Loeys-Dietz syndrome (LDS) is a newly described (2005)1,2 autosomal dominant connective-tissue disorder caused by heterozygous mutations in the genes that encode transforming growth factor-beta receptor 1 or 2 (TGFBR1 or TGFBR2). Given the fact that this syndrome is so newly described, its incidence is currently unknown. Physical stigmata of LDS include the triad of arterial/aortic tortuosity and aneurysmal disease, hypertelorism, and bifid uvula or cleft palate. Approximately 75% of LDS patients present with typical facial dysmorphic features, including cleft palate, craniosynostosis, or hypertelorism, and are termed LDS type 1.3 Type 1 patients have many features that overlap with those of Marfan syndrome (MFS).4 Type 2 patients lack those major craniofacial features but have cutaneous manifestations that include velvety/translucent skin, easy bruising, and atrophic scars. As a consequence, the features of LDS type 2 overlap those of Ehlers-Danlos syndrome.4 However, it appears that both types form a clinical continuum,3 and, in our experience,5 it is not uncommon for these patients to be labeled, at first, as MFS patients. Both LDS types are characterized by aggressive arterial/aortic disease with aneurysm formation and dissection/rupture at a young age. The median survival was 37 years in the initially reported clinical series, with thoracic dissection the leading cause of death2; the mean age at first dissection was 26.7 years (range, 0.5–47 yr). A correlation has been observed between the severity of craniofacial and cardiovascular abnormalities, to the extent that the mean age at death is lower for patients with LDS type 1.2
The diagnosis of LDS is confirmed by molecular genetic testing of TGFBR1 or TGFBR2; one third of patients have a TGFBR1 mutation, whereas two thirds have a mutation in TGFBR2. Only 25% of newly diagnosed patients have an affected parent (that is, the mutations are de novo in 75%), and there is wide intrafamilial variability with apparent nonpenetrance.2,3 There is no clinical difference between patients with mutations in TGFBR1 versus TGFBR2, and LDS types 1 and 2 can be caused by a mutation in either gene. The overall effect of the gene mutation at the tissue level (aortic wall) is increased TGF-β signaling, which results in diffuse medial degeneration with disorganization of elastic fibers and increased collagen deposits; this leads to vascular dilation and dissection.2–4
In comparison with MFS, the aortic disease is more aggressive in LDS; for example, dissection has been observed to occur at aortic diameters as small as 3.9 cm in adult patients.2 Further, with LDS, unlike MFS, the vascular disease can affect any part of the arterial tree from the head6 to pelvis, which necessitates distinguishing between the 2 syndromes for surveillance imaging upon follow-up. Although the aortic disease is more aggressive and the rate of death greater in LDS than in MFS families overall, survival is similar once LDS has been diagnosed, which again emphasizes the need for early diagnosis.7 Other distinguishing features between LDS and MFS include (in LDS) less frequent mitral valve disease, absence of ectopia lentis, and variable and milder skeletal features with no excessive height.2,7
In LDS aortic disease, aortic root aneurysms are the most common finding: two thirds of LDS patients have a root aneurysm at the time of diagnosis3,8 and almost all eventually develop dilation of the aortic root.3 Aneurysms of the ascending or descending aorta are less often seen and seldom are isolated. Aortic dilation is most commonly established by the teenage years, and 20% of patients have had an aortic dissection at the time of diagnosis. Finally, congenital heart defects such as bicuspid aortic valve, atrial septal defect, and patent ductus arteriosus occur more frequently in LDS patients than in the general population.3,7
Current criteria for elective intervention for asymptomatic aneurysms in adults with LDS include an aortic diameter >4.0–4.6 cm for the aortic root and abdominal aorta,8, 9 >5.0 cm for the descending thoracic aorta, and/or rapid expansion (>0.5 cm/yr) regardless of location.8 However, given that dissection has been reported2 at diameters smaller than 4 cm, even these criteria might not eliminate the risk of dissection or death; and earlier intervention could be indicated, depending on family history or an evaluation of the risks and benefits of surgery.8
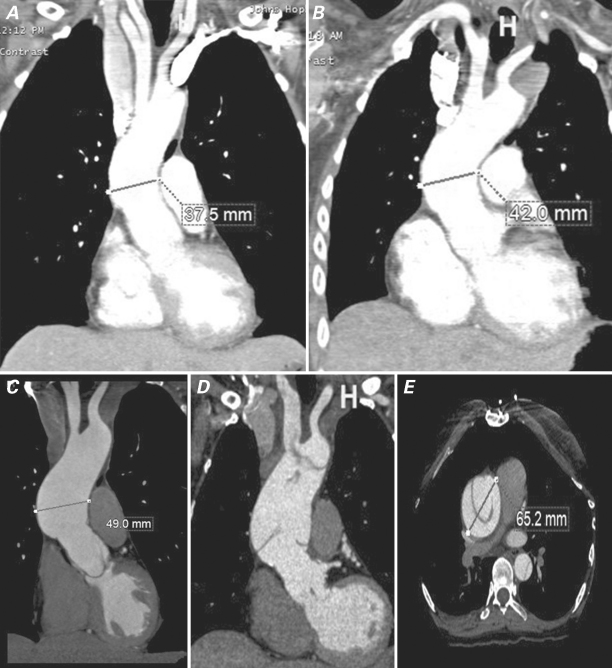
Consequent to the preponderance of root aneurysms, aortic root replacement has been the most commonly performed procedure in LDS patients.8,10–12 Given the young age of the typical patient, valve-sparing aortic root replacement (VSRR) via the David reimplantation technique appears to be the preferred option for root replacement, although the reported surgical experience to date is quite small.8 Whether prophylactic proximal arch replacement should be performed at the time of VSRR is not known,12 although we personally recommend this approach after having witnessed, in LDS patients, the late development of acute type A dissection in residual native ascending aorta after VSRR (Fig. 1).
Fig. 1 Contrast-enhanced computed tomographic angiographic (CTA) images of a Loeys-Dietz patient who underwent valve-sparing aortic root replacement (VSRR) at age 16 for root aneurysm. A) CTA 5 years after VSRR shows mild dilation of the residual native ascending aorta above the Dacron root graft to a diameter of 3.8 cm. B) CTA 8 years after VSRR shows further enlargement to 4.2 cm. C) Aorta measures 4.9 cm by CTA 10 years after VSRR. Surgical repair was recommended at this point but the patient requested delay for several months for personal reasons. D) Coronal and E) axial CTA images 6 months after scan shown in panel C show new acute type A aortic dissection of the residual native ascending aorta with further dilation of the aorta to 6.5 cm, necessitating emergent repair.
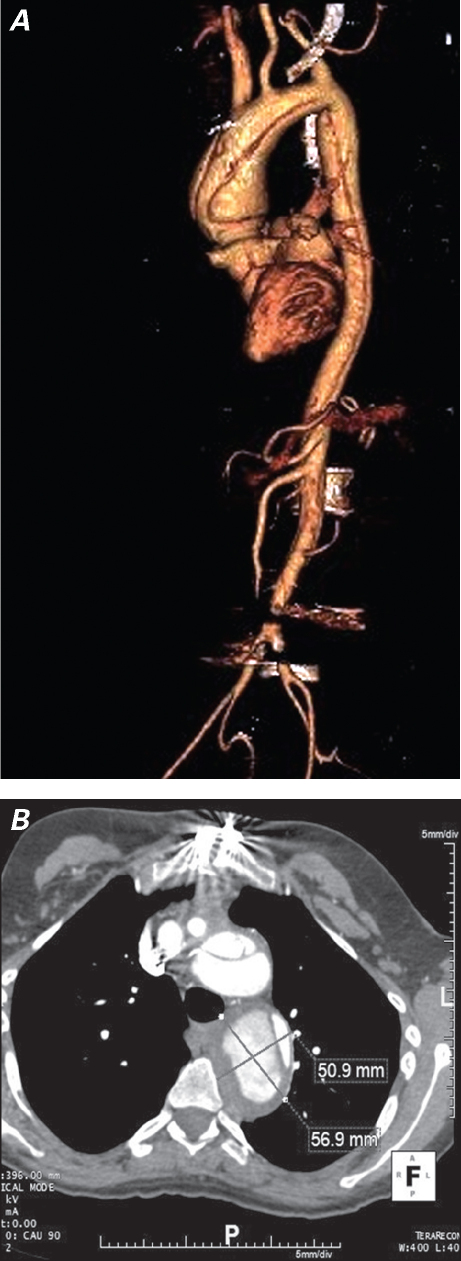
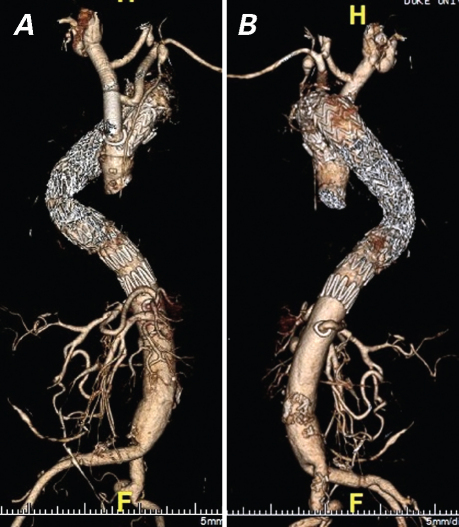
Because approximately 20% of LDS patients are not diagnosed until aortic dissection has developed,3 replacement of the aortic arch, the thoracoabdominal aorta, or both is commonly required. Further, total aortic replacement is certain to become a recognized treatment of this disorder, especially in patients who are not diagnosed until aortic dissection has occurred; in our experience, the LDS aorta dilates rapidly after dissection (Fig. 2).10,11 With regard to arch and thoracoabdominal replacement, the side-branch technique for arch and visceral vessel reimplantation should be used to avoid the known hazard (in other high-risk connective-tissue disease populations) of patch pseudoaneurysm formation.10,11 Similarly, to minimize the risk of patch aneurysm formation, we favor the creation of multiple small buttons of paired intercostal vessels, similar to those created with the coronary arteries in a button Bentall operation, for reimplantation during thoracoabdominal repair. Finally, the risk of continued arterial dilation due to the progressive nature of LDS predisposes the patient to stent-graft failure if native aorta is used for landing/fixation zones. Therefore, we consider LDS to be a contraindication to endovascular aortic repair unless both proximal and distal stent-graft landing zones are within existing Dacron grafts (Fig. 3).10
Fig. 2 A) Three-dimensional computed tomographic angiographic (CTA) reconstruction of the aorta at the time of acute type A dissection in a Loeys-Dietz patient and B) axial CTA image 2 months after type A dissection repair show rapid enlargement of the dissected descending aorta in a short interval.
Fig. 3 A) Anteroposterior and B) lateral view 3-dimensional computed tomographic angiographic reconstructions of the aorta after “hybrid” repair in a patient with Loeys-Dietz syndrome (LDS). Six years after aortic root replacement for repair of an acute type A dissection at age 45, the patient underwent Crawford extent III replacement of a thoracoabdominal aortic aneurysm (TAAA) that enlarged secondary to chronic dissection. A year later, repeat sternotomy for total aortic arch replacement via the elephant-trunk technique was performed for an enlarging transverse arch/proximal descending aneurysm; to complete treatment of the LDS aortic disease, staged endovascular exclusion of the remainder of the chronically dissected aorta was performed during the same hospitalization. Long-segment landing zones for the endografts lay proximally within the Dacron elephant-trunk graft and distally within the Dacron TAAA graft above the takeoff of the side branch to the celiac axis. A hybrid technique was chosen because of the patient's severe scoliosis in union with an extremely tortuous descending aorta that crossed over into the right side of the chest during part of its course.
In our experience of operating on LDS patients, we have encountered 2 anatomic anomalies that appear to be more common in this population and that can have surgical significance for aortic reconstruction. The first is that the left subclavian artery frequently arises from the very distal aortic arch in the left chest (Fig. 2A). During total arch replacement via median sternotomy, this often precludes reimplantation with the side-branch technique. In these instances, we have generally found it necessary to reimplant, via left thoracotomy, the left subclavian artery into the descending aorta during the second-stage repair. The second common anomaly is the presence of multiple renal arteries, which need to be preserved individually during thoracoabdominal repair using the side-graft technique. These anomalies should be sought out preoperatively, by careful examination of computed tomographic angiograms.
In conclusion, early and aggressive surgical intervention for LDS is mandatory and, given the typically young age of these patients, generally well tolerated. A valve-sparing technique is preferred for root replacement when feasible, and, to prevent late type A dissection, strong consideration should be given to routine prophylactic proximal arch replacement at the time of root replacement. The goal of surgery for an LDS patient who has already experienced aortic dissection should be the replacement of all dissected aorta, as feasible, with use of the side-branch technique for total aortic arch and thoracoabdominal aortic replacement. Both before and after repair, LDS patients need close lifelong imaging surveillance from head through pelvis, yearly at minimum. Finally, given the emerging evidence supporting improved outcomes for thoracic aortic surgery in higher-volume centers,13 these patients are probably best treated in specialty referral aortic centers by a multidisciplinary team.
Footnotes
Address for reprints: G. Chad Hughes, MD, Box 3051 DUMC, Durham, NC 27710
E-mail: gchad.hughes@duke.edu
★ CME Credit
Presented at the 8th Current Trends in Aortic and Cardiothoracic Surgery Conference; Houston, 29–30 April 2011.
References
- 1.Loeys BL, Chen J, Neptune ER, Judge DP, Podowski M, Holm T, et al. A syndrome of altered cardiovascular, craniofacial, neurocognitive and skeletal development caused by mutations in TGFBR1 or TGFBR2. Nat Genet 2005;37(3): 275–81. [DOI] [PubMed]
- 2.Loeys BL, Schwarze U, Holm T, Callewaert BL, Thomas GH, Pannu H, et al. Aneurysm syndromes caused by mutations in the TGF-beta receptor. N Engl J Med 2006;355(8):788–98. [DOI] [PubMed]
- 3.Van Hemelrijk C, Renard M, Loeys B. The Loeys-Dietz syndrome: an update for the clinician. Curr Opin Cardiol 2010; 25(6):546–51. [DOI] [PubMed]
- 4.Cozijnsen L, Braam RL, Waalewijn RA, Schepens MA, Loeys BL, van Oosterhout MF, et al. What is new in dilatation of the ascending aorta? Review of current literature and practical advice for the cardiologist. Circulation 2011;123(8):924–8. [DOI] [PubMed]
- 5.Rajagopal K, Rogers JG, Lodge AJ, Gaca JG, McCann RL, Milano CA, Hughes GC. Two-stage total cardioaortic replacement for end-stage heart and aortic disease in Marfan syndrome: case report and review of the literature. J Heart Lung Transplant 2009;28(9):958–63. [DOI] [PubMed]
- 6.Rodrigues VJ, Elsayed S, Loeys BL, Dietz HC, Yousem DM. Neuroradiologic manifestations of Loeys-Dietz syndrome type 1. AJNR Am J Neuroradiol 2009;30(8):1614–9. [DOI] [PMC free article] [PubMed]
- 7.Attias D, Stheneur C, Roy C, Collod-Beroud G, Detaint D, Faivre L, et al. Comparison of clinical presentations and outcomes between patients with TGFBR2 and FBN1 mutations in Marfan syndrome and related disorders. Circulation 2009; 120(25):2541–9. [DOI] [PubMed]
- 8.Williams JA, Loeys BL, Nwakanma LU, Dietz HC, Spevak PJ, Patel ND, et al. Early surgical experience with Loeys-Dietz: a new syndrome of aggressive thoracic aortic aneurysm disease. Ann Thorac Surg 2007;83(2):S757–63. [DOI] [PubMed]
- 9.Hiratzka LF, Bakris GL, Beckman JA, Bersin RM, Carr VF, Casey DE Jr, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine [published erratum appears in Circulation 2010; 122(4):e410]. Circulation 2010;121(13):e266–369. [DOI] [PubMed]
- 10.Williams JB, McCann RL, Hughes GC. Total aortic replacement in Loeys-Dietz syndrome. J Card Surg 2011;26(3):304–8. [DOI] [PMC free article] [PubMed]
- 11.Williams ML, Wechsler SB, Hughes GC. Two-stage total aortic replacement for Loeys-Dietz syndrome. J Card Surg 2010; 25(2):223–4. [DOI] [PubMed]
- 12.Augoustides JG, Plappert T, Bavaria JE. Aortic decision-making in the Loeys-Dietz syndrome: aortic root aneurysm and a normal-caliber ascending aorta and aortic arch. J Thorac Cardiovasc Surg 2009;138(2):502–3. [DOI] [PubMed]
- 13.Hughes GC, Zhao Y, Rankin JS, O'Brien S, Bavaria JE, Wolfe WG, et al. Effects of institutional volumes on operative outcomes for aortic root replacement in North America. J Thorac Cardiovasc Surg. In press. [DOI] [PubMed]