Current options for the management of hot flashes are addressed, key endpoints from recent clinical trials are examined, and future directions are reviewed.
Keywords: Vasomotor symptoms, Hot flashes, Menopause, Therapy, Quality of life
Abstract
Many therapies are being studied for the treatment of hot flashes for individuals with cancer, yet few studies have demonstrated safe and effective clinical benefit for those who suffer from this distressing symptom. The purpose of this paper is to assess the current options for the management of hot flashes, examining key endpoints from recent clinical trials and reviewing future directions. Hot flashes are a common stressful symptom for individuals with cancer, particularly women with a history of breast cancer and men with prostate cancer. Lifestyle modifications are proposed as the first step in the management of less severe hot flashes. Several publications have addressed nonhormonal agents as a treatment option for hot flashes. Newer antidepressant and anticonvulsant agents have been studied and show potential in treating vasomotor symptoms. Although many complementary and alternative therapies, including herbal medications and phytoestrogens, have been studied for the treatment of hot flashes, none are clinically recommended at this time. Additionally, further evidence is needed for supportive exercise such as yoga and relaxation techniques. Acupuncture may warrant further investigation in the reduction and severity of hot flashes in both men and women. Hormonal therapies, including estrogens and progestogens, are the most well-known and efficient agents in alleviating hot flashes; however, the safety of these agents is disputable.
Introduction
Menopause, an expected transitional life occurrence in women, is generally defined as the 12-month period of amenorrhea that occurs after the final menstrual period. This phase, which typically begins at a median age of 51 years, reflects ovarian follicular depletion and marked reduction in ovarian estrogen secretion. Clinical manifestations associated with menopause include, but are not limited to, vasomotor symptoms, genitourinary symptoms, sleep disturbance, and mood changes [1, 2]. One of the well-known symptoms of menopause is the occurrence of hot flashes, which occur in >75% of menopausal women [1]. Hot flashes are often described as episodic sensations of heat, intense sweating, and flushing affecting the face and chest, which are often accompanied by palpitations and anxiety [3]. Each particular episode lasts 3–10 minutes and episodes can recur with varying frequency [4]. Some women experience hot flashes hourly or daily, whereas for others they may occur occasionally. Similar to the variability in frequency of this symptom, the age at onset of hot flashes also varies from woman to woman. Although most women develop hot flashes during the perimenopausal and early postmenopausal periods, a minority of patients develop hot flashes while menstrual cycles remain regular. Furthermore, the majority of women have hot flashes for 1–2 years, but ∼15% may have persistent hot flashes for up to 30 years.
Hot flashes are common among breast cancer patients resulting from multiple treatment options that can induce an estrogen deprivation state, including antiestrogenic medications, chemical or medical oophorectomy, and certain cytotoxic agents [1]. The average incidence of hot flashes in women treated with an antiestrogenic agent such as tamoxifen is 60%–70% [5, 6]. A placebo-controlled trial, specifically designed to evaluate the symptoms associated with tamoxifen treatment in postmenopausal women, demonstrated that tamoxifen was associated with hot flashes in up to 67% of patients [7, 8]. More recently, however, aromatase inhibitors have become widely used for the treatment of breast cancer in postmenopausal women. Studies comparing exemestane with tamoxifen for first-line hormonal therapy of metastatic breast cancer in postmenopausal women have shown that the incidence of hot flashes of any grade was slightly lower with exemestane (35.1%) than with tamoxifen (38.1%) [9, 10]. This difference between aromatase inhibitors and tamoxifen was echoed in the Breast International Group (BIG) 1–98 and the Arimidex, Tamoxifen, Alone, or in Combination trials [11, 12]. For example, in the BIG 1–98 clinical trial, hot flashes were more common among women who were randomized to a tamoxifen-containing regimen than among women who received letrozole monotherapy (41.7%–44% versus 37.7%; p = .003) [11, 13].
In addition to endocrine therapy, certain chemotherapeutic agents may increase the likelihood of hot flashes by inducing premature menopause. One prospective study demonstrated that, when compared with women who had undergone natural menopause, a greater proportion of patients who had undergone chemotherapy-induced menopause developed moderate to severe hot flashes [14]. The likelihood of chemotherapy-induced menopause is related to age at treatment, type of cytotoxic agent, and dose of chemotherapy. For example, ∼40% of 40-year-old premenopausal women who receive chemotherapy undergo premature menopause, whereas >90% of 50-year-old women are likely to undergo early menopause after chemotherapy [15]. Additionally, a higher risk for menopause is associated with larger cumulative doses and with longer durations of cytotoxic therapy [16–17]. This chemically induced menopause thus leads to a higher incidence of vasomotor symptoms, including hot flashes.
For men, the phenomenon of hot flashes often occurs as a result of medical or surgical treatment for prostate cancer. Although up to 75% of men treated with androgen deprivation therapy may experience hot flashes, there are limited therapeutic options for the treatment of hot flashes [18–20].
This article focuses on the pathophysiology and management of hot flashes in cancer patients, with particular focus on the breast cancer and prostate cancer populations.
Methods
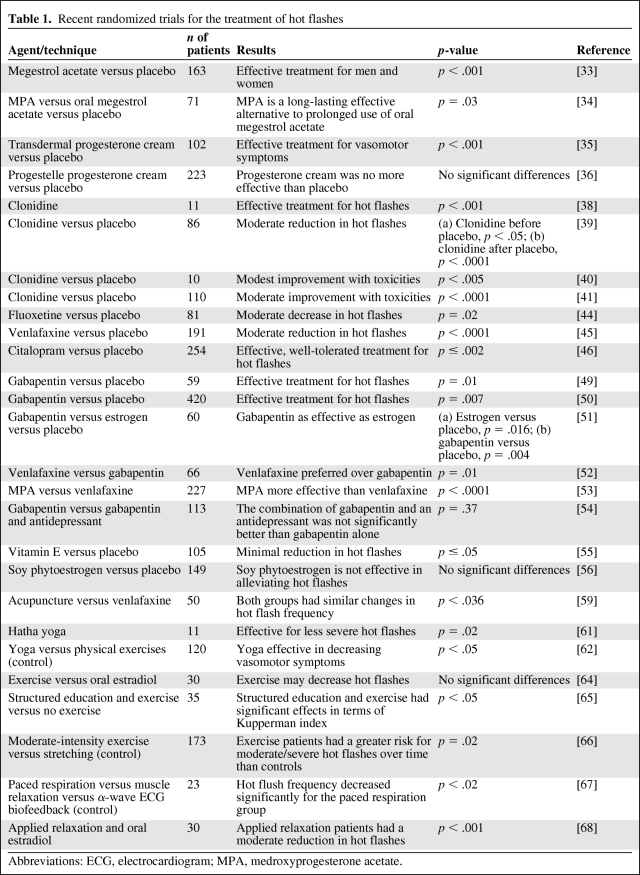
We used the following keywords in our search of the literature: vasomotor symptoms, hot flashes, hot flush, menopause, and quality of life. Recognizing that research in hot flashes is limited by the subjectivity of the patient and researcher, diverse methods of measuring hot flash improvement, and placebo effects, we focused primarily on placebo-controlled, randomized trials. We summarized significant clinical trials in Table 1.
Table 1.
Recent randomized trials for the treatment of hot flashes
Abbreviations: ECG, electrocardiogram; MPA, medroxyprogesterone acetate.
Pathophysiology
Despite extensive research, the pathophysiology of hot flashes is not entirely understood. The onset of hot flashes is hypothesized to be related to dysfunction of the thermoregulatory nucleus, which is essential in regulating the homeostatic range and the core body temperature [1, 21]. The thermoregulatory nucleus maintains the core body temperature within a homeostatic range termed the thermoregulatory zone. Sweating occurs when the body's core temperature increases above the upper threshold of the thermoregulatory zone, whereas chills occur when the core temperature dips below the lower threshold of this zone. Freedman found that women who experience hot flashes demonstrated a smaller thermoregulatory zone, leading to a greater likelihood of crossing these thresholds and thus developing the sweats and chills that are associated with hot flashes [22].
In addition, as estrogen levels decline in menopause, norepinephrine levels rise, leading to an upregulation of hypothalamic serotonin receptors, which are involved in temperature regulation [3]. Central α-2 receptors may be affected by estrogen depletion, leading to higher central norepinephrine levels [23]. This activation of the noradrenergic and serotonin pathways may further narrow the upper threshold of the thermoregulatory zone, leading to a greater propensity for hot flashes [21]. However, absolute estrogen levels are not solely responsible for hot flashes, because researchers have found no significant correlation between plasma, urinary, or vaginal estrogen levels and the appearance of this symptom [23]. Instead, it is the relative decline in estrogen levels that appears to mediate these central changes in norepinephrine and serotonin. This theory was supported by the finding of a greater prevalence of hot flashes in women who develop acute estrogen withdrawal after bilateral oophorectomy than in those who experience gradual ovarian failure related to natural menopause [24]. Interestingly, involvement of the opioidergic system has been explored; however, there is no consistent evidence for opiate interaction [23].
Treatment Interventions
Hormonal Therapies
Estrogen Replacement Therapies
Hormone replacement therapy (HRT) is widely known as an effective therapeutic means for alleviating hot flashes. However, a number of endocrine factors have been linked to the incidence of breast cancer [25, 26]. Estrogen is effective in improving hot flashes caused by not only natural menopause but also by chemotherapy-induced ovarian failure, tamoxifen use, androgen ablation therapy, and the use of leuteinizing hormone-releasing hormone agonists such as goserelin [21]. To reduce the possible harmful effects of estrogen therapy, recent trials have focused on the use of lower estrogen doses in order to decrease hot flash symptomatology. For example, Bachmann and colleagues randomized healthy postmenopausal women who were experiencing hot flashes to either low-dose transdermal estrogen, microdose transdermal estradiol, or placebo. They found that even those patients who received the microdose estradiol had a 75% lower hot flash frequency than those receiving placebo (p = .003) [27]. However, although numerous randomized trials have demonstrated that estrogen therapy markedly reduces the frequency and intensity of hot flashes, results of the Women's Health Initiative (WHI) studies have raised concern about long-term adverse effects pertaining to receiving HRT. The results of the WHI trial identified net harm for combined hormonal therapy use [28]. In particular, estrogen plus progestin was associated with a higher incidence of breast cancers, many of which were higher stage. Breast cancer mortality also appears to be amplified with the combined use of estrogen and progestin [29]. Additionally, following the initial report of results from the WHI, a significant decrease in breast cancer incidence in the U.S. was noted, which was hypothesized to be related to a decline in HRT intake resulting from awareness of the WHI findings. On the other hand, long-term follow-up from that study demonstrated that estrogen replacement therapy alone did not increase the risk for breast cancer recurrence [30]. However, data from a meta-analysis of 16 studies evaluating the use of estrogen therapy alone demonstrated that long-term estrogen replacement therapy was associated with a 30% higher relative risk for breast cancer [31, 32]. Thus, the use of any HRT following a breast cancer diagnosis continues to be discouraged.
Progestational Agents
Recent studies have demonstrated that progestational agents have the ability to reduce the frequency and intensity of hot flashes. In a double-blinded, placebo-controlled, randomized clinical trial evaluating 97 women with a history of breast cancer and 66 men receiving androgen deprivation therapy for prostate cancer, the use of megestrol acetate resulted in a 85% reduction in hot flashes, versus a 21% reduction in the placebo group [33]. In addition, the use of an i.m. depot formulation of medroxyprogesterone acetate (MPA) was demonstrated to have comparable effects on hot flashes [34]. Indeed, when MPA was compared with oral megestrol in a randomized study, 89% of responders continued to show a decrease in hot flashes at week 24, compared with 45% in the megestrol group.
While the use of oral and i.m. progestational agents has demonstrated significant efficacy in randomized trials, the role of progesterone cream for alleviating hot flashes remains a controversial topic, because placebo-controlled, randomized trials involving these agents have provided conflicting evidence [35, 36].
Furthermore, although oral and i.m. progestational agents have been shown to have efficacy in the treatment of hot flashes, researchers have found that the use of these agents may stimulate epithelial proliferation in the breast and thus increase the risk for the development of breast cancer [37]. Thus, the use of these agents is controversial and is not recommended in patients with a history of breast cancer.
Neuroactive Agents
Clonidine
Given the proposed noradrenergic mechanism of hot flashes, the use of clonidine, a centrally acting α-adrenergic agonist, was explored for the treatment of this symptom [38–40]. However, this agent is associated with significant side effects, such as dry mouth, constipation, and drowsiness [41]. In addition, a meta-analysis of 10 trials involving clonidine found that <50% of the trials showed a benefit with the use of clonidine, when compared with placebo [42]. Thus, this agent is not recommended for treating hot flashes.
Serotonin Reuptake Inhibitors
Loprinzi and colleagues reported results from randomized, placebo-controlled clinical trials evaluating the treatment of hot flashes in women [43]. Two placebo-controlled trials involving fluoxetine and venlafaxine demonstrated a 50%–60% reduction in the incidence of hot flashes [44, 45]. In addition, a randomized, placebo-controlled trial demonstrated that citalopram led to significantly better hot flashes scores than with placebo [46]. However, the use of selective serotonin reuptake inhibitors (SSRIs), such as fluoxetine, in patients taking tamoxifen therapy must be evaluated with caution, given previous studies that have demonstrated that SSRI antidepressants, such as paroxetine, inhibit the cytochrome P450 2D (CYP2D6) pathway and may be associated with higher mortality from breast cancer in patients who are taking tamoxifen [47].
Gabapentin
In aggregate, three gabapentin trials have demonstrated a relatively lower incidence of hot flashes, by 35%–38%, in patients treated with this agent than in patients treated with placebo [48–51]. Furthermore, to evaluate patient preference for gabapentin versus venlafaxine, Bordeleau et al. [52] conducted a group-sequential, open-label, randomized, crossover trial of 4 weeks of venlafaxine (37.5 mg daily for 7 days followed by 75 mg daily for 21 days) versus gabapentin (300 mg once per day for 3 days, then 300 mg twice per day for 3 days, then 300 mg three times per day for 22 days) for breast cancer survivors, with a primary end result of patient preference. The study demonstrated that, of 66 patients who were randomly assigned to treatment with venlafaxine or gabapentin, 32% of the patients preferred gabapentin, versus 68% of the patients who preferred venlafaxine [52]. Interestingly, when comparing MPA with venlafaxine following the sixth week after random assignment, hot flash scores were 55% lower in the venlafaxine arm and 79% lower in the MPA arm [53]. In a phase III trial of gabapentin alone or in combination with an antidepressant in women who do benefit from an antidepressant alone, the combination of the two agents was not significantly better than gabapentin alone [54].
Complementary and Alternative Medicine Approaches
Given the paucity of U.S. Food and Drug Administration–approved regimens for the treatment of hot flashes, patients and providers have explored complementary and alternative regimens in an attempt to find a solution for these symptoms. However, many of these therapies have shown marginal, or no, benefit. For example, a placebo-controlled, randomized trial involving the use of vitamin E demonstrated that vitamin E administration resulted in a minimal decrease in hot flashes and was not preferred over placebo [43, 55]. Additionally, placebo-controlled trials investigating soy phytoestrogen and black cohosh found no significant benefit from these agents [43, 56].
Furthermore, acupuncture has been studied as a nonpharmacologic method for the alleviation of hot flashes. Although several trials have evaluated the use of acupuncture for this symptom [57, 58], a recent meta-analysis of 11 studies (including five placebo-controlled trials) failed to confirm the benefit of acupuncture in the treatment of hot flashes [59, 60]. However, a subsequent trial comparing acupuncture with venlafaxine demonstrated that, although both groups had improvements in symptoms, the acupuncture group exhibited a sustained improvement at 2 weeks post-treatment, whereas the venlafaxine group developed more hot flashes post-treatment [57].
Behavioral Modifications
Although there have been multiple trials involving diverse medications for the treatment of hot flashes, the backbone of hot flash treatment involves behavioral modification. The North American Menopause Society recommends that, for mild hot flashes, patients should maintain a low core body temperature through dressing in layers, consuming cool or cold food or drinks, and using a fan as needed. In addition, further behavioral modifications are detailed below [61].
Yoga
Yoga has been examined as a technique for controlling hot flashes, and several trials have suggested yoga to be beneficial in alleviating hot flashes [62, 63]. However, a systematic review of seven clinical studies evaluating the use of yoga for menopausal symptoms found that there is currently insufficient evidence to suggest that yoga is an effective means for controlling hot flashes [60, 64].
Exercise
To explore the impact of exercise on hot flashes, several randomized trials compared the effect of exercise with other modalities (stretching, estrogen, or observation) and failed to demonstrate a significant benefit [65, 66]. In fact, exercise has been associated with an increase in the severity of these symptoms in postmenopausal, overweight women, a finding that is theorized to be a result of the fact that exercise increases the core body temperature, thus resulting in hot flashes in patients with a narrow thermoneutral zone [67].
Relaxation Techniques
Freedman et al. [68] studied the practice of slow-breathing techniques and found that these techniques may reduce small overall sympathetic tone, reducing the frequency of hot flashes 35% more than muscle relaxation alone. Another trial compared the use of applied relaxation with estradiol therapy; although estrogen therapy reduced hot flashes more quickly, climacteric symptoms improved in both groups over time [69]. A review of 14 studies, involving 475 patients who had received psychoeducational interventions, including relaxation, demonstrated an overall improvement in vasomotor symptoms. However, the experimental group in two studies, which had the greatest number of patients, also received pharmacologic therapies [70].
Conclusion
Research in the field of hot flashes has resulted in multiple options for the patient who is suffering from this significant symptom. A practical approach to these patients must first address behavior modification, including maintenance of a cool core body temperature (i.e., by drinking cool liquids, avoiding hot/spicy foods, and wearing looser clothing). Beyond these lifestyle interventions, there are diverse options for these patients. Given the potential long-term risks of hormonal therapies, patients should attempt nonhormonal options first. Although SSRIs have shown great promise in the treatment of hot flashes, the choice of agent should also include consideration of the patient's concomitant medications. If the patient is taking tamoxifen or other agents that are metabolized by the CYP2D6 pathway, the choice of SSRI should include venlafaxine, with careful avoidance of agents such as fluoxetine or paroxetine, which are potent inhibitors of the CYP2D6 pathway. For those who are not taking agents such as tamoxifen or other CYP2D6-dependent medications, the choice of SSRI is not limited by this consideration. Furthermore, although gabapentin was found to be less preferred than venlafaxine for the treatment of hot flashes, it remains an option for patients, especially for those who find nighttime hot flashes to be a greater concern, given the sedating effect of gabapentin. Finally, relaxation techniques should be considered as an alternative, or complementary, regimen for the treatment of this distressing symptom. Current trials involve the use of flaxseed, stellate ganglion injections, hypnosis, relaxation, and combined modalities for the treatment of hot flashes.
Footnotes
- (C/A)
- consulting/advisory relationship
- (RF)
- Research funding
- (E)
- Employment
- (H)
- Honoraria received
- (OI)
- Ownership interests
- (IP)
- Intellectual property rights/inventor/patent holder
Author Contributions
Conception/Design: Phuong Khanh H. Morrow, Danielle N. Mattair, Gabriel N. Hortobagyi
Provision of study material or patients: Phuong Khanh H. Morrow, Gabriel N. Hortobagyi
Collection and/or assembly of data: Phuong Khanh H. Morrow, Danielle N. Mattair
Data analysis and interpretation: Phuong Khanh H. Morrow, Danielle N. Mattair, Gabriel N. Hortobagyi
Manuscript writing: Phuong Khanh H. Morrow, Danielle N. Mattair
Final approval of manuscript: Phuong Khanh H. Morrow, Gabriel N. Hortobagyi
References
- 1.Pachman DR, Jones JM, Loprinzi CL. Management of menopause-associated vasomotor symptoms: Current treatment options, challenges and future directions. Int J Womens Health. 2010;2:123–135. doi: 10.2147/ijwh.s7721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grady D. Clinical practice. Management of menopausal symptoms. N Engl J Med. 2006;355:2338–2347. doi: 10.1056/NEJMcp054015. [DOI] [PubMed] [Google Scholar]
- 3.Dalal S, Zhukovsky DS. Pathophysiology and management of hot flashes. J Support Oncol. 2006;4:315–320. 325. [PubMed] [Google Scholar]
- 4.Kronenberg F. Hot flashes: Epidemiology and physiology. Ann N Y Acad Sci. 1990;592:52–86. doi: 10.1111/j.1749-6632.1990.tb30316.x. discussion 123–133. [DOI] [PubMed] [Google Scholar]
- 5.Shapiro CL, Recht A. Side effects of adjuvant treatment of breast cancer. N Engl J Med. 2001;344:1997–2008. doi: 10.1056/NEJM200106283442607. [DOI] [PubMed] [Google Scholar]
- 6.Fisher B, Dignam J, Wolmark N, et al. Tamoxifen in treatment of intraductal breast cancer: National Surgical Adjuvant Breast and Bowel Project B-24 randomised controlled trial. Lancet. 1999;353:1993–2000. doi: 10.1016/S0140-6736(99)05036-9. [DOI] [PubMed] [Google Scholar]
- 7.Loprinzi CL, Zahasky KM, Sloan JA, et al. Tamoxifen-induced hot flashes. Clin Breast Cancer. 2000;1:52–56. doi: 10.3816/cbc.2000.n.004. [DOI] [PubMed] [Google Scholar]
- 8.Love RR, Cameron L, Connell BL, et al. Symptoms associated with tamoxifen treatment in postmenopausal women. Arch Intern Med. 1991;151:1842–1847. [PubMed] [Google Scholar]
- 9.Campos SM. Aromatase inhibitors for breast cancer in postmenopausal women. The Oncologist. 2004;9:126–136. doi: 10.1634/theoncologist.9-2-126. [DOI] [PubMed] [Google Scholar]
- 10.Paridaens RJ, Dirix LY, Beex LV, et al. Phase III study comparing exemestane with tamoxifen as first-line hormonal treatment of metastatic breast cancer in postmenopausal women: The European Organisation for Research and Treatment of Cancer Breast Cancer Cooperative Group. J Clin Oncol. 2008;26:4883–4890. doi: 10.1200/JCO.2007.14.4659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mouridsen H, Giobbie-Hurder A, Goldhirsch A, et al. BIG 1–98 Collaborative Group. Letrozole therapy alone or in sequence with tamoxifen in women with breast cancer. N Engl J Med. 2009;361:766–776. doi: 10.1056/NEJMoa0810818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cuzick J, Sestak I, Cella D, et al. ATAC Trialists' Group. Treatment-emergent endocrine symptoms and the risk of breast cancer recurrence: A retrospective analysis of the ATAC trial. Lancet Oncol. 2008;9:1143–1148. doi: 10.1016/S1470-2045(08)70259-6. [DOI] [PubMed] [Google Scholar]
- 13.Koeberle D, Thuerlimann B. Letrozole as upfront endocrine therapy for postmenopausal women with hormone-sensitive breast cancer: BIG 1–98. Breast Cancer Res Treat. 2007;105:55–66. doi: 10.1007/s10549-007-9700-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mar Fan HG, Houédé-Tchen N, Chemerynsky I, et al. Menopausal symptoms in women undergoing chemotherapy-induced and natural menopause: A prospective controlled study. Ann Oncol. 2010;21:983–987. doi: 10.1093/annonc/mdp394. [DOI] [PubMed] [Google Scholar]
- 15.Goodwin PJ, Ennis M, Pritchard KI, et al. Risk of menopause during the first year after breast cancer diagnosis. J Clin Oncol. 1999;17:2365–2370. doi: 10.1200/JCO.1999.17.8.2365. [DOI] [PubMed] [Google Scholar]
- 16.Levine MN, Bramwell VH, Pritchard KI, et al. Randomized trial of intensive cyclophosphamide, epirubicin, and fluorouracil chemotherapy compared with cyclophosphamide, methotrexate, and fluorouracil in premenopausal women with node-positive breast cancer. National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 1998;16:2651–2658. doi: 10.1200/JCO.1998.16.8.2651. [DOI] [PubMed] [Google Scholar]
- 17.Goldhirsch A, Gelber RD, Castiglione M. The magnitude of endocrine effects of adjuvant chemotherapy for premenopausal breast cancer patients. The International Breast Cancer Study Group. Ann Oncol. 1990;1:183–188. doi: 10.1093/oxfordjournals.annonc.a057718. [DOI] [PubMed] [Google Scholar]
- 18.Loprinzi CL, Dueck AC, Khoyratty BS, et al. A phase III randomized, double-blind, placebo-controlled trial of gabapentin in the management of hot flashes in men (N00CB) Ann Oncol. 2009;20:542–549. doi: 10.1093/annonc/mdn644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schow DA, Renfer LG, Rozanski TA, et al. Prevalence of hot flushes during and after neoadjuvant hormonal therapy for localized prostate cancer. South Med J. 1998;91:855–857. doi: 10.1097/00007611-199809000-00010. [DOI] [PubMed] [Google Scholar]
- 20.Charig CR, Rundle JS. Flushing. Long-term side effect of orchiectomy in treatment of prostatic carcinoma. Urology. 1989;33:175–178. doi: 10.1016/0090-4295(89)90385-3. [DOI] [PubMed] [Google Scholar]
- 21.Shanafelt TD, Barton DL, Adjei AA, et al. Pathophysiology and treatment of hot flashes. Mayo Clin Proc. 2002;77:1207–1218. doi: 10.4065/77.11.1207. [DOI] [PubMed] [Google Scholar]
- 22.Freedman RR. Hot flashes: behavioral treatments, mechanisms, and relation to sleep. Am J Med. 2005;118(suppl 12B):124–130. doi: 10.1016/j.amjmed.2005.09.046. [DOI] [PubMed] [Google Scholar]
- 23.Freedman RR. Physiology of hot flashes. Am J Hum Biol. 2001;13:453–464. doi: 10.1002/ajhb.1077. [DOI] [PubMed] [Google Scholar]
- 24.Sturdee DW. The menopausal hot flush—anything new? Maturitas. 2008;60:42–49. doi: 10.1016/j.maturitas.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 25.Clemons M, Goss P. Estrogen and the risk of breast cancer. N Engl J Med. 2001;344:276–285. doi: 10.1056/NEJM200101253440407. [DOI] [PubMed] [Google Scholar]
- 26.Velie EM, Nechuta S, Osuch JR. Lifetime reproductive and anthropometric risk factors for breast cancer in postmenopausal women. Breast Dis. 2005;24:17–35. doi: 10.3233/bd-2006-24103. [DOI] [PubMed] [Google Scholar]
- 27.Bachmann GA, Schaefers M, Uddin A, et al. Lowest effective transdermal 17β-estradiol dose for relief of hot flushes in postmenopausal women: A randomized controlled trial. Obstet Gynecol. 2007;110:771–779. doi: 10.1097/01.AOG.0000284450.51264.31. [DOI] [PubMed] [Google Scholar]
- 28.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results from the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 29.Chlebowski RT, Anderson GL, Gass M, et al. Estrogen plus progestin and breast cancer incidence and mortality in postmenopausal women. JAMA. 2010;304:1684–1692. doi: 10.1001/jama.2010.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.LaCroix AZ, Chlebowski RT, Manson JE, et al. Health outcomes after stopping conjugated equine estrogens among postmenopausal women with prior hysterectomy: A randomized controlled trial. JAMA. 2011;305:1305–1314. doi: 10.1001/jama.2011.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jungheim ES, Colditz GA. Short-term use of unopposed estrogen: A balance of inferred risks and benefits. JAMA. 2011;305:1354–1355. doi: 10.1001/jama.2011.405. [DOI] [PubMed] [Google Scholar]
- 32.Steinberg KK, Thacker SB, Smith SJ, et al. A meta-analysis of the effect of estrogen replacement therapy on the risk of breast cancer. JAMA. 1991;265:1985–1990. [PubMed] [Google Scholar]
- 33.Loprinzi CL, Michalak JC, Quella SK, et al. Megestrol acetate for the prevention of hot flashes. N Engl J Med. 1994;331:347–352. doi: 10.1056/NEJM199408113310602. [DOI] [PubMed] [Google Scholar]
- 34.Bertelli G, Venturini M, Del Mastro L, et al. Intramuscular depot medroxyprogesterone versus oral megestrol for the control of postmenopausal hot flashes in breast cancer patients: A randomized study. Ann Oncol. 2002;13:883–888. doi: 10.1093/annonc/mdf151. [DOI] [PubMed] [Google Scholar]
- 35.Leonetti HB, Longo S, Anasti JN. Transdermal progesterone cream for vasomotor symptoms and postmenopausal bone loss. Obstet Gynecol. 1999;94:225–228. doi: 10.1016/s0029-7844(99)00266-5. [DOI] [PubMed] [Google Scholar]
- 36.Benster B, Carey A, Wadsworth F, et al. A double-blind placebo-controlled study to evaluate the effect of progestelle progesterone cream on postmenopausal women. Menopause Int. 2009;15:63–69. doi: 10.1258/mi.2009.009014. [DOI] [PubMed] [Google Scholar]
- 37.Hofseth LJ, Raafat AM, Osuch JR, et al. Hormone replacement therapy with estrogen or estrogen plus medroxyprogesterone acetate is associated with increased epithelial proliferation in the normal postmenopausal breast. J Clin Endocrinol Metab. 1999;84:4559–4565. doi: 10.1210/jcem.84.12.6194. [DOI] [PubMed] [Google Scholar]
- 38.Schindler AE, Müller D, Keller E, et al. Studies with clonidine (Dixarit) in menopausal women. Arch Gynecol. 1979;227:341–347. doi: 10.1007/BF02109923. [DOI] [PubMed] [Google Scholar]
- 39.Clayden JR, Bell JW, Pollard P. Menopausal flushing: Double-blind trial of a non-hormonal medication. Br Med J. 1974;1:409–412. doi: 10.1136/bmj.1.5905.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Laufer LR, Erlik Y, Meldrum DR, et al. Effect of clonidine on hot flashes in postmenopausal women. Obst Gynecol. 1982;60:583–586. [PubMed] [Google Scholar]
- 41.Goldberg RM, Loprinzi CL, O'Fallon JR, et al. Transdermal clonidine for ameliorating tamoxifen-induced hot flashes. J Clin Oncol. 1994;12:155–158. doi: 10.1200/JCO.1994.12.1.155. [DOI] [PubMed] [Google Scholar]
- 42.Nelson HD, Vesco KK, Haney E, et al. Nonhormonal therapies for menopausal hot flashes: Systematic review and meta-analysis. JAMA. 2006;295:2057–2071. doi: 10.1001/jama.295.17.2057. [DOI] [PubMed] [Google Scholar]
- 43.Loprinzi CL, Barton DL, Sloan JA, et al. Mayo Clinic and North Central Cancer Treatment Group hot flash studies: A 20-year experience. Menopause. 2008;15:655–660. doi: 10.1097/gme.0b013e3181679150. [DOI] [PubMed] [Google Scholar]
- 44.Loprinzi CL, Sloan JA, Perez EA, et al. Phase III evaluation of fluoxetine for treatment of hot flashes. J Clin Oncol. 2002;20:1578–1583. doi: 10.1200/JCO.2002.20.6.1578. [DOI] [PubMed] [Google Scholar]
- 45.Loprinzi CL, Kugler JW, Sloan JA, et al. Venlafaxine in management of hot flashes in survivors of breast cancer: A randomised controlled trial. Lancet. 2000;356:2059–2063. doi: 10.1016/S0140-6736(00)03403-6. [DOI] [PubMed] [Google Scholar]
- 46.Barton DL, LaVasseur BI, Sloan JA, et al. Phase III, placebo-controlled trial of three doses of citalopram for the treatment of hot flashes: NCCTG Trial N05C9. J Clin Oncol. 2010;28:3278–3283. doi: 10.1200/JCO.2009.26.6379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kelly CM, Juurlink DN, Gomes T, et al. Selective serotonin reuptake inhibitors and breast cancer mortality in women receiving tamoxifen: A population based cohort study. BMJ. 2010;340:c693. doi: 10.1136/bmj.c693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Loprinzi CL, Sloan J, Stearns V, et al. Newer antidepressants and gabapentin for hot flashes: An individual patient pooled analysis. J Clin Oncol. 2009;27:2831–2837. doi: 10.1200/JCO.2008.19.6253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guttuso T, Jr, Kurlan R, McDermott MP, et al. Gabapentin's effects on hot flashes in postmenopausal women: A randomized controlled trial. Obstet Gynecol. 2003;101:337–345. doi: 10.1016/s0029-7844(02)02712-6. [DOI] [PubMed] [Google Scholar]
- 50.Pandya KJ, Morrow GR, Roscoe JA, et al. Gabapentin for hot flashes in 420 women with breast cancer: A randomised double-blind placebo-controlled trial. Lancet. 2005;366:818–824. doi: 10.1016/S0140-6736(05)67215-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reddy SY, Warner H, Guttuso T, Jr, et al. Gabapentin, estrogen, and placebo for treating hot flushes: A randomized controlled trial. Obstet Gynecol. 2006;108:41–48. doi: 10.1097/01.AOG.0000222383.43913.ed. [DOI] [PubMed] [Google Scholar]
- 52.Bordeleau L, Pritchard KI, Loprinzi CL, et al. Multicenter, randomized, cross-over clinical trial of venlafaxine versus gabapentin for the management of hot flashes in breast cancer survivors. J Clin Oncol. 2010;28:5147–5152. doi: 10.1200/JCO.2010.29.9230. [DOI] [PubMed] [Google Scholar]
- 53.Loprinzi CL, Levitt R, Barton D, et al. Phase III comparison of depomedroxyprogesterone acetate to venlafaxine for managing hot flashes: North Central Cancer Treatment Group Trial N99C7. J Clin Oncol. 2006;24:1409–1414. doi: 10.1200/JCO.2005.04.7324. [DOI] [PubMed] [Google Scholar]
- 54.Loprinzi CL, Kugler JW, Barton DL, et al. Phase III trial of gabapentin alone or in conjunction with an antidepressant in the management of hot flashes in women who have inadequate control with an antidepressant alone: NCCTG N03C5. J Clin Oncol. 2007;25:308–312. doi: 10.1200/JCO.2006.07.5390. [DOI] [PubMed] [Google Scholar]
- 55.Barton DL, Loprinzi CL, Quella SK, et al. Prospective evaluation of vitamin E for hot flashes in breast cancer survivors. J Clin Oncol. 1998;16:495–500. doi: 10.1200/JCO.1998.16.2.495. [DOI] [PubMed] [Google Scholar]
- 56.Quella SK, Loprinzi CL, Barton DL, et al. Evaluation of soy phytoestrogens for the treatment of hot flashes in breast cancer survivors: A North Central Cancer Treatment Group trial. J Clin Oncol. 2000;18:1068–1074. doi: 10.1200/JCO.2000.18.5.1068. [DOI] [PubMed] [Google Scholar]
- 57.Walker EM, Rodriguez AI, Kohn B, et al. Acupuncture versus venlafaxine for the management of vasomotor symptoms in patients with hormone receptor-positive breast cancer: A randomized controlled trial. J Clin Oncol. 2010;28:634–640. doi: 10.1200/JCO.2009.23.5150. [DOI] [PubMed] [Google Scholar]
- 58.Harding C, Harris A, Chadwick D. Auricular acupuncture: A novel treatment for vasomotor symptoms associated with luteinizing-hormone releasing hormone agonist treatment for prostate cancer. BJU Int. 2009;103:186–190. doi: 10.1111/j.1464-410X.2008.07884.x. [DOI] [PubMed] [Google Scholar]
- 59.Cho SH, Whang WW. Acupuncture for vasomotor menopausal symptoms: A systematic review. Menopause. 2009;16:1065–1073. doi: 10.1097/gme.0b013e3181a48abd. [DOI] [PubMed] [Google Scholar]
- 60.Sideras K, Loprinzi CL. Nonhormonal management of hot flashes for women on risk reduction therapy. J Natl Compr Canc Netw. 2010;8:1171–1179. doi: 10.6004/jnccn.2010.0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.North American Menopause Society. Treatment of menopause-associated vasomotor symptoms: Position statement of The North American Menopause Society. Menopause. 2004;11:11–33. doi: 10.1097/01.GME.0000108177.85442.71. [DOI] [PubMed] [Google Scholar]
- 62.Booth-LaForce C, Thurston RC, Taylor MR. A pilot study of a Hatha yoga treatment for menopausal symptoms. Maturitas. 2007;57:286–295. doi: 10.1016/j.maturitas.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 63.Chattha R, Raghuram N, Venkatram P, et al. Treating the climacteric symptoms in Indian women with an integrated approach to yoga therapy: A randomized control study. Menopause. 2008;15:862–870. doi: 10.1097/gme.0b013e318167b902. [DOI] [PubMed] [Google Scholar]
- 64.Lee MS, Kim JI, Ha JY, et al. Yoga for menopausal symptoms: A systematic review. Menopause. 2009;16:602–608. doi: 10.1097/gme.0b013e31818ffe39. [DOI] [PubMed] [Google Scholar]
- 65.Lindh-Astrand L, Nedstrand E, Wyon Y, et al. Vasomotor symptoms and quality of life in previously sedentary postmenopausal women randomised to physical activity or estrogen therapy. Maturitas. 2004;48:97–105. doi: 10.1016/S0378-5122(03)00187-7. [DOI] [PubMed] [Google Scholar]
- 66.Ueda M. A 12-week structured education and exercise program improved climacteric symptoms in middle-aged women. J Physiol Anthropol Appl Human Sci. 2004;23:143–148. doi: 10.2114/jpa.23.143. [DOI] [PubMed] [Google Scholar]
- 67.Aiello EJ, Yasui Y, Tworoger SS, et al. Effect of a yearlong, moderate-intensity exercise intervention on the occurrence and severity of menopause symptoms in postmenopausal women. Menopause. 2004;11:382–388. doi: 10.1097/01.gme.0000113932.56832.27. [DOI] [PubMed] [Google Scholar]
- 68.Freedman RR, Woodward S. Behavioral treatment of menopausal hot flushes–evaluation by ambulatory monitoring. Am J Obstet Gynecol. 1992;167:436–439. doi: 10.1016/s0002-9378(11)91425-2. [DOI] [PubMed] [Google Scholar]
- 69.Nedstrand E, Wijma K, Wyon Y, et al. Applied relaxation and oral estradiol treatment of vasomotor symptoms in postmenopausal women. Maturitas. 2005;51:154–162. doi: 10.1016/j.maturitas.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 70.Tremblay A, Sheeran L, Aranda SK. Psychoeducational interventions to alleviate hot flashes: A systematic review. Menopause. 2008;15:193–202. doi: 10.1097/gme.0b013e31805c08dc. [DOI] [PubMed] [Google Scholar]