The tragedies of life are largely arterial. 1
— Sir William Osler
Acute aortic dissection (AD) is the most frequent and catastrophic manifestation of the so-called acute aortic syndrome (which also includes intramural hematoma, penetrating aortic ulcer, and ruptured thoracic aortic aneurysm).2 The incidence is said to be no less than 30 cases per million individuals per year. In its natural evolution, without treatment, acute type A aortic dissection reportedly has a mortality rate of about 1% per hour initially, with half of the patients expected to be dead by the 3rd day, and almost 80% by the end of the 2nd week.3 Death rates are lower but still significant in acute type B aortic dissection: 10% minimum at 30 days, and 70% or more in the highest-risk groups.4
The typical clinical presentation of acute type B aortic dissection is that of a man in his 60s or 70s who presents at the emergency department with sudden-onset severe or “ripping” chest pain and in obvious acute distress. Hypertension is quite common; however, low blood pressure can also be seen when the acute AD has ruptured. Physical findings may include pulse deficit and blood pressure discrepancies, and perhaps a diastolic heart murmur. Focal neurologic manifestations, including paraplegia or paraparesis, might also be detected. Variability and some inconsistency are the rule and not the exception for many such symptoms: some patients experience few or even no symptoms as acute AD begins and evolves. The diagnosis of AD and the characterization of its type and precise extent have been refined to an exquisite degree by virtue of computed tomography (CT), especially when performed with intravenous contrast administration and 3-dimensional reconstruction that can very clearly depict the entire aorta and its branches. Magnetic resonance imaging is quite useful as well and is expected to become more competitive with CT in the future.
With few exceptions, the management of acute type A aortic dissection continues to be a prime example of life-saving, emergent open-heart surgery. The operation often involves graft replacement of the dissected ascending aorta, with or without aortic valve repair or replacement. In patients presenting with extensive type A aortic dissection, cardiac surgeons have more recently been considering more extensive operations, when possible and appropriate. These may include arch repair, or “arch debranching” with a side-graft bypass to the brachiocephalic and left common carotid arteries to facilitate subsequent endovascular repair of the more distal dissected thoracic aorta.
Treatment approaches for type B aortic dissection are quite different and more varied. Approximately 70% of patients present with uncomplicated dissection.5 They are best managed medically with anti-impulse and antihypertensive pharmacotherapy—especially today, because optimal medical therapy is reportedly yielding an impressively low 30-day mortality rate of 10% or less. On the other hand, patients presenting with complicated dissection are at substantial risk of major sequelae or death and must be considered for surgical or endovascular intervention.
This brief article attempts to review the historical evolution of AD and the current knowledge and available evidence surrounding some of the most important aspects of the disease, including present-day treatment guidelines for acute type B aortic dissection.
The History of Aortic Dissection
“On the 25th of October he rose as usual at six, and drank his chocolate; for all his actions were invariably methodic. A quarter after seven he went into a little closet. His German valet de chamber in waiting heard a noise and, running in, found the King dead on the floor.” Frank Nicholls, who was the King's personal physician, was instructed to open and embalm the royal body. This provided an opportunity for Nicholls to uncover and meticulously document some of the most interesting findings. His description constitutes the very first clear account of the disease that we presently recognize as AD: “… the pericardium was found distended with a quantity of coagulated blood, nearly a pint …; the whole heart was so compressed as to prevent any blood contained in the veins from being forced into the auricles; therefore the ventricles were found absolutely void of blood …; and in the trunk of the aorta we found a transverse fissure on its inner side, about an inch and a half long, through which some blood had recently passed under its external coat and formed an elevated ecchymosis.”6 George II, King of England, died at Kensington palace “while straining on the toilet.” The year was 1760, and the King had sustained fatal cardiac tamponade caused by an acute type A aortic dissection that had ruptured into the pericardial sac.
Nearly 60 years later, in 1819, René Laennec, who had invented the stethoscope and become a medical celebrity in Europe, was apparently the first to use the term “dissecting aneurysm.” Unknown to him and totally unforeseen at the time, this label proved to be counterproductive and, in many ways, a great disservice to the “AD cause.” It contributed, more than anything else, to a state of confusion regarding the nature of AD and thoracic aortic aneurysm that persists to this day. More regrettably still, Maunoir (in 1802) had already proposed the correct term “aortic dissection.” Unfortunately, he could not match Laennec's “star power” and notoriety, so his description and terminology went largely unnoticed for many years to come.7
The next major breakthrough was to take place in Houston more than a century later, on 7 July 1954, when the all-star team of DeBakey, Cooley, and Creech performed the first successful surgical resection of a dissecting thoracic aortic aneurysm.8 DeBakey and his associates went on to accumulate a vast clinical and surgical experience in the management of AD patients, reporting on a 20-year follow-up on 527 surgically treated patients as early as 1980.9 Ironically, Michael DeBakey himself underwent and survived open surgery for type A aortic dissection at the age of 97.10
The contemporary recognition and treatment of AD was ushered in by 2 major developments. The first was the creation of the International Registry of Acute Aortic Dissection (IRAD) in 1996, which proved to be crucially important as the collaboration of 20 international centers of excellence and dedicated clinical investigators in 9 countries produced an astonishing amount of information and solid data. Their contributions on many levels constitute the biggest share of knowledge and understanding that we have gained about AD during the last 15 years.
The second was the 2 May 1999 issue of the New England Journal of Medicine, which contained 2 back-to-back landmark papers,11,12 reporting some of the earliest clinical experiences with endovascular stent-graft intervention for acute type B aortic dissection, including how this treatment might surmount the time-tested open surgical approach. These papers heralded the endovascular era in AD management.
The Cause and Pathogenesis of Aortic Dissection
The aorta is a rather complex organ. Its wall has a 3-layered anatomic configuration. The intima can be described as a metabolically intensive, monolayered endothelial liner that is supported by a fairly loose connective-tissue sublayer. This sublayer permits motion of the intima relative to the media when the aorta expands and contracts during the cardiac cycle. The media is composed of some 50 layers of fenestrated, lamellar elastic fibers. Collagenous fibers and smooth-muscle cells are interposed. Elastin is highly stretchable: this enables its fibers to lengthen 2 to 3 times without rupturing, and permits the aorta to exhibit its impressive distensibility and elasticity. Both characteristics are essential to optimal aortic function. Quite opposite are the collagenous fibers, which have an estimated stiffness 5,000 times that of elastin. Their role is to support aortic integrity and resist shearing forces as flowing blood is pumped powerfully by the left ventricle. Outermost is the adventitia, a tough layer of collagen and connective tissue that also contributes substantially to aortic integrity. The vasa vasorum within this adventitial layer provide nutritional circulation to a thick vascular wall that cannot rely solely on the diffusion of nutrients from the flowing blood in the lumen.
It is widely accepted that AD occurs when an intimomedial tear, or entry tear, allows blood flow to enter the aortic wall, thereby creating a new secondary channel: the false lumen (FL). The FL propagates distally in a spiraled (most often) or straight manner. The FL can also propagate proximally all the way to the aortic valve. Not infrequently, the true lumen (TL) becomes compressed by the pressurized FL, sometimes to the point of collapse that can lead to ischemic complications below (malperfusion). Whereas the proximal thoracic aorta is almost always the site of the entry tear, secondary or re-entry tears (fenestrations) can occur either distally in the thoracic aorta or in the abdominal aorta or iliac arteries. Why and how all this occurs is somewhat mysterious and incompletely understood. However, a diseased or weakened vessel wall is a probable prerequisite, rendering the aorta vulnerable when exposed to the tremendous burden of severe or uncontrolled hypertension. The well-documented increased risk of AD in several inherited aortic diseases (such as Marfan syndrome) supports this assumption.
Data from the IRAD4 have identified several well-defined risk factors for the development of acute AD: male sex, age in the 60s and 70s, hypertension, prior cardiac surgery (particularly aortic valve repair), bicuspid aortic valve, and a history of Marfan syndrome. Less than 10% of the time, acute AD occurs in individuals younger than age 40: they are often normotensive, but they typically have a history of cardiac surgery or a bicuspid aortic valve, Marfan syndrome, Ehlers-Danlos syndrome, or similar conditions.
Probably belonging in the same disease spectrum are intramural hematoma and penetrating aortic ulcer, which often present with symptoms similar to those of acute AD. They may be linked through a common pathogenesis.
Intramural hematoma originates from a hemorrhage within the wall of the aorta, but without a demonstrable intimomedial tear or flap. Many experts think of it as a precursor of AD. In fact, intramural hematoma evolves into full AD (with a double-barrel aorta) in nearly 20% of cases. Two thirds of intramural hematomas involve the descending aorta (rather than the ascending aorta). The converse is true in AD. Of note, the overall prognosis and 25% risk of death at 1 year are about the same for both intramural hematoma and AD.13
Penetrating aortic ulcers can form anywhere along the aorta; however, most develop in the descending thoracic portion. Patients tend to be elderly and show evidence of significant atherosclerosis. The ulcer can precede AD and be associated with intramural hematoma. The concomitant occurrence of penetrating aortic ulcer and intramural hematoma is dangerous and may warrant early intervention and repair. Penetrating aortic ulcers behave unpredictably and can lead to rupture and catastrophic hemorrhage. Thoracic endovascular aortic repair (TEVAR) is rapidly emerging as a feasible treatment option, because most ulcers develop in areas that are anatomically suitable for endovascular repair and endografting. It is increasingly agreed that intervention is justified and should be pursued (if reasonable and feasible) for penetrating aortic ulcers larger than 3 cm in diameter, as well as for all symptomatic aortic ulcers of any size.14
Clinical Classification
Acute dissection is diagnosed when the clinical symptoms have lasted 14 days or less. Beyond the 2nd week, the dissection is classified as chronic. It is difficult to explain the origin of these largely arbitrary, inaccurate definitions. Before effective therapy became available, perhaps patients still alive beyond the 2nd week were considered to be “chronic survivors.” Obviously, it would be desirable to revise these definitions to conform with present-day knowledge and needs. Even more valuable would be the development of a clinical classification that would serve as a helpful guide to therapy—TEVAR in particular. The elasticity and mobility of the dissected septum (lamella) tend to decrease over time through a process of fibrosis and gradually increasing stiffness. Acute and chronic AD differ substantially in this regard. The impact on the outcome of endograft repair can be enormous, because a freely mobile and flexible septum may enable complete or near-complete re-expansion of the TL all the way to the outer wall, thereby obliterating the FL. To the contrary, a compressed TL may re-expand little or not at all in the presence of a stiff or immobile septum (as seen in chronic AD) and lead to unsatisfactory outcomes because of partial FL thrombosis and failure of the dissected aorta to remodel. Distinguishing acute from chronic AD in terms of septal mobility and stiffness is therefore crucial when considering TEVAR intervention. When doubt arises as to the acute or chronic nature of a case, septal mobility can be evaluated by means of intravascular ultrasound or transesophageal echocardiography. Dynamic CT or dynamic magnetic resonance imaging can also provide diagnostic information in this regard.
Anatomic Classification
The extent of the dissection process along the aorta defines the type. The DeBakey classification was the first to be proposed, in 1965.15 Three main types were recognized: types I and II affect the ascending aorta; type III, distal dissection, begins distal to the left subclavian artery, sparing the proximal arch and ascending aorta. DeBakey's prescience in distinguishing types IIIa (down to or ending above the visceral segment) and IIIb (extending downward to involve the abdominal aorta and iliac arteries) has proved to be extremely valuable in the 21st century because of the substantial impact on the prognosis and long-term results after TEVAR. The simpler and more recent Stanford Classification16 has also become well established, especially outside the cardiothoracic surgical community. It describes only 2 types of AD: type A, which signifies involvement of the ascending aorta; and type B, in which the ascending aorta is not affected. Stanford type A is equivalent to DeBakey types I and II, and Stanford type B is equivalent to DeBakey types IIIa and IIIb. Approximately two thirds of cases of acute AD are type A, and the rest are type B.
Type B Aortic Dissection: Complicated versus Uncomplicated
Complicated dissection refers to evidence of thoracic aortic rupture (blood outside the aortic wall), malperfusion (ischemia that involves the viscera, kidneys, spinal cord, or lower extremities), or rapid expansion in the distal arch or proximal descending aorta to a total aortic diameter of 4.5 cm or greater. These findings constitute a clinical imperative for intervention, because they immediately threaten life or limb.
Approximately 30% of patients who present with acute type B aortic dissection have a complicated dissection. Malperfusion is perhaps one of the most intriguing and unique complications of acute AD, especially when it affects the visceral and renal vascular beds. The classic descriptions of the alleged pathogenesis of aortic-branch closure included static mechanisms (branch-vessel compression by the pressurized FL) and dynamic mechanisms (protrusion of a dissection flap into the branch-vessel origin). Various remedial techniques and approaches were developed, such as surgical and endovascular fenestrations. Today, we know that malperfusion is largely the result of severe proximal compression or collapse of the TL in the chest by the pressurized and bulging FL. This understanding has enabled the present-day treatment of most patients with the relatively simpler, seemingly more effective approach of relining the TL in the proximal (and mid) descending aorta with a stent-graft to obliterate the entry site and redirect all blood flow down the TL exclusively. Another result of such new understanding and modern therapy has been the greatly diminished role of direct branch-vessel revascularization (stenting), and the use of fenestrations only rarely.
In addition to the unequivocal and crucial diagnostic components of complicated dissection, it is unfortunately not unusual to see or hear mentions of other findings and softer criteria that only doubtfully justify intervention. These include unrelenting pain, uncontrolled hypertension, extension of the dissection, and image worsening. Most or all of the 70% of patients who present with uncomplicated dissection should be treated medically, in adherence with currently available scientific evidence. Modern anti-impulse and antihypertensive pharmacologic therapy produces very satisfactory results in the acute stage, with an expected 30-day mortality rate of 10% or less at present. However, subsequent clinical follow-up and serial aortic imaging over time are crucial, because AD patients are exposed to long-term, life-threatening risks—including the formation of dissecting thoracic aneurysms in 20% to 30% of such patients.
Treating Acute Type B Aortic Dissection: Current Trends and Emerging Evidence
The management of acute type B aortic dissection is quite varied at present. Although substantial progress has been made over the last several years, many important issues remain unclear or controversial. A concise summary of the overall picture as of mid-2011 is as follows17:
Open surgical treatment of the acutely dissected descending aorta achieves suboptimal results because of continuing major morbidity associated with it, and has a 30-day operative mortality rate in excess of 25% overall
Clinical outcomes and mortality rates of optimal medical therapy for uncomplicated acute type B aortic dissection have improved considerably in the recent past: the in-hospital mortality rate with optimal medical care is now less than 10%18
TEVAR intervention has added an entirely new dimension to the management of AD and is now emerging as a promising and probably preferable approach for patients who present with interventional imperatives and unstable situations. However, TEVAR intervention for uncomplicated AD is not supported by currently available evidence.
The ascent of TEVAR as the interventional approach of choice for most patients with complicated dissections has been relatively rapid since the initial reports were published in 1999.11,12 It is likely that TEVAR will supplant open surgical treatment in the near future. However, pronouncements of a total paradigm shift are somewhat premature and should be tempered by the notion that we still lack level-1 evidence in support of TEVAR for this clinical indication. Information on late outcomes is scant, at best.
A current area of intense focus pertains to whether early TEVAR repair may not be beneficial and best for most patients, whether or not they present with overt complications. Intervening early would undoubtedly produce high rates of TL re-expansion and FL thrombosis, and would promote rapid and complete aortic remodeling in many cases. On the other hand, permitting an extensive type IIIb acute AD to evolve into a chronic condition often results in difficult or impossible repairs, regardless of whether an open surgical or endovascular approach is used. The Investigation of Stent Grafts in Aortic Dissection (INSTEAD) trial failed to provide clarification, because the study focused on chronic-AD patients.19 The ongoing ADSORB randomized trial20 might provide useful data, but these will not be available for a few years. In the meantime, some TEVAR and AD experts are beginning to suggest that a high-risk group exists among patients with uncomplicated dissections, and that this group may do poorly on medical therapy alone. This realization would be helpful at the time of selecting patients for early intervention and thoracic repair even in the absence of overt complications. The 3 most important findings would appear to be a FL diameter greater than 22 mm, a large entry tear, and a severely compressed TL in the chest. A partially thrombosed thoracic FL may also fall into the same category.21,22
Unresolved Issues Related to TEVAR in Type B Dissection
Unresolved issues surrounding TEVAR in acute type B aortic dissection include:
Timing of Intervention in the Absence of an Urgent Indication Such as Critical Malperfusion or Rupture. Traditional surgical teaching endorsed waiting for a certain period of time in order to allow the dissected septum (flap) to “mature” so that it would hold sutures better. This precept does not apply to TEVAR, and no evidence of any kind supports a waiting period before the undertaking of stent-graft repair. In fact, there are very good reasons to wait no longer than 1 to 2 days, in general—a period reasonable and necessary to stabilize the patient and complete the evaluation. Deaths from acute type B aortic dissection are most likely to occur within the first 7 to 10 days,20 so it does not make sense to postpone repair for 2 to 3 weeks.
Extent of Endograft Coverage and Relining of the Thoracic True Lumen. Extent of coverage is also a rather important issue, and one in which disagreements and uncertainties persist. It now seems clearer that the pendulum is swinging in the direction of more coverage, not less: half to two thirds the length of the descending thoracic aorta is thought to be appropriate, in most cases. Covering the entry site only with a short endograft is conceptually sound but often unrealistic, given the frequent occurrence of multiple entries and re-entries and the desirability of achieving prompt, complete thrombosis of the FL above the diaphragm. Furthermore, when rupture is the indication for TEVAR, complete or nearly complete coverage of the entire descending thoracic aorta is unequivocally required. Concern about excessive coverage is justified, because no one wants to compromise the critical blood supply to the spinal cord. In the setting of AD, however, the risk of TEVAR-related paraplegia is quite low.23
Limitations of TEVAR
Although it is true that stent-graft repair is an important advance in the treatment of AD,24 current systems and techniques continue to be challenged by several limitations and shortcomings. The following deserve emphasis:
Most if not all devices in current use were primarily tested and designed for the treatment of aortic aneurysms, a disease quite distinct from AD. Dissection-specific endografts must and will be developed in the foreseeable future.
Many current devices involve a proximal bare (uncovered) stent. This configuration has been associated with complications and has become the focus of some concern, particularly in the treatment of acute AD. Retrograde type A aortic dissection, which occurs mostly after procedures performed for treatment of acute AD, continues to be mentioned as a risk of the use of endografts with proximal bare stents.25 The results of such a complication can be devastating, and the outcome is often fatal. However, the same complication has reportedly occurred after the implantation of devices of all types—with and without a proximal bare stent. The only truly predictable risk factor found so far is acute AD itself when TEVAR is performed to treat the condition, as opposed to situations in which TEVAR is used for aneurysms or other lesions.26 Future thoracic-device designs for treatment of acute AD will most likely not feature uncovered proximal stents. In the meantime, preoperative planning and procedural techniques might substantially minimize the risk of retrograde type A aortic dissection: not excessively oversizing the stent-graft diameter (up to 2 mm only, or not at all); avoiding post-ballooning after endograft deployment; and targeting an aortic segment that is healthy and intact for proximal endograft fixation—well above the dissection process. Patients with Marfan syndrome and other connective-tissue disorders may also be at increased risk of retrograde AD.26
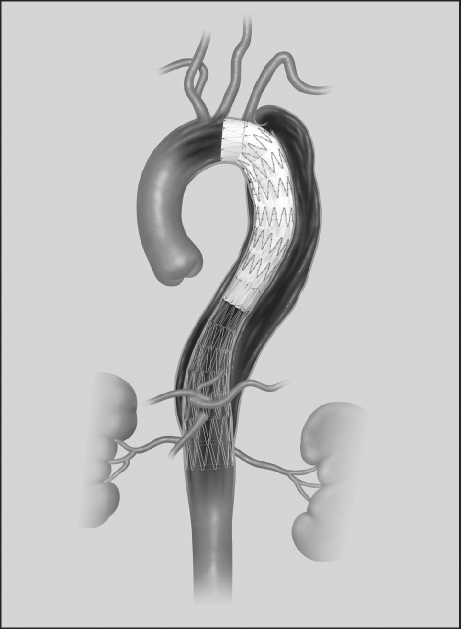
Type IIIb dissections present a real problem, because continued flow in and from the FL in the abdominal segment is typically to be expected after endograft relining of the TL in the chest. This is an important limitation of TEVAR, because extensive distal dissections are the rule rather than the exception. An intriguing concept27 is that of an endograft system with a series of uncovered metal stents that can be used to extend the proximal thoracic stent-graft repair, and TL relining that can encompass much or all of the dissection. Cook Medical, Inc. (Indianapolis, Ind) has developed and tested such a design, and the device was recently approved for European commercialization (Fig. 1). The company is pursuing regulatory approval in the United States. The concept holds promise and has been well received in the endovascular community. Early results are encouraging,28 but proof of efficacy must be established.
Fig. 1 A device for the treatment of type B aortic dissection, consisting of a proximal standard stent-graft extended distally with a series of self-expanding, uncovered metal stents. (Photo courtesy of Cook Medical, Inc.; Indianapolis, Ind)
Summary
Two hundred fifty years have passed since Frank Nicholls' history-making, accurate observations on the anatomic findings and cause of death of King George II were published.6 Several decades later, the disease was named, using—for the first time—the terms dissection and dissecting attached to an aortic disease process. Another century went by before effective surgical treatment was developed. In sharp contrast, the evolution of the last 20 years has been nothing short of amazing. Our understanding of AD, while not yet complete, has improved dramatically. In addition, the introduction of nonsurgical endovascular therapy has had a profoundly transformative impact—and we are just at the beginning! It would not be unreasonable to predict that stent-graft repair will likely replace (or nearly replace) open surgery in the treatment of complicated type B dissection in the near future,29,30 especially as technologies continue to improve and indication-specific designs are developed and tested in the clinical setting. Moreover, it is predictable that endovascular solutions for some patients with type A aortic dissection will become available in the years to come as surgical results continue to be suboptimal.31 Finally, and amidst this plethora of “good news,” it is appropriate to reflect on the formidable challenge that endovascular therapies face as they gear to “compete” with optimal medical therapy in the management of patients with acute uncomplicated type B dissection, because it will obviously be difficult (if not impossible) to improve on the already-achieved 30-day mortality rate of less than 10%. Long-term gains may well become the winning card when and if the late results of TEVAR can be shown to improve on the rather compromised outlook of medically treated dissection patients. Stay tuned.
Footnotes
Address for reprints: Frank J. Criado, MD, FACS, 3333 N. Calvert St., Suite 570, Baltimore, MD 21218
E-mail: frank.criado@medstar.net
★ CME Credit
Presented at the 8th Current Trends in Aortic and Cardiothoracic Surgery Conference; Houston, 29–30 April 2011.
References
- 1.Silverman ME, Murray TJ, Bryan CS, editors. The quotable Osler. Deluxe ed. Philadelphia: American College of Physicians; 2008.
- 2.Vilacosta I, Ramon JA. Acute aortic syndrome. Heart 2001; 85(4):365–8. [DOI] [PMC free article] [PubMed]
- 3.Coady MA, Rizzo JA, Goldstein LJ, Elefteriades JA. Natural history, pathogenesis, and etiology of thoracic aortic aneurysms and dissections. Cardiol Clin 1999;17(4):615–35; vii. [DOI] [PubMed]
- 4.Hagan PG, Nienaber CA, Isselbacher EM, Bruckman D, Karavite DJ, Russman PL, et al. The International Registry of Acute Aortic Dissection (IRAD): new insights into an old disease. JAMA 2000;283(7):897–903. [DOI] [PubMed]
- 5.Suzuki T, Mehta RH, Ince H, Nagai R, Sakomura Y, Weber F, et al. Clinical profiles and outcomes of acute type B aortic dissection in the current era: lessons from the International Registry of Aortic Dissection (IRAD). Circulation 2003;108 Suppl 1:II312–7. [DOI] [PubMed]
- 6.Nicholls F. Observations concerning the body of His Late Majesty, October 26, 1760. Phil Trans 1761;52:265–75.
- 7.Leonard JC. Thomas Bevill Peacock and the early history of dissecting aneurysm. Br Med J 1979;2(6184):260–2. [DOI] [PMC free article] [PubMed]
- 8.DeBakey ME, Cooley DA, Creech O Jr. Surgical considerations of dissecting aneurysm of the aorta. Ann Surg 1955;142 (4):586–612. [DOI] [PMC free article] [PubMed]
- 9.DeBakey ME, McCollum CH, Crawford ES, Morris GC Jr, Howell J, Noon GP, Lawrie G. Dissection and dissecting aneurysms of the aorta: twenty-year follow-up of five hundred twenty-seven patients treated surgically. Surgery 1982;92(6): 1118–34. [PubMed]
- 10.Altman LK. The man on the table devised the surgery. The New York Times; 2006 Dec 25. Available from: http://www.nytimes.com/2006/12/25/health/25surgeon.html?pagewanted=all.
- 11.Nienaber CA, Fattori R, Lund G, Dieckmann C, Wolf W, von Kodolitsch Y, et al. Nonsurgical reconstruction of thoracic aortic dissection by stent-graft placement. N Engl J Med 1999;340(20):1539–45. [DOI] [PubMed]
- 12.Dake MD, Kato N, Mitchell RS, Semba CP, Razavi MK, Shimono T, et al. Endovascular stent-graft placement for the treatment of acute aortic dissection. N Engl J Med 1999;340 (20):1546–52. [DOI] [PubMed]
- 13.Evangelista A, Mukherjee D, Mehta RH, O'Gara PT, Fattori R, Cooper JV, et al. Acute intramural hematoma of the aorta: a mystery in evolution. Circulation 2005;111(8):1063–70. [DOI] [PubMed]
- 14.Botta L, Buttazzi K, Russo V, Parlapiano M, Gostoli V, Di Bartolomeo R, Fattori R. Endovascular repair for penetrating atherosclerotic ulcers of the descending thoracic aorta: early and mid-term results. Ann Thorac Surg 2008;85(3):987–92. [DOI] [PubMed]
- 15.DeBakey ME, Henly WS, Cooley DA, Morris GC Jr, Crawford ES, Beall AC Jr. Surgical management of dissecting aneurysms of the aorta. J Thorac Cardiovasc Surg 1965;49:130–49. [PubMed]
- 16.Daily PO, Trueblood HW, Stinson EB, Wuerflein RD, Shumway NE. Management of acute aortic dissections. Ann Thorac Surg 1970;10(3):237–47. [DOI] [PubMed]
- 17.Fattori R, Tsai TT, Myrmel T, Evangelista A, Cooper JV, Trimarchi S, et al. Complicated acute type B dissection: is surgery still the best option? A report from the International Registry of Acute Aortic Dissection. JACC Cardiovasc Interv 2008;1(4):395–402. [DOI] [PubMed]
- 18.Estrera AL, Miller CC, Goodrick J, Porat EE, Achouh PE, Dhareshwar J, et al. Update on outcomes of acute type B aortic dissection. Ann Thorac Surg 2007;83(2):S842–5. [DOI] [PubMed]
- 19.Nienaber CA, Rousseau H, Eggebrecht H, Kische S, Fattori R, Rehders TC, et al. Randomized comparison of strategies for type B aortic dissection: the INvestigation of STEnt Grafts in Aortic Dissection (INSTEAD) trial. Circulation 2009;120(25):2519–28. [DOI] [PubMed]
- 20.Tang DG, Dake MD. TEVAR for acute uncomplicated aortic dissection: immediate repair versus medical therapy. Semin Vasc Surg 2009;22(3):145–51. [DOI] [PubMed]
- 21.Song JM, Kim SD, Kim JH, Kim MJ, Kang DH, Seo JB, et al. Long-term predictors of descending aorta aneurysmal change in patients with aortic dissection. J Am Coll Cardiol 2007;50(8):799–804. [DOI] [PubMed]
- 22.Tsai TT, Evangelista A, Nienaber CA, Myrmel T, Meinhardt G, Cooper JV, et al. Partial thrombosis of the false lumen in patients with acute type B aortic dissection. N Engl J Med 2007;357(4):349–59. [DOI] [PubMed]
- 23.Szeto WY, McGarvey M, Pochettino A, Moser GW, Hoboken A, Cornelius K, et al. Results of a new surgical paradigm: endovascular repair for acute complicated type B aortic dissection. Ann Thorac Surg 2008;86(1):87–94. [DOI] [PubMed]
- 24.Sachs T, Pomposelli F, Hagberg R, Hamdan A, Wyers M, Giles K, Schermerhorn M. Open and endovascular repair of type B aortic dissection in the Nationwide Inpatient Sample. J Vasc Surg 2010;52(4):860–6. [DOI] [PubMed]
- 25.Eggebrecht H, Thompson M, Rousseau H, Czerny M, Lonn L, Mehta RH, et al. Retrograde ascending aortic dissection during or after thoracic aortic stent graft placement: insight from the European registry on endovascular aortic repair complications. Circulation 2009;120(11 Suppl):S276–81. [DOI] [PubMed]
- 26.Dong ZH, Fu WG, Wang YQ, Guo da Q, Xu X, Ji Y, et al. Retrograde type A aortic dissection after endovascular stent graft placement for treatment of type B dissection. Circulation 2009;119(5):735–41. [DOI] [PubMed]
- 27.Nienaber CA, Kische S, Zeller T, Rehders TC, Schneider H, Lorenzen B, et al. Provisional extension to induce complete attachment after stent-graft placement in type B aortic dissection: the PETTICOAT concept. J Endovasc Ther 2006;13 (6):738–46. [DOI] [PubMed]
- 28.Lombardi JV, Nienaber CA, Cambria R, Chiesa R, Teekben O, Lee A, et al. SS18. Endovascular treatment of complicated type B aortic dissection using a composite device design: initial results of a prospective multicenter clinical trial (STABLE) [abstract]. J Vasc Surg 2011;53(6 Suppl):25S. [DOI] [PubMed]
- 29.Steingruber IE, Chemelli A, Glodny B, Hugl B, Bonatti J, Hiemetzbeger R, et al. Endovascular repair of acute type B aortic dissection: midterm results. J Endovasc Ther 2008;15 (2):150–60. [DOI] [PubMed]
- 30.Sayer D, Bratby M, Brooks M, Loftus I, Morgan R, Thompson M. Aortic morphology following endovascular repair of acute and chronic type B aortic dissection: implications for management. Eur J Vasc Endovasc Surg 2008;36(5):522–9. [DOI] [PubMed]
- 31.Trimarchi S, Nienaber CA, Rampoldi V, Myrmel T, Suzuki T, Mehta RH, et al. Contemporary results of surgery in acute type A aortic dissection: the International Registry of Acute Aortic Dissection experience. J Thorac Cardiovasc Surg 2005; 129(1):112–22. [DOI] [PubMed]