The clinical development of irreversible tyrosine kinase inhibitors that target the human epidermal growth factor receptor family in non-small cell lung cancer is reviewed.
Keywords: Irreversible EGFR/HER-2 tyrosine kinase inhibitors, Non-small cell lung cancer, Resistance, Afatinib (BIBW 2992), PF00299804
Abstract
Small-molecule tyrosine kinase inhibitors (TKIs) of the human epidermal growth factor receptor (HER) include the reversible epidermal growth factor receptor (EGFR/HER-1) inhibitors gefitinib and erlotinib. EGFR TKIs have demonstrated activity in the treatment of patients with non-small cell lung cancer (NSCLC) harboring activating EGFR mutations; however, multiple mechanisms of resistance limit the benefit of these drugs. Although resistance to EGFR TKIs can be intrinsic and correlated with molecular lesions such as in Kirsten rat sarcoma viral oncogene homolog (KRAS; generally observed in a wild-type EGFR background), acquired resistance to EGFR TKIs can evolve in the setting of activating EGFR mutations, such as in the case of EGFR T790M mutations. Several irreversible inhibitors that target multiple members of the HER family simultaneously are currently in clinical development for NSCLC and may have a role in the treatment of TKI-sensitive and TKI-resistant disease. These include PF00299804, an inhibitor of EGFR/HER-1, HER-2, and HER-4, and afatinib (BIBW 2992), an inhibitor of EGFR/HER-1, HER-2, and HER-4. Results of large, randomized trials of these agents may help to determine their potential for the treatment of NSCLC.
Introduction
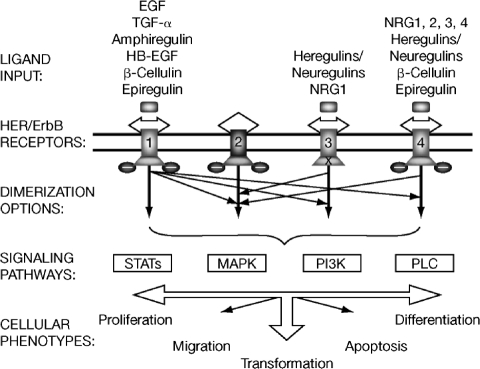
The human epidermal growth factor receptor (HER) family of receptor tyrosine kinases (RTKs) is a well-established target for anticancer therapies. Composed of four members—epidermal growth factor receptor (EGFR, HER-1, ErbB-1), HER-2 (ErbB-2), HER-3 (ErbB-3), and HER-4 (ErbB-4)—the HER family controls various signaling pathways leading to cell growth, proliferation, differentiation, and survival throughout development and during adult physiologic homeostasis [1]. HER family ligands (e.g., EGF, transforming growth factor α, amphiregulin, epiregulin, b-cellulin, heparin-binding EGF) each bind to at least one HER family member [2]. Ligand binding leads to receptor dimerization and phosphorylation; homodimerization and heterodimerization among HER family members trigger cellular responses through signal diversification and amplification (Fig. 1) [3, 4]. Upon ligand-induced receptor dimerization, autophosphorylation of key tyrosine residues results in the stimulation of tyrosine kinase (TK) activity. HER-2 itself has no known ligand but possesses strong TK activity [5] and is the preferred binding partner for other HER receptors [6]. HER-3 can bind ligand but has an inactive TK domain, so phosphorylation and subsequent downstream signaling occur only when dimerized with a partner (e.g., HER-2) [5]. Although HER-4 signaling in normal cells has been well characterized, its role in carcinogenesis is poorly understood.
Figure 1.
The diverse HER signaling network. Receptor-specific ligands for HER-1/EGFR, HER-3, and HER-4 have been identified, but not for HER-2. Receptor engagement leads to tyrosine phosphorylation and activation of signaling pathways (boxes) depending upon the arrangements of ligand–ErbB engagement (thick arrows denote homodimerization and thin arrows denote heterodimerization; “X” represents the absence of intrinsic TK activity).
Abbreviations: EGF, epidermal growth factor; EGFR, epidermal growth factor receptor; HB, heparin-binding; HER, human epidermal growth factor receptor; MAPK, mitogen-activated protein kinase; NRG, neuregulin; PI3K, phosphatidyl inositol 3-kinase; PLC, phospholipase C; STAT, signal transducers and activators of transcription; TGF, transforming growth factor; TK, tyrosine kinase.
Numerous studies have indicated that aberrant signaling from the HER family of RTKs can lead to the development and progression of cancer [7–9], providing a rationale for targeting this family for cancer treatment. Drugs targeting the HER family play an important role in the management of many cancer types, including non-small cell lung cancer (NSCLC) [7, 8]. This review discusses the clinical development of irreversible tyrosine kinase inhibitors (TKIs) that target the HER family in NSCLC.
A History of EGFR TKIs in NSCLC and the Rationale for Irreversible Inhibition of EGFR
Erlotinib and Gefitinib
EGFR overexpression has been detected in a variety of epithelial malignancies, including NSCLC [10]. This observation spurred the study of EGFR inhibitors, such as gefitinib (Iressa®; AstraZeneca, Wilmington, DE) and erlotinib (Tarceva®; Genentech, South San Francisco, CA), in patients with NSCLC. Both agents are orally available, reversible, small-molecule inhibitors of the TK portion of the receptor. They inhibit ATP binding and subsequent signal transduction and downstream effector functions [11]. In phase II trials, activity was observed with gefitinib in patients with advanced NSCLC and prior chemotherapy. Gefitinib dosed at 250 mg and 500 mg daily yielded response rates (RRs) of 18% and 19%, respectively, in a multicenter trial conducted in the European Union and Japan (Iressa® Dose Evaluation in Advanced Lung Cancer [IDEAL] 1) [12], and 9% and 12% in a multicenter trial conducted in the U.S. (IDEAL 2) [13]. A multicenter phase II trial studying erlotinib in previously treated patients with advanced NSCLC reported an RR of 12.3% [14].
Gefitinib was subsequently conditionally approved by the U.S. Food and Drug Administration (FDA) in May 2003 as monotherapy for patients with advanced NSCLC who failed to respond to conventional chemotherapy [15]. However, phase III trials combining gefitinib with platinum-based chemotherapy (carboplatin plus paclitaxel or gemcitabine plus cisplatin) in chemotherapy-naive patients with advanced NSCLC (Iressa® NSCLC Trial Assessing Combination Therapy [INTACT] 1 and INTACT 2) [16, 17] failed to show an overall survival (OS) advantage with gefitinib, nor did a single-agent trial of gefitinib compared with placebo in previously treated patients (Iressa® Survival Evaluation in Lung Cancer [ISEL]) [18]. Based on these results, in 2005 the U.S. FDA recommended a label restriction limiting continued gefitinib use to patients with advanced or metastatic NSCLC who had failed both platinum- and docetaxel-based chemotherapies who are benefiting or have benefited from gefitinib [19]. Similarly, results from two large phase III trials of erlotinib in unselected chemotherapy-naive patients with advanced NSCLC (Tarceva® Lung Cancer Investigation [TALENT] and Tarceva® Responses in Conjunction with Paclitaxel and Carboplatin [TRIBUTE]) failed to show a significantly longer OS time when used in combination with platinum-based chemotherapy [20, 21]. However, in the pivotal phase III BR.21 trial [22], single-agent erlotinib produced a significantly longer OS time than with placebo (6.7 months versus 4.7 months; hazard ratio [HR], 0.70; 95% confidence interval [CI], 0.58–0.85; p < .001) in previously treated patients with NSCLC. In November 2004, erlotinib was approved by the U.S. FDA for the treatment of patients with locally advanced or metastatic NSCLC after the failure of at least one prior chemotherapy regimen [23]. Based on results from the phase III Sequential Tarceva® in Unresectable NSCLC (SATURN) trial, erlotinib is approved as maintenance therapy in the U.S. in patients with locally advanced or metastatic NSCLC whose disease has not progressed after four cycles of platinum-based therapy [24, 25].
The landmark discovery that a subset of NSCLCs harbor activating mutations in the TK domain of EGFR elucidated the determinant of the dramatic responses observed in small percentages of patients treated with single-agent gefitinib or erlotinib [26–28]. These heterozygous somatic mutations most frequently consist of a point mutation within exon 21, leading to an amino acid substitution (e.g., L858R) or in-frame deletions within exon 19. Kinase domain mutations lead to constitutive activation of EGFR by destabilization of the autoinhibited conformation of the receptor [29, 30]. In mutant EGFR tumors, cell survival is dependent on EGFR signaling, a phenomenon termed “oncogene addiction” [15]. Interestingly, although mutant EGFRs are constitutively activated, they possess lesser affinity for ATP [31]. Furthermore, mutant EGFR binds gefitinib more tightly than wild-type EGFR; therefore, TKIs outcompete ATP in interactions with mutant EGFR, effectively inhibiting the oncogene-addicted state [30, 31].
Among patients with NSCLC, the presence of EGFR mutations correlates with certain clinical characteristics (female gender, nonsmoking status, Asian ethnicity, and adenocarcinoma histology) [32], several of which had been previously associated with greater clinical benefit with EGFR TKIs [12, 13, 22]. Prospective clinical trials of patients with tumors harboring activating EGFR mutations have been performed, reporting RRs ≥55% and indicating first-line activity of EGFR TKIs in genetically selected tumors [33–35]. Despite these impressive RRs in mutant EGFR NSCLCs, in a randomized phase III trial (Iressa® Non-small-cell lung cancer Trial Evaluating REsponse and Survival against Taxotere®) of previously treated patients with NSCLC that demonstrated the noninferiority of gefitinib compared with docetaxel with respect to the OS time (median, 7.6 months versus 8.0 months; HR, 1.020; 96% CI, 0.905–1.150), there was no difference in the OS times noted in subgroups with a higher EGFR gene copy number or EGFR mutation [36]. These results called into question the role of patient selection by EGFR mutation status prior to initiation of gefitinib therapy.
The rationale of prospective genotyping and patient selection was subsequently supported by the results of the phase III Iressa® Pan-Asia Study (IPASS) trial [37], which included >1,200 genetically unselected patients with advanced lung adenocarcinoma who received first-line gefitinib or carboplatin plus paclitaxel. The progression-free survival (PFS) interval was significantly longer with gefitinib than with chemotherapy in the overall population (HR, 0.74; 95% CI, 0.65–0.85; p < .001). Notably, in a preplanned exploratory subgroup analysis of 261 patients whose tumors possessed EGFR mutations, the PFS duration was significantly longer for patients receiving gefitinib than for those receiving carboplatin plus paclitaxel (HR, 0.48; 95% CI, 0.36–0.64; p < .001), whereas in patients whose tumors did not have an EGFR mutation (n = 176), the PFS interval was significantly shorter with gefitinib than with chemotherapy (HR, 2.85; 95% CI, 2.05–3.98; p < .001) [37]. In 2009, gefitinib was approved in Europe for all lines of therapy in patients with locally advanced or metastatic NSCLC with an EGFR-activating mutation [38]. Two Japanese phase III trials published in 2010 confirmed the activity of gefitinib in chemotherapy-naive patients with advanced NSCLC harboring an EGFR mutation [39, 40]. In the first trial (West Japan Thoracic Oncology Group 3405) [39], gefitinib resulted in a longer PFS duration (9.2 months versus 6.3 months; HR, 0.489; 95% CI, 0.336–0.710; p < .0001) and a higher objective RR (62.1% versus 32.2%; p < .0001) than with cisplatin plus docetaxel; OS data were not available at the time of this review. Similarly, in a second trial conducted by the North-East Japan Study Group [40], gefitinib was associated with a longer PFS time (10.8 months versus 5.4 months; HR, 0.30; 95% CI, 0.22–0.41; p < .001) and a higher RR (73.7% versus 30.7%; p < .001) than with carboplatin plus paclitaxel. However, the OS time was not significantly different between the two arms (23.6 months, versus 30.5 months with gefitinib; p = 0.31). This lack of a significant OS difference was also reported in the IPASS trial—the OS times were similar for gefitinib and chemotherapy in the overall population (HR, 0.901; 95% CI, 0.793–1.023; p = .109), in the subgroup of patients with EGFR mutations (HR, 1.002; 95% CI, 0.756–1.328; p = .990), and in the subgroup of patients without EGFR mutations (HR, 1.181; 95% CI, 0.857–1.628; p = .309) [41].
The similarity in OS times for gefitinib- and chemotherapy-treated patients with mutant EGFR tumors is likely a result of crossover and the effectiveness of EGFR inhibitors whether given in the first- or second-line setting [42]. Interestingly, a subgroup analysis of never-smokers from the TRIBUTE trial demonstrated that the survival duration of patients randomized to erlotinib plus carboplatin and paclitaxel was 22.5 months, compared with 10.1 months for those randomized to placebo plus chemotherapy (HR, 0.49; 95% CI, 0.28–0.85), suggesting that, in the absence of crossover, EGFR inhibition would likely produce superior outcomes in patients with mutant EGFR tumors [21].
Resistance to Currently Approved EGFR TKIs
The most prevalent determinant of de novo resistance to EGFR TKIs is the presence of a Kirsten rat sarcoma viral oncogene homolog (KRAS) mutation, associated primarily with NSCLC patients having a history of smoking [15, 43]. Most studies have found that EGFR-activating mutations and KRAS mutations, found in approximately one third of NSCLCs of adenocarcinoma histology [44], are mutually exclusive. Retrospective analyses suggest that KRAS mutations may be associated with poorer survival with erlotinib in patients with NSCLC [45, 46]. However, in a retrospective analysis of the BR.21 trial, correlation of KRAS status with erlotinib treatment outcome did not reach statistical significance (p = .09), and the RR was 5% (one of 20) among patients with KRAS mutations (RR among patients with wild-type KRAS, 10%) [46]. Even among tumors with activated EGFR, a subset of mutations, such as exon 20 insertions, is inherently resistant to erlotinib or gefitinib [47].
For those cases in which primary resistance is not the obstacle to EGFR TKI benefit, acquired resistance becomes the challenge. Despite initial response to EGFR TKIs, patients with mutant EGFR NSCLC experience disease progression within ∼12 months of treatment [48]. The most common mechanism of acquired resistance is the emergence of a secondary mutation in exon 20, T790M, within the catalytic cleft of EGFR, detectable in approximately 50% of NSCLCs that become resistant to first-generation EGFR TKIs [15]. Interestingly, although the T790M mutation is associated with acquired resistance, it has also been detected in circulating tumor cells from TKI treatment–naive patients [49]. In addition, the T790M mutation was identified in the germline of a family predisposed to NSCLC, indicating an additional role in NSCLC susceptibility [50]. An analysis of pretreatment biopsies from NSCLC patients with EGFR mutations who subsequently received erlotinib reported that the incidence of double EGFR mutations (L858R or exon 19 deletion as well as T790M) was 35% (45 of 129) when using an ultrasensitive assay, with no difference in the initial response to erlotinib (63.6% versus 72.3%) in patients with or without T790M mutations, but with a shorter PFS interval in cases in which pretreatment T790M was identified [51]. These findings suggest that the T790M mutation may be present with other EGFR mutations in some patients prior to TKI therapy and may be selected during therapy because of the treatment resistance associated with the mutation.
Steric hindrance of TKIs by the “gatekeeper” T790M mutation has been hypothesized as the basis for T790M-induced TKI resistance. However, in vitro, the T790M mutant remains sensitive to irreversible TKIs that are structurally similar to erlotinib and gefitinib, and therefore would be expected to be subject to the same steric hindrance [52, 53]. Yun et al. [54] showed that, although the L858R mutation is activating, it also possesses less affinity for ATP than wild-type EGFR. Furthermore, the presence of the T790M mutation increases the ATP affinity of the oncogenic L858R mutant by approximately five-fold. Therefore, enhanced ATP affinity reduces the ability of reversible TKIs such as gefitinib and erlotinib to effectively compete with ATP binding, resulting in a lower potency of reversible TKIs in the setting of the L858R and T790M double mutation [54]. Interestingly, the T790M mutation alone increases the catalytic turnover of EGFR to that of approximately six-fold of the wild-type receptor [54], indicating that T790M in isolation has oncogenic potential, as reflected by reports of inherited susceptibility to lung cancer and the germline presence of T790M [49, 50].
Less frequent mechanisms of acquired resistance in mutant EGFR NSCLC include amplification of the mesenchymal-epithelial transition factor (MET) proto-oncogene [55] and phosphatidylinositol-3-kinase (PI3K)/Akt activation [52, 53]. MET amplification has been identified in approximately 20% of mutant EGFR NSCLC tumor specimens that were resistant to erlotinib or gefitinib [56]. Sequist et al. [48] recently described other mechanisms of acquired resistance to EGFR inhibitors, including acquisition of PIK3CA mutations. In addition, striking examples of histologic transformation to small cell histology and epithelial-to-mesenchymal transition were reported [48].
Clinical Evaluation of Investigational Irreversible HER Family TKIs in NSCLC
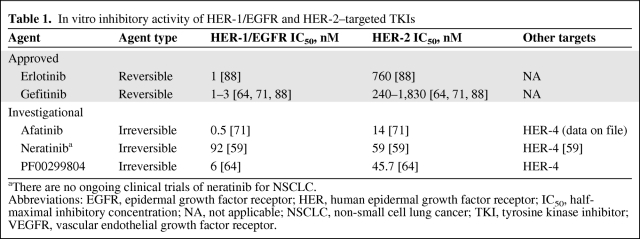
Multiple strategies, including the development of agents that bind irreversibly and/or inhibit multiple targets simultaneously, are being investigated to treat NSCLCs that are resistant to first-generation EGFR TKIs [15]. Unlike reversible TKIs, irreversible TKIs contain a reactive Michael-acceptor group that binds covalently with Cys797 present at the ATP-binding cleft of mutant EGFR, thus providing greater presence at the ATP site and overcoming the competition with ATP that becomes unfavorable to reversible TKIs in the presence of the T790M mutation [54, 57]. The ability of an irreversible TKI to overcome resistance was demonstrated in vitro in mutant EGFR cell lines either clonally selected for resistance by growth in gefitinib or known to harbor the T790M mutation [53]. Several investigational irreversible multitargeted HER family TKIs (Table 1) are being evaluated in patients with NSCLC (Table 2). These include neratinib or HKI-272 (Wyeth, which was acquired by Pfizer in 2009, New London, CT), PF00299804 (Pfizer), and afatinib or BIBW 2992 (Boehringer Ingelheim, Ingelheim, Germany).
Table 1.
In vitro inhibitory activity of HER-1/EGFR and HER-2–targeted TKIs
aThere are no ongoing clinical trials of neratinib for NSCLC.
Abbreviations: EGFR, epidermal growth factor receptor; HER, human epidermal growth factor receptor; IC50, half-maximal inhibitory concentration; NA, not applicable; NSCLC, non-small cell lung cancer; TKI, tyrosine kinase inhibitor; VEGFR, vascular endothelial growth factor receptor.
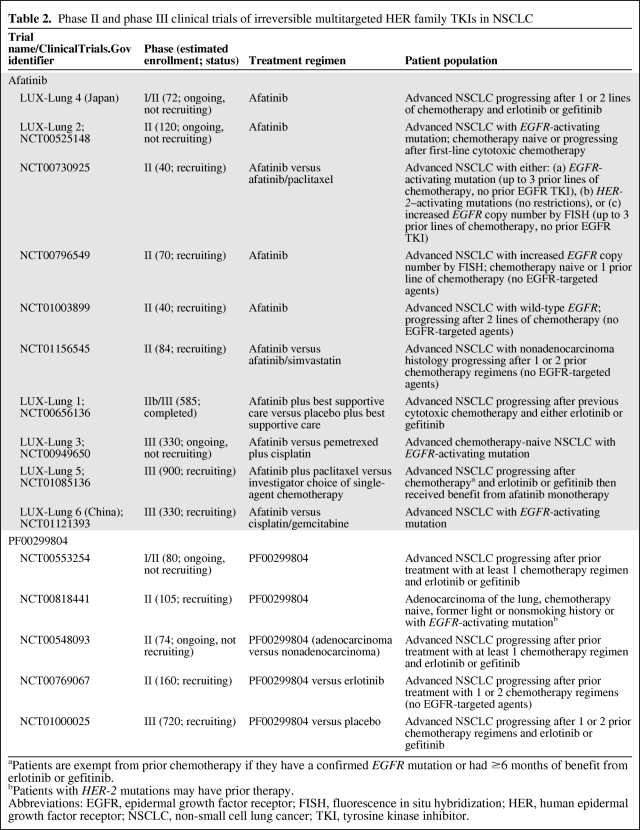
Table 2.
Phase II and phase III clinical trials of irreversible multitargeted HER family TKIs in NSCLC
aPatients are exempt from prior chemotherapy if they have a confirmed EGFR mutation or had ≥6 months of benefit from erlotinib or gefitinib.
bPatients with HER-2 mutations may have prior therapy.
Abbreviations: EGFR, epidermal growth factor receptor; FISH, fluorescence in situ hybridization; HER, human epidermal growth factor receptor; NSCLC, non-small cell lung cancer; TKI, tyrosine kinase inhibitor.
Neratinib (HKI-272)
Neratinib, an irreversible HER family inhibitor that targets EGFR/HER-1, HER-2, and HER-4 [58, 59] (Table 1), was evaluated in a phase I trial of patients with advanced solid tumors [60]. Neratinib was administered as a single dose followed by a 1-week observation period and then as continuous, once-daily treatment with doses in the range of 40–500 mg. Grade 3 diarrhea was observed as a dose-limiting toxicity, and the maximum-tolerated dose of neratinib was determined to be 320 mg. Of 14 evaluable patients with NSCLC, stable disease (SD) ≥24 weeks was observed in six (43%). Eight partial responses (PRs) and one patient with SD were also reported among 24 evaluable patients with breast cancer. Grade 3 treatment-emergent and treatment-related adverse events (AEs) included diarrhea (32%), fatigue (8%), vomiting (7%), dehydration (6%), nausea (4%), asthenia (1%), and anorexia (1%); no grade 4 toxicities were reported.
A phase II trial [61] of 172 patients with advanced NSCLC who progressed following erlotinib or gefitinib studied three subgroups of patients with tumors thought likely to benefit from neratinib: arm A, EGFR mutation (n = 91); arm B, wild-type EGFR (n = 48); and arm C, EGFR TKI naive but with adenocarcinoma and a light smoking history (n = 28). Patients initially received neratinib at a dose of 320 mg/day, but the dose was decreased to 240 mg/day because of dose delays and reductions associated with diarrhea. Of 158 evaluable patients, three (1.9%) had an objective response and 14 (9%) had SD for six or more cycles, resulting in an objective RR of 3.4% for arm A and 0% for arms B and C. The median PFS intervals were 15.3 weeks overall and 15.3, 16.1, and 9.3 weeks in arms A, B, and C, respectively. The overall RRs observed in patients with an EGFR mutation (all 91 patients in arm A and 11 of 28 patients in arm C) were disappointing. However, three of four patients with an exon 18 G719X EGFR point mutation experienced a PR and the fourth had SD ≥40 weeks; among these patients, the median PFS interval was 52.7 weeks (90% CI, 25.6–57.0 weeks). Therefore, neratinib provided benefit in this small subgroup of patients with exon 18 G719X mutant EGFR tumors that had become refractory to reversible TKIs. Despite preclinical data suggesting a role for neratinib in overcoming resistance mediated by T790M [53], no patients with a known T790M mutation responded. Based on these results, neratinib is no longer in development for NSCLC (http://www.clinicaltrials.gov), although it is being investigated in HER-2+ breast cancer [62].
PF00299804
PF00299804, an irreversible HER family inhibitor that targets EGFR/HER-1, HER-3, and HER-4 [63] (Table 1), has demonstrated preclinical activity in gefitinib-resistant NSCLC models both in vitro and in vivo [64]. In a phase I trial in patients with advanced solid tumors, PF00299804 (0.5–60 mg/day) was administered on two dosing schedules (once daily continuously, n = 111; intermittently, n = 10) [63]. In total, 121 patients were enrolled, with 47% of tumors being NSCLCs. Dose-limiting toxicities observed at the 60-mg/day dose were stomatitis, palmar–plantar erythema, and dehydration (n = 1 for each, all grade 3). The maximum-tolerated dose was established at 45 mg/day. Four patients, each with NSCLC previously treated with erlotinib and/or gefitinib had a PR, and an additional 28 patients with NSCLC had SD ≥6 weeks. Interestingly, of five evaluable patients with an exon 20 mutation (typically erlotinib/gefitinib resistant), one patient had a PR and two patients had SD. Four patients with documented T790M mutations did not respond to PF00299804. The most common nonhematologic AE occurring in ≥15% of patients on both dosing schedules was diarrhea.
PF00299804 has been evaluated in clinical trials in patients with NSCLC following treatment with a first-generation EGFR TKI. In a phase I trial [65], PF00299804 was evaluated in 44 NSCLC patients, most of whom had received prior EGFR inhibitors (94%) and prior chemotherapy (79%). Of 29 evaluable patients, two had PRs and eight had SD, resulting in a clinical benefit rate of 34%. Both patients who achieved a PR had previously received three or more lines of chemotherapy and either erlotinib or gefitinib. The most frequently reported AEs of any grade were diarrhea (78%) and rash (65%). Based on these results, trials of PF00299804 in patients with NSCLC refractory to chemotherapy and first-generation EGFR TKIs were initiated. In a phase I/II trial of PF00299804 in patients with NSCLC who progressed following one or two prior chemotherapy regimens and erlotinib [66], 36 patients with adenocarcinoma and five patients with nonadenocarcinoma histology were evaluable for efficacy. Among patients with adenocarcinoma, 67% had a clinical benefit (response or SD), and among those with nonadenocarcinoma histology, the clinical benefit rate was 40%. In another phase I/II study of PF00299804 in Korean patients with wild-type KRAS NSCLC who failed one or more chemotherapy regimen and erlotinib or gefitinib [67], preliminary phase II data from 42 patients demonstrated an objective RR of 15%, a clinical benefit rate (PR or SD ≥24 weeks) of 25%, and 4- and 6-month PFS rates of 48% and 32%, respectively. In a similar phase II study in patients with wild-type KRAS NSCLC who had failed one or more chemotherapy regimen and erlotinib [68], of 62 evaluable patients, three achieved PRs and 35 had SD ≥6 weeks.
PF00299804 was evaluated versus erlotinib in a phase II study of 188 previously treated patients with NSCLC [69]. Some imbalance existed between treatment arms in the trial with regard to the percentage of patients with a performance status score of 2 (19.1%, versus 3.2% with erlotinib) and with EGFR mutations (20.2%, versus 11.7% with erlotinib). Overall, the PFS interval was longer (HR, 0.681; 95% CI, 0.490–0.945; p = .019), the objective RR was higher (17.0% versus 4.3%; p = .009), and the clinical benefit rate (response or SD ≥24 weeks) was higher (27.7% versus 13.8%; p = .03) with PF00299804 than with erlotinib. However, diarrhea and acne were more common with PF00299804 than with erlotinib. First-line therapy with PF00299804 is being evaluated in a phase II study of patients with NSCLC harboring an EGFR mutation [70]. Preliminary results indicated that, of 29 patients, one had a complete response (CR), six had PRs, and 16 had SD ≥6 weeks. These and other ongoing trials, including a phase III trial of PF00299804 compared with placebo (ClinicalTrials.gov identifier, NCT01000025) in patients with refractory NSCLC, are summarized in Table 2.
Afatinib (BIBW 2992)
Afatinib is an oral irreversible HER family inhibitor that targets EGFR/HER-1, HER-2 [71], and HER-4 (data on file and Table 1) with preclinical data supporting a role in overcoming resistance to reversible EGFR TKIs [71]. Afatinib has been studied in multiple phase I clinical trials [71–76], one of which enrolled 53 patients with advanced solid tumors who received once-daily afatinib, 10–50 mg [76]. Dose-limiting toxicities included rash and reversible dyspnea secondary to pneumonitis; the recommended phase II dose of afatinib was 50 mg. Three patients with NSCLC experienced PRs lasting 24, 18, and 34 months; their tumors were found to have mutations in EGFR, although none had received prior EGFR TKI treatment. Two additional patients (one with NSCLC and one with esophageal cancer) had unconfirmed PRs. One of the NSCLC patients with an activating exon 19 mutation who had a PR was initially treated with afatinib (10 mg/day) but subsequently progressed and developed brain metastases. That patient then experienced regression after a dose increase to 40 mg/day. No grade 4 or 5 AEs were reported; grade 3 AEs observed included skin-related effects, diarrhea, and fatigue.
The role of afatinib in patients with NSCLC resistant to reversible TKIs is being explored in a number of clinical trials. LUX-Lung 1 was a phase IIb/III, randomized, double-blinded trial in patients with stage IIIB/IV lung adenocarcinoma who failed one or two chemotherapy treatments and progressed following ≥12 weeks of treatment with erlotinib or gefitinib [77]. LUX-Lung 1 patients (N = 585) were randomized in a 2:1 ratio to best supportive care (BSC) plus afatinib (50 mg/day) or BSC plus placebo; the primary endpoint was OS. The study was enriched for tumors with EGFR-activating mutations, with 58% Asian and 60% female patients, although prospective sequencing was not performed. In addition, 81% of patients were previously treated with erlotinib or gefitinib for ≥24 weeks, with 45% having responded (PR or CR) to prior treatment. Primary analysis revealed median OS times of 10.8 months for afatinib plus BSC and 12.0 months for placebo plus BSC (HR, 1.08; 95% CI, 0.86–1.35). Despite the lack of OS benefit, afatinib provided significantly better results in the secondary endpoints of PFS time (3.3 months versus 1.1 months; HR, 0.38; p < .0001), disease control rate (DCR) at 8 weeks (58% versus 19%; p < .0001), and objective RR (7.4% versus 0.5% by independent analysis; p <.01) than with placebo [77].
Afatinib has also been evaluated as first-line and second-line therapy in patients who have not received a first-generation EGFR TKI. LUX-Lung 2 is a single-arm, multicenter, phase II trial evaluating the efficacy of afatinib (50 mg/day or 40 mg/day) in patients with stage IIIB/IV mutant EGFR adenocarcinoma and no prior EGFR-targeted therapy. Of 129 patients who received treatment (first line, n = 61; second line, n = 68), 54 had L858R EGFR mutations, 52 had exon 19 deletions in EGFR, and 23 had other EGFR mutations [79]. By investigator assessment, the objective RR, DCR, median PFS interval, and median OS time were 60%, 86%, 14 months, and 24 months, respectively, for all patients [73]. The objective RR, DCR, and median PFS were 59%, 83%, and 16.1 months, respectively, for patients with L858R mutations and 69%, 93%, and 13.7 months, respectively, for patients with exon 19 deletions. Additional trials of afatinib in NSCLC are ongoing and summarized in Table 2.
Clinical Perspective
Expectations have been high for irreversible HER family inhibitors in the treatment of NSCLC, and results are awaited from ongoing large randomized clinical trials evaluating these agents in NSCLC, particularly in clinically and/or molecularly selected populations. The optimal role of irreversible HER inhibitors in the treatment of NSCLC has yet to be determined; however, their potential potency in the first-line setting and ability to bind covalently to block the ATP-binding site of mutant EGFR could potentially improve upon outcomes seen with gefitinib and erlotinib. This may be true particularly for specific activating mutations. In NSCLCs with the most common EGFR activators, exon 19 deletions and L858R mutations (85% of known mutations), outcomes are better after reversible TKI treatment for patients with exon 19 mutations than for patients with L858R mutations [80, 81], perhaps because of less effective inhibition of the L858R mutant. In vitro, PF00299804 was more effective at inhibiting exon 19 deletions and L858R compared with gefitinib [64]. Similar activity has also been observed with afatinib compared with gefitinib against exon 19 mutations [82]. Therefore, potent irreversible inhibitors may improve outcomes and delay the onset of resistance than with reversible TKIs, particularly for patients with L858R-mutant NSCLCs. Randomized trials of first-line irreversible inhibitors versus erlotinib or gefitinib in prospectively identified mutant EGFR NSCLCs are required to explore this concept. An ongoing trial (ClinicalTrials.gov identifier, NCT00769067) of PF00299804 versus erlotinib in patients previously treated with chemotherapy may answer these questions in part, although that trial does not include prospective identification of EGFR mutations.
Results of irreversible inhibitors in erlotinib- or gefitinib-resistant, mutant EGFR NSCLCs have been disappointing to date and suggest that the ability of irreversible inhibitors to overcome acquired resistance may have limitations that were not predicted in preclinical studies. This may be a result of an inability to attain the drug concentrations in humans that were effective in preclinical studies. In the case of neratinib, grade 3 diarrhea in half of the patients necessitated a dose reduction in the three-arm phase II trial. Although not measured, it was proposed that dose reduction of neratinib to 240 mg daily resulted in steady-state neratinib concentrations that may have been insufficient to inhibit exon 19 deletions or T790M mutations based on the concentrations required for inhibition in preclinical models (60 nmol/L for exon 19 deletion and 90–800 nmol/L for T790M mutation). In contrast, the much lower dose of neratinib required to inhibit the G719S mutation (3 nmol/L) may have been achievable, leading to the PRs observed in that small subgroup of patients refractory to reversible TKIs [61]. Similar to neratinib, the half-maximal inhibitory concentration of PF00299804 required for growth inhibition in NSCLC cell lines with the T790M resistance mutation is 100–900 nM. The inability to achieve these concentrations with doses administered clinically may explain the lack of efficacy in tumors with a T790M mutation [83]. Because T790M-mutant EGFR has an affinity for ATP that is similar to the affinity of wild-type EGFR for ATP, concentrations of irreversible inhibitors that overcome the resistance mutation in vitro are not clinically achievable because of toxicities related to systemic wild-type EGFR inhibition, such as diarrhea and rash. EGFR T790M mutations notwithstanding, there are glimpses into the potential for irreversible inhibitors in gefitinib- or erlotinib-refractory disease. The PRs and SD seen in PF00299804-treated NSCLC patients with exon 20 insertions (typically resistant to reversible EGFR TKIs) and the PRs seen in neratinib-treated NSCLC patients with exon 18 G719X-mutant tumors previously treated with a reversible EGFR TKI suggest that specific EGFR mutations have differential sensitivities to TKI inhibition and that, similar to the situation noted for exon 19 deletions and L858R mutations, irreversible inhibitors are better able to address those relative sensitivities [61, 63].
One approach to expand upon the utility of clinically available 4-anilinoquinazoline irreversible EGFR inhibitors is to pair them with downstream pathway inhibitors or other types of EGFR inhibitors. For instance, afatinib has been combined in vitro with a PI3K/mammalian target of rapamycin (mTOR) inhibitor, a mitogen-activated protein kinase/extracellular signal–related kinase kinase (MEK) inhibitor, and a v-src sarcoma viral oncogene homolog (Src) inhibitor, yielding greater apoptosis in T790M cell lines than with afatinib alone [84]. In another experiment, the combination of afatinib plus the mTOR inhibitor rapamycin was studied in a mouse model of de novo EGFR L858R/T790M-driven lung cancer. Although single-agent afatinib produced a >50% reduction in tumor volume, the addition of rapamycin to afatinib led to nearly complete tumor regression [71]. In the clinic, the combination of an irreversible inhibitor and mTOR inhibitor is being explored in a phase I study of neratinib plus temsirolimus [85]. Results from a phase Ib/II trial of afatinib in combination with the anti-EGFR antibody cetuximab (Erbitux®; Bristol-Myers Squibb, New York) [86] were recently reported. In that trial, patients with mutant EGFR NSCLC and clinically defined acquired resistance to reversible EGFR TKIs were treated with daily afatinib (40 mg) plus biweekly cetuximab (500 mg/m2). Confirmed PRs were observed in 36% of evaluable patients (eight of 22; 95% CI, 0.17–0.59) including in 29% of patients with T790M-mutant tumors (four of 13). These promising results will be further explored in a larger study. Reported AEs included rash (grade 1, 35%; grade 2, 46%; grade 3, 11.5%) and diarrhea (grade 1, 50%; grade 2, 19%).
Despite the potential of drug combinations, the 4-anilinoquinazoline core structure that is common to the clinically available irreversible inhibitors may not provide optimal molecular interactions or binding kinetics in the setting of T790M mutation. Nonetheless, new structurally distinct irreversible HER family inhibitors, such as the pyrimidine-based inhibitors described by Zhou et al. [87], indicate that the concept of irreversible HER family inhibition is a sound one. Those investigators screened a library of compounds to identify agents that inhibited growth of gefitinib-resistant and gefitinib-sensitive cell lines without producing toxicity in mutant KRAS cells at high concentrations. One such compound, WZ4002, is an irreversible inhibitor with chemical properties that favor 100-fold greater binding to the T790M mutant and 100-fold weaker binding to wild-type EGFR than with neratinib and other quinazoline-based EGFR inhibitors. WZ4002 inhibited L858R/T790M EGFR kinase activity more potently than wild-type EGFR protein activity, whereas the opposite was true for neratinib and gefitinib (i.e., they inhibited wild-type EGFR more potently than the L858R/T790M mutant). Interestingly, the importance of irreversibility was demonstrated by the markedly lesser efficacy of a reversible WZ4002 analog against T790M-mutant cell lines, as well as by the markedly lesser efficacy of the irreversible WZ4002 against cell lines with an EGFR mutation at Cys 797 that prevented covalent interaction of drug and protein. Such findings indicate that the concept of irreversible HER family inhibition is a sound one that may yet provide a solution to the problem of acquired resistance.
Conclusions
Preclinical and early clinical data suggest that there is a valid rationale for the development of irreversible HER family TKIs for the treatment of NSCLC. In vitro studies have demonstrated activity of these agents in preclinical models of first-generation EGFR TKI–resistant NSCLC, and several multitargeted HER family TKIs have provided responses in phase I trials in patients with NSCLC. Clinical trials are under way to evaluate the efficacy of these agents in patients with advanced NSCLC in a variety of settings, both alone and in combination with chemotherapy and in chemotherapy-naive and previously treated patients. Clinical trials of specific patient subgroups (e.g., those with EGFR-activating mutations or a higher EGFR copy number) are also ongoing to evaluate irreversible HER family TKIs in selected patient populations. In addition, some clinical trials are evaluating these agents compared with reversible EGFR TKIs. Whereas results of these trials will help determine the potential for the currently available irreversible HER family TKIs in the treatment paradigm for NSCLC, combination therapy and newer preclinical irreversible inhibitors are building upon lessons learned from previous scientific and current clinical data, with a promise to improve upon the ability to treat EGFR TKI resistance.
Acknowledgments
The author would like to acknowledge Lecia Sequist, M.D., M.P.H., for her insightful comments and careful review.
This work was supported by Boehringer Ingelheim Pharmaceuticals, Inc (BIPI). The author received no compensation related to the development of the manuscript.
Footnotes
- (C/A)
- consulting/advisory relationship
- (RF)
- Research funding
- (E)
- Employment
- (H)
- Honoraria received
- (OI)
- Ownership interests
- (IP)
- Intellectual property rights/inventor/patent holder
Medical Writer Acknowledgment
The author takes full responsibility for the content of the paper but thanks Staci Heise, Ph.D. (MedErgy, supported by BIPI) for her writing and editorial assistance. The author meets criteria for authorship as recommended by the International Committee of Medical Journal Editors (ICMJE), was fully responsible for all content and editorial decisions, and was involved at all stages of manuscript development.
References
- 1.Roskoski R., Jr The ErbB/HER receptor protein-tyrosine kinases and cancer. Biochem Biophys Res Commun. 2004;319:1–11. doi: 10.1016/j.bbrc.2004.04.150. [DOI] [PubMed] [Google Scholar]
- 2.Sibilia M, Kroismayr R, Lichtenberger BM, et al. The epidermal growth factor receptor: From development to tumorigenesis. Differentiation. 2007;75:770–787. doi: 10.1111/j.1432-0436.2007.00238.x. [DOI] [PubMed] [Google Scholar]
- 3.Britten CD. Targeting ErbB receptor signaling: A pan-ErbB approach to cancer. Mol Cancer Ther. 2004;3:1335–1342. [PubMed] [Google Scholar]
- 4.Grandis JR, Sok JC. Signaling through the epidermal growth factor receptor during the development of malignancy. Pharmacol Ther. 2004;102:37–46. doi: 10.1016/j.pharmthera.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 5.Citri A, Skaria KB, Yarden Y. The deaf and the dumb: The biology of ErbB-2 and ErbB-3. Exp Cell Res. 2003;284:54–65. doi: 10.1016/s0014-4827(02)00101-5. [DOI] [PubMed] [Google Scholar]
- 6.Olayioye MA, Neve RM, Lane HA, et al. The ErbB signaling network: Receptor heterodimerization in development and cancer. EMBO J. 2000;19:3159–3167. doi: 10.1093/emboj/19.13.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gschwind A, Fischer OM, Ullrich A. The discovery of receptor tyrosine kinases: Targets for cancer therapy. Nat Rev Cancer. 2004;4:361–370. doi: 10.1038/nrc1360. [DOI] [PubMed] [Google Scholar]
- 8.Hynes NE, Schlange T. Targeting ADAMS and ERBBs in lung cancer. Cancer Cell. 2006;10:7–11. doi: 10.1016/j.ccr.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 9.Araújo A, Ribeiro R, Azevedo I, et al. Genetic polymorphisms of the epidermal growth factor and related receptor in non-small cell lung cancer—a review of the literature. The Oncologist. 2007;12:201–210. doi: 10.1634/theoncologist.12-2-201. [DOI] [PubMed] [Google Scholar]
- 10.Joy AA, Butts CA. Extending outcomes: Epidermal growth factor receptor-targeted monoclonal antibodies in non-small-cell lung cancer. Clin Lung Cancer. 2009;10(suppl 1):S24–S29. doi: 10.3816/CLC.2009.s.004. [DOI] [PubMed] [Google Scholar]
- 11.Ganjoo KN, Wakelee H. Review of erlotinib in the treatment of advanced non-small cell lung cancer. Biologics. 2007;1:335–346. [PMC free article] [PubMed] [Google Scholar]
- 12.Fukuoka M, Yano S, Giaccone G, et al. Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer (The IDEAL 1 Trial) [corrected] J Clin Oncol. 2003;21:2237–2246. doi: 10.1200/JCO.2003.10.038. [DOI] [PubMed] [Google Scholar]
- 13.Kris MG, Natale RB, Herbst RS, et al. Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer: A randomized trial. JAMA. 2003;290:2149–2158. doi: 10.1001/jama.290.16.2149. [DOI] [PubMed] [Google Scholar]
- 14.Pérez-Soler R, Chachoua A, Hammond LA, et al. Determinants of tumor response and survival with erlotinib in patients with non–small-cell lung cancer. J Clin Oncol. 2004;22:3238–3247. doi: 10.1200/JCO.2004.11.057. [DOI] [PubMed] [Google Scholar]
- 15.Sharma SV, Bell DW, Settleman J, et al. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer. 2007;7:169–181. doi: 10.1038/nrc2088. [DOI] [PubMed] [Google Scholar]
- 16.Giaccone G, Herbst RS, Manegold C, et al. Gefitinib in combination with gemcitabine and cisplatin in advanced non-small-cell lung cancer: A phase III trial—INTACT 1. J Clin Oncol. 2004;22:777–784. doi: 10.1200/JCO.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 17.Herbst RS, Giaccone G, Schiller JH, et al. Gefitinib in combination with paclitaxel and carboplatin in advanced non-small-cell lung cancer: A phase III trial—INTACT 2. J Clin Oncol. 2004;22:785–794. doi: 10.1200/JCO.2004.07.215. [DOI] [PubMed] [Google Scholar]
- 18.Thatcher N, Chang A, Parikh P, et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: Results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer) Lancet. 2005;366:1527–1537. doi: 10.1016/S0140-6736(05)67625-8. [DOI] [PubMed] [Google Scholar]
- 19.AstraZeneca Pharmaceuticals LP. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2005. Iressa® (gefitinib tablets) [package insert] [Google Scholar]
- 20.Gatzemeier U, Pluzanska A, Szczesna A, et al. Phase III study of erlotinib in combination with cisplatin and gemcitabine in advanced non-small-cell lung cancer: The Tarceva Lung Cancer Investigation Trial. J Clin Oncol. 2007;25:1545–1552. doi: 10.1200/JCO.2005.05.1474. [DOI] [PubMed] [Google Scholar]
- 21.Herbst RS, Prager D, Hermann R, et al. TRIBUTE: A phase III trial of erlotinib hydrochloride (OSI-774) combined with carboplatin and paclitaxel chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol. 2005;23:5892–5899. doi: 10.1200/JCO.2005.02.840. [DOI] [PubMed] [Google Scholar]
- 22.Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–132. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 23.Genentech, Inc. South San Francisco, CA: Genentech, Inc.; 2010. Tarceva® (erlotinib tablets) [package insert] [Google Scholar]
- 24.Genentech Inc. FDA Approves Tarceva as a Maintenance Therapy for Advanced Non-Small Cell Lung Cancer. [accessed April 21, 2010]. Available at http://www.gene.com/gene/news/press-releases/displaydo?method=detail&id=12727.
- 25.Roche. Roche Announces Filing of Tarceva in EU as First-Line Maintenance Therapy in Advanced Non-Small Cell Lung Cancer. 2009. Mar 19, [accessed May 12, 2010]. Available at http://www.roche.com/investors/ir_update/inv-update-2009-03-19.htm.
- 26.Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 27.Paez JG, Jänne PA, Lee JC, et al. EGFR mutations in lung cancer: Correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 28.Pao W, Miller V, Zakowski M, et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci U S A. 2004;101:13306–13311. doi: 10.1073/pnas.0405220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang X, Gureasko J, Shen K, et al. An allosteric mechanism for activation of the kinase domain of epidermal growth factor receptor. Cell. 2006;125:1137–1149. doi: 10.1016/j.cell.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 30.Yun CH, Boggon TJ, Li Y, et al. Structures of lung cancer-derived EGFR mutants and inhibitor complexes: Mechanism of activation and insights into differential inhibitor sensitivity. Cancer Cell. 2007;11:217–227. doi: 10.1016/j.ccr.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carey KD, Garton AJ, Romero MS, et al. Kinetic analysis of epidermal growth factor receptor somatic mutant proteins shows increased sensitivity to the epidermal growth factor receptor tyrosine kinase inhibitor, erlotinib. Cancer Res. 2006;66:8163–8171. doi: 10.1158/0008-5472.CAN-06-0453. [DOI] [PubMed] [Google Scholar]
- 32.Shigematsu H, Lin L, Takahashi T, et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst. 2005;97:339–346. doi: 10.1093/jnci/dji055. [DOI] [PubMed] [Google Scholar]
- 33.Inoue A, Kobayashi K, Usui K, et al. First-line gefitinib for patients with advanced non-small-cell lung cancer harboring epidermal growth factor receptor mutations without indication for chemotherapy. J Clin Oncol. 2009;27:1394–1400. doi: 10.1200/JCO.2008.18.7658. [DOI] [PubMed] [Google Scholar]
- 34.Porta R, Queralt C, Cardenal F, et al. Erlotinib customization based on epidermal growth factor receptor (EGFR) mutations in stage IV non-small-cell lung cancer (NSCLC) patients [abstract 8038] J Clin Oncol. 2008;26(suppl 15):333. [Google Scholar]
- 35.Sequist LV, Martins RG, Spigel D, et al. First-line gefitinib in patients with advanced non-small-cell lung cancer harboring somatic EGFR mutations. J Clin Oncol. 2008;26:2442–2449. doi: 10.1200/JCO.2007.14.8494. [DOI] [PubMed] [Google Scholar]
- 36.Kim ES, Hirsh V, Mok T, et al. Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): A randomised phase III trial. Lancet. 2008;372:1809–1818. doi: 10.1016/S0140-6736(08)61758-4. [DOI] [PubMed] [Google Scholar]
- 37.Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 38.AstraZeneca. Iressa (Gefitinib) Receives Marketing Authorisation for the Treatment of Non-Small Cell Lung Cancer in Europe. [accessed April 8, 2010]. Available at http://www.astrazeneca.com/Media/Press-releases/Article/20090701–IRESSA-Gefitinib-Receives-Marketing-Authorisation-f.
- 39.Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): An open label, randomised phase 3 trial. Lancet Oncol. 2010;11:121–128. doi: 10.1016/S1470-2045(09)70364-X. [DOI] [PubMed] [Google Scholar]
- 40.Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–2388. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 41.Yang CH, Fukuoka M, Mok TS, et al. Final overall survival (OS) results from a phase III, randomised, open-label, first-line study of gefitinib (G) V carboplatin/paclitaxel (C/P) in clinically selected patients with advanced non-small cell lung cancer (NSCLC) in Asia (iPASS) [abstract LBA2] Ann Oncol. 2010;21(suppl 8):viii1–viii2. [Google Scholar]
- 42.Rosell R, Moran T, Queralt C, et al. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med. 2009;361:958–967. doi: 10.1056/NEJMoa0904554. [DOI] [PubMed] [Google Scholar]
- 43.Mack PC, Redman MW, Chansky K, et al. KRAS and EGFR mutations in the molecular epidemiology of NSCLC: Interim analysis of S0424. J Clin Oncol. 2010;28(15 suppl):7013. [Google Scholar]
- 44.Bos JL. ras oncogenes in human cancer: A review. Cancer Res. 1989;49:4682–4689. [PubMed] [Google Scholar]
- 45.Eberhard DA, Johnson BE, Amler LC, et al. Mutations in the epidermal growth factor receptor and in KRAS are predictive and prognostic indicators in patients with non-small-cell lung cancer treated with chemotherapy alone and in combination with erlotinib. J Clin Oncol. 2005;23:5900–5909. doi: 10.1200/JCO.2005.02.857. [DOI] [PubMed] [Google Scholar]
- 46.Zhu CQ, da Cunha SG, Ding K, et al. Role of KRAS and EGFR as biomarkers of response to erlotinib in National Cancer Institute of Canada Clinical Trials Group Study BR.21. J Clin Oncol. 2008;26:4268–4275. doi: 10.1200/JCO.2007.14.8924. [DOI] [PubMed] [Google Scholar]
- 47.Greulich H, Chen TH, Feng W, et al. Oncogenic transformation by inhibitor-sensitive and -resistant EGFR mutants. PLoS Med. 2005;2:e313. doi: 10.1371/journal.pmed.0020313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sequist LV, Waltman B, Dias-Santagata D, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med. 2011;3:75ra26. doi: 10.1126/scitranslmed.3002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maheswaran S, Sequist LV, Nagrath S, et al. Detection of mutations in EGFR in circulating lung-cancer cells. N Engl J Med. 2008;359:366–377. doi: 10.1056/NEJMoa0800668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bell DW, Gore I, Okimoto RA, et al. Inherited susceptibility to lung cancer may be associated with the T790M drug resistance mutation in EGFR. Nat Genet. 2005;37:1315–1316. doi: 10.1038/ng1671. [DOI] [PubMed] [Google Scholar]
- 51.Rosell R, Molina MA, Costa C, et al. Pretreatment EGFR T790M mutation and BRCA1 mRNA expression in erlotinib-treated advanced non-small-cell lung cancer patients with EGFR mutations. Clin Cancer Res. 2011;17:1160–1168. doi: 10.1158/1078-0432.CCR-10-2158. [DOI] [PubMed] [Google Scholar]
- 52.Kobayashi S, Boggon TJ, Dayaram T, et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med. 2005;352:786–792. doi: 10.1056/NEJMoa044238. [DOI] [PubMed] [Google Scholar]
- 53.Kwak EL, Sordella R, Bell DW, et al. Irreversible inhibitors of the EGF receptor may circumvent acquired resistance to gefitinib. Proc Natl Acad Sci U S A. 2005;102:7665–7670. doi: 10.1073/pnas.0502860102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yun CH, Mengwasser KE, Toms AV, et al. The T790M mutation in EGFR kinase causes drug resistance by increasing the affinity for ATP. Proc Natl Acad Sci U S A. 2008;105:2070–2075. doi: 10.1073/pnas.0709662105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Engelman JA, Zejnullahu K, Mitsudomi T, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039–1043. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 56.Engelman JA, Jänne PA. Mechanisms of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small cell lung cancer. Clin Cancer Res. 2008;14:2895–2899. doi: 10.1158/1078-0432.CCR-07-2248. [DOI] [PubMed] [Google Scholar]
- 57.Suda K, Onozato R, Yatabe Y, et al. EGFR T790M mutation: A double role in lung cancer cell survival? J Thorac Oncol. 2009;4:1–4. doi: 10.1097/JTO.0b013e3181913c9f. [DOI] [PubMed] [Google Scholar]
- 58.Bose P, Ozer H. Neratinib: An oral, irreversible dual EGFR/HER2 inhibitor for breast and non-small cell lung cancer. Expert Opin Investig Drugs. 2009;18:1735–1751. doi: 10.1517/13543780903305428. [DOI] [PubMed] [Google Scholar]
- 59.Rabindran SK, Discafani CM, Rosfjord EC, et al. Antitumor activity of HKI-272, an orally active, irreversible inhibitor of the HER-2 tyrosine kinase. Cancer Res. 2004;64:3958–3965. doi: 10.1158/0008-5472.CAN-03-2868. [DOI] [PubMed] [Google Scholar]
- 60.Wong KK, Fracasso PM, Bukowski RM, et al. A phase I study with neratinib (HKI-272), an irreversible pan ErbB receptor tyrosine kinase inhibitor, in patients with solid tumors. Clin Cancer Res. 2009;15:2552–2558. doi: 10.1158/1078-0432.CCR-08-1978. [DOI] [PubMed] [Google Scholar]
- 61.Sequist LV, Besse B, Lynch TJ, et al. Neratinib, an irreversible pan-ErbB receptor tyrosine kinase inhibitor: Results of a phase II trial in patients with advanced non-small-cell lung cancer. J Clin Oncol. 2010;28:3076–3083. doi: 10.1200/JCO.2009.27.9414. [DOI] [PubMed] [Google Scholar]
- 62.Burstein HJ, Sun Y, Dirix LY, et al. Neratinib, an irreversible ErbB receptor tyrosine kinase inhibitor, in patients with advanced ErbB2-positive breast cancer. J Clin Oncol. 2010;28:1301–1307. doi: 10.1200/JCO.2009.25.8707. [DOI] [PubMed] [Google Scholar]
- 63.Janne PA, Boss DS, Camidge DR, et al. Phase I dose-escalation study of the pan-HER inhibitor, PF299804, in patients with advanced malignant solid tumors. Clin Cancer Res. 2011;17:1131–1139. doi: 10.1158/1078-0432.CCR-10-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Engelman JA, Zejnullahu K, Gale CM, et al. PF00299804, an irreversible pan-ERBB inhibitor, is effective in lung cancer models with EGFR and ERBB2 mutations that are resistant to gefitinib. Cancer Res. 2007;67:11924–11932. doi: 10.1158/0008-5472.CAN-07-1885. [DOI] [PubMed] [Google Scholar]
- 65.Janne PA, Schellens JH, Engelman JA, et al. Preliminary activity and safety results from a phase I clinical trial of PF-00299804, an irreversible pan-HER inhibitor, in patients (pts) with NSCLC. J Clin Oncol. 2008;26:8027. [Google Scholar]
- 66.Janne PA, Reckamp K, Koczywas M, et al. A phase 2 trial of PF-00299804 (PF299), an oral irreversible HER tyrosine kinase inhibitor (TKI), in patients (pts) with advanced NSCLC after failure of prior chemotherapy and erlotinib: Preliminary efficacy and safety results [abstract A3.1] J Thorac Oncol. 2009;4(suppl 1):S293–S294. [Google Scholar]
- 67.Park K, Heo DS, Cho BC, et al. PF299804 in Asian patients with non-small cell lung cancer refractory to chemotherapy and erlotinib or gefitinib: A phase I/II study. Presented at the 46th Annual Meeting of the American Society of Clinical Oncology; June 4–8, 2010; Chicago, IL. [Google Scholar]
- 68.Campbell A, Reckamp KL, Camidge DR, et al. PF-00299804 (PF299) patient (pt)-reported outcomes (PROs) and efficacy in adenocarcinoma (adeno) and nonadeno non-small cell lung cancer (NSCLC): A phase (P) II trial in advanced NSCLC after failure of chemotherapy (CT) and erlotinib (E) J Clin Oncol. 2010;28(15 suppl):7596. [Google Scholar]
- 69.Boyer MJ, Blackhall FH, Park K, et al. Efficacy and Safety of PF299804 Versus Erlotinib: A Global, Randomized Phase 2 Trial in Patients with Advanced Non-small Cell Lung Cancer After Failure of Chemotherapy. Presented at the 46th Annual Meeting of the American Society of Clinical Oncology; June 4–8, 2010; Chicago, IL. [Google Scholar]
- 70.Mok T, Spigel DR, Park K, et al. Efficacy and safety of PF-00299804 (PF299), an oral, irreversible, pan-human epidermal growth factor receptor (pan-HER) tyrosine kinase inhibitor (TKI), as first-line treatment (tx) of selected patients (pts) with advanced (adv) non-small cell lung cancer (NSCLC) J Clin Oncol. 2010;28(15 suppl):7537. [Google Scholar]
- 71.Li D, Ambrogio L, Shimamura T, et al. BIBW2992, an irreversible EGFR/HER2 inhibitor highly effective in preclinical lung cancer models. Oncogene. 2008;27:4702–4711. doi: 10.1038/onc.2008.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Eskens FA, Mom CH, Planting AS, et al. A phase I dose escalation study of BIBW 2992, an irreversible dual inhibitor of epidermal growth factor receptor 1 (EGFR) and 2 (HER2) tyrosine kinase in a 2-week on, 2-week off schedule in patients with advanced solid tumours. Br J Cancer. 2008;98:80–85. doi: 10.1038/sj.bjc.6604108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Awada A, Dumez H, Wolter P, et al. A phase I dose finding study of the 3-day administration of BIBW 2992, an irreversible dual EGFR/HER2 inhibitor, in combination with 3-weekly docetaxel in patients with advanced solid tumors [abstract 3556]. Presented at the 45th Annual Meeting of the American Society of Clinical Oncology; May 29 to June 2, 2009; Orlando, FL. [Google Scholar]
- 74.Stavridi F, Kristeleit R, Forster M, et al. Activity of BIBW 2992, an oral irreversible EGFR/HER2 dual kinase inhibitor, in combination with weekly paclitaxel in non-small cell lung cancer [abstract PD3.1.5] J Thorac Oncol. 2009;4(suppl 1):S444. [Google Scholar]
- 75.Vermorken JB, Machiels JH, Rottey S, et al. Phase Ib study evaluating the combination of BIBW 2992 with two different standard chemotherapy regimens, cisplatin/paclitaxel (PT) and cisplatin/5-FU (PF), in patients with advanced solid tumors. J Clin Oncol. 2010;28:e13521. [Google Scholar]
- 76.Yap TA, Vidal L, Adam J, et al. Phase I trial of the irreversible EGFR and HER2 kinase inhibitor BIBW 2992 in patients with advanced solid tumors. J Clin Oncol. 2010;28:3965–3972. doi: 10.1200/JCO.2009.26.7278. [DOI] [PubMed] [Google Scholar]
- 77.Miller VA, Hirsh V, Cadranel J, et al. Phase IIB/III double-blind randomized trial of afatinib (BIBW 2992, an irreversible inhibitor of EGFR/HER1 and HER2) + best supportive care (BSC) versus placebo + BSC in patients with NSCLC failing 1–2 lines of chemotherapy and erlotinib or gefitinib (LUX-LUNG 1) [abstract LBA1] Ann Oncol. 2010;21(suppl 8):viii1. [Google Scholar]
- 78.Yang C, Shih J, Su W, et al. A phase II study of BIBW 2992 in patients with adenocarcinoma of the lung and activating EGFR mutations (LUX-Lung 2). Presented at the 46th Annual Meeting of the American Society of Clinical Oncology; Chicago, IL; June 4–8, 2010. [Google Scholar]
- 79.Yang C, Shih J, Su W, et al. A phase II study of BIBW 2992 in patients with adenocarcinoma of the lung and activating EGFR/HER1 mutations (LUX-LUNG 2) [abstract 367PD] Ann Oncol. 2010;21(suppl 8):viii123. [Google Scholar]
- 80.Jackman DM, Yeap BY, Sequist LV, et al. Exon 19 deletion mutations of epidermal growth factor receptor are associated with prolonged survival in non-small cell lung cancer patients treated with gefitinib or erlotinib. Clin Cancer Res. 2006;12:3908–3914. doi: 10.1158/1078-0432.CCR-06-0462. [DOI] [PubMed] [Google Scholar]
- 81.Riely GJ, Pao W, Pham D, et al. Clinical course of patients with non-small cell lung cancer and epidermal growth factor receptor exon 19 and exon 21 mutations treated with gefitinib or erlotinib. Clin Cancer Res. 2006;12:839–844. doi: 10.1158/1078-0432.CCR-05-1846. [DOI] [PubMed] [Google Scholar]
- 82.Ninomiya T, Takigawa N, Kubo T, et al. Effect of afatinib on lung cancer burden induced by an exon 19 EGFR mutation in transgenic mice [abstract 3566]. Presented at the 102nd Annual Meeting of the American Association for Cancer Research; Orlando, Florida; April 2–11, 2011. [Google Scholar]
- 83.Janne PA, Boss DS, Camidge DR, et al. Phase I dose-escalation study of the pan-HER inhibitor, PF299804, in patients with advanced malignant solid tumors. Clin Cancer Res. 2011;17(5):1131–1139. doi: 10.1158/1078-0432.CCR-10-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sos ML, Rode HB, Heynck S, et al. Chemogenomic profiling provides insights into the limited activity of irreversible EGFR Inhibitors in tumor cells expressing the T790M EGFR resistance mutation. Cancer Res. 2010;70:868–874. doi: 10.1158/0008-5472.CAN-09-3106. [DOI] [PubMed] [Google Scholar]
- 85.Gandhi L, Bahleda R, Cleary JM, et al. Two-dimensional phase I study of neratinib (NER) combined with temsirolimus (TEM) in patients (Pts) with solid tumors. J Clin Oncol. 2011;29(suppl) Abstract 3027. [Google Scholar]
- 86.Janjigian YY, Groen HJ, Horn L, et al. Activity and tolerability of afatinib (BIBW 2992) and cetuximab in NSCLC patients with acquired resistance to erlotinib or gefitinib. J Clin Oncol. 2011;29(suppl) Abstract 7525. [Google Scholar]
- 87.Zhou W, Ercan D, Chen L, et al. Novel mutant-selective EGFR kinase inhibitors against EGFR T790M. Nature. 2009;462:1070–1074. doi: 10.1038/nature08622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sharma PS, Sharma R, Tyagi T. Receptor tyrosine kinase inhibitors as potent weapons in war against cancers. Curr Pharm Des. 2009;15:758–776. doi: 10.2174/138161209787582219. [DOI] [PubMed] [Google Scholar]