Health-related quality of life was examined in long-term colorectal cancer survivors, 5, 10, and 15 years after diagnosis, in comparison with a control group from the general population. Effects were found up to 10 years after diagnosis, particularly for rectal cancer survivors.
Keywords: Colorectal cancer, Long-term survivors, Quality of life, Population-based study, SF-36, QLQ-C30
Learning Objectives
After completing this course, the reader will be able to:
Compare quality of life in long-term colorectal cancer survivors with quality of life in the general population.
Identify cancer complications that affect quality of life in long-term colorectal cancer survivors.
This article is available for continuing medical education credit at CME.TheOncologist.com
Abstract
Background.
The number of long-term colorectal cancer survivors is increasing. Cancer and its treatment can cause physical and psychological complications, but little is known about how it impacts quality of life (QOL) over the long term—5, 10, and 15 years after diagnosis.
Methods.
Cancer survivors were randomly selected from three tumor registries in France, diagnosed in 1990 (±1 year), 1995 (±1 year), and 2000 (±1 year). Controls were randomly selected from electoral rolls, stratifying on gender, age group, and residence area. Participants completed two QOL questionnaires, a fatigue questionnaire, an anxiety questionnaire, and a life conditions questionnaire. An analysis of variance was used to compare QOL scores of cancer survivors by period of diagnosis (5, 10, and 15 years) with those of controls, adjusted for sociodemographic data and comorbidities.
Results.
We included 344 colon cancer and 198 rectal cancer survivors and 1,181 controls. In a global analysis, survivors reported a statistically and clinically significant lower score in social functioning 5 years after diagnosis and higher scores in diarrhea symptoms 5 and 10 years after diagnosis. In subgroup analyses, rectal cancer affected QOL in the physical dimensions at 5 years and in the fatigue dimensions at 5 and 10 years.
Conclusion.
Survivors of colorectal cancer may experience the effects of cancer and its treatment up to 10 years after diagnosis, particularly for rectal cancer. Clinicians, psychologists, and social workers must pay special attention to rectal cancer survivors to improve overall management.
Introduction
Colorectal cancer is the third most common malignancy in France, with an estimated 40,000 new cases in 2009, and it is the second leading cause of cancer deaths after lung cancer, with 19,500 deaths in 2007 [1, 2]. This figure is similar in western Europe and in other high-resource countries like the U.S., Canada, and Australia [3, 4]. Thanks to advances in screening, earlier detection, and treatment, and also as a result of the aging population, the number of survivors of colorectal cancer is increasing [5].
The treatments that have helped to improve the cancer survival rate can cause several physical and psychological repercussions, which negatively impact health-related quality of life (HRQOL), particularly during the postoperative period. Some of these effects may even persist after the treatment period, and other problems can appear years later [6–8]. HRQOL information on long-term colorectal cancer survivors is important in order to evaluate the full spectrum of impact of the disease on patients, their family, and society. However, only during the last decade has understanding the long-term effects of cancer become a priority. In France, this was enshrined in the objectives of the National Cancer Plan in order to improve global management of cancer patients. Some studies have investigated the effects of cancer on QOL at least 5 years after diagnosis [9–14]. However, few were population-based studies, focused on colorectal cancer QOL, or performed on a large scale. No such study has been performed in France to date.
We performed a multicenter population-based study on patients randomly selected from the French regional cancer registries of the Calvados, Doubs, and Bas-Rhin departments to compare QOL of colorectal cancer survivors, considered as cured, 5, 10, and 15 years after diagnosis with that of healthy controls.
Materials and Methods
Survey Participants
All the colorectal cancer survivors were randomly selected from files of three population-based cancer registries in the Calvados, Doubs, and Bas-Rhin departments in France. Patients were eligible if they were disease free and not under treatment (except hormone therapy) for a period ≥5 years after diagnosis. They had to be >18 years old and be able to provide written informed consent. To evaluate the time effect on QOL, we defined three survival periods: 5, 10, and 15 years after diagnosis. We thus selected three colorectal cancer patient groups, diagnosed in 2000 (±1 year), in 1995 (±1 year), and in 1990 (±1 year).
Cancer survivors were compared with controls who had no prior history of cancer and were selected from the general population covered by the three cancer registry areas, that is, a total of 2.2 million inhabitants. In each registry area, controls were randomly selected from electoral rolls, were matched with cancer patients for gender, and were stratified on age (±10 years) and residence area (urban, ≥2,000 inhabitants; rural, <2,000 inhabitants) at the time of the survey. Controls were selected for each tumor location (colon or rectum) and for each of the three surviving periods (5, 10, or 15 years). Two controls were selected per case.
Survey Procedure
The project was approved by the Ethics Committee of the University Hospital of Besançon (Doubs, France) as well as by the National French Data Protection Authority.
After selection, we checked with the cancer registries database and the treating physician that the patients had not died or experienced relapse, metastasis, or another type of cancer or undergone treatment in the previous 5 years. Similarly, we verified in the registries data that controls did not have a prior history of cancer.
Data collection started in 2006. Selected subjects were mailed a package including: (a) a letter presenting the study aim signed by the medical department physician (for cancer patients) or by the coinvestigator in charge of the study in the relevant registry area (for the controls), (b) an informed consent form, (c) the questionnaires, and (d) a stamped return envelope. Persons who refused to participate had the opportunity to give their reasons on the consent form when they returned it. Nonrespondents were sent a reminder letter after 1 month.
Questionnaires
Participants completed standardized validated French language instruments addressing QOL, fatigue, and anxiety, plus a living conditions questionnaire.
The Medical Outcomes Study 36-item Short Form Health Survey (hereafter, SF-36) has 36 items measuring eight dimensions of health status: physical functioning, role limitation resulting from physical problems, role limitation resulting from emotional problems, social functioning, mental health, bodily pain, vitality, and general health perceptions. The eight dimensions can be combined into the physical component summary and mental component summary scores [15–17].
The European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire-Core 30 (QLQ-C30) assesses QOL with 30 items measuring 15 dimensions: a global health scale, five functioning scales (physical, daily activities, cognitive, emotional, and social), three symptoms scales (fatigue, pain, and nausea/vomiting) and six single-item scales measuring symptoms or problems (dyspnea, insomnia, appetite loss, constipation, diarrhea, and financial difficulties) [18, 19].
The French version of the Multidimensional Fatigue Inventory (MFI-20) was used to evaluate participants' fatigue. It is a 20-item questionnaire measuring five aspects of fatigue: general fatigue, physical fatigue, reduced activity, reduced motivation, and mental fatigue [20, 21].
Anxiety was assessed using the French version of the Spielberger State-Trait Anxiety Inventory (STAI). It contains two 20-item forms that measure state anxiety (the level of present anxiety) and trait anxiety (the general level of anxiety experienced and linked to personality). The global score for each form was calculated as the sum of the 20 items. Only state anxiety was analyzed because it was more appropriate for the present study [22].
A linear transformation was used to standardize raw scores to a 0–100 scale. For functional scales, higher scores indicate better perceived heath (SF-36, five dimensions in the QLQ-C30). For symptoms or problems scales, higher scores indicate a higher level of problems (nine dimensions in the QLQ-C30, MFI, and STAI).
In addition to the aforementioned questionnaires, we collected information about family, social, professional, and comorbid conditions using a questionnaire entitled Living Conditions, created by the research team of Calvados and used in previous French surveys [23–25]. This questionnaire records data on educational level, marital status, number of children, social and family relationships, professional status, monthly income, and use of medical services (health insurance, type and number of comorbidities, treatments, number of visits to treating physician or specialists, hospitalization). Prior to the survey, the five questionnaires were tested in 30 subjects (15 cases and 15 controls, 10 per registry area) not subsequently enrolled in the population study.
For cancer survivors, information on clinical variables (date of diagnosis, tumor extension, surgery, radiotherapy, chemotherapy, and stoma) was retrieved from medical records.
Statistical Analysis
Descriptive analysis was performed using the χ2 test for categorical variables and Kruskal-Wallis nonparametric test for QOL scores. Missing data for component items of QOL scores were treated according to published recommendations. Briefly, for the QLQ-C30, SF-36, and MFI questionnaires, missing items were attributed values equal to the average of the items that were present, provided at least half the items on the scale were answered. For the STAI questionnaire, 17 of the 20 items at least had to be answered to calculate the score, and missing items (maximum of three) were attributed a value equal to the average of the 17 items present [17, 19, 21, 22].
In order to identify potential confounding factors (sociodemographic and health variables) in the relation between QOL scores and cancer, we performed multivariate analysis of variance in controls separately for each QOL instrument.
For multivariate analyses, a four-level categorical variable to compare each of three survivor groups with controls was created. Analysis of variance was performed adjusting for confounding variables found to be significantly linked to QOL scores in the previous step. Considering the study design, registry areas (Calvados, Doubs, Bas-Rhin), age group (18–54 years, 55–64 years, 65–74 years, ≥75 years), gender, and residence area (urban versus rural) were systematically included as explanatory variables. Following this analysis, score adjusted means were computed. Statistical score mean differences and their confidence limits were considered significant if p ≤ .01 (accounting for numerous tests performed). Regarding clinical significance, we relied on the values generally in use, according to Osoba et al. [26]. That is, on a scale of 0–100, a difference of 5–10 units was considered small, a difference of 10–20 units was considered moderate, and a difference >20 units was considered large. A difference <5 units was considered as not clinically significant.
First, global analysis was performed in the whole sample (both tumor locations and both genders taken together). Then, analyses by subgroups were conducted according to tumor location (colon and rectum) and gender. All analyses were performed using the same methodology. The statistical analysis software SAS, version 9.1 (SAS Institute Inc., Cary, NC) was used to analyze data.
Considering the general results of QOL studies regarding score variability, the present study was designed to be able to detect a difference ≥10 points on a scale of 0–100 when the standard deviation of the difference was equal to 60 (in a matched setting with two controls per case). With a first-type error of 0.01 and a power of 90%, this required 536 cases to be recruited. Consequently, approximately 670 cases, with an expected participation rate of 80%, and 2,100 controls, with an expected participation rate of 50%, needed to be selected.
Results
Participation Rate and Participant Characteristics
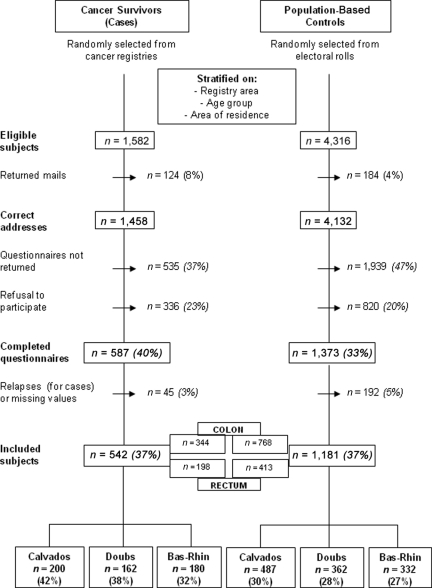
Taking into account the participation rate, 1,582 eligible colorectal cancer survivors were selected. Of these, 1,458 were contacted and 542 accepted participation and fully completed the questionnaires—64% colon cancer and 36% rectal cancer. The response rate averaged 37% over the three regions (42% in Calvados, 38% in Doubs, and 32% in Bas-Rhin) and decreased gradually with increasing time since diagnosis (from 39% at 5 years to 27% at 15 years). Among the 4,132 controls contacted, 1,181 completed the questionnaires. The response rate averaged 28% over the three regions (30% in Calvados, 28% in Doubs, and 27% in Bas-Rhin) (Fig. 1).
Figure 1.
Flowchart of the study population.
The main reasons given for refusal to participate were that the questionnaires were too long or the subjects were too old or too disabled. Indeed, nonparticipants were older than participants—75.9 years versus 70.8 years (p < .0001) for cases and 72.9 years versus 70.2 years (p < .0001) for controls—and the percentage of women was higher among nonparticipants (52.1% versus 43.1%; p = .0007). More of the nonparticipants had survived ≥15 years than the participants in the same period (26.5% versus 18.3%; p = .0003).
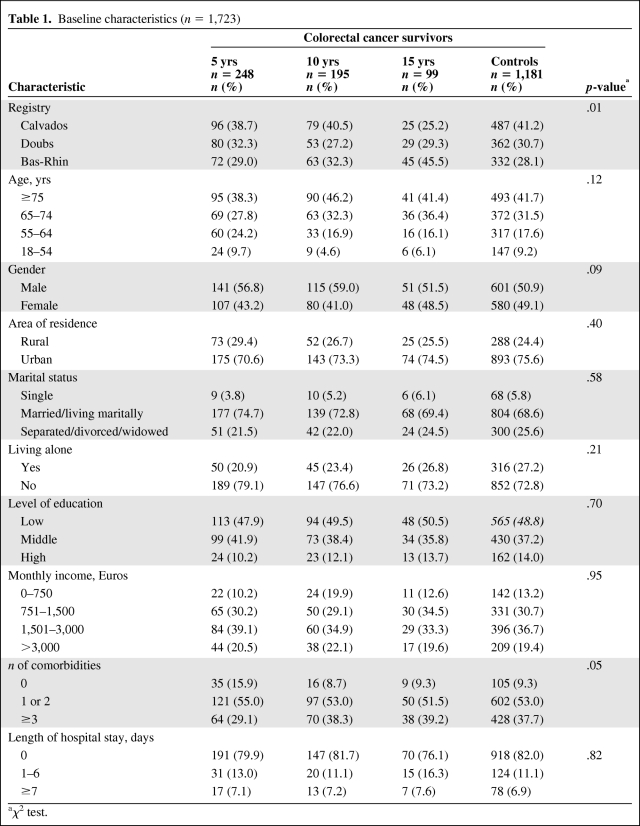
Baseline sociodemographic characteristics of cases and controls are presented in Table 1. There were no statistically significant differences among the four groups, except for a significant difference in the distribution of cases in the 15-year survivors group between Calvados and Bas-Rhin.
Table 1.
Baseline characteristics (n = 1,723)
aχ2 test.
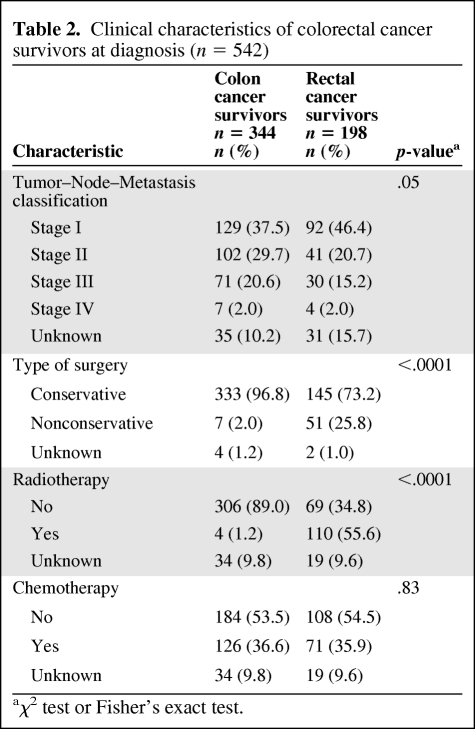
Clinical characteristics are presented in Table 2. All cancer survivors had surgery, but more rectal cancer survivors maintained a permanent colostomy. They received more radiotherapy, but not significantly more chemotherapy, than colon cancer survivors. According to the tumor–node–metastasis classification, colon cancer survivors were diagnosed with a higher stage of tumor than rectal cancer survivors.
Table 2.
Clinical characteristics of colorectal cancer survivors at diagnosis (n = 542)
aχ2 test or Fisher's exact test.
Variables Related to QOL in Controls
According to the results of the multivariate analysis of variance, age group, gender, marital status, living alone, level of education, employment status, income, comorbid conditions and length of hospital stay significantly influenced all scales (p ≤ .01). As a result, all these variables were included in the subsequent analysis of variance.
Comparison of QOL Between Survivors and Controls
Global Analysis
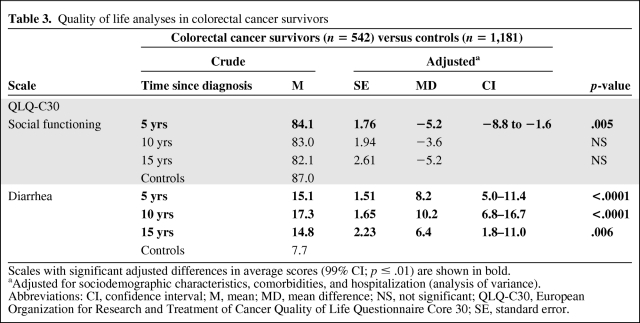
On the EORTC QLQ-C30 questionnaire, cancer survivors showed significantly different adjusted mean scores than controls for two scales, namely, social functioning and diarrhea. We found a clinically significantly lower score at 5 years after diagnosis for the social functioning scale in cancer survivors (Table 3). On the diarrhea scale, we observed a clinically significantly higher score, qualified as moderate at 10 years and small at 5 years and 15 years after diagnosis, in cancer survivors than in controls (Table 3).
Table 3.
Quality of life analyses in colorectal cancer survivors
Scales with significant adjusted differences in average scores (99% CI; p ≤ .01) are shown in bold.
aAdjusted for sociodemographic characteristics, comorbidities, and hospitalization (analysis of variance).
Abbreviations: CI, confidence interval; M, mean; MD, mean difference; NS, not significant; QLQ-C30, European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 30; SE, standard error.
On the SF-36, MFI-20, and STAI questionnaires, there was no clinically significant difference in any of the QOL scores between cancer survivors and controls at any time point after diagnosis.
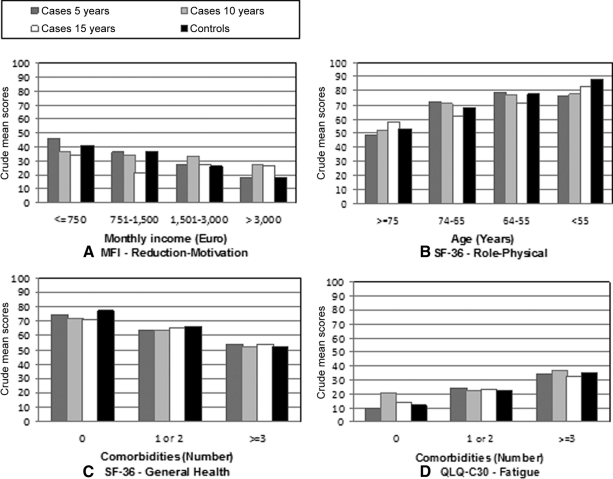
Monthly income, age, and comorbid conditions influenced most of the QOL scores irrespective of time since diagnosis. Low income, advanced age, and the presence of more than three comorbidities negatively impacted QOL (Fig. 2).
Figure 2.
Quality of life crude scores according to monthly income (A), age group (B), and comorbidities (C, D). Higher scores indicate a better health status, except on the fatigue scale where higher scores indicate a higher level of symptoms.
Abbreviations: QLQ-C30, Quality of Life Questionnaire Core 30; SF-36, Medical Outcomes Study 36-Item Short Form; MFI, Multidimensional Fatigue Inventory.
Results According to Cancer Location
In colon cancer survivors, we observed clinically small but statistically significantly higher scores on the diarrhea scale (QLQ-C30) than in controls at 5 years and 10 years after diagnosis (data not shown). There were no clinically significant differences in other QOL scores between colon cancer survivors and controls at any time point after diagnosis.
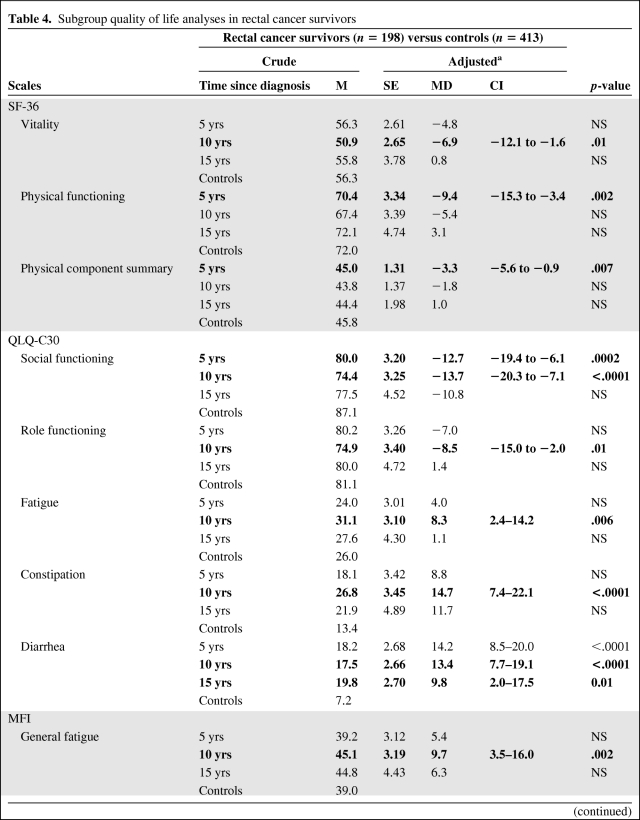
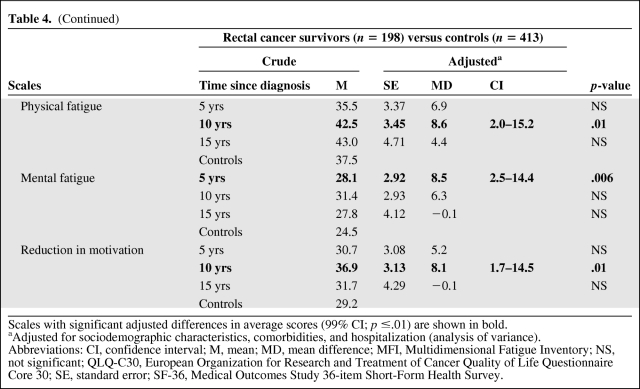
In rectal cancer survivors, we observed statistically significant differences in functioning scales, particularly in the physical and social dimensions (Table 4). The vitality and physical functioning scores were lower, respectively, at 10 years and at 5 years after diagnosis, as was the physical component summary score (SF-36) at 5 years, in comparison with those of controls. On the social functioning scale, the score was lower at 5 years and 10 years after diagnosis and on the role functioning scale (QLQ-C30), the score was lower only at 5 years, compared with controls. Rectal cancer survivors perceived more problems in terms of symptoms: their score on the diarrhea scale (QLQ-C30) was higher at 5 years, 10 years, and 15 years after diagnosis than in controls. Constipation problems (QLQ-C30) were also more frequently reported by rectal cancer survivors at 10 years after diagnosis (Table 4). Similarly, rectal cancer also affected most of the fatigue scales: on the fatigue scale (QLQ-C30), the general and physical fatigue scales, and the reduction of motivation scale (MFI), scores were higher at 10 years after diagnosis, whereas the mental fatigue score was higher at 5 years, in comparison with controls. The fatigue score differences, clinically qualified as small, are shown in Table 4.
Table 4.
Subgroup quality of life analyses in rectal cancer survivors
Table 4a.
(Continued)
Scales with significant adjusted differences in average scores (99% CI; p ≤.01) are shown in bold.
aAdjusted for sociodemographic characteristics, comorbidities, and hospitalization (analysis of variance).
Abbreviations: CI, confidence interval; M, mean; MD, mean difference; MFI, Multidimensional Fatigue Inventory; NS, not significant; QLQ-C30, European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 30; SE, standard error; SF-36, Medical Outcomes Study 36-item Short-Form Health Survey.
Results According to Sex
Male and female survivors of colorectal cancer showed statistically and clinically significantly higher scores on the diarrhea scale (QLQ-C30) at 5 years and 10 years after diagnosis than controls. We observed a statistically and clinically significant lower score at 5 years after diagnosis on the social functioning scale in male survivors than in the overall population (data not shown). In female survivors, this dimension seemed to be affected at 15 years after diagnosis, but the statistical significance was borderline. Concerning the vitality scale (QLQ-C30), we observed a clinically significant lower score for male cancer survivors at 10 years after diagnosis. On the mental fatigue scale (MFI), the score was lower in male cancer survivors at 5 years after diagnosis, results that were not replicated in female cancer survivors (data not shown). In female cancer survivors only, colorectal cancer did not especially influence QOL dimensions.
Discussion
In the overall study population, the QOL of colorectal cancer survivors seemed to be relatively satisfactory 15 years after diagnosis. Our data showed that QOL reached quite high levels in this group, comparable with those of the general population. These results are consistent with most previous research on QOL among survivors of colorectal and other cancers. Furthermore, overall QOL increased over time, with long-term survivors having the best QOL [8, 9]. Conversely, one previous study demonstrated that health status perceived by cancer survivors remained poor, even ≥11 years after diagnosis [27]. However, these results could be a result of the inclusion of patients with recurrence or metastasis, who have a worse prognosis than those in our study (staging in Table 2). Three explanations can be proposed for the few statistical or clinical differences detected in overall QOL, especially at 15 years after diagnosis, between colorectal cancer survivors and controls. First, these could be a result of the “response shift” phenomenon, which suggests that an individual may change his internal standards or redefine his concept of HRQOL over time [28, 29]. Second, in our study, several QOL score differences were borderline significant. We performed our analyses in a sample of colorectal cancer survivors including both colon and rectal cancer survivors and both men and women, and the weight of certain subgroups may not have been strong enough to show differences in a global analysis. Third, the response rate in the 15-year time period was very low and we cannot exclude the possibility that the most disabled survivors with the worst QOL did not respond.
On the other hand, we found a very statistically and clinically significant score difference in the diarrhea symptom. Diarrhea is a common specific symptom in colorectal cancer and is reported in several studies [8, 9, 30]. It is also a specific symptom in other abdominal cancers treated using radiotherapy such as prostate and gynecological cancers [6, 11, 31–33]. This symptom can appear in the acute period, or weeks to years after radiotherapy.
In the overall study population, colorectal cancer survivors reported perceiving a problem in social relations 5 years after diagnosis, but not thereafter. Depending on the instrument used, the social dimension is not always measured in QOL surveys. For colorectal cancer, various authors have shown that this dimension is impacted more within the first few years following diagnosis, with a possible long-lasting effect [6, 8, 9, 34].
To date, there has been little research among long-term colon cancer survivors only. Research has focused either on rectal cancer survivors alone or on colorectal cancer survivors overall. In the latter case, few studies have directly compared colon cancer and rectal cancer long-term survivors. In 2001, Phipps et al. [30], in a study of QOL among long-term colon cancer survivors, detected intestinal, emotional, and sexual problems as well as pain in this group. These findings were relatively consistent with our data. Phipps et al. [30] excluded rectal cancer patients to evaluate the effect of colon cancer proper, because treatment differences and a possible permanent colostomy could bias the results. In our survey, rectal cancer survivors perceived more disabilities and difficulties than controls in numerous QOL dimensions. Because we performed comparisons between colon cancer survivors and controls, and between rectal cancer survivors and controls, we cannot infer any differences in QOL between colon and rectal cancer. Thus, our results suggest that possible differences might be a result of different treatment and, indeed, rectal cancer survivors received radiotherapy and kept a permanent stoma significantly more frequently than colon cancer survivors (Table 2). This suggestion was confirmed by Stein et al. [35], who demonstrated that radiotherapy impacted QOL in the physical dimension and mainly diarrhea symptoms and fatigue, results that were adjusted for tumor location and disease stage. Regarding the effect of a permanent stoma on QOL, in rectal cancer only studies, some authors have shown that the overall QOL of long-term rectal cancer survivors was similar to that of population controls, with the presence of stoma having no real impact on QOL [7, 36]. Studies that have directly compared QOL between colon and rectal cancer survivors have shown conflicting findings. Some noted no relevant difference with respect to QOL, but QOL was evaluated 1 year after diagnosis [6]. Others observed that rectal cancer patients reported a lower QOL than colon cancer survivors regarding bowel symptoms at an average of 3 years after diagnosis [37]. It is possible that relatively early information on cancer complications may not always predict long-term complications, and sequelae of rectum cancer may persist or worsen over time, whereas colon cancer complications may be reduced or disappear.
Few statistically and clinically significant differences were found between male cancer survivors and male controls, and between female cancer survivors and female controls, except with regard to diarrhea problems. Male cancer survivors perceived some difficulties, such as lack of vitality, mental fatigue, and social problems, difficulties that were not perceived by female cancer survivors. These results seem to be consistent with previous studies reporting that QOL was better among female cancer survivors than among male cancer survivors for colorectal cancer or for cancer in general, whereas long-term QOL in female cancer survivors was similar to that of the general population [13, 14, 38, 39].
Our study is the first in France to gather information about the QOL of a population-based group of long-term colorectal cancer survivors. It includes a large sample of cancer survivors and was of sufficient statistical power to assess QOL score differences. We used validated and complementary questionnaires, with good psychometric properties. The QLQ-C30 specific cancer questionnaire assesses the degree of perceived symptoms or problems, which is not evaluated in the SF-36 generic health questionnaire. Fatigue and anxiety are common symptoms in cancer and need to be measured through the use of more thorough questionnaires. In our study, we accounted for socioeconomic factors likely to impact QOL in colorectal cancer survivors, such as monthly income, age, and chronic comorbidities [6, 9, 32, 40]. Despite the weight of these factors, cancer survivors perceived difficulties that we can only attribute to cancer. Nevertheless, the study also had some limitations. We sought to evaluate the time effect on QOL transversally in three different groups of cancer survivors. Although the three time-since-diagnosis groups were comparable, some individual differences might persist within a group. Moreover, the number of cases in each of the three groups decreased substantially with increasing time since diagnosis. This type of design is relatively infrequent in this type of research, and a longitudinal study would likely have been more appropriate to assess QOL evolution over time. Furthermore, the low response rate may undermine the representativeness of the study sample, but the magnitude and the orientation of this bias are uncertain. If nonrespondents were more disabled than respondents, QOL differences between colorectal cancer survivors and controls would be underestimated (and vice versa). Finally, although our results seem to be consistent with those of the literature, we are not able to confirm them in indirect comparisons between colon and rectal cancer survivors, or between males and females.
Conclusion
Our study focused on colorectal cancer survivors, considered as cured (without recurrence or metastasis ≥5 years after diagnosis) and showed that QOL in long-term colorectal cancer survivors seems to be globally satisfactory 15 years after diagnosis, in comparison with that of population controls. Nevertheless, some cancer complications may persist for ≥10 years after diagnosis, such as bowel problems, fatigue, and social relations problems. Our results need to be confirmed in a longitudinal survey, focusing on how prior treatment impacts QOL in the long term, and separating analyses of colon and rectal cancer.
Clinicians, psychologists, and social workers must pay special attention to colorectal cancer patients during this period, in particular, survivors of rectal cancer and older survivors. Moreover, management of comorbidities is essential to avoid worsening of the cancer's effects.
Acknowledgments
The authors thank all physicians from the three cancer registries who kindly agreed to ask cases treated to participate in the study. We also thank Fiona Ecarnot for editorial assistance and Marc Puyraveau for methodological support.
This work was supported by the French Programme Hospitalier de Recherche Clinique of the National Institut of Cancer (INCa), the Fondation de France, and the Ligue Contre le Cancer du Doubs.
Footnotes
- (C/A)
- consulting/advisory relationship
- (RF)
- Research funding
- (E)
- Employment
- (H)
- Honoraria received
- (OI)
- Ownership interests
- (IP)
- Intellectual property rights/inventor/patent holder
Author Contributions
Conception/Design: Michel Henry-Amar, Michel Velten, Mariette Mercier
Provision of study material or patients: Guy Launoy, Michel Henry-Amar, Arlette Danzon, Michel Velten, Mariette Mercier
Collection and/or assembly of data: Agnès Caravati-Jouvenceaux, Guy Launoy, Delphine Klein, Edwige Abeilard, Arlette Danzon, Astrid Pozet
Data analysis and interpretation: Agnès Caravati-Jouvenceaux
Manuscript writing: Agnès Caravati-Jouvenceaux
Final approval of manuscript: Agnès Caravati-Jouvenceaux, Guy Launoy, Delphine Klein, Michel Henry-Amar, Edwige Abeilard, Arlette Danzon, Astrid Pozet, Michel Velten, Mariette Mercier
References
- 1.Guérin S, Hill C. Cancer epidemiology in France in 2010, comparison with the USA. Bull Cancer. 2010;97:47–54. doi: 10.1684/bdc.2010.1013. In French. [DOI] [PubMed] [Google Scholar]
- 2.Belot A, Grosclaude P, Bossard N, et al. Cancer incidence and mortality in France over the period 1980–2005. Rev Epidemiol Sante Publique. 2008;56:159–175. doi: 10.1016/j.respe.2008.03.117. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Murray T, Samuels A, et al. Cancer statistics, 2003. CA Cancer J Clin. 2003;53:5–26. doi: 10.3322/canjclin.53.1.5. [DOI] [PubMed] [Google Scholar]
- 4.Parkin DM. Global cancer statistics in the year 2000. Lancet Oncol. 2001;2:533–543. doi: 10.1016/S1470-2045(01)00486-7. [DOI] [PubMed] [Google Scholar]
- 5.Faivre-Finn C, Bouvier-Benhamiche AM, Phelip JM, et al. Colon cancer in France: Evidence for improvement in management and survival. Gut. 2002;51:60–64. doi: 10.1136/gut.51.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arndt V, Merx H, Stegmaier C, et al. Quality of life in patients with colorectal cancer 1 year after diagnosis compared with the general population: A population-based study. J Clin Oncol. 2004;22:4829–4836. doi: 10.1200/JCO.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 7.Rauch P, Miny J, Conroy T, et al. Quality of life among disease-free survivors of rectal cancer. J Clin Oncol. 2004;22:354–360. doi: 10.1200/JCO.2004.03.137. [DOI] [PubMed] [Google Scholar]
- 8.Schneider EC, Malin JL, Khan KL, et al. Surviving colorectal cancer: Patient-reported symptoms 4 years after diagnosis. Cancer. 2007;110:2075–2082. doi: 10.1002/cncr.23021. [DOI] [PubMed] [Google Scholar]
- 9.Ramsey SD, Berry K, Moinpour C, et al. Quality of life in long term survivors of colorectal cancer. Am J Gastroenterol. 2002;97:1228–1234. doi: 10.1111/j.1572-0241.2002.05694.x. [DOI] [PubMed] [Google Scholar]
- 10.Zebrack BJ, Yi J, Petersen L, et al. The impact of cancer and quality of life for long-term survivors. Psychooncology. 2008;17:891–900. doi: 10.1002/pon.1300. [DOI] [PubMed] [Google Scholar]
- 11.Nord C, Mykletun A, Thorsen L, et al. Self-reported health and use of health care services in long-term cancer survivors. Int J Cancer. 2005;114:307–316. doi: 10.1002/ijc.20713. [DOI] [PubMed] [Google Scholar]
- 12.Eakin EG, Youlden DR, Baade PD, et al. Health status of long-term cancer survivors: Results from an Australian population-based sample. Cancer Epidemiol Biomarkers Prev. 2006;15:1969–1976. doi: 10.1158/1055-9965.EPI-06-0122. [DOI] [PubMed] [Google Scholar]
- 13.Trentham-Dietz A, Remington PL, Moinpour CM, et al. Health-related quality of life in female long-term colorectal cancer survivors. The Oncologist. 2003;8:342–349. doi: 10.1634/theoncologist.8-4-342. [DOI] [PubMed] [Google Scholar]
- 14.Schultz PN, Beck ML, Stava C, et al. Health profiles in 5836 long-term cancer survivors. Int J Cancer. 2003;104:488–495. doi: 10.1002/ijc.10981. [DOI] [PubMed] [Google Scholar]
- 15.McHorney CA, Ware JE, Jr, Lu JF, et al. The MOS 36-item Short Form Health Survey (SF-36): III. Tests of data quality, scaling assumptions, and reliability across diverse patients groups. Med Care. 1994;32:40–66. doi: 10.1097/00005650-199401000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Leplège A, Mesbah M, Marquis P. [Preliminary analysis of the psychometric properties of the French version of an international questionnaire measuring the quality of life: The MOS SF-36 (version 1.1).] Rev Epidemiol Sante Publique. 1995;43:371–379. In French. [PubMed] [Google Scholar]
- 17.Leplège A, Ecosse E, Pouchot J, et al. Paris: Editions ESTEM; 2001. Le Questionnaire MOS SF-36: Manuel de l'Utilisateur et Guide d'Interprétation des Scores; pp. 1–155. [Google Scholar]
- 18.Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 19.Fayers P, Aaronson N, Bjordal K, et al. EORTC QLQ-C30 Scoring Manuel. Third Edition. Brussels, Belgium: EORTC Quality of Life Group; 2001. pp. 1–49. [Google Scholar]
- 20.Smets EM, Garssen B, Bonke B, et al. The Multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. J Psychosom Res. 1995;39:315–325. doi: 10.1016/0022-3999(94)00125-o. [DOI] [PubMed] [Google Scholar]
- 21.Gentile S, Delarozière JC, Favre F, et al. Validation of the French ‘Multidimensional Fatigue Inventory’ (MFI-20) Eur J Cancer Care (Engl) 2003;12:58–64. doi: 10.1046/j.1365-2354.2003.00295.x. [DOI] [PubMed] [Google Scholar]
- 22.Spielberger CD. Paris: Centre de Psychologie Appliquée; 1993. Inventaire d'Anxiété tat-Trait: Adaptation Frana¸ise de Bruchon-Schweitzer M, Paulhan I; pp. 1–68. In French. [Google Scholar]
- 23.Joly F, Héron JF, Kalusinski L, et al. Quality of life in long-term survivors of testicular cancer: A population-based case-control study. J Clin Oncol. 2002;20:73–80. doi: 10.1200/JCO.2002.20.1.73. [DOI] [PubMed] [Google Scholar]
- 24.Joly F, Henry-Amar M, Arveux P, et al. Late psychosocial sequelae in Hodgkin's disease survivors: A French population-based case-control study. J Clin Oncol. 1996;14:2444–2453. doi: 10.1200/JCO.1996.14.9.2444. [DOI] [PubMed] [Google Scholar]
- 25.Joly F, Espié M, Marty M, et al. Long-term quality of life in premenopausal women with node-negative localized breast cancer treated with or without adjuvant chemotherapy. Br J Cancer. 2000;83:577–582. doi: 10.1054/bjoc.2000.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Osoba D, Rodrigues G, Myles J, et al. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol. 1998;16:139–144. doi: 10.1200/JCO.1998.16.1.139. [DOI] [PubMed] [Google Scholar]
- 27.Yabroff KR, Lawrence WF, Clauser S, et al. Burden of illness in cancer survivors: Findings from a population-based national sample. J Natl Cancer Inst. 2004;96:1322–1330. doi: 10.1093/jnci/djh255. [DOI] [PubMed] [Google Scholar]
- 28.Sprangers MA, Schwartz CE. Integrating response shift into health-related quality of life research: A theoretical model. Soc Sci Med. 1999;48:1507–1515. doi: 10.1016/s0277-9536(99)00045-3. [DOI] [PubMed] [Google Scholar]
- 29.Mols F, van de Poll-Franse LV, Vingerhoets AJ, et al. Long-term quality of life among Dutch prostate cancer survivors: Results of a population-based study. Cancer. 2006;107:2186–2196. doi: 10.1002/cncr.22231. [DOI] [PubMed] [Google Scholar]
- 30.Phipps E, Braitman LE, Stites S, et al. Quality of life and symptom attribution in long-term colon cancer survivors. J Eval Clin Pract. 2008;14:254–258. doi: 10.1111/j.1365-2753.2007.00842.x. [DOI] [PubMed] [Google Scholar]
- 31.Bloom JR, Petersen DM, Kang SH. Multi-dimensional quality of life among long-term (5+ years) adult cancer survivors. Psychooncology. 2007;16:691–706. doi: 10.1002/pon.1208. [DOI] [PubMed] [Google Scholar]
- 32.Miller DC, Sanda MG, Dunn RL, et al. Long-term outcomes among localized prostate cancer survivors: Health-related quality-of-life changes after radical prostatectomy, external radiation, and brachytherapy. J Clin Oncol. 2005;23:2772–2780. doi: 10.1200/JCO.2005.07.116. [DOI] [PubMed] [Google Scholar]
- 33.Wenzel LB, Donnelly JP, Fowler JM, et al. Resilience, reflection, and residual stress in ovarian cancer survivorship: A Gynecologic Oncology Group Study. Psychooncology. 2002;11:142–153. doi: 10.1002/pon.567. [DOI] [PubMed] [Google Scholar]
- 34.Ramsey SD, Andersen MR, Etzioni R, et al. Quality of life in survivors of colorectal carcinoma. Cancer. 2000;88:1294–1303. [PubMed] [Google Scholar]
- 35.Stein KD, Syrjale KL, Andrykowski MA. Physical and psychological long-term and late effects of cancer. Cancer. 2008;112(11 suppl):2577–2592. doi: 10.1002/cncr.23448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu L, Herrington LJ, Hornbrook MC, et al. Early and late complications among colorectal cancer survivors with ostomy or anastomosis. Dis Colon Rectum. 2010;53:200–212. doi: 10.1007/DCR.0b013e3181bdc408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Di Fabio F, Koller M, Nascimbeni R, et al. Long-term outcome after colorectal cancer resection. Patients' self-reported quality of life, sexual dysfunction and surgeons' awareness of patients' needs. Tumori. 2008;94:30–35. doi: 10.1177/030089160809400107. [DOI] [PubMed] [Google Scholar]
- 38.Yost KJ, Hahn EA, Zaslavski AM, et al. Predictors of health-related quality of life in patients with colorectal cancer. Health Quality Life Outcomes. 2008;6:66. doi: 10.1186/1477-7525-6-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sapp AL, Trentham-Dietz A, Newcomb PA, et al. Social networks and quality of life among female long-term colorectal cancer survivors. Cancer. 2003;98:1749–1758. doi: 10.1002/cncr.11717. [DOI] [PubMed] [Google Scholar]
- 40.Baker F, Denniston M, Smith T, et al. Adult cancer survivors: How are they faring? Cancer. 2005;104(11 suppl):2565–2576. doi: 10.1002/cncr.21488. [DOI] [PubMed] [Google Scholar]