The use of sorafenib in combination with selected chemotherapies in patients with advanced breast cancer is examined and the incidence, prevention, and management of hand–foot skin reaction are considered.
Keywords: Breast cancer, Hand–foot skin reaction, Hand–foot syndrome, Sorafenib, Capecitabine, Paclitaxel
Abstract
Current combination therapies for advanced breast cancer provide a modest survival benefit but with greater toxicity than with monotherapies. New combinations are needed that improve the efficacy of current treatments and have acceptable tolerability profiles. Recent clinical trials have assessed the efficacy and safety of the multikinase inhibitor sorafenib in combination with common treatments for advanced breast cancer. Sorafenib has both antiangiogenic and antiproliferative activities and is indicated for patients with unresectable hepatocellular and advanced renal cell carcinoma. Generally, sorafenib is associated with manageable, non–life-threatening adverse events. One of the more common adverse events seen with sorafenib is hand–foot skin reaction, a dermatologic toxicity usually localized to the pressure points of the palms and soles. Although hand–foot skin reaction is reversible and not life threatening, it can have a significant impact on a patient's quality of life and may necessitate dose modification. Moreover, sorafenib is being evaluated in combination with breast cancer treatments that are associated with a similar dermatologic toxicity (e.g., capecitabine-induced hand–foot syndrome). This review looks at the use of sorafenib in combination with selected chemotherapies in patients with advanced breast cancer and considers the incidence, prevention, and management of hand–foot skin reaction.
Introduction
Survival outcomes for patients with metastatic breast cancer (MBC) over recent decades have steadily improved with the introduction of new therapeutic agents [1, 2]. Combinations of cytotoxic agents have also provided modest advances in survival outcomes and disease control compared with single-agent regimens, but with greater toxicity. However, combination regimens have not shown a survival benefit compared with the sequential use of agents [3–7].
Targeted therapies have become practical candidates for use in combination therapies. Generally, targeted therapies have better hematopoietic and nonspecific toxicity profiles than chemotherapy [8, 9]. In phase III trials, bevacizumab, an antiangiogenic monoclonal antibody, provided a progression-free survival (PFS) benefit in human epidermal growth factor (HER)-2− MBC patients when added to standard chemotherapies (e.g., paclitaxel, docetaxel, capecitabine) [10–13]. Treatment was tolerable, but bevacizumab did not produce a longer overall survival (OS) time.
Recent studies support a potential role for sorafenib in the treatment of HER-2− MBC patients. Sorafenib is a multikinase inhibitor approved for hepatocellular and renal cell carcinoma. It has a multifaceted mechanism of action, with both antiproliferative and antiangiogenic activities. Investigational studies of sorafenib monotherapy demonstrated modest activity in heavily pretreated patients with MBC [14, 15]. Subsequently, a program of four double-blinded, randomized, placebo-controlled, phase IIb clinical trials—Trials to Investigate the Efficacy of Sorafenib (TIES)—was initiated to assess sorafenib in combination regimens for HER-2− advanced breast cancer. Efficacy and safety data have been reported for three of these studies. Compared with the control arm (placebo plus chemotherapy), the addition of sorafenib to capecitabine provided a PFS benefit [16, 17], a PFS trend favored the addition of sorafenib to paclitaxel as first-line therapy [18, 19], and the addition of sorafenib to gemcitabine or capecitabine resulted in a longer PFS interval in patients previously treated with bevacizumab [20]. Generally, these regimens were tolerable, with manageable toxicity and no unexpected safety issues. The most common high-grade toxicity with combination therapy was palmar–plantar erythrodysesthesia, commonly known as hand–foot skin reaction (HFSR) when associated with sorafenib and other multikinase inhibitors and hand–foot syndrome (HFS) when associated with chemotherapy agents.
Although other toxicities were observed in the TIES studies, including other mucocutaneous events, the incidence and severity of HFSR with these combination regimens warrants consideration [16–20]. The incidence of HFSR in the TIES studies was notably higher than those reported in studies of single-agent sorafenib in patients with MBC [14, 15] and other tumor types [21–23]. Although HFSR is not life threatening (per the National Cancer Institute's Common Terminology Criteria for Adverse Events, version 3.0 or 4.0, the most severe HFSR is grade 3 [24, 25]) and is reversible, sorafenib-induced HFSR can significantly impact quality of life (QoL) and may necessitate dose modifications or treatment discontinuation [26–28]. Furthermore, sorafenib treatment is continuous, so even grade 1 or 2 toxicities can impact QoL if not properly managed over the long term. Clinicians should closely monitor patients receiving sorafenib for HFSR and be aware of prevention and management strategies that may reduce its incidence, duration, and severity.
This review provides an overview of sorafenib-based combinations in MBC patients with reference to its safety profile, focusing on HFSR and its management strategies.
Sorafenib in Solid Tumors
In general, sorafenib has a favorable safety profile. Side effects are manageable and rarely life threatening [21–23]. Three pivotal phase III, placebo-controlled monotherapy trials were conducted in patients with advanced renal cell carcinoma (the Treatment Approaches in Renal Cancer Global Evaluation Trial [TARGET] [22]) and with hepatocellular carcinoma (the Sorafenib Hepatocellular Carcinoma Assessment Randomized Protocol [SHARP] trial [23] and the Asia-Pacific trial [21]). Sorafenib provided a PFS benefit in the TARGET trial over placebo (median PFS interval, 5.5 months versus 2.8 months; hazard ratio [HR], 0.44; 95% confidence interval [CI], 0.35–0.55; p < .01) and a survival benefit in both the SHARP trial (median survival time, 10.7 months versus 7.9 months; HR, 0.69; 95% CI, 0.55–0.87; p < .001) and the Asia-Pacific trial (median survival time, 6.5 months versus 4.2 months; HR, 0.68; 95% CI, 0.50–0.93; p = .14) [21]. During these studies, sorafenib was shown to have an acceptable safety profile, with most events of grade 1 or 2 severity being gastrointestinal, constitutional, or dermatologic in nature. Grade 3 or 4 events associated with sorafenib included hypertension, diarrhea, weight loss, hypophosphatemia, thrombocytopenia, and HFSR [21–23].
Generally, HFSR was manageable, with some patients requiring dose modifications. The incidence of HFSR (any grade) in the sorafenib arms of these studies was in the range of 21%–45%, compared with 3%–7% in the placebo arms [21–23]. Grade 3 HFSR was infrequent in the sorafenib arms (6%–10.7%) but the rate was greater than in the placebo arms (0% to <1%). In the SHARP study, HFSR-related dose reductions occurred in 5% of patients in the sorafenib arm [23], and 1.3% discontinued sorafenib as a result of HFSR (data on file). HFSR-related dose reductions occurred in 11.4% of patients receiving sorafenib in the Asia-Pacific study [21].
A meta-analysis of sorafenib monotherapy for solid tumors reported HFSR rates in the range of 9%–61.9%, with a summary incidence of 33.8% (95% CI, 24.5%–44.7%) for HFSR of any grade and 8.9% (95% CI, 7.3%–10.7%) for grade 3 [29]. Sorafenib use in renal cancer patients was associated with a greater risk for HFSR of any grade than in other solid tumors (relative risk [RR], 1.52; 95% CI, 1.32–1.75), but this difference was not observed for grade 3 HFSR (RR, 0.98; 95%, CI 0.76–1.26). Overall, the risk for HFSR was 6.6 times greater with sorafenib than with placebo or interferon controls (p < .001).
HFSR has also been described in studies assessing sorafenib in combination regimens. A placebo-controlled phase III trial in advanced melanoma patients showed a 7% rate of grade 3 HFSR for patients treated with sorafenib in combination with second-line carboplatin plus paclitaxel (a rate comparable with that of sorafenib monotherapy) and a 0% rate for those given placebo plus carboplatin and paclitaxel [30]. On the other hand, a retrospective analysis of three trials in patients with solid tumors showed a higher HFSR rate for patients treated with the combination of sorafenib plus bevacizumab than for those treated with sorafenib alone [31]. Although bevacizumab is not associated with HFSR/HFS, the rates of any grade HFSR were 79% for sorafenib plus bevacizumab and 52% for sorafenib monotherapy, and the rates for grade 2 or 3 were 57% and 30%, respectively.
Clinical Features and Pathophysiology of HFSR
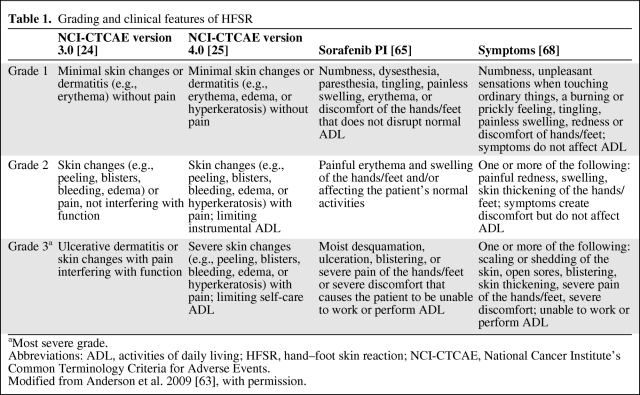
As the development of sorafenib in combination with cytotoxic therapies continues, it will be important for clinicians to understand the clinical features of HFSR and distinguish between sorafenib-associated HFSR and chemotherapy-associated HFS (Table 1 and Fig. 1) in order to modify the dose of the specific causative agent(s) and maintain dose intensity.
Table 1.
Grading and clinical features of HFSR
aMost severe grade.
Abbreviations: ADL, activities of daily living; HFSR, hand–foot skin reaction; NCI-CTCAE, National Cancer Institute's Common Terminology Criteria for Adverse Events.
Modified from Anderson et al. 2009 [63], with permission.
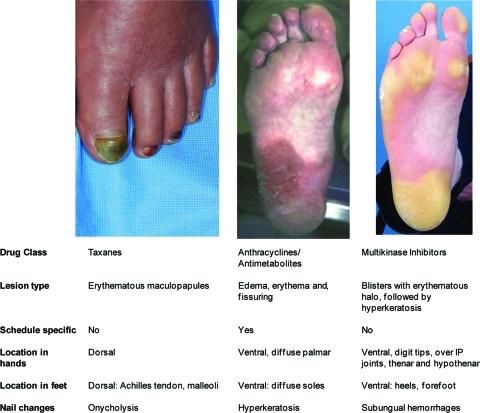
Figure 1.
Differentiation of HFSR and HFS [26, 27, 33, 53, 58, 59, 69].
Abbreviations: HFS, hand–foot syndrome; HFSR, hand–foot skin reaction; IP, interphalangeal.
Modified with permission from (i) Childress J, Lokich J. Cutaneous hand and foot toxicity associated with cancer chemotherapy. Am J Clin Oncol 2003;26:435–436, with permission. (ii) Photographs reprinted from Autier J, Escudier B, Wechsler J et al. Prospective study of the cutaneous adverse effects of sorafenib, a novel multikinase inhibitor. Arch Dermatol 2008;144:886–892 (multikinase inhibitors). Copyright © 2008 American Medical Association. All rights reserved. (iii) Lassere Y, Hoff P. Management of hand-foot syndrome in patients treated with capecitabine (Xeloda). Eur J Oncol Nurs 2004;8(suppl 1):S31–S40 (anthracyclines/antimetabolites), with permission, and photograph courtesy of Mario Lacouture, M.D. (taxane).
A dermatologic substudy of the TARGET trial (n = 85) described the incidence and severity of HFSR in greater detail [26]. HFSR was the most common cutaneous toxicity in the sorafenib arm (60% for any grade and 5% for grade 3) with no HFSR events in the placebo arm. Symptoms of HFSR included paresthesia, tingling, burning or painful sensations on the palms and/or soles, and a decreased tolerance for touching hot objects. These symptoms usually occurred before cutaneous lesions emerged and negatively affected walking in 35% of patients. Symmetric acral erythematous and edematous lesions were associated with desquamation and fissures, usually developing on the palms and soles but could involve the lateral sides of the fingers and toes. Hyperkeratosis was reported in 54% of patients with HFSR, usually as yellowish, painful plaques on pressure areas of the sole, sometimes encompassed by an erythematous/edematous halo [26]. Hyperkeratosis usually develops after blister formation and is a defining feature of HFSR compared with HFS (Fig. 1).
The incidence and severity of HFSR appear to be dose dependent. A pooled safety analysis of sorafenib in patients with advanced solid tumors showed that HFSR was infrequent for doses <300 mg twice daily (BID), but the incidence and severity increased for doses of 300–600 mg BID, with dose-limiting toxicity at 600 mg BID [32]. The rate of grade 3 HFSR was 3% in the cohort receiving the standard 400-mg BID dose (n = 37), whereas the rate was 31% in the 600-mg BID dose cohort (n = 39). The rate in the 800-mg BID cohort was 8%, but the sample size was small (n = 13). The continuous regimen might also increase drug concentrations, prolong exposure, and lead to accumulation in the skin [26]. Biopsies in patients with HFSR showed keratinocyte necrosis that correlated with time of drug initiation, as well as vessel ectasia and eccrine gland cystic degeneration [27].
The exact mechanism by which HFSR develops during sorafenib use is unknown [33]. The key may lie in the multiplicity of the drug's targeting mechanism because drugs inhibiting single pathways (e.g., vascular endothelial growth factor [VEGF] with bevacizumab) are not associated with HFSR [33]. In preclinical studies, sorafenib decreased both tumor cell proliferation and angiogenesis by inhibiting multiple signaling pathways. Proliferation was inhibited in some cell lines by sorafenib blockade of RAF-1 of the RAF/mitogen-activated protein kinase kinase (MEK)/extracellular signal-regulated kinase (ERK), or RAF/MEK/ERK, signaling pathway, as well as inhibition of the tyrosine kinases Flt-3 and c-KIT [34]. Upstream, sorafenib interfered with signaling via VEGF receptors (VEGFRs), platelet-derived growth factor receptor (PDGFR)-β and serine/threonine kinases in the RAF/MEK/ERK pathway, thus decreasing abnormal blood vessel proliferation.
It has been theorized that HFSR may occur because keratinocytes in the epidermis synthesize PDGF-α and PDGF-β, which activate PDGFRs located on dermal fibroblasts, capillaries, and eccrine glands [33]. Dermal eccrine glands also express c-KIT and PDGFR, targets of sorafenib. The higher incidence of HFSR when sorafenib is combined with bevacizumab [31] suggests that blockade of both the VEGF and PDGF pathways may hinder vascular repair, thereby triggering HFSR in areas that undergo repeated high-pressure insult, such as palms and soles.
Sorafenib in MBC
Phase II investigations in MBC patients demonstrated moderate activity with sorafenib as monotherapy [14, 15]. In a study of 54 patients with MBC who had received prior MBC therapy (78% with three or more regimens), treatment with sorafenib (400 mg BID) resulted in a partial response in one patient (2%) and 20 patients achieved stable disease (37%) [14]. The most common grade 3 treatment-related adverse events (AEs) were rash/desquamation (6%), fatigue (4%), and HFSR (4%). These response data prompted further investigation of sorafenib in MBC patients, but as part of combination regimens.
Initial studies in patients with solid tumors demonstrated the feasibility and safety of combining sorafenib with cytotoxic agents [35]. Promising anticancer activity was observed in some tumor types, including MBC. In general, the combination treatments were tolerable, although dose-limiting toxicities, including HFSR, were reported. This was not unexpected, because a number of chemotherapies are associated with HFS [36–41].
A phase I/II study in MBC patients evaluated sorafenib in combination with anastrozole (1 mg daily) to overcome aromatase inhibitor (AI) resistance [42]. The study included 35 postmenopausal patients with hormone-positive MBC and disease recurrence or progression during AI treatment. The 400-mg BID sorafenib dose was selected as the phase II dose. One patient achieved a partial response and seven had stable disease for ≥24 weeks, resulting in a clinical benefit rate of 23%. The most common grade 3 or 4 AEs were HFSR (34%), fatigue (17%), rash (11%), emesis (11%), and hypertension (11%). Other grade 3 or 4 mucocutaneous events were infrequent (e.g., 3% for mucositis). The rate of dose reduction was 77% and the rate of discontinuation because of toxicity was 31%. The investigators concluded that future trials were warranted but with a lower sorafenib dose.
TIES
The TIES program was initiated to evaluate a potential role for sorafenib in combination with common chemotherapies for patients with HER-2− MBC and determine whether phase III trials were warranted. These four phase IIb, double-blinded, randomized, placebo-controlled trials aimed to assess sorafenib in combination with capecitabine, paclitaxel, gemcitabine or capecitabine, and docetaxel and/or letrozole. The primary endpoint in each study was the PFS interval and the estimated sample size was 220 patients with locally advanced or metastatic disease. Three of the TIES studies have reported efficacy and safety data for the addition of sorafenib to capecitabine (the SOLTI-0701 study), paclitaxel (the NU07B1 study), and gemcitabine or paclitaxel (the AC01B07 study).
Sorafenib Plus Capecitabine
The SOLTI-0701 trial enrolled patients who had received no more than one prior metastatic treatment. Capecitabine, a prodrug of 5-fluorouracil, is indicated for MBC treatment either alone or with docetaxel [38–40]. Side effects associated with capecitabine include gastrointestinal events, fatigue, and HFS. Rates of HFS in breast cancer patients receiving capecitabine monotherapy have been in the range of 43%–63% for any grade and 11%–24% for grade 3 [39–41, 43–46]. In a phase II study of patients with MBC (n = 126) receiving capecitabine (1,250 mg/m2 BID), the incidence of grade 3 HFS was 21%, with 17% of patients requiring dose reductions as a result of HFS [41].
In the SOLTI-0701 trial, patients were randomized to receive capecitabine (1,000 mg/m2 BID, days 1–14 of a 21-day cycle) in combination with placebo or sorafenib (400 mg BID). The protocol outlined dosing algorithms to help manage persistent or high-grade AEs, including HFSR/HFS (Table 2). In total, 229 patients were randomized to treatment. The addition of sorafenib to capecitabine provided a significant PFS benefit over capecitabine monotherapy (median PFS time, 6.4 months versus 4.1 months; HR, 0.58; 95% CI, 0.41–0.81; one-sided p = .0006), supporting a phase III study of similar design [16, 17]. The overall response rates (ORRs) were 38.3% and 30.7% (one-sided p = .12), respectively, with median durations of response of 6.2 months and 4.1 months (one-sided p = .048), respectively. The OS duration did not differ statistically between treatment arms (median, 22.2 months versus 20.9 months, respectively; HR, 0.86; 95% CI, 0.61–1.23; one-sided p = .21). There was a nonsignificant trend in the OS time favoring sorafenib plus capecitabine over placebo plus capecitabine in the first-line subgroup (median, 22.8 months versus 18.6 months, respectively; HR, 0.67; 95% CI, 0.40–1.11; one-sided p = .06) that was not observed in the second-line subgroup (median, 19.0 months versus 23.4 months, respectively; HR, 1.08; 95% CI, 0.65–1.78; one-sided p = .40).
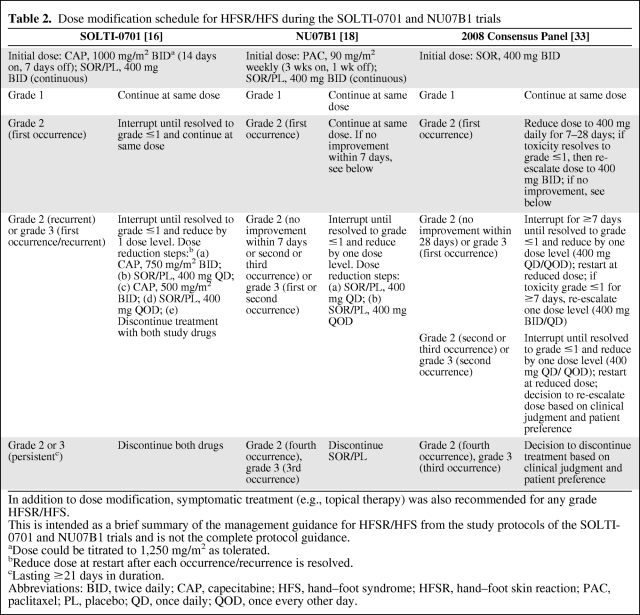
Table 2.
Dose modification schedule for HFSR/HFS during the SOLTI-0701 and NU07B1 trials
In addition to dose modification, symptomatic treatment (e.g., topical therapy) was also recommended for any grade HFSR/HFS.
This is intended as a brief summary of the management guidance for HFSR/HFS from the study protocols of the SOLTI-0701 and NU07B1 trials and is not the complete protocol guidance.
aDose could be titrated to 1,250 mg/m2 as tolerated.
bReduce dose at restart after each occurrence/recurrence is resolved.
cLasting ≥21 days in duration.
Abbreviations: BID, twice daily; CAP, capecitabine; HFS, hand–foot syndrome; HFSR, hand–foot skin reaction; PAC, paclitaxel; PL, placebo; QD, once daily; QOD, once every other day.
Overall, sorafenib plus capecitabine was tolerable with a manageable side-effect profile and no unexpected AEs [16, 17]. In the combination arm, the average duration of treatment was 33.8 weeks for sorafenib and 10.7 cycles for capecitabine. On average, the duration of treatment was shorter for placebo (22.5 weeks) plus capecitabine (7.4 cycles). Most AEs were grade ≤2. For AEs of any grade, rates were higher for sorafenib plus capecitabine than for placebo plus capecitabine: diarrhea (58% versus 30%), mucosal inflammation (33% versus 21%), rash (22% versus 8%), neutropenia (13% versus 4%), and HFSR/HFS (90% versus 66%). Generally, grade 3 or 4 AE rates were comparable between treatment arms, except for grade 3 HFSR/HFS (44% versus 14%). The incidences of grade 3 or 4 rash were 4% and 0%, respectively.
Dose modifications were common, but few patients discontinued treatment because of HFSR/HFS. In the combination arm, 53% of patients had their sorafenib dose reduced and 78% had their capecitabine dose reduced. In the control arm, the corresponding values for dose reductions were 14% for placebo and 33% for capecitabine. The rates of treatment discontinuation resulting from AEs were 20% in the combination arm and 9% in the control arm, with rates of 8% and 4%, respectively, specific to HFSR/HFS [16, 17].
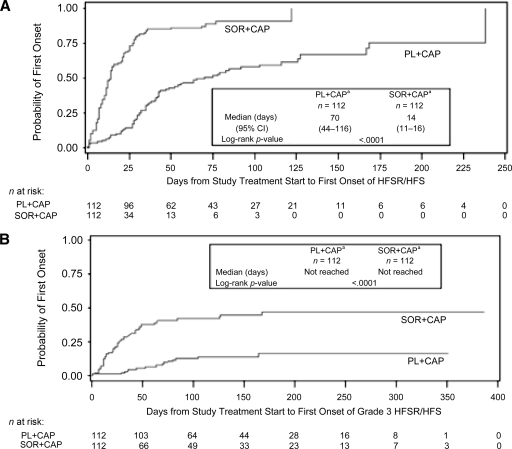
The original SOLTI-0701 protocol required patient follow-up for compliance and safety every 3 weeks for the first 24 weeks of treatment. However, shortly after the start of the trial, HFSR/HFS was found to emerge within the first few months of treatment. Therefore, the protocol was amended to weekly follow-up visits for the first 6 weeks to closely monitor patients during the time of greatest risk. In a secondary analysis of the SOLTI-0701 study [47], a Kaplan–Meier analysis of HFSR/HFS showed a shorter median time to onset with sorafenib plus capecitabine than with placebo plus capecitabine for any grade (median, 14 days versus 70 days; p < .0001) and for grade 3 (median not reached for either arm; p < .0001) (Fig. 2), with the highest incidence in the combination arm during cycle 1 for any grade (63%) and in cycle 2 for grade 3 (22%). The incidence of HFSR/HFS declined over the course of treatment, corresponding to an incremental reduction in the mean dose of study drugs, although a direct correlation could not be established.
Figure 2.
Time to first onset of HFSR/HFS by the Kaplan–Meier method during the SOLTI-0701 trial [47]. (A): Any grade event. (B): Grade 3 events.
aSafety population (patients who received any study drug[s]).
Abbreviations: CAP, capecitabine; HFS, hand–foot syndrome; HFSR, hand–foot skin reaction; PL, placebo; SOR, sorafenib.
Sorafenib Plus Paclitaxel
The NU07B1 study evaluated first-line therapy for advanced disease by comparing the combination of sorafenib plus paclitaxel with placebo plus paclitaxel in 237 patients [18]. Studies have demonstrated the clinical activity of paclitaxel in MBC patients and better survival outcomes than with other standard treatments [48–51]. Side effects associated with paclitaxel include neutropenia, leukopenia, alopecia, anemia, peripheral neuropathy, myalgia/arthralgia, nausea, and vomiting. Taxanes are not associated with HFS, although a syndrome characterized by periarticular thenar erythema with onycholysis (PATEO syndrome) with pain has been observed in up to 50% of patients receiving a taxane [52]. Because of the appearance of PATEO on the hands and feet, and its association with pain, it is frequently misdiagnosed as HFS [53]. Taxane-induced nail changes may be prevented with cold glove and sock therapy. In a study of 45 patients with solid tumors treated with docetaxel that compared the use of a frozen glove on one hand with no protection on the other, grade 1 or 2 onycholysis occurred in 11% and 51% of patients, respectively, and grade 1 or 2 skin toxicity occurred in 27% versus 59% of cases, respectively [52]. Similar benefits were observed with a frozen sock [54].
During the NU07B1 trial, paclitaxel was administered at a dose of 90 mg/m2 per week (3 weeks on/1 week off) in combination with placebo or sorafenib (400 mg BID) [18, 19], with dosing algorithms to limit toxicity, including dermatologic events (Table 2). Assessment of the PFS intervals indicated a nonsignificant trend favoring sorafenib plus paclitaxel over placebo plus paclitaxel (median PFS time, 6.9 months versus 5.6 months; HR, 0.79; 95% CI, 0.56–1.11; one-sided p = .09), but this did not reach the predefined critical effect size. For secondary endpoints, sorafenib plus paclitaxel resulted in a greater median time to progression (8.1 months versus 5.6 months, respectively; one-sided p = .017), longer duration of response (5.6 months versus 3.7 months, respectively; one-sided p = .008), and higher ORR (67% versus 54%, respectively; one-sided p = .023). There was no significant difference between treatment arms in the OS times (median, 16.8 months versus 17.4 months, respectively; HR, 1.02; 95% CI, 0.72–1.46; one-sided p = .45) [18, 19].
Some AEs were more frequent in the combination arm than in the placebo arm [19]. The incidences of HFSR of any grade were 55% and 7%, respectively, and the incidences of grade 3 HFSR were 31% and 3%, respectively. Other AEs (any grade) that occurred more frequently in patients treated with sorafenib plus paclitaxel than for those given placebo plus paclitaxel included rash (19% versus 11%), stomatitis (17% versus 3%), diarrhea (37% versus 25%), and vomiting (29% versus 16%). The incidences of grade 3 or 4 AEs were generally comparable for sorafenib plus paclitaxel and placebo plus paclitaxel, with moderately higher rates for asthenia (7% versus 4%), neutropenia (13% versus 7%), anemia (11% versus 6%), and stomatitis (4% versus 0%). The incidences of grade 3 or 4 rash were 2% and 0%, respectively.
Patients receiving sorafenib plus paclitaxel required more dose modifications to manage toxicity than those receiving placebo plus paclitaxel. In the combination arm, sorafenib was interrupted for 55% of patients and reduced for 50%, with corresponding rates of 54% and 33% for paclitaxel. In the control group, the placebo dose was interrupted for 16% of patients and reduced for 9%, with corresponding values of 47% and 22% for paclitaxel [19]. Overall, 22% of patients in the sorafenib–paclitaxel arm and 6% of patients in the placebo–paclitaxel arm discontinued treatment as a result of AEs.
The incidence of grade 3 HFSR (31%) in the sorafenib arm during the NU07B1 trial appears higher than expected, given that paclitaxel is not associated with HFSR/HFS and that the rate of grade 3 HFSR for sorafenib, carboplatin, and paclitaxel in a melanoma study was 7% [30]. The higher incidence may be a result of greater awareness of sorafenib-induced HFSR during the NU07B1 trial than in previous studies or differences in the study populations. In a NU07B1 subgroup analysis, the rates of grade 3 HFSR in the sorafenib–paclitaxel arm were 34% for patients enrolled in centers in India and 21% for patients enrolled in centers outside India (U.S. or Brazil) [55]. Other studies have suggested differences in the incidence of HFSR by ethnicity, with Asian patients more frequently affected [56].
Sorafenib Plus Gemcitabine or Capecitabine
The AC01B07 trial enrolled 180 patients who had experienced disease progression during or after treatment with bevacizumab and randomized them to treatment with gemcitabine (1,000 mg/m2 i.v., days 1 and 8 of a 21-day cycle) in combination with sorafenib (400 mg BID) or placebo [20]. After the start of the trial, the protocol was amended and capecitabine (1,000 mg/m2 BID, days 1–14 of a 21-day cycle) was allowed as an alternative to gemcitabine at the treating physician's discretion. Most patients (83%) received gemcitabine. Gemcitabine is approved for use in MBC patients in combination with paclitaxel but is also used as monotherapy within sequential regimens [57]. The most common side effects associated with gemcitabine include nausea/vomiting, myelosuppression, renal and hepatic toxicities, fever, rash, and dyspnea.
Primary analysis of the AC01B07 trial demonstrated that the addition of sorafenib to chemotherapy provided a statistically significant longer PFS interval than with placebo (median, 3.4 months versus 2.7 months; HR, 0.65; 95% CI, 0.45–0.95; one-sided p = .01) and longer time to progression (median, 3.6 months versus 2.7 months; HR, 0.64; 95% CI, 0.44–0.93; one-sided p = .009). There was no statistical difference between treatment arms for the ORR (19.8% versus 12.7%, respectively; one-sided p = .12). The addition of sorafenib was associated with higher rates of some grade 3 or 4 AEs, including HFSR/HFS (39% versus 5%), stomatitis (10% versus 0%), thrombocytopenia (10% versus 1%), fatigue (18% versus 9%), anemia (5% versus 0%), and rash (4% versus 0%). Updated safety data, including more detailed analyses of HFSR/HFS, and an analysis of OS outcomes are expected after the data mature.
Management Strategies for HFSR
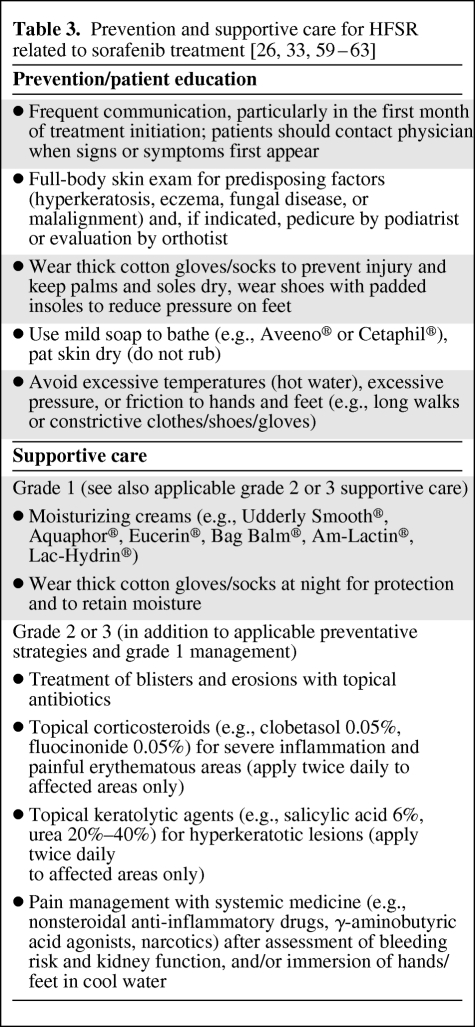
The goals of prevention and management strategies for sorafenib-induced HFSR are to reduce the incidence, duration, and severity, minimize the impact on QoL, and maximize treatment potency and duration. Table 3 outlines prevention and management strategies that are also applicable to HFS [58].
Table 3.
Although preventive measures have not been assessed formally, a number of practical approaches are recommended [33, 59–63]. Patients should be aware of the signs and symptoms of HFSR and of prevention steps, and be advised to stay in close contact with their physicians, particularly in the first 6 weeks of sorafenib treatment when HFSR usually emerges. Before sorafenib treatment begins, a qualified health care professional should conduct a baseline skin check for predisposing factors [33, 61, 63]. Hyperkeratotic areas or calluses should be removed via pedicure or manicure [33, 61, 63]. Feet and hands should be examined for areas under excessive friction or undue stress, and cushioning these areas is recommended [33, 63]. Although anecdotal evidence suggests that prophylactic pyridoxine (vitamin B6) is beneficial for preventing HFSR [63], studies found pyridoxine to be ineffective for managing capecitabine-induced HFS [64].
If HFSR does occur, grade 1 can be managed with symptomatic interventions. Management strategies should be escalated with increasing grades of severity (Fig. 3). If symptoms reach grade ≥2, the mainstay of management is dose modification, but supportive treatment should continue with other options considered. Severe inflammation and painful erythematous areas can be treated with topical corticosteroids [33]. Pain is the most significant HFSR symptom affecting QoL [28], therefore analgesia is of paramount importance. Once acute erythema improves, tender hyperkeratotic lesions may develop, which can be treated with topical keratolytics [33].
Figure 3.
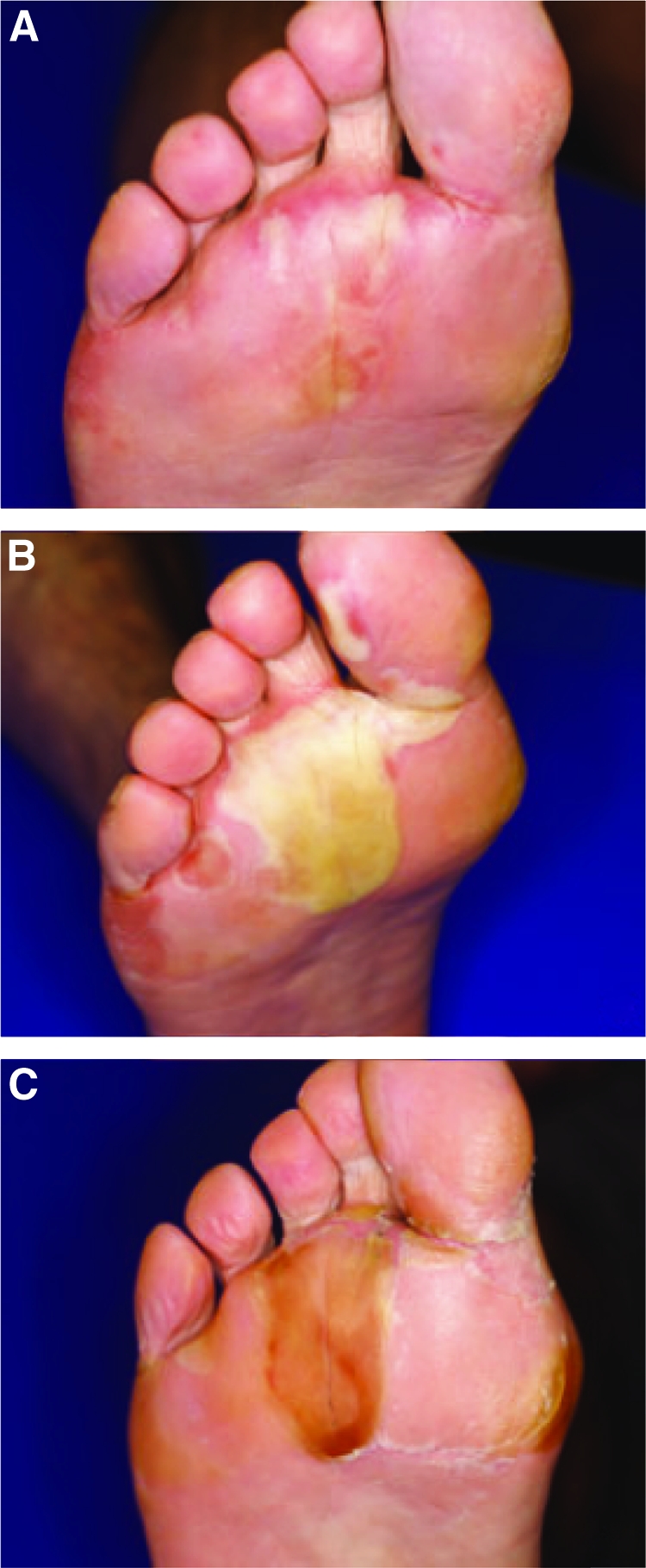
Time course of hand–foot skin reaction (HFSR) associated with sorafenib. (A): Early HFSR after 1 week of 400 mg sorafenib twice daily. (B): Progression of HFSR despite reduction to 400 mg daily and topical mometasone. (C): Sorafenib dosing interrupted, improvement in HFSR within 4 days. After 1 week of interruption, sorafenib was restarted at 400 mg daily without further complications. Reprinted from Degen A, Alter M, Schenck F et al. The hand-foot-syndrome associated with medical tumor therapy—classification and management. J Dtsch Dermatol Ges 2010;8:652–661, with permission.
The U.S. prescribing information for sorafenib recommends dose modifications to manage treatment-related skin toxicity based on severity and occurrence (initial or subsequent) [65]. Elsewhere in the literature, experts have detailed similar dosing algorithms but with variations for dose interruption and reduction, as well as dose re-escalation as tolerated (Table 2) [33].
Although supportive care data are limited, formal studies are under way to improve the management of sorafenib-induced HFSR. A double-blinded, randomized, phase II study is assessing four different treatments for HFSR (urea 40% cream, fluocinonide 0.05% cream, tazarotene 0.1% cream, and bland emollient cream) in patients receiving sorafenib either alone or in combination with agents not associated with HFSR/HFS (ClinicalTrials.gov identifier, NCT00667589).
As demonstrated in the TIES studies, the management of HFSR in the MBC setting needs to be aggressive at all stages, particularly for sorafenib in combination with agents associated with HFS, such as capecitabine. Management strategies for capecitabine-induced HFS that have shown benefit in the uncontrolled setting include celecoxib [66] and high-potency topical steroids [58]. On the other hand, pyridoxine [64] and urea/lactic acid containing preparations [67] have not shown benefit.
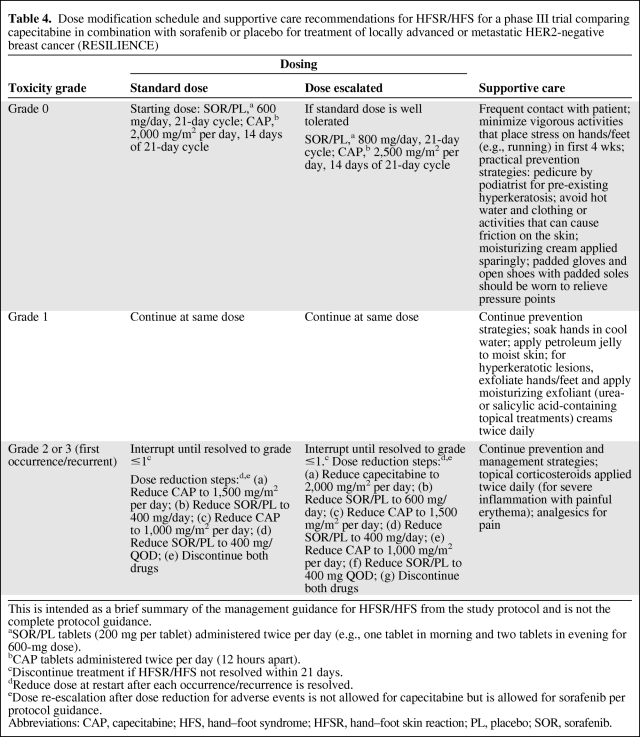
In view of the SOLTI-0701 experience, a phase III trial comparing capecitabine in combination with sorafenib or placebo for treatment of locally advanced or metastatic HER2-negative breast cancer (RESILIENCE) has been initiated (ClinicalTrials.gov identifier, NCT01234337), with a similar design but with a number of important modifications. Patients will start treatment with sorafenib at a lower dose of 600 mg per day in combination with capecitabine at 2,000 mg/m2 per day. The dosing algorithm of that study will allow dose escalation after the first cycle if fatigue and dermatologic and gastrointestinal toxicities are grade ≤1. Furthermore, the study protocol provides detailed measures for HFSR/HFS prevention and supportive care (Table 4).
Table 4.
Dose modification schedule and supportive care recommendations for HFSR/HFS for a phase III trial comparing capecitabine in combination with sorafenib or placebo for treatment of locally advanced or metastatic HER2-negative breast cancer (RESILIENCE)
This is intended as a brief summary of the management guidance for HFSR/HFS from the study protocol and is not the complete protocol guidance.
aSOR/PL tablets (200 mg per tablet) administered twice per day (e.g., one tablet in morning and two tablets in evening for 600-mg dose).
bCAP tablets administered twice per day (12 hours apart).
cDiscontinue treatment if HFSR/HFS not resolved within 21 days.
dReduce dose at restart after each occurrence/recurrence is resolved.
eDose re-escalation after dose reduction for adverse events is not allowed for capecitabine but is allowed for sorafenib per protocol guidance.
Abbreviations: CAP, capecitabine; HFS, hand–foot syndrome; HFSR, hand–foot skin reaction; PL, placebo; SOR, sorafenib.
Conclusions
Results from the TIES studies demonstrated clinical activity for sorafenib in HER-2− advanced breast cancer patients when combined with selected chemotherapies, compared with a placebo control. Sorafenib plus capecitabine provided a significant PFS benefit, supporting further study of this combination in the phase III setting (the SOLTI-0701 trial). A statistically significant longer PFS interval was also demonstrated when sorafenib was combined with gemcitabine or capecitabine in patients who had progressed during or after bevacizumab treatment (the AC01B07 trial), and sorafenib with first-line paclitaxel showed a trend of a longer PFS interval (the NU07B1 trial). The rate of grade 3 HFSR/HFS was high in the combination therapy arms of these studies. In the SOLTI-0701 trial, HFSR/HFS was both manageable and tolerable, but the high incidence necessitated modifications to the dosing schema and management strategies of the phase III RESILIENCE study.
As the potential role for sorafenib advances into novel therapeutic areas and as part of combination regimens, the management of HFSR will need to improve. Prevention strategies, proactive management, and, when necessary, dose modification should help patients maintain QoL as well as the clinical benefits of sorafenib treatment.
Acknowledgments
We acknowledge critical review by Sarah Guadagno (Onyx Pharmaceuticals).
Footnotes
- (C/A)
- consulting/advisory relationship
- (RF)
- Research funding
- (E)
- Employment
- (H)
- Honoraria received
- (OI)
- Ownership interests
- (IP)
- Intellectual property rights/inventor/patent holder
Author Contributions
Conception/Design: Patricia Gomez, Mario E. Lacouture
Data analysis and interpretation: Mario E. Lacouture
Manuscript writing: Patricia Gomez, Mario E. Lacouture
Final approval of manuscript: Patricia Gomez, Mario E. Lacouture
The authors take full responsibility for the content of the paper but thank Michael Raffin (Fishawack Communications), supported by Bayer HealthCare Pharmaceuticals and Onyx Pharmaceuticals, for his writing and editorial assistance.
References
- 1.Chia SK, Speers CH, D'Yachkova Y, et al. The impact of new chemotherapeutic and hormone agents on survival in a population-based cohort of women with metastatic breast cancer. Cancer. 2007;110:973–979. doi: 10.1002/cncr.22867. [DOI] [PubMed] [Google Scholar]
- 2.Mauri D, Polyzos NP, Salanti G, et al. Multiple-treatments meta-analysis of chemotherapy and targeted therapies in advanced breast cancer. J Natl Cancer Inst. 2008;100:1780–1791. doi: 10.1093/jnci/djn414. [DOI] [PubMed] [Google Scholar]
- 3.Joensuu H, Holli K, Heikkinen M, et al. Combination chemotherapy versus single-agent therapy as first- and second-line treatment in metastatic breast cancer: A prospective randomized trial. J Clin Oncol. 1998;16:3720–3730. doi: 10.1200/JCO.1998.16.12.3720. [DOI] [PubMed] [Google Scholar]
- 4.Sledge GW, Neuberg D, Bernardo P, et al. Phase III trial of doxorubicin, paclitaxel, and the combination of doxorubicin and paclitaxel as front-line chemotherapy for metastatic breast cancer: An intergroup trial (E1193) J Clin Oncol. 2003;21:588–592. doi: 10.1200/JCO.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 5.Cardoso F, Bedard PL, Winer EP, et al. International guidelines for management of metastatic breast cancer: Combination vs sequential single-agent chemotherapy. J Natl Cancer Inst. 2009;101:1174–1181. doi: 10.1093/jnci/djp235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carrick S, Parker S, Thornton CE, et al. Single agent versus combination chemotherapy for metastatic breast cancer. Cochrane Database Syst Rev. 2009;(2):CD003372. doi: 10.1002/14651858.CD003372.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tomova A, Bartsch R, Brodowicz T, et al. Concomitant docetaxel plus gemcitabine versus sequential docetaxel followed by gemcitabine in anthracycline-pretreated metastatic or locally recurrent inoperable breast cancer patients: A prospective multicentre trial of the Central European Cooperative Oncology Group (CECOG) Breast Cancer Res Treat. 2010;119:169–176. doi: 10.1007/s10549-009-0553-4. [DOI] [PubMed] [Google Scholar]
- 8.Beslija S, Bonneterre J, Burstein HJ, et al. Third consensus on medical treatment of metastatic breast cancer. Ann Oncol. 2009;20:1771–1785. doi: 10.1093/annonc/mdp261. [DOI] [PubMed] [Google Scholar]
- 9.Muss HB. Targeted therapy for metastatic breast cancer. N Engl J Med. 2006;355:2783–2785. doi: 10.1056/NEJMe068260. [DOI] [PubMed] [Google Scholar]
- 10.Miles DW, Chan A, Dirix LY, et al. Phase III study of bevacizumab plus docetaxel compared with placebo plus docetaxel for the first-line treatment of human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol. 2010;28:3239–3247. doi: 10.1200/JCO.2008.21.6457. [DOI] [PubMed] [Google Scholar]
- 11.Miller K, Wang M, Gralow J, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666–2676. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 12.Brufsky A, Bondarenko I, Smirnov V, et al. RIBBON-2: A randomized, double-blind, placebo-controlled, phase III trial evaluating the efficacy and safety of bevacizumab in combination with chemotherapy for second-line treatment of HER2-negative metastatic breast cancer [abstract nr 42] Cancer Res. 2009;69(24 suppl) doi: 10.1200/JCO.2010.34.1255. [DOI] [PubMed] [Google Scholar]
- 13.Robert NJ, Diéras V, Glaspy J, et al. RIBBON-1: Randomized, double-blind, placebo-controlled, phase III trial of chemotherapy with or without bevacizumab for first-line treatment of human epidermal growth factor receptor 2-negative, locally recurrent or metastatic breast cancer. J Clin Oncol. 2011;29:1252–1260. doi: 10.1200/JCO.2010.28.0982. [DOI] [PubMed] [Google Scholar]
- 14.Bianchi G, Loibl S, Zamagni C, et al. Phase II multicenter, uncontrolled trial of sorafenib in patients with metastatic breast cancer. Anticancer Drugs. 2009;20:616–624. [PubMed] [Google Scholar]
- 15.Moreno-Aspitia A, Morton RF, Hillman DW, et al. Phase II trial of sorafenib in patients with metastatic breast cancer previously exposed to anthracyclines or taxanes: North Central Cancer Treatment Group and Mayo Clinic Trial N0336. J Clin Oncol. 2009;27:11–15. doi: 10.1200/JCO.2007.15.5242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baselga J, Segalla JGM, Roché H, et al. SOLTI-0701: A double-blind, randomized phase 2b study evaluating the efficacy and safety of sorafenib (SOR) compared to placebo (PL) when administered in combination with capecitabine (CAP) in patients (pts) with locally advanced (adv) or metastatic (met) breast cancer (BC) [abstract 3LBA] Eur J Cancer Suppl. 2009;7(3 suppl):3–4. [Google Scholar]
- 17.Gomez P, Roché H, Costa F, et al. Overall survival data from SOLTI-0701: A multinational, double-blind, placebo-controlled, randomized phase 2b study evaluating the oral combination of sorafenib and capecitabine in patients with locally advanced or metastatic HER2-negative breast cancer [abstract P2–16-01]. Presented at the 33rd Annual San Antonio Breast Cancer Symposium; San Antonio, TX; December 8–12, 2010. [Google Scholar]
- 18.Gradishar W, Kaklamani V, Prasad Sahoo T, et al. A double-blind, randomized, placebo-controlled, phase 2b study evaluating the efficacy and safety of sorafenib (SOR) in combination with paclitaxel (PAC) as a first-line therapy in patients (pts) with locally recurrent or metastatic breast cancer (BC) [abstract 44] Cancer Res. 2009;69(24 suppl) [Google Scholar]
- 19.Bondarde S, Kaklamani V, Prasad Sahoo T, et al. Sorafenib in combination with paclitaxel as a first-line therapy in patients with locally recurrent or metastatic breast cancer: Overall survival results from a double-blind, randomized, placebo-controlled, phase 2b trial [abstract P2–16-03]. Presented at the 33rd Annual San Antonio Breast Cancer Symposium; December 8–12, 2010; San Antonio, TX. [Google Scholar]
- 20.Hudis C, Tauer KW, Hermann RC, et al. Sorafenib (SOR) plus chemotherapy (CRx) for patients (pts) with advanced (adv) breast cancer (BC) previously treated with bevacizumab (BEV) [abstract 1009]. Presented at the 2011 American Society of Clinical Oncology Annual Meeting; June 3–7, 2011; Chicago, IL. [Google Scholar]
- 21.Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: A phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 22.Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 23.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 24.National Cancer Institute. Bethesda, MD: National Cancer Institute; 2006. [accessed September 19, 2011]. Cancer Therapy Evaluation Program, Common Terminology Criteria for Adverse Events, Version 3.0. Available at http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf. [Google Scholar]
- 25.National Cancer Institute. Bethesda, MD: National Cancer Institute; 2009. [accessed September 19, 2011]. Cancer Therapy Evaluation Program, Common Terminology Criteria for Adverse Events, Version 4.0. Available at: http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf. [Google Scholar]
- 26.Autier J, Escudier B, Wechsler J, et al. Prospective study of the cutaneous adverse effects of sorafenib, a novel multikinase inhibitor. Arch Dermatol. 2008;144:886–892. doi: 10.1001/archderm.144.7.886. [DOI] [PubMed] [Google Scholar]
- 27.Lacouture ME, Reilly LM, Gerami P, et al. Hand foot skin reaction in cancer patients treated with the multikinase inhibitors sorafenib and sunitinib. Ann Oncol. 2008;19:1955–1961. doi: 10.1093/annonc/mdn389. [DOI] [PubMed] [Google Scholar]
- 28.Huggins RH, Kuzel TM, Anderson RT, et al. Hand foot skin reaction (HFSR) by the multikinase inhibitors (MKIs) sorafenib and sunitinib: Impact on quality of life (QoL) J Clin Oncol (Meeting Abstracts) 2008;26(15 suppl):16122. [Google Scholar]
- 29.Chu D, Lacouture ME, Fillos T, et al. Risk of hand-foot skin reaction with sorafenib: A systematic review and meta-analysis. Acta Oncol. 2008;47:176–186. doi: 10.1080/02841860701765675. [DOI] [PubMed] [Google Scholar]
- 30.Hauschild A, Agarwala SS, Trefzer U, et al. Results of a phase III, randomized, placebo-controlled study of sorafenib in combination with carboplatin and paclitaxel as second-line treatment in patients with unresectable stage III or stage IV melanoma. J Clin Oncol. 2009;27:2823–2830. doi: 10.1200/JCO.2007.15.7636. [DOI] [PubMed] [Google Scholar]
- 31.Azad NS, Aragon-Ching JB, Dahut WL, et al. Hand-foot skin reaction increases with cumulative sorafenib dose and with combination anti-vascular endothelial growth factor therapy. Clin Cancer Res. 2009;15:1411–1416. doi: 10.1158/1078-0432.CCR-08-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Strumberg D, Awada A, Hirte H, et al. Pooled safety analysis of BAY 43–9006 (sorafenib) monotherapy in patients with advanced solid tumours: Is rash associated with treatment outcome? Eur J Cancer. 2006;42:548–556. doi: 10.1016/j.ejca.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 33.Lacouture ME, Wu S, Robert C, et al. Evolving strategies for the management of hand-foot skin reaction associated with the multitargeted kinase inhibitors sorafenib and sunitinib. The Oncologist. 2008;13:1001–1011. doi: 10.1634/theoncologist.2008-0131. [DOI] [PubMed] [Google Scholar]
- 34.Wilhelm SM, Carter C, Tang LY, et al. BAY 43–9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. J Cancer Res. 2004;64:7099–7109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 35.Dal Lago L, D'Hondt V, Awada A. Selected combination therapy with sorafenib: A review of clinical data and perspectives in advanced solid tumors. The Oncologist. 2008;13:845–858. doi: 10.1634/theoncologist.2007-0233. [DOI] [PubMed] [Google Scholar]
- 36.O'Brien ME, Wigler N, Inbar M, et al. Reduced cardiotoxicity and comparable efficacy in a phase III trial of pegylated liposomal doxorubicin HCl (CAELYX/Doxil) versus conventional doxorubicin for first-line treatment of metastatic breast cancer. Ann Oncol. 2004;15:440–449. doi: 10.1093/annonc/mdh097. [DOI] [PubMed] [Google Scholar]
- 37.Keller AM, Mennel RG, Georgoulias VA, et al. Randomized phase III trial of pegylated liposomal doxorubicin versus vinorelbine or mitomycin C plus vinblastine in women with taxane-refractory advanced breast cancer. J Clin Oncol. 2004;22:3893–3901. doi: 10.1200/JCO.2004.08.157. [DOI] [PubMed] [Google Scholar]
- 38.O'Shaughnessy J, Miles D, Vukelja S, et al. Superior survival with capecitabine plus docetaxel combination therapy in anthracycline-pretreated patients with advanced breast cancer: Phase III trial results. J Clin Oncol. 2002;20:2812–2823. doi: 10.1200/JCO.2002.09.002. [DOI] [PubMed] [Google Scholar]
- 39.O'Shaughnessy JA, Blum J, Moiseyenko V, et al. Randomized, open-label, phase II trial of oral capecitabine (Xeloda) versus. a reference arm of intravenous CMF (cyclophosphamide, methotrexate and 5-fluorouracil) as first-line therapy for advanced/metastatic breast cancer. Ann Oncol. 2001;12:1247–1254. doi: 10.1023/a:1012281104865. [DOI] [PubMed] [Google Scholar]
- 40.Blum JL, Jones SE, Buzdar AU, et al. Multicenter phase II study of capecitabine in paclitaxel-refractory metastatic breast cancer. J Clin Oncol. 1999;17:485–493. doi: 10.1200/JCO.1999.17.2.485. [DOI] [PubMed] [Google Scholar]
- 41.Fumoleau P, Largillier R, Clippe C, et al. Multicentre, phase II study evaluating capecitabine monotherapy in patients with anthracycline- and taxane-pretreated metastatic breast cancer. Eur J Cancer. 2004;40:536–542. doi: 10.1016/j.ejca.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 42.Isaacs C, Herbolsheimer P, Liu MC, et al. Phase I/II study of sorafenib with anastrozole in patients with hormone receptor positive aromatase inhibitor resistant metastatic breast cancer. Breast Cancer Res Treat. 2011;125:137–143. doi: 10.1007/s10549-010-1226-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barrios CH, Liu MC, Lee SC, et al. Phase III randomized trial of sunitinib versus capecitabine in patients with previously treated HER2-negative advanced breast cancer. Breast Cancer Res Treat. 2010;121:121–131. doi: 10.1007/s10549-010-0788-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thomas ES, Gomez HL, Li RK, et al. Ixabepilone plus capecitabine for metastatic breast cancer progressing after anthracycline and taxane treatment. J Clin Oncol. 2007;25:5210–5217. doi: 10.1200/JCO.2007.12.6557. [DOI] [PubMed] [Google Scholar]
- 45.Geyer CE, Forster J, Lindquist D, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355:2733–2743. doi: 10.1056/NEJMoa064320. [DOI] [PubMed] [Google Scholar]
- 46.Miller KD, Chap LI, Holmes FA, et al. Randomized phase III trial of capecitabine compared with bevacizumab plus capecitabine in patients with previously treated metastatic breast cancer. J Clin Oncol. 2005;23:792–799. doi: 10.1200/JCO.2005.05.098. [DOI] [PubMed] [Google Scholar]
- 47.Gomez P, Roche HH, Costa F, et al. Management of hand-foot skin reaction/hand-foot syndrome in SOLTI-0701: A double-blind, randomized phase 2b study comparing sorafenib versus placebo in combination with capecitabine in patients with advanced breast cancer [abstract 1083]. Presented at the 2010 American Society of Clinical Oncology Annual Meeting; June 4–8, 2010; Chicago, IL. [Google Scholar]
- 48.Seidman AD, Tiersten A, Hudis C, et al. Phase II trial of paclitaxel by 3-hour infusion as initial and salvage chemotherapy for metastatic breast cancer. J Clin Oncol. 1995;13:2575–2581. doi: 10.1200/JCO.1995.13.10.2575. [DOI] [PubMed] [Google Scholar]
- 49.Nabholtz JM, Gelmon K, Bontenbal M, et al. Multicenter, randomized comparative study of two doses of paclitaxel in patients with metastatic breast cancer. J Clin Oncol. 1996;14:1858–1867. doi: 10.1200/JCO.1996.14.6.1858. [DOI] [PubMed] [Google Scholar]
- 50.Bishop JF, Dewar J, Toner GC, et al. Initial paclitaxel improves outcome compared with CMFP combination chemotherapy as front-line therapy in untreated metastatic breast cancer. J Clin Oncol. 1999;17:2355–2364. doi: 10.1200/JCO.1999.17.8.2355. [DOI] [PubMed] [Google Scholar]
- 51.Donadio M, Manzin E, Berruti A, et al. Paclitaxel administration on days 1 and 8 every 21 days in anthracycline-pretreated metastatic breast cancer patients. A multicenter phase II trial. Cancer Chemother Pharmacol. 2001;47:391–396. doi: 10.1007/s002800000247. [DOI] [PubMed] [Google Scholar]
- 52.Scotté F, Tourani JM, Banu E, et al. Multicenter study of a frozen glove to prevent docetaxel-induced onycholysis and cutaneous toxicity of the hand. J Clin Oncol. 2005;23:4424–4429. doi: 10.1200/JCO.2005.15.651. [DOI] [PubMed] [Google Scholar]
- 53.Childress J, Lokich J. Cutaneous hand and foot toxicity associated with cancer chemotherapy. Am J Clin Oncol. 2003;26:435–436. doi: 10.1097/01.coc.0000026486.56886.18. [DOI] [PubMed] [Google Scholar]
- 54.Scotté F, Banu E, Medioni J, et al. Matched case-control phase 2 study to evaluate the use of a frozen sock to prevent docetaxel-induced onycholysis and cutaneous toxicity of the foot. Cancer. 2008;112:1625–1631. doi: 10.1002/cncr.23333. [DOI] [PubMed] [Google Scholar]
- 55.Sahoo TP, Kaklamani VG, Lokanatha D, et al. A regional subgroup analysis of a multinational, double-blind, randomized, placebo-controlled, phase IIb study evaluating sorafenib (SOR) with paclitaxel (PAC) in patients (pts) with advanced breast cancer (BC) [abstract 1114]. Presented at the 2010 American Society of Clinical Oncology Annual Meeting; June 4–8, 2010; Chicago, IL. [Google Scholar]
- 56.Zhang H, Dong B, Lu JJ, et al. Efficacy of sorafenib on metastatic renal cell carcinoma in Asian patients: Results from a multicenter study. BMC Cancer. 2009;9:249. doi: 10.1186/1471-2407-9-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Breast Cancer Version 2.2011. [accessed July 11, 2011]. Available at: http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf.
- 58.Lassere Y, Hoff P. Management of hand-foot syndrome in patients treated with capecitabine (Xeloda) Eur J Oncol Nurs. 2004;8(suppl 1):S31–S40. doi: 10.1016/j.ejon.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 59.Degen A, Alter M, Schenck F, et al. The hand-foot-syndrome associated with medical tumor therapy—classification and management. J Dtsch Dermatol Ges. 2010;8:652–661. doi: 10.1111/j.1610-0387.2010.07449.x. [DOI] [PubMed] [Google Scholar]
- 60.Lipworth AD, Robert C, Zhu AX. Hand-foot syndrome (hand-foot skin reaction, palmar-plantar erythrodysesthesia): Focus on sorafenib and sunitinib. Oncology. 2009;77:257–271. doi: 10.1159/000258880. [DOI] [PubMed] [Google Scholar]
- 61.Wood LS. Management of vascular endothelial growth factor and multikinase inhibitor side effects. Clin J Oncol Nurs. 2009;13(suppl):13–18. doi: 10.1188/09.CJON.S2.13-18. [DOI] [PubMed] [Google Scholar]
- 62.Bellmunt J, Eisen T, Fishman M, et al. Experience with sorafenib and adverse event management. Crit Rev Oncol Hematol. 2010;78:24–32. doi: 10.1016/j.critrevonc.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 63.Anderson R, Jatoi A, Robert C, et al. Search for evidence-based approaches for the prevention and palliation of hand-foot skin reaction (HFSR) caused by the multikinase inhibitors (MKIs) The Oncologist. 2009;14:291–302. doi: 10.1634/theoncologist.2008-0237. [DOI] [PubMed] [Google Scholar]
- 64.Kang YK, Lee SS, Yoon DH, et al. Pyridoxine is not effective to prevent hand-foot syndrome associated with capecitabine therapy: Results of a randomized, double-blind, placebo-controlled study. J Clin Oncol. 2010;28:3824–3829. doi: 10.1200/JCO.2010.29.1807. [DOI] [PubMed] [Google Scholar]
- 65.Wayne, NJ: Bayer HealthCare Pharmaceuticals Inc; 2009. Nexavar [prescribing information] [Google Scholar]
- 66.Lin E, Morris J, Chau N, et al. Celecoxib attenuated capecitabine induced hand-and-foot syndrome (HFS) and diarrhea and improved time to tumor progression in metastatic colorectal cancer (MCRC) [abstract 2364] Proc Am Soc Clin Oncol. 2002;21(part 2):138B. [Google Scholar]
- 67.Wolf SL, Qin R, Menon SP, et al. Placebo-controlled trial to determine the effectiveness of a urea/lactic acid-based topical keratolytic agent for prevention of capecitabine-induced hand-foot syndrome: North Central Cancer Treatment Group Study N05C5. J Clin Oncol. 2010;28:5182–5187. doi: 10.1200/JCO.2010.31.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.West Haven, CT; Emeryville, CA: Bayer Healthcare Pharmaceuticals, Onyx Pharmaceuticals; 2006. Skin reactions and cancer therapy—Guidelines for identifying and managing skin-related side effects during your therapy; pp. 1–12. [Google Scholar]
- 69.Janusch M, Fischer M, Marsch W, et al. The hand-foot syndrome—a frequent secondary manifestation in antineoplastic chemotherapy. Eur J Dermatol. 2006;16:494–499. [PubMed] [Google Scholar]