Since Cooley and DeBakey first replaced the transverse aortic arch over 50 years ago,1 outcomes from arch repair have improved. Despite these advances, challenges in preventing complications, such as neurologic injury, remain. Historically, various adjuncts have been developed to prevent neurologic injury, including passive shunting and cardiopulmonary bypass, as described by Cooley and DeBakey in the 1960s.2 Griepp's report3 on profound hypothermic circulatory arrest (PHCA) for arch repair led to a dramatic shift in the field by providing a simple approach to managing the aortic arch. Many adopted this approach for its simplicity and its ability to provide both cerebral and multiorgan protection. Crawford and associates reported respectable results when using PHCA,4 but drew attention to its limitation—its duration. They showed that increasing circulatory arrest times led to a greater risk of stroke and death.
The following is a brief overview of current trends in adjunctive cerebral perfusion, which highlights antegrade and retrograde perfusion. It must be recognized, however, that cerebral perfusion is only one of many aspects to be considered in the prevention of neurologic injury; therefore, determining the superiority of one approach to perfusion over another is difficult.
General Information
On average, the brain weighs 1,400 g, constituting 5% of the body weight, but it receives 15% of the entire cardiac output. Autoregulation is responsible for maintaining constant flow to the cerebral tissue, despite systemic blood pressures that vary from a low of 50 to a high of 150 mmHg at normal body temperature (37 °C). This window narrows as core body temperature decreases, in such a manner that at 20 °C the systemic pressure ranges from 30 to 100 mmHg. Complete loss of autoregulation occurs at core body temperatures of less than 12 °C, and cerebral blood flow is then directly dependent upon systemic blood pressure and flow.5 Average cerebral flow at normal body temperature provides 55 mL of blood per 100 g of brain tissue per minute, and oligemia occurs when flow decreases to between 20 and 55 mL/100 g/min. Frank ischemia occurs when flow decreases to below 20 mL/100 g/min, and neuronal dysfunction results. At flows of less than 10 mL/100 g/min, electrolyte release and neuronal death ensue.6 Hypothermia extends ischemic tolerance by reducing the brain's metabolic demands.
Neurologic Injury
Neurologic injury is categorized in accordance with the severity and duration of clinical injury, without consideration of cause or origin. According to Roach,7 type 1 injury includes stroke (whether fatal or nonfatal), transient ischemic attack, hypoxic encephalopathy, stupor, or coma. Type 2 includes decreased intellectual function, confusion, agitation, disorientation, memory deficit, or seizure (without focal deficit).7 Ergin's classification8 accounts for reversibility: those who sustain permanent injury (stroke and coma) are differentiated from those whose condition can possibly be reversed (temporary neurologic dysfunction).
Malperfusion or Emboli?
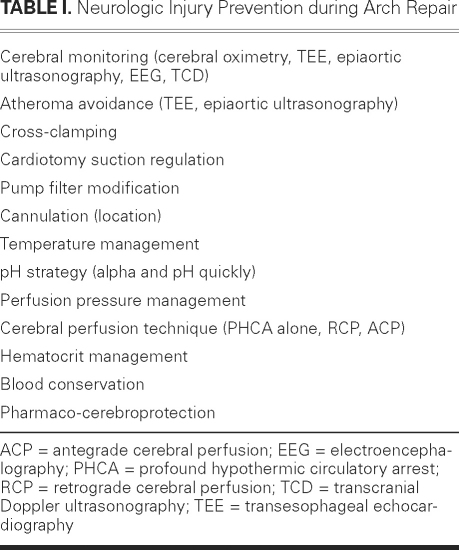
Historically, many reports have implicated cerebral malperfusion as the cause of neurologic injury during aortic arch surgery. For this reason, much emphasis has been placed on the type of cerebral perfusion used (that is, antegrade or retrograde). Undoubtedly, warm ischemia leads to neurologic dysfunction, but most neurologic injuries (strokes) occur as the result of emboli (60% of the time) rather than hypoperfusion, which accounts for only 10% of strokes.9,10 Therefore, in addition to the type of cerebral perfusion implemented during PHCA, many other variables need to be considered and optimized during aortic arch repair (Table I). Each cardiovascular unit's approach to arch surgery is unique. Although each unit's particular combination of cerebral perfusion adjuncts, cannulation approaches, monitoring techniques, and anesthetic methods has been standardized within the unit, its overall approach is unlike that of any other unit. In any attempt to determine equivalence, this uniqueness renders comparison difficult.
Table I. Neurologic Injury Prevention during Arch Repair
Profound Hypothermic Circulatory Arrest
Ever since profound hypothermic circulatory arrest was popularized by Griepp and colleagues in 1975,3 it has remained the primary approach (or an adjunct) during aortic arch repair. Although profound hypothermia reduces metabolic demand, its duration of protection is limited because demand never reaches nil. At normal body temperature, the oxygen demand of the brain is 2.9 mL/g/min. At 25 °C, the demand drops to 0.9 mL/g/min, and, at 20 °C, it drops to 0.2 mL/g/min.5 Animal studies have shown that even at very low systemic temperatures (8 °C), metabolic demand remains at 11% of baseline.11
Recently, Elefteriades and colleagues12 have reported respectable results with the use of PHCA alone during arch repairs. In a series of 394 patients undergoing ascending aortic and aortic arch repair (both elective and emergent), the early mortality rate was 6.3% (25/394). The stroke rate for ascending and arch repair was 3.1% (11/356), when 38 patients with descending and thoracoabdominal aortic aneurysms were excluded. As expected, patients with PHCA times of greater than 40 minutes experienced a higher stroke rate (13.1%, or 8/61). This supports the earlier report of Crawford and associates,4 wherein a PHCA time of longer than 40 minutes was associated with increased risk of stroke. In a separate report, Elefteriades' group also showed that the use of PHCA alone could be performed without late neurologic sequelae even in patients with demanding cognitive professions.12
The only randomized, controlled trial in human beings that compared PHCA alone with antegrade cerebral perfusion (ACP) revealed no difference in early and late neurologic outcomes, despite improved jugular venous oxygen saturation when ACP was used. Admittedly, the authors acknowledged that the study was underpowered for clinical outcomes.13
Because few clinical data counter the use of PHCA alone during arch repairs, especially when PHCA time takes less than 30 minutes, PHCA alone or in combination with other perfusion techniques remains acceptable for transverse arch repairs that take less than 30 minutes.
Antegrade Cerebral Perfusion
Antegrade cerebral perfusion in combination with PHCA was first reported by Frist and colleagues14 in 1986 and later was popularized independently by Bachet15 and Kazui16 and their respective colleagues in the early 1990s. The technique used direct cannulation of either cold or moderately cold blood in combination with PHCA. Subsequent animal data, together with clinical experience, led to the increased adoption of ACP.
Although many experimental animal and patient cohort studies have been performed with ACP, only 3 prospective randomized controlled trials have compared ACP with retrograde cerebral perfusion (RCP). Okita and associates17 studied a total of 60 patients (30 with ACP and 30 with RCP) and found a decreased rate of total neurologic deficit in the ACP group (33% vs 13%, P <0.05) but found no difference between groups in rates of death, stroke, or neurocognitive deficit. Verification of adequacy of the RCP was not performed in this study. In an earlier report, Tanoue and co-authors18 used transcranial Doppler ultrasonography to verify cerebral blood flow in 32 patients (15 with RCP and 17 with ACP). This study found improved cerebral blood flow in the ACP group. Only 3 patients in the RCP group showed evidence of reversal of cerebral blood. This low incidence of identification of flow reversal can be attributed to the technique of RCP used in the study: the superior vena cava pressure was only 15 to 25 mmHg. In addition, the cerebral perfusion time was 71 minutes in the ACP group, but only 38 minutes in the RCP group (P=0.0047). No difference in clinical outcomes was noted; probably, the study was not sufficiently powered to show a difference.18 A third prospective randomized controlled trial was performed by Harrington and colleagues,13 who compared ACP to PHCA alone and noted improved jugular venous oxygenation but no improvement in early or late clinical outcomes.
Recent studies with antegrade cerebral perfusion have reported excellent clinical outcomes, but variations in technique make it difficult to determine if ACP alone was responsible.19 The limitations, in common with those of most such clinical studies, included differences in cannulation, delivery of perfusate (unilateral vs bilateral), amount of perfusate, and temperature of perfusate.20 In addition, moderate hypothermia has now been adopted, and this varies the technique still more.21 The use of moderate hypothermia with circulatory arrest has been suggested for the purpose of reducing the deleterious effects of profound hypothermia, specifically coagulopathy, but the potential for multiorgan dysfunction, such as spinal cord or renal dysfunction, is worrisome. Results are preliminary at this point.
Retrograde Cerebral Perfusion
The suggested benefits of RCP include evenly distributed cooling (70% of the cerebral blood volume resides in the venous system), flushing of atheromatous débris, and potential for providing nutritive flow. Although several animal and clinical studies have reported the superiority of ACP to RCP, only 3 prospective randomized controlled trials, albeit with small cohorts, have been performed, and they have reported no differences in clinical outcomes.17,18,22 Whether outcomes would have been different with larger cohorts remains unclear, but it must be recognized that the optimal approach to delivery of either ACP or RCP might not have been achieved.
We adopted the use of Power Mode Transcranial Doppler (PM-TCD) and identified reversal of flow in the middle cerebral artery, which suggests that flow can be supplied to the cerebrum.23 The adequacy of this flow in human beings, however, was not studied and remains a question. At any rate, we learned that cerebral perfusion could be achieved clinically, provided that the technique was modified. The standard approach of infusing 500 cc/min at a jugular pressure of between 15 and 25 mmHg was not adequate for all patients. Using PM-TCD, we found that an “opening” pressure and flow was required to identify reversal of flow, after which a decreased or “dial-down” flow could be performed. The “maintenance” flow would then maintain cerebral flow at a jugular pressure close to what had been used as the standard (that is, 15–25 mmHg).23
The clinical benefit of RCP has been shown in both animal and clinical studies.24,25 In the largest series (more than 1,200 cases) reporting use of RCP, the overall rate of early death was 9.9%, with a stroke rate of 2.7%. It is worth noting that the only independent factor protective against both stroke and early death was RCP.26
It is intuitive that ACP may be superior to RCP in providing cerebral perfusion, but the clinical proof has not been apparent and the reasons for this are unclear. Although ACP may provide superior cerebral perfusion, it requires manipulation of the head vessels, which increases the risk of emboli. Retrograde cerebral perfusion requires no manipulation of head vessels and provides, further, the ability to flush atheromatous débris from the cerebral circulation. This is the probable reason that the superiority of one approach over another has been difficult to demonstrate.
Integrated Cerebral Perfusion
Conklin and colleagues27 first reported the integration of both perfusion approaches in order to combine their advantages. The small pilot series reported 1 stroke in 11 patients.
We performed a prospective cohort study that used an integrated cerebral perfusion approach similar to Conklin's, in patients who needed more than 40 minutes of PHCA. This study in 56 patients did not reveal any significant clinical differences, although trends in higher total neurologic deficit, stroke, and death were observed in the integrated approach.28 For this reason, we continued to use RCP.
Recommendations
The superiority of one perfusion approach over another will be very difficult to determine without multicenter randomized prospective controlled trials. Furthermore, the complexity of performing arch surgery makes such a trial inherently difficult. Controlling all of the variables required, including the surgeon-specific variables, is probably impossible; therefore a clinical difference will always be difficult to identify in a definitive manner.
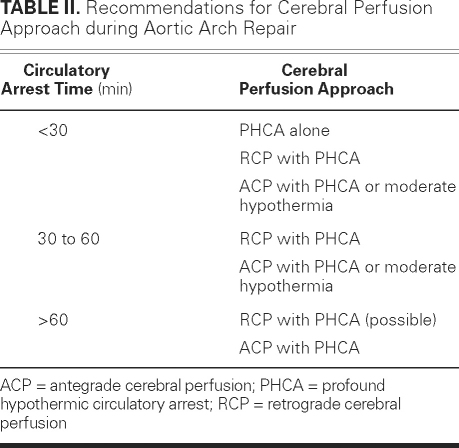
At any rate, the current clinical evidence suggests that any approach, whether PHCA alone, RCP, or ACP can be used if cerebral circulatory arrest time remains under 30 minutes; that the addition of RCP or ACP might improve outcomes if circulatory arrest time is longer than 30 minutes but shorter than 60 minutes; and that ACP and RCP may provide respectable outcomes when circulatory arrest time is longer than 60 minutes (Table II).
Table II. Recommendations for Cerebral Perfusion Approach during Aortic Arch Repair
Footnotes
Address for reprints: Anthony L. Estrera, MD, 6400 Fannin St., Suite 2850, Houston, TX 77030
E-mail: Anthony.L.Estrera@uth.tmc.edu
Presented at the 8th Current Trends in Aortic and Cardiothoracic Surgery Conference; Houston, 29–30 April 2011.
References
- 1.Cooley DA, DeBakey ME. Resection of the thoracic aorta with replacement by homograft for aneurysms and constrictive lesions. J Thorac Surg 1955;29(1):66–104. [PubMed]
- 2.DeBakey ME, Henly WS, Cooley DA, Crawford ES, Morris GC Jr, Beall AC Jr. Aneurysms of the aortic arch: factors influencing operative risk. Surg Clin North Am 1962;42:1543–54. [DOI] [PubMed]
- 3.Griepp RB, Stinson EB, Hollingsworth JF, Buehler D. Prosthetic replacement of the aortic arch. J Thorac Cardiovasc Surg 1975;70(6):1051–63. [PubMed]
- 4.Svensson LG, Crawford ES, Hess KR, Coselli JS, Raskin S, Shenaq SA, Safi HJ. Deep hypothermia with circulatory arrest. Determinants of stroke and early mortality in 656 patients. J Thorac Cardiovasc Surg 1993;106(1):19–31. [PubMed]
- 5.Bachet J. What is the best method for brain protection in surgery of the aortic arch? Selective antegrade cerebral perfusion. Cardiol Clin 2010;28(2):389–401. [DOI] [PubMed]
- 6.Astrup J, Symon L, Branston NM, Lassen NA. Cortical evoked potential and extracellular K+ and H+ at critical levels of brain ischemia. Stroke 1977;8(1):51–7. [DOI] [PubMed]
- 7.Roach GW, Kanchuger M, Mangano CM, Newman M, Nussmeier N, Wolman R, et al. Adverse cerebral outcomes after coronary bypass surgery. Multicenter Study of Perioperative Ischemia Research Group and the Ischemia Research and Education Foundation Investigators. N Engl J Med 1996;335 (25):1857–63. [DOI] [PubMed]
- 8.Ergin MA, Uysal S, Reich DL, Apaydin A, Lansman SL, McCullough JN, Griepp RB. Temporary neurological dysfunction after deep hypothermic circulatory arrest: a clinical marker of long-term functional deficit. Ann Thorac Surg 1999;67(6):1887–94. [DOI] [PubMed]
- 9.Likosky DS, Marrin CA, Caplan LR, Baribeau YR, Morton JR, Weintraub RM, et al. Determination of etiologic mechanisms of strokes secondary to coronary artery bypass graft surgery. Stroke 2003;34(12):2830–4. [DOI] [PubMed]
- 10.Gega A, Rizzo JA, Johnson MH, Tranquilli M, Farkas EA, Elefteriades JA. Straight deep hypothermic arrest: experience in 394 patients supports its effectiveness as a sole means of brain preservation. Ann Thorac Surg 2007;84(3):759–67. [DOI] [PubMed]
- 11.Ehrlich MP, McCullough JN, Zhang N, Weisz DJ, Juvonen T, Bodian CA, Griepp RB. Effect of hypothermia on cerebral blood flow and metabolism in the pig. Ann Thorac Surg 2002;73(1):191–7. [DOI] [PubMed]
- 12.Percy A, Widman S, Rizzo JA, Tranquilli M, Elefteriades JA. Deep hypothermic circulatory arrest in patients with high cognitive needs: full preservation of cognitive abilities. Ann Thorac Surg 2009;87(1):117–23. [DOI] [PubMed]
- 13.Harrington DK, Walker AS, Kaukuntla H, Bracewell RM, Clutton-Brock TH, Faroqui M, et al. Selective antegrade cerebral perfusion attenuates brain metabolic deficit in aortic arch surgery: a prospective randomized trial. Circulation 2004;110 (11 Suppl 1):II231–6. [DOI] [PubMed]
- 14.Frist WH, Baldwin JC, Starnes VA, Stinson EB, Oyer PE, Miller DC, et al. A reconsideration of cerebral perfusion in aortic arch replacement. Ann Thorac Surg 1986;42(3):273–81. [DOI] [PubMed]
- 15.Bachet J, Guilmet D, Goudot B, Termignon JL, Teodori G, Dreyfus G, et al. Cold cerebroplegia. A new technique of cerebral protection during operations on the transverse aortic arch. J Thorac Cardiovasc Surg 1991;102(1):85–94. [PubMed]
- 16.Kazui T, Inoue N, Yamada O, Komatsu S. Selective cerebral perfusion during operation for aneurysms of the aortic arch: a reassessment. Ann Thorac Surg 1992;53(1):109–14. [DOI] [PubMed]
- 17.Okita Y, Minatoya K, Tagusari O, Ando M, Nagatsuka K, Kitamura S. Prospective comparative study of brain protection in total aortic arch replacement: deep hypothermic circulatory arrest with retrograde cerebral perfusion or selective antegrade cerebral perfusion. Ann Thorac Surg 2001;72(1):72–9. [DOI] [PubMed]
- 18.Tanoue Y, Tominaga R, Ochiai Y, Fukae K, Morita S, Kawachi Y, Yasui H. Comparative study of retrograde and selective cerebral perfusion with transcranial Doppler. Ann Thorac Surg 1999;67(3):672–5. [DOI] [PubMed]
- 19.Spielvogel D, Strauch JT, Minanov OP, Lansman SL, Griepp RB. Aortic arch replacement using a trifurcated graft and selective cerebral antegrade perfusion. Ann Thorac Surg 2002; 74(5):S1810–4. [DOI] [PubMed]
- 20.Harrington DK, Fragomeni F, Bonser RS. Cerebral perfusion. Ann Thorac Surg 2007;83(2):S799–804. [DOI] [PubMed]
- 21.Leshnower BG, Myung RJ, Kilgo PD, Vassiliades TA, Vega JD, Thourani VH, et al. Moderate hypothermia and unilateral selective antegrade cerebral perfusion: a contemporary cerebral protection strategy for aortic arch surgery. Ann Thorac Surg 2010;90(2):547–54. [DOI] [PubMed]
- 22.Harrington DK, Bonser M, Moss A, Heafield MT, Riddoch MJ, Bonser RS. Neuropsychometric outcome following aortic arch surgery: a prospective randomized trial of retrograde cerebral perfusion. J Thorac Cardiovasc Surg 2003;126(3):638–44. [DOI] [PubMed]
- 23.Estrera AL, Garami Z, Miller CC 3rd, Sheinbaum R, Huynh TT, Porat EE, et al. Determination of cerebral blood flow dynamics during retrograde cerebral perfusion using power M-mode transcranial Doppler. Ann Thorac Surg 2003;76(3): 704–10. [DOI] [PubMed]
- 24.Safi HJ, Iliopoulos DC, Gopinath SP, Hess KR, Asimacopoulos PJ, Bartoli S, et al. Retrograde cerebral perfusion during profound hypothermia and circulatory arrest in pigs. Ann Thorac Surg 1995;59(5):1107–12. [DOI] [PubMed]
- 25.Safi HJ, Letsou GV, Iliopoulos DC, Subramaniam MH, Miller CC 3rd, Hassoun H, et al. Impact of retrograde cerebral perfusion on ascending aortic and arch aneurysm repair. Ann Thorac Surg 1997;63(6):1601–7. [DOI] [PubMed]
- 26.Estrera AL, Miller CC 3rd, Lee TY, Shah P, Safi HJ. Ascending and transverse aortic arch repair: the impact of retrograde cerebral perfusion. Circulation 2008;118(14 Suppl):S160–6. [DOI] [PubMed]
- 27.Conklin LD, LeMaire SA, Raskin SA, Pisimisis GT, Coselli JS. Aortic arch repair with a new cerebral protection strategy: combined intermittent antegrade and retrograde cerebral perfusion [abstract]. Outcomes Key West 2002. Available from: http://outcomeskeywest.com/Portals/16/Abstracts/2002/a2002-27.pdf.
- 28.Estrera AL, Miller CC, Lee TY, Shah P, Irani AD, Ganim N, et al. Integrated cerebral perfusion for hypothermic circulatory arrest during transverse aortic arch repairs. Eur J Cardiothorac Surg 2010;38(3):293–8. [DOI] [PubMed]