The pathologic complete response rate, delivered dose intensity, disease-free survival and overall survival rates, and toxicity of breast cancer patients treated with 5-fluorouracil, doxorubicin, and cyclophosphamide (FAC) versus dose-intense FAC plus G-CSF in the neoadjuvant setting were compared.
Keywords: Breast cancer, Locally advanced, Dose intensity, Disease-free survival, Overall survival, G-CSF
Learning Objectives
After completing this course, the reader will be able to:
Compare outcomes in patients treated with standard fluorouracil, doxorubicin, and cyclophosphamide (FAC) and those treated with dose-intense FAC.
Describe toxicity profiles in patients treated with standard fluorouracil, doxorubicin, and cyclophosphamide (FAC) and those treated with dose-intense FAC.
This article is available for continuing medical education credit at CME.TheOncologist.com
Abstract
Objective.
To compare the pathologic complete response (pCR) rate of patients treated with 5-fluorouracil (5-FU), doxorubicin, and cyclophosphamide (FAC) versus dose-intense FAC plus G-CSF in the neoadjuvant setting and to compare the delivered dose intensity, disease-free survival (DFS) and overall survival (OS) times, and toxicity between treatment arms in patients with breast cancer.
Methods.
Patients were randomized to receive preoperative FAC (5-FU, 500 mg/m2; doxorubicin, 50 mg/m2; cyclophosphamide, 500 mg/m2) every 21 days for four cycles or dose-intense FAC (5-FU, 600 mg/m2; doxorubicin, 60 mg/m2; cyclophosphamide, 1,000 mg/m2) plus G-CSF every 18 days for four cycles.
Results.
Two hundred two patients were randomly assigned. The median follow-up was 7.5 years. Patients randomized to FAC plus G-CSF had a higher pCR rate as well as clinical complete response rate; however, these differences were not statistically different from those with the FAC arm. Patients in the FAC + G-CSF arm had a higher delivered dose intensity of doxorubicin in the neoadjuvant and adjuvant settings than those in the standard FAC arm. DFS and OS times were not significantly different between the two groups. However, the OS and DFS rates were significantly higher for patients who achieved a pCR than for those who did not. Thrombocytopenia, febrile neutropenia, and infection rates were higher in the FAC + G-CSF arm.
Conclusions.
A higher delivered dose intensity of doxorubicin with the FAC + G-CSF regimen did not result in a statistically significant higher pCR rate. However, patients who achieved a pCR experienced longer DFS and OS times.
Introduction
Adjuvant chemotherapy reduces the risk for disease recurrence and death in women who have operable breast cancer [1]. Even though the introduction of anthracyclines and taxanes has improved the risk for recurrence over cyclophosphamide, methotrexate, and 5-fluorouracil (5-FU) containing regimens, the risk for relapse still remains high, especially for patients with lymph node–positive or high-risk, lymph node–negative disease [1]. Therefore, in order to further improve outcome, several studies evaluated the dose intensity (DI) of cyclophosphamide and/or doxorubicin in the adjuvant setting and reported conflicting results [2–8].
To further evaluate the role of DI in the neoadjuvant therapy of breast cancer, in 1992 we initiated a prospective, randomized trial to compare the pathologic complete response (pCR) rate, delivered DI, incidence of myelosuppression, disease-free survival (DFS) rate, and overall survival (OS) rate between patients treated with standard fluorouracil, doxorubicin, and cyclophosphamide (FAC) or dose-intense FAC. Here, we report long-term results.
Patients and Methods
Patient Population
Patients were enrolled from the University of Texas MD Anderson Cancer Center (MDACC) in 1992–1997, and all signed an institutional review board–approved (local trial # ID91-0156, copy available at Office of Protocol Research) informed consent form. Patients with a histologic diagnosis of breast cancer, stage IIA–IV (ipsilateral supraclavicular disease as the sole evidence of metastatic disease), aged 16–75 years, with no prior chemotherapy, radiation therapy, or definitive surgical therapy for breast cancer, and with adequate organ function were eligible.
Treatment Schema
Randomization was done using the Institutional Protocol Management System. Patients randomized to arm A were to receive 5-FU, 500 mg/m2 (days 1 and 4), doxorubicin, 50 mg/m2 (48- to 72-hour continuous i.v. infusion, starting on day 1), and cyclophosphamide, 500 mg/m2 (day 1) in the neoadjuvant setting for four cycles, every 21 days. Patients in arm B were to receive 5-FU, 600 mg/m2 (days 1 and 4), doxorubicin, 60 mg/m2 (48- to 72-hour continuous i.v. infusion), and cyclophosphamide, 1,000 mg/m2 (day 1) for four cycles, every 18 days. G-CSF (5 μg/kg per day) was started on day 4 (counting 48 hours for doxorubicin infusion followed by 5-FU administration and starting G-CSF 24 hours after that, which, by the MDACC counting method at that time, was 4 days) in the high-dose arm and continued for 14 days or until the absolute granulocyte count. (AGC) increased to 10,000/μL, whichever was earlier. The next cycle of chemotherapy was initiated 24 hours after cessation of G-CSF therapy (target day, day 18 in arm B) or when the AGC recovered to ≥1,500/μL (arm A).
The rationale for varying both the dose and dose density at the same time was based on the Hryniuk and Bush DI analysis showing a steep dose response for survival in breast cancer [9]. DI across the various regimens at that time was defined as mg/m2 per week for each of the drugs, then the intensities of all the drugs were averaged. We tried to take advantage of the fact that G-CSF would allow delivery of higher doses of the drug and that the faster kinetics of recovery would allow us to give the next cycle sooner, thereby allowing for a pretty substantial increase in DI. The projected DI, calculated in terms of mg/m2 per week was almost 67% higher. The term dose density was not a widely used concept at the time.
Postoperative Chemotherapy
Patients who achieved a pCR after neoadjuvant chemotherapy and those with residual tumor <1 cm3 and fewer than four positive lymph nodes after chemotherapy received four additional cycles of FAC at the same doses and schedule. Patients with >1 cm3 residual disease or four or more positive lymph nodes received four additional cycles of FAC followed by four cycles of methotrexate (120 mg/m2 i.v. on day 1) plus vinblastine (8.5 mg/m2 over 72 hours) every 14 days. Patients with progressive disease after four cycles FAC received radiation therapy followed by definitive surgery and six cycles of methotrexate plus vinblastine.
Tamoxifen (20 mg daily) was recommended to all patients aged >50 years or whose tumors were positive for estrogen receptors (ERs).
Surgery and Radiation Therapy
The decision to perform a mastectomy or breast-conserving surgery was based on the technical feasibility and preference of the patient. All patients underwent at least a level I and level II axillary dissection. All patients had the axillary apex, supraclavicular fossa, and internal mammary nodes irradiated with 50 Gy at a rate of 9–10 Gy per week, 5 days per week. All patients were treated with a boost of 10–15 Gy using external-beam or interstitial techniques.
Endpoints and Definitions
The primary objective of this study was to compare the pCR rate between the two arms—FAC and FAC plus G-CSF. Secondary objectives included comparing the delivered DI, tumor downstaging, DFS rate, OS rate, and toxicity.
Complete clinical remission was defined as the complete disappearance of all clinical evidence of tumor by clinical evaluation, mammogram, and ultrasound. Partial remission was defined as a ≥50% decrease for a minimum of 4 weeks in the measurable lesion as determined by the product of the longest perpendicular diameters of the lesion.
pCR was defined as no residual invasive tumor in the breast and regional lymph nodes. OS was measured from the start of neoadjuvant chemotherapy to the date of death from any cause or last follow-up. Relapse-free survival was measured from the start of neoadjuvant chemotherapy to the date of disease recurrence or last follow-up. Patients who died before experiencing a disease recurrence were considered censored at their date of death. For descriptive purposes, overall response was defined as clinical CR or pCR.
Patients were followed up according to institutional guidelines every 3 months with physical examinations for 2 years, then every 6 months for 3 years, and annually thereafter. Patients followed at outside institutions were contacted yearly via telephone for follow-up.
Statistical Analysis
This phase III study was designed to enroll a minimum of 112 evaluable patients randomized equally to receive either standard FAC therapy or dose-intense FAC therapy with G-CSF support. Fifty-six evaluable patients in each arm would provide 80% power to detect a 20% difference in the CR rate (from 15% to 35%). An interim analysis was planned when half the targeted accrual was completed. The protocol was later amended in April of 1995 in order to allow for the detection of a difference in the 5-year survival rate from 35% (historical experience with the regimen used in the control arm of the current protocol) to 55% (in the escalated arm), to allow a minimum of 94 evaluable patients to be enrolled in each arm. This would provide a power of 80% to detect the desired difference with a p-value < .05.
Patient characteristics were tabulated and compared between the two groups with a χ2 test, Fisher's exact test, or Wilcoxon's rank sum test as appropriate. OS and DFS rates were estimated using the Kaplan–Meier product limit method and compared between treatment groups with the log-rank test. The association of OS and DFS with DI was estimated with Cox proportional hazards models. The median follow-up was calculated as the median observation time among all patients.
Delivered DI was calculated as described in Ang et al. [10]. Neoadjuvant and adjuvant chemotherapy were considered separately. The neoadjuvant delivered DI (NDI4) was calculated as the total amount of drug received during the first four cycles of neoadjuvant therapy divided by the number of weeks from day 1 of cycle 1 to either day 18 or day 21 of cycle 4, depending on randomization assignment. For patients who received fewer than four cycles, treatment duration was taken to be the projected treatment duration, which was 12 weeks for patients randomized to the FAC arm and 10.3 weeks for patients randomized to the FAC plus G-CSF arm. Missing doses were assumed to be zero. Adjuvant delivered DI (ADI4) was calculated similarly. Total delivered DI was calculated as the sum of the total amount of drug received in the first four neoadjuvant and the first four adjuvant cycles, divided by the sum of the number of weeks calculated for NDI4 and ADI4. Although the treatment regimen consisted of three drugs, we considered only the delivered DI of doxorubicin.
Results
Response to Neoadjuvant Chemotherapy and Planned Dose Delivery
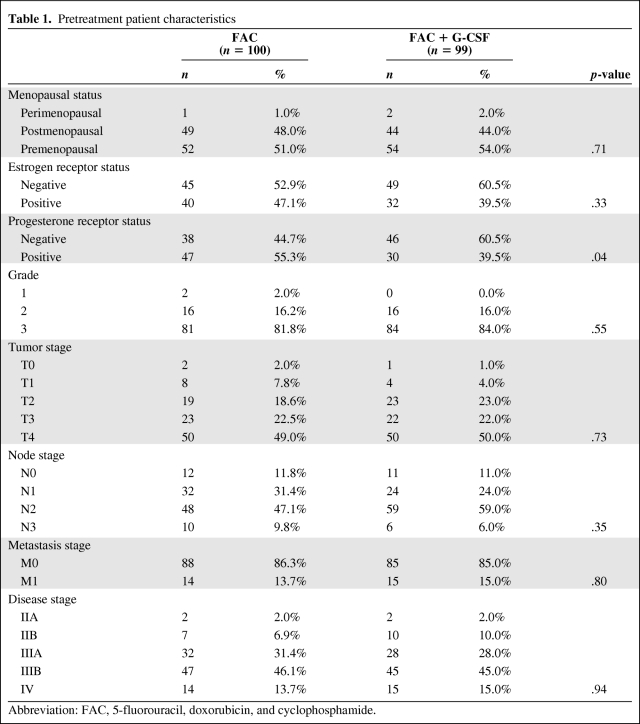
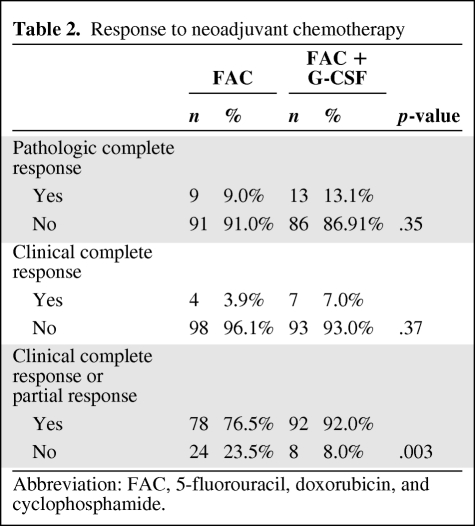
Pretreatment patient characteristics are shown in Table 1. Table 2 shows the pCR and clinical response rates to neoadjuvant chemotherapy. Patients in the FAC plus G-CSF arm had a higher pCR rate (13.1% versus 9%) and clinical CR rate (7% versus 3.9%), although these were not statistically different from those in the FAC arm. The rate of overall response (clinical or pCR) was significantly higher for patients in the FAC plus G-CSF arm (76.5% versus 92%; p = .003).
Table 1.
Pretreatment patient characteristics
Abbreviation: FAC, 5-fluorouracil, doxorubicin, and cyclophosphamide.
Table 2.
Response to neoadjuvant chemotherapy
Abbreviation: FAC, 5-fluorouracil, doxorubicin, and cyclophosphamide.
Downstaging rates following neoadjuvant chemotherapy were similar in the two groups. Sixty-four patients (62.7%) in the FAC arm and 69 patients (69%) in the FAC plus G-CSF arm were downstaged by at least one tumor–node–metastasis stage. Also, a similar proportion of patients in each treatment group underwent breast-conserving surgery after neoadjuvant chemotherapy.
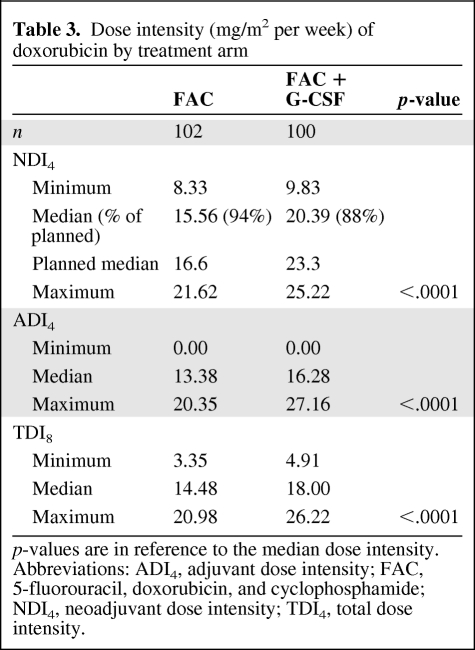
The projected DI of doxorubicin was 16.67 mg/m2 per week in the FAC arm and 23.33 mg/m2 per week in the FAC plus G-CSF arm. Patients in the FAC plus G-CSF arm had higher delivered DIs of doxorubicin in the neoadjuvant and adjuvant settings than patients in the standard FAC arm (median, 15.56 mg/m2 per week and 20.39 mg/m2 per week, respectively; p < .0001) (Table 3). Even though the neoadjuvant delivered DI of doxorubicin was not significantly different between the two pCR groups, there was a trend toward pCR in patients who had a higher delivered DI of doxorubicin (16.79 mg/m2 per week versus 19.45 mg/m2 per week; p = .053). The median interval between treatments was 22 days (range, 18–39 days) in the standard FAC arm and 18 days (range, 13–42 days) in the FAC plus G-CSF arm. Thirty-nine percent of the patients achieved the every-18-day administration.
Table 3.
Dose intensity (mg/m2 per week) of doxorubicin by treatment arm
p-values are in reference to the median dose intensity.
Abbreviations: ADI4, adjuvant dose intensity; FAC, 5-fluorouracil, doxorubicin, and cyclophosphamide; NDI4, neoadjuvant dose intensity; TDI4, total dose intensity.
An exploratory descriptive analysis was performed to evaluate if more pCRs were seen in patients with ER− tumors. In the FAC arm, there were nine patients who had a pCR (9%), with five having ER− disease and four unknown cases. In the FAC plus G-CSF arm, there were 12 patients who had a pCR (13%), and of these, eight had ER− tumors, one had an ER+ tumor, and three were unknown. Because there were slightly more ER− cases in the FAC plus G-CSF arm, it could be hypothesized that the ER− status was the driving force. However, the very small number of patients with a pCR with known ER status precluded us from performing a statistical analysis that would yield clinical meaningful results.
DFS and OS
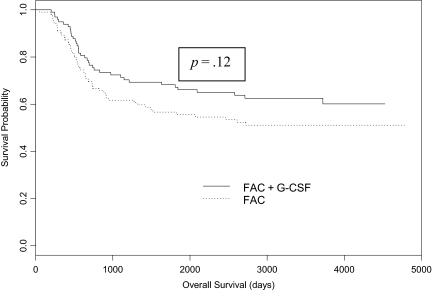
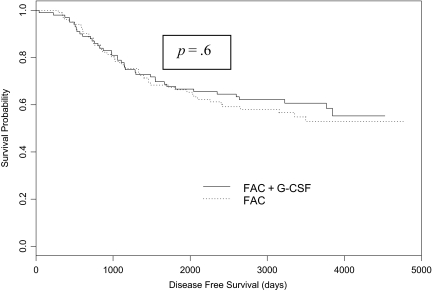
The median follow-up was 91.5 months (range, 1.3–157.0 months). The OS results by treatment group are shown in Figure 1. In the FAC arm, 45 patients had died and the OS rate at 5 years was 66.3% (95% confidence interval [CI], 57.7%–76.2%). In the FAC plus G-CSF arm, 40 patients had died and the OS rate at 5 years was 66.6% (95% CI, 58.0%–76.6%). The OS rates were not significantly different between the two treatment groups (p = .61). DFS results by treatment group are shown in Figure 2. In the FAC arm, 49 patients experienced a disease recurrence and the DFS rate at 5 years was 55.6% (95% CI, 46.7%–66.2%). In the FAC plus G-CSF arm, 37 patients experienced a disease recurrence and the DFS at 5 years was 67.2% (95% CI, 58.5%–77.2%). The DFS rates were not significantly different between the two treatment groups (p = .12). The neoadjuvant delivered DI of doxorubicin was not significantly associated with either OS or DFS. However, higher adjuvant and total delivered DI of doxorubicin were significantly associated with higher DFS and OS rates (p = 0.01 and p = .001, respectively).
Figure 1.
Overall survival by treatment group.
Abbreviation: FAC, 5-fluorouracil, doxorubicin, and cyclophosphamide.
Figure 2.
Disease-free survival by treatment group.
Abbreviation: FAC, 5-fluorouracil, doxorubicin, and cyclophosphamide.
DFS and OS in Patients Achieving pCR Versus No pCR
Twenty-two patients achieved a pCR and 177 did not. Among the patients who achieved a pCR, three experienced recurrence and four died. Among patients who did not achieve a pCR, 81 experienced disease recurrence and 78 died. At 5 years, the OS rates were 86.4% (95% CI, 73.2%–100%) and 65.1% (95% CI, 58.4%–72.6%) among patients who did and did not experience a pCR, respectively (p = 0.03). At 5 years, the DFS rates were 86.4% (95% CI, 73.2%–100%) and 58.8% (95% CI, 51.9%–66.6%) among patients who did and did not experience a pCR, respectively (p = .007).
Among patients who did not achieve a pCR, there was not a statistically significant difference between the two treatment arms for OS (p = .89) or RFS (p = 0.32). There were not enough events among patients who did achieve a pCR to compare treatment arms.
Overall, the compliance in the FAC plus G-CSF arm was good, and only five patients did not receive the planned number of cycles and withdrew from the study.
Toxicity
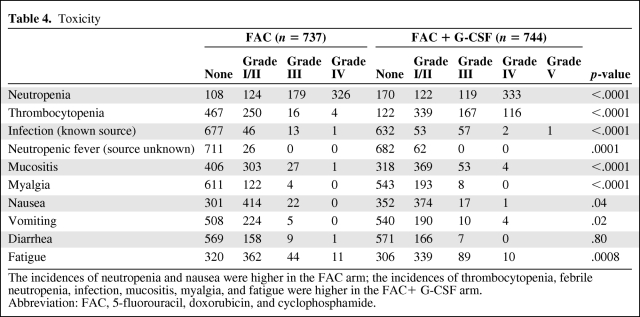
There were statistically significantly higher rates of grade 3 and 4 neutropenia in the FAC arm (Table 4). However, the rates of thrombocytopenia, febrile neutropenia, and infection were higher in the FAC plus G-CSF arm and one patient died as a result of infectious complications. More than half of the patients in the FAC plus G-CSF arm required packed RBC transfusions, compared with 5% of patients in the FAC arm. Platelet transfusions were required during 43 courses in 22 patients in the FAC plus G-CSF arm. None of the patients in the FAC arm required a platelet transfusion.
Table 4.
Toxicity
The incidences of neutropenia and nausea were higher in the FAC arm; the incidences of thrombocytopenia, febrile neutropenia, infection, mucositis, myalgia, and fatigue were higher in the FAC+ G-CSF arm.
Abbreviation: FAC, 5-fluorouracil, doxorubicin, and cyclophosphamide.
More patients in the FAC arm experienced nausea and more patients in the FAC plus G-CSF arm experienced mucositis, myalgia, and fatigue. There were no treatment-related deaths in either treatment arm. The rates of cardiotoxicity or secondary malignancies did not differ between the two arms.
Discussion
The dose escalation concept is based on the Skipper-Schabel/log-kill model [11], which assumes that a dose of drug active against a uniformly sensitive cell population will always kill a constant proportion of cells. Therefore, higher doses of that drug given for several cycles should be able to kill a higher percentage of cells. On the basis of this hypothesis, a number of clinical trials have evaluated the dose escalation of cyclophosphamide and/or doxorubicin in the adjuvant setting and reported conflicting results [2–7]. The Cancer and Leukemia Group B (CALGB), for example, randomized 1,572 women with node-positive, stage II breast cancer to three treatment groups. One group received cyclophosphamide at 400 mg/m2 and doxorubicin at 40 mg/m2 once every 28 days and 5-FU at 400 mg/m2 twice every 28 days, for six cycles. Another group received 50% higher doses of the three drugs (600 mg/m2, 60 mg/m2, and 600 mg/m2, respectively), but for only four cycles. The third group of women received half the total dose used in the other two groups and at half the DI used in the second group. After a median of 3.4 years of follow-up, the women treated with a high or moderate DI had significantly longer DFS (p < .001) and OS (p = .004) times than those treated with a low DI. However, the difference in survival between the two groups treated with a moderate or high DI was not significant [3].
In another study, by the French Adjuvant Study Group, patients received either 5-FU at 500 mg/m2, epirubicin at 50 mg/m2, and cyclophosphamide at 500 mg/m2 every 21 days for six cycles or the same regimen except with an epirubicin dose of 100 mg/m2. That study showed significantly greater 5-year DFS and OS rates in the group given epirubicin at 100 mg/m2 [7].
However, several studies did not report a better outcome with dose escalation. Dose intensification of cyclophosphamide in the National Surgical Adjuvant Breast and Bowel Project B-22 and B-25 studies and dose escalation of doxorubicin in the CALGB 9344 study were not associated with better DFS or OS results [4–6].
It appears from these studies that dose escalation at conventional intervals may not significantly improve outcome. According to the refined Norton-Simon model, delivering treatments at a greater dose rate (dose density) could optimize chemotherapy efficacy, minimize regrowth between cycles, and increase cumulative cell kill [12].
One study took such an approach and revealed that six cycles of 5-FU, epirubicin, and cyclophosphamide (FEC) at 600 mg/m2 and 60 mg/m2, respectively, given every 21 days versus 14 days resulted in similar DFS and OS outcomes [13]. Another study further evaluated the addition of a taxane and showed that the DFS and OS rates were significantly higher for the dose-dense (every 2 weeks) arm than for the conventional 3-week schedule [14].
Whereas the studies discussed above evaluated DI in the adjuvant setting, some studies were undertaken in the neoadjuvant setting. In one of those studies, locally advanced breast cancer patients were randomly assigned to cyclophosphamide, 75 mg/m2 orally (days 1–14), epirubicin, 60 mg/m2 i.v. days 1 and 8, and 5-FU, 500 mg/m2 i.v. days 1 and 8, for six cycles every 28 days versus epirubicin, 120 mg/m2 i.v. day 1, cyclophosphamide, 830 mg/m2 i.v. day 1, and G-CSF, for six cycles every 14 days. The pCR rates were 14% and 10%, respectively, and there was no difference in DFS and OS outcomes [15].
Further dose-dense studies were carried out with regimens that also included taxanes, and most of them revealed that dose-dense regimens induced a higher pCR rate [16, 17]. However, it is difficult to compare these neoadjuvant studies because although some studies had the same agents in both arms, other studies used different agents in their study and control arms.
In our current study, the pCR rate was not different between the two arms; however, patients who achieved a pCR had higher DFS and OS rates. In the overall population, there was no difference in the DFS and OS rates. This study is most probably underpowered to detect significant differences. The DFS and OS difference seen in the CALGB 9741 study, for example, required 2,000 patients [14].
Regarding acute toxicities, in our current study, more patients in the FAC arm experienced nausea and more patients in the FAC plus G-CSF arm reported mucositis, myalgia, and fatigue. Higher rates of grade 3 and 4 neutropenia were observed in the FAC arm. However, rates of thrombocytopenia, febrile neutropenia, and infection were higher in the FAC plus G-CSF arm. More patients in the FAC plus G-CSF arm required packed RBC transfusions than patients in the FAC arm. The long median follow-up in our study allowed us to obtain information about the long-term safety of dose-intense chemotherapy with filgrastim support. The use of filgrastim has been hypothesized to be associated with a higher risk for leukemia and myelodysplastic syndrome (MDS) [18, 19]. However, no patients in our study developed acute myelogenous leukemia (AML) or MDS. In a study reported by Bergh et al. [20], patients received tailored and dose-escalated FEC chemotherapy with G-CSF support in comparison with high-dose chemotherapy supported by autologous stem cells. The occurrence of AML or MDS was 3.6% in the tailored FEC arm at the 3-year follow-up [20]. Several studies have reported that it is feasible to use filgrastim in biweekly treatments [13, 7, 21, 22].
In our current study, there were no treatment-related deaths and no difference in cardiotoxicity rates between the treatment arms. The relationship between cumulative anthracycline dose and long-term cardiac effects has been evaluated in several studies, with a reported rate of congestive heart failure in the range of 0%–2%, with a median follow-up time of 39–74 months [23–28]. Recently, a study reported on long-term cardiac outcome in patients treated with dose-dense and dose-intense sequential doxorubicin, paclitaxel, and cyclophosphamide in the adjuvant setting, wherein doxorubicin was given at 90 mg/m2 every 14 days for three cycles, and no cardiac-related deaths were seen [29].
In conclusion, dose-intense and dose-dense therapy with FAC is feasible; however, it is not possible to administer this regimen at 2-week intervals because of limiting toxicity. We have shown that, in the neoadjuvant setting, higher delivered DIs of doxorubicin in the FAC regimen resulted in higher pCR rates. Furthermore, the DFS and OS rates were significantly greater in patients who achieved a pCR. Furthermore, whether or not outcome can be further improved with the addition of noncrossresistant sequential agents and targeted therapies needs to be explored.
Acknowledgment
Supported, in part, by NIH-NCI: 2P30 CA016672 28(PP-4) and the Nellie B. Connally Breast Cancer Research Fund.
Footnotes
- (C/A)
- consulting/advisory relationship
- (RF)
- Research funding
- (E)
- Employment
- (H)
- Honoraria received
- (OI)
- Ownership interests
- (IP)
- Intellectual property rights/inventor/patent holder
Author Contributions
Conception/Design: Vicente Valero, Aman U. Buzdar, Eric Strom, Saroj Vadhan-Raj, Gabriel N. Hortobagyi
Provision of study material or patients: Banu K. Arun, Vicente Valero, Daniel Booser, Ronald Walters, Nuhad Ibrahim, Aman U. Buzdar, Merrick Ross, Richard L. Theriault, Gabriel N. Hortobagyi
Collection and/or assembly of data: Banu K. Arun, Kapil Dhingra, Shu-Wan Kau, Laura Guerra, Debbie Frye
Data analysis and interpretation: Banu K. Arun, Kapil Dhingra, Vicente Valero, Shu-Wan Kau, Kristine Broglio, Laura Guerra, Guosheng Yin, Debbie Frye
Manuscript writing: Banu K. Arun, Kapil Dhingra, Vicente Valero, Gabriel N. Hortobagyi
Final approval of manuscript: Banu K. Arun, Kapil Dhingra, Vicente Valero, Shu-Wan Kau, Kristine Broglio, Aysegul Sahin, Laura Guerra, Daniel Booser, Guosheng Yin, Ronald Walters, Nuhad Ibrahim, Aman U. Buzdar, Debbie Frye, Nour Sneige, Eric Strom, Merrick Ross, Richard L. Theriault, Saroj Vadhan-Raj, Gabriel N. Hortobagyi
References
- 1.Early Breast Cancer Trialists' Collaborative Group (EBCTCG) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 2.Hryniuk W, Levine MN. Analysis of dose intensity for adjuvant chemotherapy trials in stage II breast cancer. J Clin Oncol. 1986;4:1162–1170. doi: 10.1200/JCO.1986.4.8.1162. [DOI] [PubMed] [Google Scholar]
- 3.Wood WC, Budman DR, Korzun AH, et al. Dose and dose intensity of adjuvant chemotherapy for stage II, node-positive breast carcinoma. N Engl J Med. 1994;330:1253–1259. doi: 10.1056/NEJM199405053301801. [DOI] [PubMed] [Google Scholar]
- 4.Fisher B, Anderson S, Wickerham DL, et al. Increased intensification and total dose of cyclophosphamide in a doxorubicin-cyclophosphamide regimen for the treatment of primary breast cancer: Findings from National Surgical Adjuvant Breast and Bowel Project B-22. J Clin Oncol. 1997;15:1858–1869. doi: 10.1200/JCO.1997.15.5.1858. [DOI] [PubMed] [Google Scholar]
- 5.Fisher B, Anderson S, DeCillis A, et al. Further evaluation of intensified and increased total dose of cyclophosphamide for the treatment of primary breast cancer: Findings from National Surgical Adjuvant Breast and Bowel Project B-25. J Clin Oncol. 1999;17:3374–3388. doi: 10.1200/JCO.1999.17.11.3374. [DOI] [PubMed] [Google Scholar]
- 6.Henderson IC, Berry DA, Demetri GD, et al. Improved outcomes from adding sequential paclitaxel but not from escalating doxorubicin dose in an adjuvant chemotherapy regimen for patients with node-positive primary breast cancer. J Clin Oncol. 2003;21:976–983. doi: 10.1200/JCO.2003.02.063. [DOI] [PubMed] [Google Scholar]
- 7.French Adjuvant Study Group. Benefit of a high-dose epirubicin regimen in adjuvant chemotherapy for node-positive breast cancer patients with poor prognostic factors: 5-year follow-up results of French Adjuvant Study Group 05 randomized trial. J Clin Oncol. 2001;19:602–611. doi: 10.1200/JCO.2001.19.3.602. [DOI] [PubMed] [Google Scholar]
- 8.Bonilla L, Ben-Aharon I, Vidal L, et al. Dose-dense chemotherapy in nonmetastatic breast cancer: A systematic review and meta-analysis of randomized controlled trials. J Natl Cancer Inst. 2010;102:1845–1854. doi: 10.1093/jnci/djq409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hryniuk W, Bush H. The importance of dose intensity in chemotherapy of metastatic breast cancer. J Clin Oncol. 1984;2:1281–1288. doi: 10.1200/JCO.1984.2.11.1281. [DOI] [PubMed] [Google Scholar]
- 10.Ang PT, Buzdar AU, Smith TL, et al. Analysis of dose intensity in doxorubicin-containing adjuvant chemotherapy in stage II and III breast carcinoma. J Clin Oncol. 1989;7:1677–1684. doi: 10.1200/JCO.1989.7.11.1677. [DOI] [PubMed] [Google Scholar]
- 11.Skipper HE, Schabel FM, Jr, Wilcox WS. Experimental evaluation of potential anticancer agents. XIII. On the criteria and kinetics associated with “curability” of experimental leukemia. Cancer Chemother Rep. 1964;35:1–111. [PubMed] [Google Scholar]
- 12.Norton L. Conceptual and practical implications of breast tissue geometry: Toward a more effective, less toxic therapy. The Oncologist. 2005;10:370–381. doi: 10.1634/theoncologist.10-6-370. [DOI] [PubMed] [Google Scholar]
- 13.Venturini M, Del Mastro L, Aitini E, et al. Dose-dense adjuvant chemotherapy in early breast cancer patients: Results from a randomized trial. J Natl Cancer Inst. 2005;97:1724–1733. doi: 10.1093/jnci/dji398. [DOI] [PubMed] [Google Scholar]
- 14.Citron ML, Berry DA, Cirrincione C, et al. Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: First report of Intergroup Trial C9741/Cancer and Leukemia Group B Trial 9741 10.1200/jco.2003.09.081. J Clin Oncol. 2003;21:1431–1439. doi: 10.1200/JCO.2003.09.081. [DOI] [PubMed] [Google Scholar]
- 15.Therasse P, Mauriac L, Welnicka-Jaskiewicz M, et al. Final results of a randomized phase III trial comparing cyclophosphamide, epirubicin, and fluorouracil with a dose-intensified epirubicin and cyclophosphamide + filgrastim as neoadjuvant treatment in locally advanced breast cancer: An EORTC-NCIC-SAKK multicenter study. J Clin Oncol. 2003;21:843–850. doi: 10.1200/JCO.2003.05.135. [DOI] [PubMed] [Google Scholar]
- 16.von Minckwitz G, Raab G, Caputo A, et al. Doxorubicin with cyclophosphamide followed by docetaxel every 21 days compared with doxorubicin and docetaxel every 14 days as preoperative treatment in operable breast cancer: The GEPARDUO study of the German Breast Group. J Clin Oncol. 2005;23:2676–2685. doi: 10.1200/JCO.2005.05.078. [DOI] [PubMed] [Google Scholar]
- 17.Untch M, Fasching PA, Konecny GE, et al. PREPARE trial: A randomized phase III trial comparing preoperative, dose-dense, dose-intensified chemotherapy with epirubicin, paclitaxel and CMF versus a standard-dosed epirubicin/cyclophosphamide followed by paclitaxel {+/-} darbepoetin alfa in primary breast cancer—results at the time of surgery. Ann Oncol. 2011;9:1988–1998. doi: 10.1093/annonc/mdq709. Epub 2011 Mar 8. [DOI] [PubMed] [Google Scholar]
- 18.Smith RE. Risk for the development of treatment-related acute myelocytic leukemia and myelodysplastic syndrome among patients with breast cancer: Review of the literature and the National Surgical Adjuvant Breast and Bowel Project experience. Clin Breast Cancer. 2003;4:273–279. doi: 10.3816/cbc.2003.n.032. [DOI] [PubMed] [Google Scholar]
- 19.Smith RE, Bryant J, DeCillis A, et al. Acute myeloid leukemia and myelodysplastic syndrome after doxorubicin-cyclophosphamide adjuvant therapy for operable breast cancer: The national surgical adjuvant breast and bowel project experience. J Clin Oncol. 2003;21:1195–1204. doi: 10.1200/JCO.2003.03.114. [DOI] [PubMed] [Google Scholar]
- 20.Bergh J, Wiklund T, Erikstein B, et al. Tailored fluorouracil, epirubicin, and cyclophosphamide compared with marrow-supported high-dose chemotherapy as adjuvant treatment for high-risk breast cancer: A randomised trial. Lancet. 2000;356:1384–1391. doi: 10.1016/s0140-6736(00)02841-5. [DOI] [PubMed] [Google Scholar]
- 21.Fornier MN, Seidman AD, Theodoulou M, et al. Doxorubicin followed by sequential paclitaxel and cyclophosphamide versus concurrent paclitaxel and cyclophosphamide: 5-year results of a phase II randomized trial of adjuvant dose-dense chemotherapy for women with node-positive breast carcinoma. Clin Cancer Res. 2001;7:3934–3941. [PubMed] [Google Scholar]
- 22.Hudis C, Seidman A, Baselga J, et al. Sequential dose-dense doxorubicin, paclitaxel, and cyclophosphamide for resectable high-risk breast cancer: Feasibility and efficacy. J Clin Oncol. 1999;17:93–100. doi: 10.1200/JCO.1999.17.1.93. [DOI] [PubMed] [Google Scholar]
- 23.Basser RL, Abraham R, To LB, et al. Cardiac effects of high-dose epirubicin and cyclophosphamide in women with poor prognosis breast cancer. Ann Oncol. 1999;10:53–58. doi: 10.1023/a:1008390203340. [DOI] [PubMed] [Google Scholar]
- 24.Levine MN, Gent M, Hryniuk WM, et al. A randomized trial comparing 12 weeks versus 36 weeks of adjuvant chemotherapy in stage II breast cancer. J Clin Oncol. 1990;8:1217–1225. doi: 10.1200/JCO.1990.8.7.1217. [DOI] [PubMed] [Google Scholar]
- 25.Piccart MJ, Di Leo A, Beauduin M, et al. Phase III trial comparing two dose levels of epirubicin combined with cyclophosphamide with cyclophosphamide, methotrexate, and fluorouracil in node-positive breast cancer. J Clin Oncol. 2001;19:3103–3110. doi: 10.1200/JCO.2001.19.12.3103. [DOI] [PubMed] [Google Scholar]
- 26.Wils JA, Bliss JM, Marty M, et al. Epirubicin plus tamoxifen versus tamoxifen alone in node-positive postmenopausal patients with breast cancer: A randomized trial of the International Collaborative Cancer Group. J Clin Oncol. 1999;17:1988–1998. doi: 10.1200/JCO.1999.17.7.1988. [DOI] [PubMed] [Google Scholar]
- 27.Bonneterre J, Roché H, Kerbrat P, et al. Long-term cardiac follow-up in relapse-free patients after six courses of fluorouracil, epirubicin, and cyclophosphamide, with either 50 or 100 mg of epirubicin, as adjuvant therapy for node-positive breast cancer: French Adjuvant Study Group. J Clin Oncol. 2004;22:3070–3079. doi: 10.1200/JCO.2004.03.098. [DOI] [PubMed] [Google Scholar]
- 28.Fumoleau P, Roché H, Kerbrat P, et al. Long-term cardiac toxicity after adjuvant epirubicin-based chemotherapy in early breast cancer: French Adjuvant Study Group results. Ann Oncol. 2006;17:85–92. doi: 10.1093/annonc/mdj034. [DOI] [PubMed] [Google Scholar]
- 29.Abu-Khalaf MM, Juneja V, Chung GG, et al. Long-term assessment of cardiac function after dose-dense and -intense sequential doxorubicin (A), paclitaxel (T), and cyclophosphamide (C) as adjuvant therapy for high risk breast cancer. Breast Cancer Res Treat. 2007;104:341–349. doi: 10.1007/s10549-006-9413-7. [DOI] [PubMed] [Google Scholar]