Patients who need cardiac surgery have become increasingly more complex, and an increasing proportion of them require reoperative cardiac surgery. Surgical techniques have significantly improved, and recent reports suggest that reoperative status is no longer an independent predictor of death.1,2
Reducing Errors and Rescue
It has been suggested that the safety processes of human-factors engineering could be applied to cardiac surgery to improve outcomes in patients undergoing pediatric cardiac operations.3 It already has been demonstrated that reoperation does not necessarily add risk for a patient undergoing isolated coronary surgery or valve replacement. We sought to determine whether reoperation status adds risk to adult cardiac surgery when all patients who require repeat surgery are considered. In reviewing the operative notes on 1,847 patients who had undergone reoperative cardiac surgery over a 2-year period, we described major life-threatening intraoperative adverse events (IAEs) and analyzed outcomes and cost.4
One hundred forty-five IAEs had occurred in 127 patients, or in 7% of the operations. Associated risk factors were the number of previous operations and a history of chest radiation. Most commonly, IAEs involved injury to bypass grafts, the chambers of the heart, or the great vessels. Other IAEs included the development of life-threatening arrhythmias, or lung injury that was severe enough to alter the course of the operation. Intraoperative adverse events occurred during every phase of operation but were most common during pre-pump dissection. This finding constitutes a change from previous reports that highlight the risk of injury during repeat sternotomy. Improved safety of redo sternotomy is in part attributable to a standardized protocol for preoperative imaging.
Standardized Preoperative Imaging Assists Planning
In preparation for repeat sternotomy, all patients undergo not only coronary catheterization to determine the patency of previous grafts but also a careful evaluation by the surgeon to determine the location and mobility of the grafts. It is important that the catheterization be complete: coronary arteries do not disappear. In the event that a graft has not been visualized on the most recent preoperative catheterization, it is not uncommon to repeat the study with selective cannulation of that graft. It is also very important to review any previous cardiac catheterizations that a patient may have had in order to understand how the native-vessel disease has progressed over time.
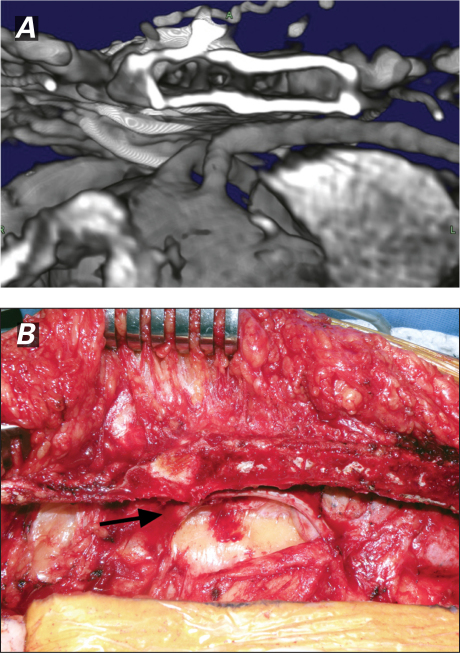
Multidetector computed tomographic (CT) scanning has increasingly played a role in the planning of reoperative cardiac surgery.5 All patients undergo a CT scan of the chest to reveal the location of all intrathoracic structures. When you use volume-rendered and multiplanar-reconstruction 3-dimensional techniques in planning, what lies under the sternum should never be a mystery (Fig. 1).
Fig. 1 A) Volume-rendered 3-dimensional reconstruction computed tomogram of a patent coronary artery bypass graft densely adherent to the sternum, and B) the intraoperative view of the same after a safe opening lateral to the graft (arrow).
Mechanical Circulatory Support Techniques
With the use of imaging, the risk of sternal opening is predictable. It is the responsibility of the surgeon to determine, on the basis of those preoperative imaging studies, the appropriate level of preparedness of the team for rescue, in the event that an injury occurs. This increases in intensity from having the perfusionist in the room (for the lowest-risk patients), to exposing the axillary artery or groin vessels, to establishing cardiopulmonary bypass (CPB) before opening, to performing hypothermic circulatory arrest (for the highest-risk patients) as described above. Early CPB results in a longer pump run, increased risk of bleeding due to coagulopathy, and higher risk of end-organ dysfunction. For these reasons, we believe that CPB or circulatory arrest (or both) should be established before opening only in patients who are at the highest risk of injury upon opening.
Checklists and the “Huddle”
All members of the surgical and anesthesia team are present for the preoperative huddle before inducing anesthesia. In addition to review of the basic safety items on the checklist such as confirming procedure, location of incision and vascular access, preoperative medications, availability of blood, and equipment needs, we discuss the conduct of rescue plans in the event of an IAE. This level of preparedness, directed by preoperative imaging, optimizes the efficiency of all team members during times of crisis.
Standardized Techniques for Opening and Dissection
After preparation for the method of circulatory support, including the selective exposure of alternative cannulation sites, we open the sternum. An oscillating saw should be used while the assistant provides anteriorly directed traction on the sternum. Sternal wires can be left in while opening the posterior table, in order to protect structures that are closely adherent.
The right sternal border is released from the heart first. Cautery should be limited and sharp dissection preferred. Sternal traction should be gentle and upward. Usually, the correct plane can be developed along the diaphragmatic surface, then up around the right atrium toward the aorta. A “no-touch” technique should be applied to patent vein grafts by dissecting adherent structures at least a few millimeters away from the grafts. Dissection of the left side of the heart should be completed while the patient is on CPB, preferably with the heart arrested. The heart should not be dissected more than is needed to safely perform the planned operation.
Outcomes of Reoperations after Intraoperative Adverse Events
In the study described, the overall hospital mortality rate was 4.5% (higher after IAE). Because the number of events was small, we calculated the composite poor outcome of death, stroke, or myocardial infarction (poor outcome 19% with IAE vs 6.2% without; P <0.0001). Propensity analysis also revealed that direct technical costs were 30% higher after IAE. However, because of confounding factors, this analysis still does not answer the question of whether reoperation increases the risk of cardiac surgery.
Rescue and Failure to Rescue
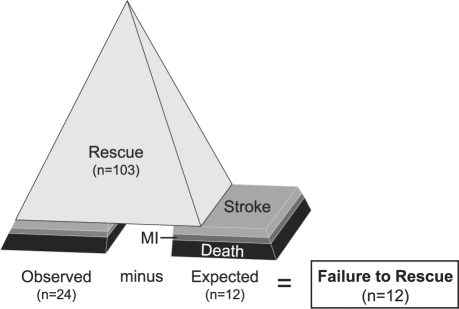
In an attempt to answer this question, we used a logistic regression model of patients without IAE (n=1,720) to determine expected poor outcome in the patients with IAE. By comparing expected (n=12) to observed (n=24) poor outcome, we derived the number of patients in whom there was a failure to rescue (n=12) (Fig. 2). In contrast, 103 patients were successfully rescued from IAE without death, stroke, or myocardial infarction. The ability to rescue patients successfully after IAE during complex reoperative cardiac surgery explains why it is difficult to describe the performance of reoperations as an independent risk factor by means of more common statistical techniques. These 12 failure-to-rescue patients comprised 0.65% of the total population under study.
Fig. 2 Diagram shows the concepts of failure to rescue from intraoperative adverse events defined as an observed occurrence of poor outcome exceeding the expected in a population of reoperative cardiac surgery patients.4
MI = myocardial infarction
Challenges around the Corner
An increasing number of patients have been treated with bioprostheses in the last decade, and more of these bioprostheses will fail, including transcatheter aortic valves. More patients with aortic disease are undergoing thoracic endovascular aortic repair (TEVAR), and the stability of these devices is dependent on a stable landing zone in healthy aorta. We can also expect to see more such patients requiring reoperations. Nearly 40% of patients who undergo heart transplantation are bridged with mechanical circulatory support, and the number is sure to grow as smaller and more reliable pumps are developed. Reoperations on all of these complex patients will present new challenges and require experienced teams to achieve success.
Conclusion
Reoperation does appear to present increased risk in selected patients, such as those who need repeated reoperations, or have a history of radiation therapy, or present with severe right-sided heart failure. Fortunately, that added risk is low, at approximately one half of one percent, because of the ability of experienced teams to rescue patients when an IAE occurs—although rescue comes at increased cost to the patient and the system. Human error can be avoided with improved systematic protocols for planning, the creation of an efficient team, and the preparation of that team with detailed checklists.
Footnotes
Address for reprints: Eric E. Roselli, MD, Department of Thoracic & Cardiovascular Surgery, Desk J4-1, The Cleveland Clinic Foundation, 9500 Euclid Ave., Cleveland, OH 44195
E-mail: Roselle@ccf.org
★ CME Credit
Presented at the 8th Current Trends in Aortic and Cardiothoracic Surgery Conference; Houston, 29–30 April 2011.
References
- 1.Davierwala PM, Maganti M, Yau TM. Decreasing significance of left ventricular dysfunction and reoperative surgery in predicting coronary artery bypass grafting-associated mortality: a twelve-year study. J Thorac Cardiovasc Surg 2003;126 (5):1335–44. [DOI] [PubMed]
- 2.Sabik JF 3rd, Blackstone EH, Houghtaling PL, Walts PA, Lytle BW. Is reoperation still a risk factor in coronary artery bypass surgery? Ann Thorac Surg 2005;80(5):1719–27. [DOI] [PubMed]
- 3.de Leval MR, Carthey J, Wright DJ, Farewell VT, Reason JT. Human factors and cardiac surgery: a multicenter study. J Thorac Cardiovasc Surg 2000;119(4 Pt 1):661–72. [DOI] [PubMed]
- 4.Roselli EE, Pettersson GB, Blackstone EH, Brizzio ME, Houghtaling PL, Hauck R, et al. Adverse events during reoperative cardiac surgery: frequency, characterization, and rescue. J Thorac Cardiovasc Surg 2008;135(2):316–23. [DOI] [PubMed]
- 5.Kamdar AR, Meadows TA, Roselli EE, Gorodeski EZ, Curtin RJ, Sabik JF, et al. Multidetector computed tomographic angiography in planning of reoperative cardiothoracic surgery. Ann Thorac Surg 2008;85(4):1239–45. [DOI] [PubMed]