedule of reinforcement and to examine the neurochemical basis of this interaction using in vivo microdialysis in awake rhesus monkeys.
Materials and methods
Cocaine (0.03–0.3 mg/kg per injection), DPH (0.3–3.0 mg/kg per injection), or a combination was available under a second-order schedule of intravenous drug reinforcement (n=3). In microdialysis studies, noncontingent cocaine (0.1–1.0 mg/kg, iv), DPH (1.7 and 3.0 mg/kg, iv), or a combination was administered and changes in extracellular dopamine levels in the caudate nucleus were examined (n=3–5).
Results
Cocaine and DPH dose-dependently maintained operant responding. Dose combinations of 1.0 or 1.7 mg/kg per injection DPH and 0.03 mg/kg per injection cocaine maintained greater rates of operant responding than 0.03 mg/kg per injection cocaine alone in the second component of the behavioral session. In microdialysis studies, cocaine dose-dependently increased extracellular dopamine levels, but no dose of DPH tested significantly increased dopamine levels above baseline. Moreover, combining DPH with cocaine did not enhance cocaine-induced dopamine increases.
Conclusions
The results support previous evidence of enhanced reinforcement with cocaine and DPH combinations and extend this finding to operant behavior maintained under a second-order schedule. However, the reinforcing effects of DPH alone or in combination with cocaine do not appear to be mediated via changes in dopamine overflow.
Keywords: Antihistamine, Cocaine, Monkey, Microdialysis, Dopamine, Self-administration
Introduction
Histamine H1 receptor antagonists (antihistamines) are available as over-the-counter medications used primarily in the treatment of allergy symptoms. Preclinical behavioral studies have suggested that antihistamines have abuse liability because they function as positive reinforcers in intravenous (iv) drug self-administration models (Bergman and Spealman 1986; Beardsley and Balster 1992; Wang and Woolverton 2007, 2009). Also, antihistamines in nonhuman primates and rodents demonstrate behavioral-stimulant properties similar to the abused drug cocaine (McKearney 1982; Bergman and Spealman 1986, 1988; Bergman 1990; Jun et al. 2004). Furthermore, like cocaine, the antihistamine diphenhydramine (DPH) has been shown to increase extracellular dopamine levels in similar brain areas (Tanda et al. 2008). DPH does show activity at the dopamine transporter, although DPH is about tenfold less effective than cocaine (Tanda et al. 2008). These preclinical studies are supported by clinical studies demonstrating that DPH functions as a positive reinforcer (Preston et al. 1992; Mumford et al. 1996) and DPH abuse has been documented in the medical literature (for review, see Thomas et al. 2009). While antihistamine abuse does not appear to be problematic, there does appear to be the potential for abuse based on the preclinical and clinical studies cited above.
Recently, Wang and Woolverton (2007) reported that the combination of DPH and cocaine in rhesus monkeys had greater reinforcing strength than was predicted based on additivity alone. These authors recently followed up on their preliminary findings by investigating another antihistamine pyrilamine and a broader range of dose combinations and found similar results (Wang and Woolverton 2009). Furthermore, when tested in combination, antihistamines have been shown to enhance the discriminative stimulus effects of cocaine (Campbell et al. 2005). However, no previous work has examined the neurochemical effects of cocaine and antihistamine combinations.
The aim of the present study was to examine the behavioral and neurochemical effects of cocaine, the antihistamine DPH, and their combination in rhesus monkeys. Self-administration experiments using a second-order schedule of reinforcement were conducted to examine the reinforcing effectiveness of cocaine and DPH alone and in combination. There are several advantages in using this behavioral schedule to study drug combinations. First, operant behavior can be rapidly and reliably shaped under this schedule and fully trained subjects exhibit highly consistent operant behavior. Second, the second-order schedule of reinforcement has been extensively used to study behavioral interactions between cocaine and acute pretreatments of candidate monoaminergic transporter inhibitors for treating cocaine dependence (Lindsey et al. 2004; Howell et al. 2007). Third, rates of operant responding under a second-order schedule are maintained by both primary (e.g., cocaine injection) and secondary reinforcers (e.g., drug-associated stimuli). This temporally spaces the delivery of the primary reinforcer and thus limits drug accumulation and possible recruitment of other confounding behaviors. We hypothesized that rates of operant responding would be greater when the combination of cocaine and DPH was available compared to when only one of the drugs was available under the second-order schedule. To investigate the role of dopamine in the cocaine and DPH interactions, in vivo microdialysis studies were conducted. We hypothesized that administration of cocaine and DPH together would produce an enhanced increase in extracellular dopamine compared to either cocaine or DPH alone. These neurochemical results would implicate an important role of dopamine in the drug interactions observed on behavioral measures related to abuse liability.
Materials and methods
Subjects
Adult male (n=1; RLk4) and female (n=7) rhesus monkeys (Macaca mulatta) served as subjects. All subjects in these experiments had a history of exposure to psychoactive compounds. Each subject was individually housed and fed Purina monkey chow (Ralston Purina, St. Louis, MO, USA), supplemented with fresh fruit and vegetables. Water was continuously available in the home cage and food restriction protocols were not used. Food intake was monitored daily throughout the study by recording the number of chow delivered during each afternoon feeding and the number of chow remaining in the home cage each morning. Animal care procedures strictly followed the National Institutes of Health Guide for the Care and Use of laboratory Animals and were approved by the Institutional Animal Care and Use Committee of Emory University.
Vascular surgery
Each subject was implanted with a chronic indwelling venous catheter into a major vein (femoral or jugular) under sterile surgical conditions as previously described (Wilcox et al. 2002). Briefly, anesthesia was induced with Telazol (tiletamine and zolazepam) and maintained with isoflurane (1.0–2.0%). One end of a silicone catheter (Access Technologies, Skokie, IL, USA) was inserted into a major vein (femoral or jugular) to the level of the vena cava. The distal end of the catheter was routed subcutaneously between the scapulae and attached to a vascular access port (Access Technologies). Catheters were flushed with heparinized saline (100 U/mL) at the end of each operant session to maintain patency.
Drugs
Cocaine HCl (National Institute on Drug Abuse, Bethesda, MD, USA) and diphenhydramine HCl (Sigma, St. Louis, MO, USA) were dissolved in 0.9% sterile saline. All doses are expressed as the salt form.
Self-administration apparatus and procedure
Three rhesus monkeys (one male and two females) with previous experience of intravenous drug self-administration under a second-order schedule of reinforcement served as subjects. The apparatus and schedule of reinforcement were as previously described (Wilcox et al. 2005) except that the limited hold was 240 s. Briefly, the second-order schedule was an [FI 10(FR 20:S)] where the first fixed ratio (FR) completed after 10 min (fixed interval, FI 10) resulted in presentation of the primary reinforcer (i.e., cocaine injection) and the illumination of a white stimulus light (CS) for 15 s. FR 20 components completed during the FI 10-min component resulted in the illumination of the CS for 2 s. There were five [FI 10(FR 20:S)] components during the operant session. Cocaine (0.03–0.3 mg/kg per injection) and DPH (0.3–3.0 mg/kg per injection) dose–effect curves were determined in each subject prior to testing the cocaine/DPH combinations. Drug doses were manipulated by changing the concentration in the syringe while maintaining a 1-mL injection volume. For the cocaine/DPH combination studies, concentrations of cocaine and DPH were combined in the same syringe to allow simultaneous administration of both drug doses available. Each dose of cocaine, DPH, or combination was available for at least 4 days and stability was determined by the mean operant response rate of the last three sessions with no more than 20% variability from the mean. Behavioral sessions were conducted 5 to 6 days per week.
In vivo microdialysis procedure
A separate group of five female rhesus monkeys served as subjects and underwent aseptic stereotaxic surgery for implantation of bilateral microdialysis CMA/11 guide cannulae (CMA, North Chelmsford, MA, USA) targeting the head of the caudate nucleus in the same coronal plane as the nucleus accumbens as previously described (Czoty et al. 2000, 2002; Wilcox et al. 2005). Bilateral microdialysis experiments were conducted no more frequently than every 2 weeks in each subject. Experiments were conducted in awake monkeys seated in a commercially available primate chair (Primate Products, Woodside, CA, USA) and started with a 1-hour equilibration period after insertion of the custom micro-dialysis probes (CMA/Microdialysis). The probes were composed of a 20-mm stainless steel shaft and a 4-mm-long membrane for a total length of 24 mm. The polyarylethersulfone membrane had an outer diameter of 0.5 mm and cutoff was 20 kDa. After the equilibration period, baseline samples were collected every 10 min for an hour. Following baseline collection, samples were continually collected every 10 for 90 min after injection of the test solutions listed below. Finally, a potassium-enriched (100 mM) solution ionically matched to cerebrospinal fluid was retrodialyzed for 10 min and an additional sample was collected to ensure the viability of the sampling site. Cocaine (0.1–1.0 mg/kg, iv), DPH (1.7 and 3.0 mg/kg, iv), and cocaine/DPH dose combinations (0.1 mg/kg cocaine/1.7 mg/kg DPH; 0.3 mg/kg cocaine/1.7 mg/kg DPH; and 0.3 mg/kg cocaine/3.0 mg/kg DPH, iv) were counterbalanced across subjects. As in the self-administration studies, concentrations of cocaine and DPH were combined in a single syringe for administration. For each bilateral experiment, the data from each hemisphere for that subject were averaged and statistically analyzed. However, if only one hemisphere was experimentally determined to be viable via postsession potassium-enriched cerebrospinal fluid challenge, only the data from that single hemisphere were utilized in the analysis.
Data analysis
For the behavioral studies, the primary dependent variable examined was the rate of operant responding in each component (responses per second). Individual data for the cocaine and DPH dose combinations were transformed to percent control of operant responding maintained by cocaine alone (0.03 or 0.1 mg/kg per injection) for each set of dose combinations during each component. Transformed data were then analyzed using a two-way repeated-measures analysis of variance (ANOVA) with both dose combination and component of the behavioral session as main factors. In the presence of a significant interaction, a Fisher least significant difference post hoc test was conducted to determine which cocaine and DPH dose combinations were significantly different from cocaine alone. For microdialysis studies, dopamine levels were determined as nanomolar concentrations in dialysate unadjusted for probe recovery. Percent increase in extracellular dopamine in the caudate nucleus relative to baseline was calculated as (extracellular dopamine level after delivery of the drug/average of the six baseline samples)×100 for each drug dose and combination. The peak effect was determined for each drug dose or dose combination tested and a one-way repeated-measures ANOVA was performed with experimental condition (drug or drug combinations) as the main factor. Thus, a separate ANOVA was performed for cocaine alone, DPH alone, and cocaine/DPH combinations. In the presence of a significant main effect, a Bonferroni multiple-comparison post hoc test was conducted to determine which treatments were significantly different from baseline levels. Significance was set at the p<0.05 level of confidence.
Results
Reinforcing effectiveness of cocaine and DPH alone or in combinations
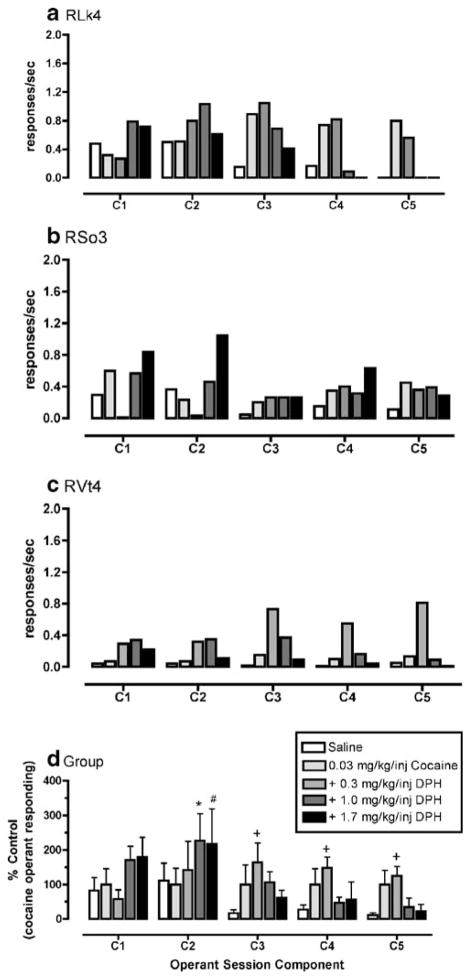
Cocaine maintained rates of operant responding under a second-order schedule of reinforcement with the peak dose of 0.1 mg/kg per injection in two monkeys (RLk4 and RVt4) and 0.3 mg/kg per injection in one monkey (RSo3; Fig. 1). DPH also maintained rates of operant responding with the peak dose of 1.0 mg/kg per injection in two monkeys (RLk4 and RVt4) and 1.7 mg/kg per injection in one monkey (RSo3; Fig. 2). Higher doses of DPH were not tested in RSo3 due to adverse effects (agitation, motor incoordination) at the end of the behavioral session of 3.0 mg/kg per injection DPH availability. For DPH dose combinations with 0.03 mg/kg per injection cocaine, there was a significant interaction between dose combination and component of the behavioral session (F16, 32=3.49, p<0.01). Post hoc analysis revealed that, in the second component, the dose combination of 0.03 mg/kg per injection cocaine and 1.0 mg/kg per injection DPH maintained significantly (p< 0.05) greater rates of operant responding compared to 0.03 mg/kg per injection cocaine alone or saline (Fig. 3). Also, in the second behavioral component, the dose combination of 1.7 mg/kg per injection DPH and 0.03 mg/kg per injection cocaine maintained significantly (p<0.05) greater rates of operant responding compared to 0.03 mg/kg per injection cocaine alone. Finally, post hoc analysis demonstrated that the dose combination of 0.3 mg/kg per injection DPH and 0.03 mg/kg per injection cocaine maintained significantly (p<0.05) higher rates of operant responding compared to saline during behavioral components 3–5. For DPH dose combinations with 0.1 mg/kg per injection cocaine, there was no significant interaction between dose combinations and component of the behavioral session (F16, 32=0.29, p >0.05; Fig. 4). However, visual examination of the data (Fig. 4) demonstrates that, in two of the three monkeys, at least one DPH dose combination with 0.1 mg/kg per injection cocaine maintained higher rates of operant responding than 0.1 mg/kg per injection cocaine alone.
Fig. 1.
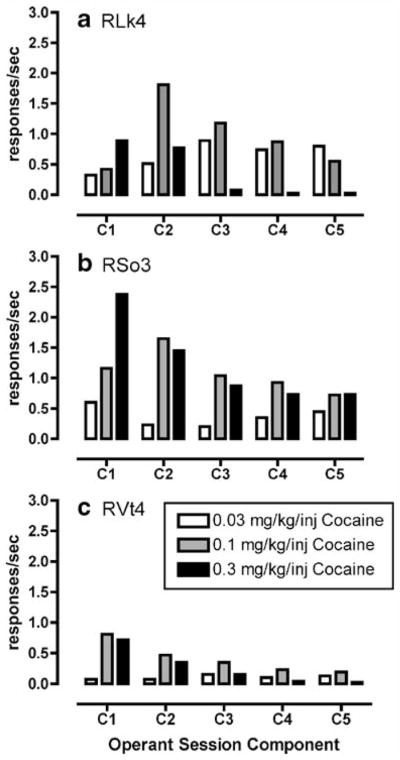
a–c Cocaine-maintained (0.03–0.3 mg/kg per injection) operant responding under a second-order schedule of reinforcement in rhesus monkeys (n=3). Data are represented as mean of the last three behavioral sessions during which the variance was less than 20%. Abscissa represents the component of the behavioral session and ordinate represents the rate of operant responding (responses per second)
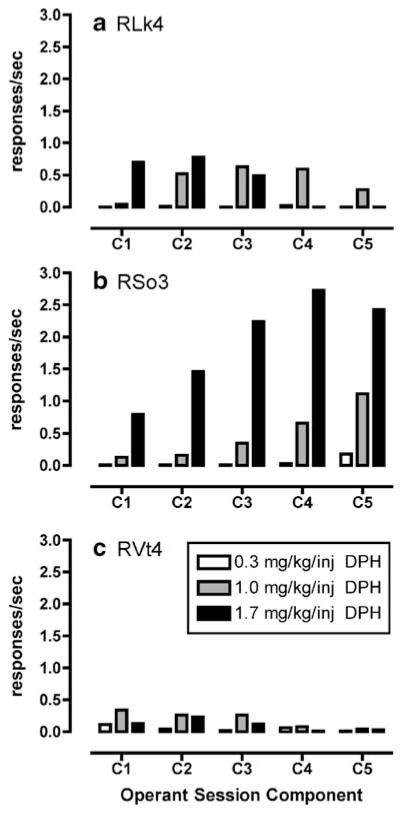
Fig. 2.
a–c Diphenhydramine-maintained (DPH; 0.3–3.0 mg/kg per injection) operant responding under a second-order schedule of reinforcement in rhesus monkeys (n=3). Data are represented as mean of the last three behavioral sessions during which the variance was less than 20%. Abscissa represents the component of the behavioral session and ordinate represents the rate of operant responding (responses per second)
Fig. 3.
Effects of different doses of diphenhydramine (DPH; 0.3–1.7 mg/kg per injection) in combination with cocaine (0.03 mg/kg per injection) on operant responding under a second-order schedule of reinforcement in rhesus monkeys (n=3). a, b, and c show individual mean data for the last three behavioral sessions during which the variance was less than 20%. d is shown as mean±SEM normalized as percent control of cocaine-maintained operant responding for each component of the behavioral sessions. Asterisk indicates significantly different from saline and cocaine alone within that component. # indicates significantly different from cocaine only, + indicates significantly different from saline only
Fig. 4.
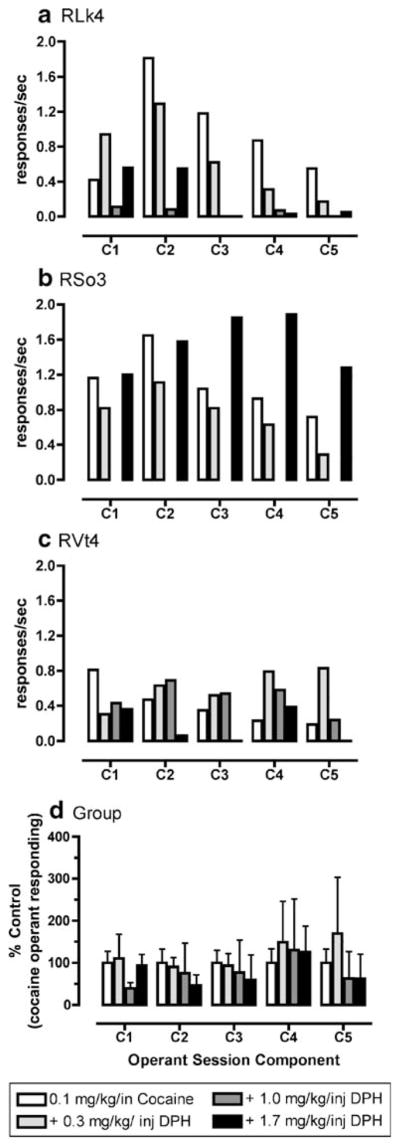
Effects of different doses of diphenhydramine (DPH; 0.3–1.7 mg/kg per injection) in combination with cocaine (0.1 mg/kg per injection) on operant responding under a second-order schedule of reinforcement in rhesus monkeys (n=3). a, b, and c show individual mean data for the last three behavioral sessions during which the variance was less than 20%. d is shown as mean±SEM normalized as percent control of cocaine-maintained operant responding for each component of the behavioral sessions
Effects of cocaine and DPH alone or in combinations on extracellular dopamine levels
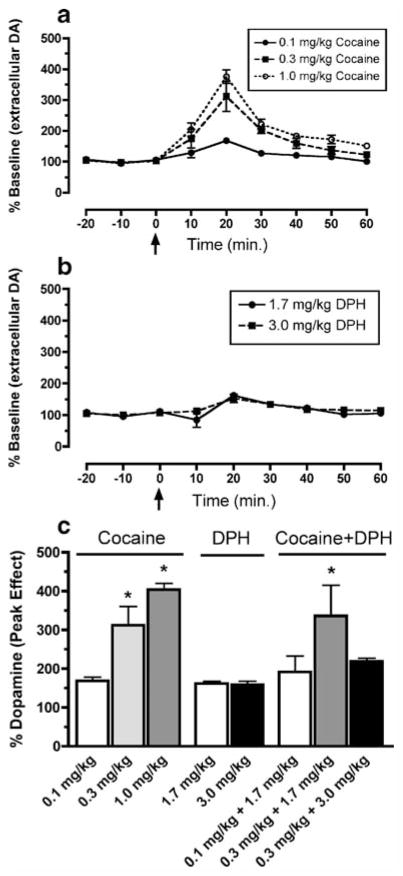
Noncontingent cocaine dose-dependently increased peak extracellular levels of dopamine in the caudate nucleus (F4, 16=21.9, p<0.01; Fig. 5). Post hoc analysis revealed that 0.3- and 1.0-mg/kg doses of cocaine significantly (p<0.01) increased extracellular dopamine levels above baseline. In contrast, no dose of DPH significantly increased dopamine levels. There was also a significant main effect (F4, 17=5.78, p<0.05) of cocaine and DPH combinations on peak extracellular dopamine levels. Post hoc analysis revealed that only the combination of 0.3 mg/kg cocaine and 1.7 mg/kg DPH significantly (p<0.01) increased dopamine levels above baseline.
Fig. 5.
Effects of cocaine (0.1–1.0 mg/kg; a) and diphenhydramine (DPH; 1.7–3.0 mg/kg; b) alone or in combination on extracellular dopamine levels in the caudate nucleus of rhesus monkeys (n=3–5). Data in a and b are expressed as percent baseline dopamine levels and plotted as mean±SEM. Average (±SEM) dopamine levels were estimated at 6.84±0.5 ng/mL. Data in c are represented as peak dopamine effect for each treatment condition. The three cocaine and DPH dose combinations tested were (from left to right): 0.1 mg/kg cocaine and 1.7 mg/kg DPH, 0.3 mg/kg cocaine and 1.7 mg/kg DPH, and 0.3 mg/kg cocaine and 3.0 mg/kg DPH. Asterisk indicates significantly different from percent baseline levels averaged during the first 60 min of the experiment before drug challenge
Discussion
In the present study, there was a significant increase in rates of operant responding maintained by several dose combinations of the 0.03 mg/kg per injection cocaine and DPH compared to cocaine alone, especially during the second component of the behavioral session. Under a second-order schedule of reinforcement, the research subject is not presented with the primary reinforcer (i.e., dose of drug solution available) until completion of the ratio requirement at the end of the first component of the behavioral session. Therefore, operant responding during the second component of the test session could be analyzed to determine the reinforcing effectiveness of dose combinations before the potential of multiple self-administered doses and recruitment of other confounding behaviors. The finding in this study that several doses of DPH in combination with both cocaine doses tested maintained higher rates of operant responding is in agreement with previous studies showing that combinations of DPH and cocaine maintain higher breakpoints under a progressive-ratio schedule of reinforcement (Wang and Woolverton 2007, 2009).
The present study also examined the capacity of DPH alone or in combination with cocaine to increase extracellular levels of dopamine in the caudate nucleus using in vivo microdialysis. Previous in vivo microdialysis studies demonstrated that like cocaine DPH dose-dependently increased extracellular dopamine levels in the rodent nucleus accumbens shell (Tanda et al. 2007, 2008). Neither dose of DPH examined significantly increased dopamine levels above baseline. In nonhuman primates, the functional and anatomical distinctions between ventral (nucleus accumbens) and dorsolateral (caudate nucleus) striatum are less pronounced and can be thought of as broader brain regions than discrete brain regions as in rodents (Haber and McFarland 1999; Haber et al. 2000). Moreover, while the present study did not sample from the nucleus accumbens directly during the microdialysis experiments, inferences between the capacity of DPH to increase extracellular dopamine levels in the caudate nucleus and DPH-maintained operant responding under a second-order schedule of reinforcement are appropriate based on the neuroanatomical literature cited above and previous studies from our laboratory demonstrating measurable effects of cocaine on extracellular dopamine levels during both noncontingent and contingent administration (Czoty et al. 2000, 2002; Kimmel et al. 2005). In conclusion, reasons for the disparate results between Tanda et al. (2008) and the present study with respect to the effects of DPH alone are not apparent, but we cannot rule out differences due to species or neuroanatomy.
Furthermore, microdialysis studies examining dose combinations of DPH and cocaine did not show an enhanced dopamine response in the caudate nucleus compared to cocaine alone. In fact, the dose combination of 3.0 mg/kg dose of DPH and 0.3 mg/kg cocaine was not significantly different from baseline, indicating that this dose combination was less effective than 0.3 mg/kg cocaine alone in increasing extracellular dopamine levels. One potential mechanism mediating this effect is DPH antagonism of muscarinic acetylcholine receptors. Antihistamines, such as DPH, have been shown to produce anticholinergic effects in vivo (Niemegeers et al. 1982). Smolders et al. (1997) reported that administration of a muscarinic M1 antagonist decreased dopamine overflow in the rodent striatum. Thus, at high doses of DPH, anticholinergic effects might be recruited resulting in an attenuation of the increased dopamine levels after cocaine administration. Future studies using specific cholinergic antagonists will be needed to test this hypothesized mechanism of action for DPH.
In conclusion, the present study demonstrates that, under a second-order schedule of reinforcement, certain dose combinations of cocaine and DPH maintain higher rates of operant responding compared to cocaine alone. Furthermore, DPH, at the doses tested, does not significantly increase extracellular levels of dopamine in the caudate nucleus of nonhuman primates nor does the combination of DPH with cocaine enhance the increases in dopamine induced by cocaine alone. Overall, the behavioral results support the notion that combinations of the antihistamine DPH and cocaine have enhanced reinforcing effect; however, the neurochemical data from in vivo microdialysis studies suggest that the reinforcing effects of DPH are not mediated by increasing extracellular dopamine levels nor are the enhanced reinforcing effects of cocaine DPH combinations mediated through an enhancement of cocaine’s effects on dopamine overflow.
Acknowledgments
We appreciate the technical assistance of Lisa Niedert, Jodi Godfrey, Juliet Brown, and Peggy Plant. This research was supported by NIH grants DA00517, DA10344, DA15040, and RR00165.
Contributor Information
Matthew L. Banks, Yerkes National Primate Research Center, Emory University, 954 Gatewood Rd, Atlanta, GA 30329, USA
Monica L. Andersen, Yerkes National Primate Research Center, Emory University, 954 Gatewood Rd, Atlanta, GA 30329, USA. Department of Psychobiology, Universidade Federal de São Paulo, Escola Paulista de Medicina (UNIFESP/EPM), São Paulo, São Paulo, Brazil
Kevin S. Murnane, Yerkes National Primate Research Center, Emory University, 954 Gatewood Rd, Atlanta, GA 30329, USA
Rebecca C. Meyer, Yerkes National Primate Research Center, Emory University, 954 Gatewood Rd, Atlanta, GA 30329, USA
Leonard L. Howell, Email: lhowell@emory.edu, Yerkes National Primate Research Center, Emory University, 954 Gatewood Rd, Atlanta, GA 30329, USA. Department of Psychiatry and Behavioral Sciences, Emory University, Atlanta, GA, USA
References
- Beardsley PM, Balster RL. The intravenous self-administration of antihistamines by rhesus monkeys. Drug Alcohol Depend. 1992;30:117–126. doi: 10.1016/0376-8716(92)90016-6. [DOI] [PubMed] [Google Scholar]
- Bergman J. Psychomotor stimulant effects of the stereoisomers of chlorpheniramine. Psychopharmacology. 1990;100:132–134. doi: 10.1007/BF02245804. [DOI] [PubMed] [Google Scholar]
- Bergman J, Spealman RD. Some behavioral effects of histamine H1 antagonists in squirrel monkeys. J Pharmacol Exp Ther. 1986;239:104–110. [PubMed] [Google Scholar]
- Bergman J, Spealman RD. Behavioral effects of histamine H1 antagonists: comparison with other drugs and modification by haloperidol. J Pharmacol Exp Ther. 1988;245:471–478. [PubMed] [Google Scholar]
- Campbell VC, Kopajtic TA, Ah N, Katz JL. Assessment of the influence of histaminergic actions on cocaine-like effects of 3alpha-diphenylmethoxytropane analogs. J Pharmacol Exp Ther. 2005;315:631–640. doi: 10.1124/jpet.105.090829. [DOI] [PubMed] [Google Scholar]
- Czoty PW, Justice JB, Jr, Howell LL. Cocaine-induced changes in extracellular dopamine determined by microdialysis in awake squirrel monkeys. Psychopharmacology. 2000;148:299–306. doi: 10.1007/s002130050054. [DOI] [PubMed] [Google Scholar]
- Czoty PW, Ginsburg BC, Howell LL. Serotonergic attenuation of the reinforcing and neurochemical effects of cocaine in squirrel monkeys. J Pharmacol Exp Ther. 2002;300:831–837. doi: 10.1124/jpet.300.3.831. [DOI] [PubMed] [Google Scholar]
- Haber SN, McFarland NR. The concept of the ventral striatum in nonhuman primates. Ann N Y Acad Sci. 1999;877:33–48. doi: 10.1111/j.1749-6632.1999.tb09259.x. [DOI] [PubMed] [Google Scholar]
- Haber SN, Fudge JL, McFarland NR. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J Neurosci. 2000;20:2369–238. doi: 10.1523/JNEUROSCI.20-06-02369.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell LL, Carroll FI, Votaw JR, Goodman MM, Kimmel HL. Effects of combined dopamine and serotonin transporter inhibitors on cocaine self-administration in rhesus monkeys. J Pharmacol Exp Ther. 2007;320:757–765. doi: 10.1124/jpet.106.108324. [DOI] [PubMed] [Google Scholar]
- Jun JH, Thorndike EB, Schindler CW. Abuse liability and stimulant properties of dextromethorphan and diphenhydramine combinations in rats. Psychopharmacology. 2004;172:277–282. doi: 10.1007/s00213-003-1650-4. [DOI] [PubMed] [Google Scholar]
- Kimmell HL, Ginsburg BC, Howell LL. Changes in extracellular dopamine during cocaine self-administration in squirrel monkeys. Synapse. 2005;56:129–134. doi: 10.1002/syn.20135. [DOI] [PubMed] [Google Scholar]
- Lindsey KP, Wilcox KM, Votaw JR, Goodman MM, Plisson C, Carroll FI, Rice KC, Howell LL. Effects of dopamine transporter inhibitors on cocaine self-administration in rhesus monkeys: relationship to transporter occupancy determined by positron emission tomography neuroimaging. J Pharmacol Exp Ther. 2004;309:959–969. doi: 10.1124/jpet.103.060293. [DOI] [PubMed] [Google Scholar]
- McKearney JW. Stimulant actions of histamine H1 antagonists on operant behavior in the squirrel monkey. Psychopharmacology. 1982;77:156–158. doi: 10.1007/BF00431939. [DOI] [PubMed] [Google Scholar]
- Mumford GK, Silverman K, Griffiths RR. Reinforcing, subjective and performance effects of lorazepam and diphenhydramine in humans. Exp Clin Psychopharm. 1996;4:421–430. [Google Scholar]
- Niemegeers CJE, Awouters FHL, Janssen PAJ. The in vivo pharmacological profile of histamine (H1) antagonists in the rat. Drug Dev REs. 1982;2:559–566. [Google Scholar]
- Preston KL, Wolf B, Guarino JJ, Griffiths RR. Subjective and behavioral effects of diphenhydramine, lorazepam and metho-carbamol: evaluation of abuse liability. J Pharmacol Exp Ther. 1992;262:707–720. [PubMed] [Google Scholar]
- Smolders I, Bogaert L, Ebinger G, Michotte Y. Muscarinic modulation of striatal dopamine, glutamate, and GABA release, as measured with in vivo microdialysis. J Neurochem. 1997;68:1942–1948. doi: 10.1046/j.1471-4159.1997.68051942.x. [DOI] [PubMed] [Google Scholar]
- Tanda G, Ebbs AL, Kopajtic TA, Elias LM, Campbell BL, Newman AH, Katz JL. Effects of muscarinic M1 receptor blockade on cocaine-induced elevations of brain dopamine levels and locomotor behavior in rats. J Pharmacol Exper Ther. 2007;321:334–344. doi: 10.1124/jpet.106.118067. [DOI] [PubMed] [Google Scholar]
- Tanda G, Kopajtic TA, Katz JL. Cocaine-like neurochemical effects of antihistaminic medications. J Neurochem. 2008;106:147–157. doi: 10.1111/j.1471-4159.2008.05361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas A, Nallur DG, Jones N, Deslandes PN. Diphenhydramine abuse and detoxification: a brief review and case report. J Psychopharmacol. 2009;23:101–105. doi: 10.1177/0269881107083809. [DOI] [PubMed] [Google Scholar]
- Wang Z, Woolverton WL. Self-administration of cocaine-antihistamine combinations: super-additive reinforcing effects. Eur J Pharmacol. 2007;557:159–160. doi: 10.1016/j.ejphar.2006.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Woolverton WL. Super-additive interaction of the reinforcing effects of cocaine and H1-antihistamines in rhesus monkeys. Pharmacol Biochem Behav. 2009;91:590–595. doi: 10.1016/j.pbb.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox KM, Lindsey KP, Votaw JR, Goodman MM, Martarello L, Carroll FI, Howell LL. Self-administration of cocaine and the cocaine analog RTI-113: relationship to dopamine transporter occupancy determined by PET neuroimaging in rhesus monkeys. Synapse. 2002;43:78–85. doi: 10.1002/syn.10018. [DOI] [PubMed] [Google Scholar]
- Wilcox KM, Kimmel HL, Lindsey KP, Votaw JR, Goodman MM, Howell LL. In vivo comparison of the reinforcing and dopamine transporter effects of local anesthetics in rhesus monkeys. Synapse. 2005;58:220–228. doi: 10.1002/syn.20199. [DOI] [PubMed] [Google Scholar]