Abstract
Persistent hypotension subsequent to percutaneous coronary intervention is attributed to access-site bleeding, re-infarction, or mechanical complications either of myocardial infarction or of the procedure itself (for example, pericardial tamponade). Dynamic left ventricular outflow tract obstruction after an uncomplicated percutaneous coronary intervention is an unusual, and to our knowledge not previously reported, complication that manifests itself as hypotension refractory to the usual therapy with inotropic agents. We discuss the clinical course, pathophysiology, diagnosis, and management of hypotension due to left ventricular outflow tract obstruction after percutaneous coronary intervention. Early recognition and accurate diagnosis that determines appropriate therapy will improve the patient's prospects.
Key words: Cardiogenic shock; hypotension; left ventricular outflow tract obstruction/etiology/physiopathology/ultrasonography; myocardial infarction/complications; percutaneous coronary intervention; ventricular function, left
Hypotension after percutaneous coronary intervention (PCI) is commonly attributed to internal or external bleeding, cardiogenic shock from stent thrombosis, new myocardial infarction (MI) with or without mechanical complications, or vasovagal response. Other causes include anaphylaxis and the use of certain medications (for example, intracoronary nitroglycerin during coronary angiography). Hypotension and increased left ventricular outflow tract (LVOT) pressure gradient are reported in the setting of MI due to increased contractility of the preserved myocardial segments. Hypotension after a successful PCI in our patient was due to a pure dynamic LVOT obstruction and hyperdynamic left ventricular (LV) systolic function.
Case Report
A 51-year-old man was brought to the emergency department after cardiopulmonary resuscitation for ventricular fibrillation. Electrocardiography showed inferior ST-segment-elevation MI. Emergent coronary angiography revealed an occlusive thrombus in the distal right coronary artery. Thrombolysis in Myocardial Infarction-III flow was restored in the right coronary artery after the placement of a drug-eluting stent without complications. Before angiography, transthoracic echocardiography (TTE) had shown the LV ejection fraction (LVEF) to be at the lower limits of normal (0.50–0.55); there was moderate-to-severe LV hypertrophy with impaired relaxation, and hypokinesis of the inferior and inferolateral walls.
The patient's subsequent hospital course in the critical care unit was complicated by cardiogenic shock that was manifested by blood pressure as low as 80/40 mmHg and by sinus tachycardia up to 135 beats/min. Despite intravenous fluid resuscitation, he remained hypotensive. He was given norepinephrine and dopamine infusions; however, his blood pressure did not improve. Clinical examination revealed a new grade 3/6 systolic ejection murmur along the left sternal border that did not radiate to the carotid arteries. The patient was uncooperative, so dynamic maneuvers could not be performed. An S4 gallop was heard. Carotid palpation revealed a brisk upstroke.
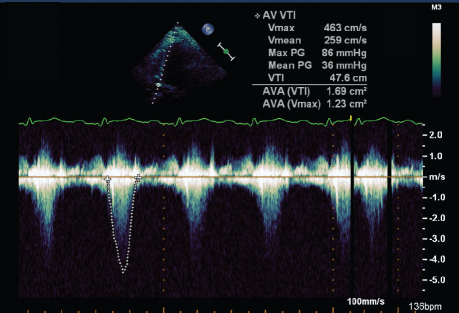
Laboratory testing revealed a 4- to 5-g/dL drop in the hemoglobin level and a rise in the serum creatinine level from 1.6 to 1.9 mg/dL. No hematomas could be felt at the catheter insertion site. We could not order a computed tomographic scan to find the bleeding source, because the patient was not hemodynamically stable. A repeat TTE revealed a hyperdynamic LV with an ejection fraction >0.65, cavity obliteration during systole, and systolic anterior motion (SAM) of the anterior mitral valve leaflet. Transthoracic continuous-wave Doppler echocardiography across the LVOT and aortic valve showed mean and peak gradients of 36 and 86 mmHg, respectively; peak velocity was 463 cm/s (Fig. 1). M-Mode Doppler TTE of the aortic valve showed no aortic stenosis that could contribute to the elevated gradients (image not shown).
Fig. 1 Transthoracic continuous-wave Doppler echocardiogram at the left ventricular outflow tract during hypotension shows increased gradients.
AVA = aortic valve area; PG = pressure gradient; Vmax = maximum velocity; Vmean = mean velocity; VTI = velocity time interval
On the basis of the TTE findings, our patient was weaned from intravenous norepinephrine and dopamine drips, and intravenous esmolol and phenylephrine drips were instituted. Aggressive intravenous fluid resuscitation was continued. The earlier cardiac physical findings started to resolve. Eventually, the blood pressure normalized and the tachycardia resolved. Only when the patient was stable enough to leave the critical care unit was detailed questioning possible. He was uncertain about whether he had a history of heart disease—in particular, cardiomyopathy. He was discharged from the hospital in stable condition.
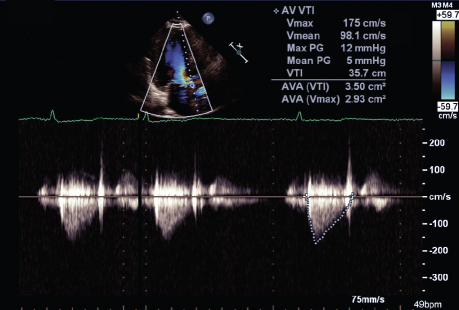
Follow-up TTE showed complete resolution of the hyperdynamic LVOT obstruction and normalization of the gradients across the LVOT and aortic valve. The mean gradient improved from 36 to 5 mmHg; the peak gradient improved from 86 to 12 mmHg; and peak velocity decreased to 175 cm/s from 463 cm/s (Fig. 2).
Fig. 2 Transthoracic continuous-wave Doppler echocardiogram at the left ventricular outflow tract shows normalization of gradients after appropriate management.
AVA = aortic valve area; PG = pressure gradient; Vmax = maximum velocity; Vmean = mean velocity; VTI = velocity time interval
Discussion
Left ventricular outflow tract obstruction is usually described within the context of hypertrophic obstructive cardiomyopathy1 and has been thought to be a hallmark of that condition—especially in cases with asymmetric septal hypertrophy, which causes structural obstruction. This obstruction can be dynamically aggravated by the abnormal SAM of the anterior mitral leaflet. However, more recently, a pure dynamic LVOT obstruction (detected with Doppler TTE) was reported as a possible complication of acute coronary syndromes.2,3 As reported by Armstrong and Marcovitz,4 the mechanism of dynamic LVOT obstruction in the setting of MI is compensatory hyperkinesis of the preserved myocardial segments. It is more commonly observed when only a single coronary artery (usually the left anterior descending) is affected. The remaining uninjured myocardial segments can become hyperdynamic while trying to compensate for the hypokinetic segment, thus causing the dynamic LVOT obstruction. Nonetheless, dynamic LVOT obstruction can occur in other situations, such as that experienced by our patient, whose successful PCI was followed by hypovolemia that resulted in hyperdynamic LV.
Pathophysiology
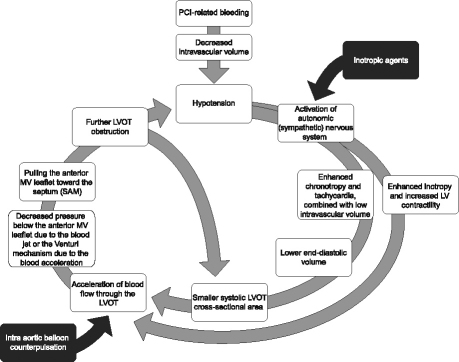
Left ventricular outflow tract obstruction is a dynamic state that is affected by fluctuations in volume status,5 autonomic nervous activity, diurnal variations, pharmacotherapy, exercise,6–8 and even the physical position of the patient. Percutaneous coronary intervention–related bleeding, as well as volume depletion resulting from fasting or the effect of diuretic agents, can lead to decreased intravascular volume and hypotension. This causes increased sympathetic nervous system output. The high level of catecholamines enhances the inotropic and chronotropic effects—both of which have deleterious effects on LVOT obstruction. The increased LV contractility, as a result of the enhanced inotropic effects, accelerates blood flow across the LVOT. On the other hand, tachycardia allows less time for LV filling, thereby resulting in less end-diastolic volume and, in turn, in less LVOT cross-sectional area during systole. This further accelerates blood flow through the narrower LVOT. The resultant high acceleration of blood flow, likely to occur in the presence of LV hypertrophy, can create a negative pressure below the anterior mitral leaflet that sucks it anteriorly toward the septum. Some investigators postulate that the SAM is caused by the Venturi mechanism, which in turn is caused by the accelerated blood jet. The SAM of the anterior mitral leaflet toward the septum further obstructs the LVOT. As a net result, cardiac output decreases and hypotension worsens and further activates the sympathetic system. This process can continue to deteriorate until the cycle is interrupted (Fig. 3).
Fig. 3 Diagram shows the pathophysiology of the dynamic left ventricular outflow tract obstruction.
PCI = percutaneous coronary intervention; MV = mitral valve; LV = left ventricular; LVOT = left ventricular outflow tract; SAM = systolic anterior motion
Our patient's LV hypertrophy might have placed him at higher risk of developing dynamic LVOT obstruction in the presence of volume depletion. We did not believe that primary cardiomyopathy was the underlying cause, so we did not order genetic testing. Although our patient had experienced an acute coronary syndrome, the LVOT obstruction was not caused by a compensatory hyperkinesis of preserved segments, as none was evident on the TTE obtained on admission. It occurred after the PCI, due to bleeding, volume depletion, and the hyperdynamic LV state.
Also, we have seen another patient (a 68-year-old woman) with a similar presentation after an elective PCI in the absence of acute coronary syndrome. She had increasing left-groin bruising at the catheter insertion site with no palpable hematoma, a 3-g/dL drop in hemoglobin level, and a rise in serum creatinine from 1.0 to 1.5 mg/dL. Left ventricular outflow tract obstruction resolved after volume expansion with blood transfusion. Similarly, that patient had reported no family history of cardiac disease. Her baseline TTE revealed mild LV hypertrophy and impaired relaxation.
Left ventricular hypertrophy, intravascular volume depletion, and hyperdynamic LV are the key components of the pathogenesis of this dynamic LVOT obstruction.
Pitfalls
After one understands the pathophysiology, it is obvious that inotropic agents (black box in Fig. 3) aggravate the hypotension as they increase the acceleration of the blood jet across the LVOT and increase the SAM of the anterior mitral leaflet. Similarly, intra-aortic balloon counterpulsation aggravates the obstruction, because the deflated balloon creates a negative pressure in the aorta during systole, causing further acceleration of the blood flow across the LVOT.9 Intracoronary or intravenous nitroglycerin used during or after coronary angiography can worsen the LVOT obstruction by decreasing the preload and the end-diastolic LV volume.
Diagnosis
Thorough cardiac examination is always imperative. Development of a new systolic murmur in the setting of an acute MI complicated by cardiogenic shock suggests the presence of LVOT obstruction, as well as the other classical mechanical complications of MI—namely, ventricular septal perforation and papillary muscle rupture. Auscultation might reveal a medium-pitched crescendo-decrescendo systolic ejection murmur along the left sternal border that rarely radiates to the carotid arteries, unlike the murmur of aortic stenosis. The intensity of the murmur is quite variable. Almost all cardiac murmurs decrease in intensity during the Valsalva maneuver. Murmur associated with LVOT obstruction is an exception, because this maneuver decreases end-diastolic volume and preload. Similarly, rising from a squatting to a standing position decreases preload and makes the murmur louder. Conversely, increasing the afterload, as occurs in a hand-grip maneuver, decreases the gradient across the LVOT, thus decreasing the intensity of the murmur.
Palpation of the carotid pulse can be helpful. The upstroke is brisk because there is little resistance during early systole, while blood is being ejected through the LVOT into the aorta. As systole continues, LVOT obstruction occurs, resulting in a collapse in the pulse; then there is a secondary increase because the gradient across the LVOT starts to decrease as most of the blood has been ejected and the blood jet is weaker—a finding termed pulsus bisferiens (spike and dome).
Left ventricular outflow tract obstruction is usually accompanied by SAM of the anterior mitral leaflet; therefore, careful auscultation can reveal an accompanying mitral regurgitation murmur. Worsening hypotension after use of inotropic agents can be highly suggestive of dynamic LVOT obstruction. Repeating the cardiac examination might reveal that the above physical findings intensified while using the inotropic agents. It is important to remember that the cardiogenic shock due to mechanical complications of MI is typically seen on the 3rd day or later, while the cardiogenic shock due to LVOT obstruction manifests itself early, during the first 24 hours. Ventricular septal rupture and ruptured papillary muscle are more likely to present with acute pulmonary edema, while they are absent in LVOT obstruction. Diminished heart sounds could indicate cardiac tamponade. The source of bleeding (retroperitoneal, for example) may not always be obvious; even the thigh can conceal a significant amount of blood that might not be visible or palpable on physical examination. Laboratory testing, including a complete blood count and renal function tests, can be helpful, because a drop in hemoglobin level together with an elevation of serum creatinine strongly suggests impaired renal perfusion due to bleeding and intravascular volume depletion. However, we should remember that the drop in hemoglobin level can lag behind. The presence of dynamic LVOT obstruction is reliably detected by TTE10 or (if the transthoracic image quality is suboptimal) by transesophageal echocardiography.11
Management
Dynamic LVOT obstruction may be common in post-PCI patients who present with hypotension, but it might go undiscovered because it can resolve with simple volume expansion, the first line of management for hypotension in most cases. In our patient, the cardiogenic shock persisted until therapy was directed toward decreasing the degree of the dynamic LVOT obstruction. Intuitively, the attending physician would expect β-blockers to be contraindicated in the treatment of cardiogenic shock; standard management includes inotropic support, the use of an intra-aortic balloon pump, or both. In this particular instance, such management is contraindicated. Aggressive volume expansion and the use of β-blockers are chief components of the treatment. Management might also include the use of a1-agonists (such as phenylephrine) and the avoidance of therapies that increase the magnitude of the LVOT obstructive pressure gradient, such as diuretic agents and vasodilators, which would reduce preload or afterload, or both.
This case highlights the significance of the early use of echocardiography in the evaluation of patients who present with hypotension after a PCI. In addition to excluding the usual causes of hypotension, such as pericardial tamponade, new wall-motion abnormalities, and mechanical complications of MI, one must keep in mind the possibility of dynamic LVOT obstruction and look for its specific cardiac physical findings and echocardiographic attributes. Accurate diagnosis will help in determining the appropriate therapy for a favorable outcome.
Acknowledgment
We would like to thank Mr. Michael Konomos, medical illustrator, Section of Cardiology, for his help with the illustrations.
Footnotes
Address for reprints: Ali Dahhan, MD, 1120 - 15th St. (BI-5070), Augusta, GA 30912-3105
E-mail: adahhan@georgiahealth.edu
References
- 1.Nishimura RA, Holmes DR Jr. Clinical practice. Hypertrophic obstructive cardiomyopathy [published erratum appears in N Engl J Med 2004;351(10):1038]. N Engl J Med 2004; 350(13):1320–7. [DOI] [PubMed]
- 2.Joffe II, Riley MF, Katz SE, Ginsburg GS, Douglas PS. Acquired dynamic left ventricular outflow tract obstruction complicating acute anterior myocardial infarction: serial echocardiographic and clinical evaluation. J Am Soc Echocardiogr 1997;10(7):717–21. [DOI] [PubMed]
- 3.Keegan MT, Sinak LJ, Nichols DA, Lanier WL. Dynamic left ventricular outflow tract obstruction in acute coronary syndromes. Mayo Clin Proc 2000;75(2):216–7. [DOI] [PubMed]
- 4.Armstrong WF, Marcovitz PA. Dynamic left ventricular outflow tract obstruction as a complication of acute myocardial infarction. Am Heart J 1996;131(4):827–30. [DOI] [PubMed]
- 5.Yang JH, Park SW, Yang JH, Cho SW, Kim HS, Choi KA, Kim HJ. Dynamic left ventricular outflow tract obstruction without basal septal hypertrophy, caused by catecholamine therapy and volume depletion. Korean J Intern Med 2008;23 (2):106–9. [DOI] [PMC free article] [PubMed]
- 6.Mingo S, Benedicto A, Jimenez MC, Perez MA, Montero M. Dynamic left ventricular outflow tract obstruction secondary to catecholamine excess in a normal ventricle. Int J Cardiol 2006;112(3):393–6. [DOI] [PubMed]
- 7.Geske JB, Sorajja P, Ommen SR, Nishimura RA. Left ventricular outflow tract gradient variability in hypertrophic cardiomyopathy. Clin Cardiol 2009;32(7):397–402. [DOI] [PMC free article] [PubMed]
- 8.Sorrentino MJ, Marcus RH, Lang RM. Left ventricular outflow tract obstruction as a cause for hypotension and symptoms during dobutamine stress echocardiography. Clin Cardiol 1996;19(3):225–30. [DOI] [PubMed]
- 9.Morewood GH, Weiss SJ. Intra-aortic balloon pump associated with dynamic left ventricular outflow tract obstruction after valve replacement for aortic stenosis. J Am Soc Echocardiogr 2000;13(3):229–31. [DOI] [PubMed]
- 10.Fukuda S, Lever HM, Stewart WJ, Tran H, Song JM, Shin MS, et al. Diagnostic value of left ventricular outflow area in patients with hypertrophic cardiomyopathy: a real-time three-dimensional echocardiographic study. J Am Soc Echocardiogr 2008;21(7):789–95. [DOI] [PubMed]
- 11.Ashidagawa M, Ohara M, Koide Y. An intraoperative diagnosis of dynamic left ventricular outflow tract obstruction using transesophageal echocardiography leads to the treatment with intravenous disopyramide. Anesth Analg 2002;94(2):310–2. [DOI] [PubMed]